Introduction
Senile dementia is a multifactorial syndrome
characterized by cognitive functional disorders and cognitive
decline (1). Alzheimer's disease
is among the most common pathogeneses leading to senile dementia,
with a highest incidence rate among the elderly (2,3).
Alzheimer's disease is a progressive neurodegenerative syndrome
characterized by the presence of neurodegenerative disorders
(4). Alzheimer's disease primarily
impairs hippocampus-associated memory and cognition, and leads to
cognitive functional disorders and impairments in cognition,
including memory, language or attention (5,6).
Previous studies have suggested that Alzheimer's may directly
affect hippocampal cells (7–9). The
association between corticocerebral and hippocampal neuronal
apoptosis has been demonstrated in a rat model of Alzheimer's
disease and in a clinical study (10,11).
Dysfunction and aberrant apoptosis of hippocampalcells has been
observed in preclinical and clinical studies (12). Inhibition of neuronal apoptosis may
attenuate the phosphorylation of microtubule-associated protein tau
(MAPT) and protect memory, which may be beneficial for the
treatment of Alzheimer's disease (13,14).
Absalon et al (15)
suggested that the activation of cell cycle entry,
MAPT-phosphorylation and the inhibition of apoptosis in
post-mitotic neurons through microRNA-regulated gene expression may
contribute to the recovery of mice with Alzheimer's disease. These
findings suggested that the apoptosis of hippocampal cells is
associated with the progression of cognitive impairment, and thus
with the onset and development of Alzheimer's disease.
The apoptosis of hippocampal cells is a diagnostic
criterion for Alzheimer's disease (16,17).
Decreased apoptosis in the hippocampus signifies an improvement in
cognitive impairment. Previous studies have supported the
hypothesis that inflammatory responses are the most important
pathogenic factors for the initiation and progression of
Alzheimer's disease (18,19). Although Alzheimer's disease has
been extensively studied, comprehensive assessments have not been
performed in an animal model. The majority of research in animal
models of Alzheimer's disease has focused on the neuroprotective
effects of anti-dementia drugs against inflammation, apoptosis and
neuronal excitability (20).
Oxidative stress has been associated with Alzheimer's disease and
the development of neurodegenerative processes (21). Therefore, the development of
pharmacological agents targeting apoptosis, inflammation and
oxidative stress represents a novel prospect for the treatment of
Alzheimer's disease.
Nicergoline is a derivative of ergot produced by
semisynthesis that exhibits the potential to selectively block α-1A
adrenergic receptors in the brain and periphery (22). Nicergoline may improve a number of
aging-associated diseases, including dysphagia, ischemia and
dizziness (23–25). Nicergoline is beneficial for the
treatment of cognitive impairment (26–28).
At present, nicergolineis a registered drug in >50 countries
and, for the last 30 years, it has been used for the treatment of
cognitive impairment and behavioral disorders in the elderly.
Therefore, nicergoline may contribute to the treatment of
Alzheimer's disease.
In the present study, it was hypothesized that
nicergoline may be able to protect neurons against apoptosis by
controlling the expression of apoptosis-associated genes. The
efficacy of nicergoline in the prevention of cognitive impairment
was evaluated in a rat model of Alzheimer's disease and determined
by the assessment of the learning memory. The molecular mechanism
of nicergoline-mediated improvement in hippocampal cells was
studied. Although nicergoline demonstrated neuroprotective effects
via stimulation of the phosphatidylinositol 3-kinase (PI3K)/RAC-α
serine/threonine-protein kinase (AKT) signaling pathway, further
analysis of larger volumes of preclinical data is required to
understand the potential efficacy and tolerability.
Materials and methods
Ethical approval
The present study was approved by the Ethics
Committee of Zhejiang University (Hangzhou, China). All surgery and
euthanasia of experimental mice was performed under sodium
pentobarbital anesthesia to minimize suffering.
Cells and reagents
Hippocampal cells were isolated from 3×Tg-AD mice
and cultured in 1640 medium (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) supplemented with 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Hippocampal cells were
treated with PI3K inhibitor LY294002 (2 mg/ml) and cultured in for
12 h a 37°C humidified atmosphere with 5% CO2.
Animal study
A total of twenty 6-week-old 3×Tg-AD mice (male,
28–35 g) with the Alzheimer's disease were purchased from Vital
River Laboratory Animal Technology Co., Ltd. (Shanghai, China). All
animals were housed in a temperature-controlled facility at 23±1°C
and relative humidity of 50±5% with a 12-h light/dark cycle with
free access to food and water. The mice were divided into two group
(n=10 in each group) and given intravenous injections of
nicergoline (10 mg/kg body weight) or PBS (10 mg/kg body weight;
both from Sigma-Aldrich; Merck KGaA) once a day. The treatment
lasted 60 days.
Histological and immunohistochemical
analysis
Hippocampal tissues were isolated from the CA1
regions of experimental mice following a 60-day treatment with
nicergoline (10 mg/kg body weight) or PBS. The brains were frozen
in liquid nitrogen and following perfusion, fixation and
cryoprotection, coronal sections were cut to 4 µm in a cryostat.
After blocking with 5% Bull Serum Albumin (Sigma-Aldrich; Merck
KGaA) for 1 h at 37°C, free-floating sections were rinsed with PBS
and placed in a solution with goat anti-mouse amyloid β (Aβ)-42
(1:1,000; cat. no. ab201060; Abcam, Cambridge, UK), Aβ-40 (1:1,000;
cat. no. ab17295; Abcam) and amyloid precursor protein (APP;
1:1,000; cat. no. ab32136; Abcam) for 12 h at 4°C. Following
incubation the sections were washed and incubated with a secondary
rabbit anti-goat antibody conjugated with horseradish peroxidase
(1:500; cat. no. A-11034; Chemicon International; Thermo Fisher
Scientific, Inc.) for Aβ-42, Aβ-40 and APP staining for 1 h at
37°C. The sections were washed and observed using a fluorescent
video microscope (BZ-9000; Keyence Corporation, Osaka, Japan) at
magnification ×40. Immunohistochemical staining was used to examine
the abundance of neuroprotection-associated proteins in the
hippocampus. Immunohistochemical procedures were performed as
previously described (29).
Immunofluorescence
Hippocampal CA1 regions were isolated from
experimental mice following treatment with nicergoline. Hippocampal
neural cells were fixed in situ with bromodeoxyuridine and
NeuN for 24 h at 4°C. The impairment of neurogenesis was analyzed
under a fluorescence microscope (Olympus BX61; Olympus Corporation,
Tokyo, Japan) at magnification 40×.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
ATUNEL assay was performed to analyze apoptotic
neurons in the hippocampus as previously described (30). Brain sections were viewed under
alight microscope and of apoptotic morphology was labeled in
sections. Magnification, ×40.
ELISA analysis
ELISA kits [Huiying Chemical Industry (Xiamen) Co.,
Ltd., Shanghai, China] were used to determine interleukin (IL)-1
(cat. no. MLB00C; Bio-Rad Laboratories, Inc., Hercules, CA, USA),
tumor necrosis factor (TNF)-α (cat. no. MTA00B; Bio-Rad
Laboratories, Inc.) and IL-6 (cat. no. M6000B; Bio-Rad
Laboratories, Inc.) serum levels in mice. The procedures were
performed according to the manufacturer's protocol. The final
results were recorded at a wavelength of 450 nm.
Flow cytometry
The apoptosis of hippocampal neural cells was
evaluated using an Annexin V-fluorescein isothiocyanate (FITC)
Apoptosis Staining/Detection kit (cat. no. ab14085; Abcam,
Cambridge, UK) and a propidium iodide (PI) apoptosis detection kit
(BD Biosciences, Franklin Lakes, NJ, USA). Hippocampal neural cells
were isolated from experimental mice and suspended with Annexin
V-FITC and PI, according to the manufacturer's protocols.
Fluorescence was measured via fluorescence-activated cell sorting
using a flow cytometer (BD Biosciences). Cell apoptosis was
analyzed using Expo32-ADC v. 1.2B software (Beckman Coulter, Inc.,
Brea, CA, USA).
Fluorescent reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from experimental mouse
hippocampal are as using RNA Easy Mini Extract kit (Sigma-Aldrich;
Merck KGaA) and the RNA served as a template for cDNA synthesis by
RT reaction using a PrimeScript RT Master mix kit (Takara
Biotechnology Co., Ltd., Dalian, China). A total of one-tenth of
the cDNA was used in the fluorescent RT-qPCR reaction using iQ
SYBR-Green (Invitrogen; Thermo Fisher Scientific, Inc.). The PCR
conditions included an initial denaturation step of 94°C for 2 min,
followed by 30 cycles of 94°C for 30 sec, 59°C for 30 sec, 72°C for
2 min and a final elongation step at 72°C for 10 min. All forward
and reverse primers were summarized in Table I. Relative mRNA expression was
calculated by the 2−ΔΔCq calculation (31). Results were presented as a fold
difference relative to the housekeeping β-actin control gene.
 | Table I.Sequences of primers used in this
study. |
Table I.
Sequences of primers used in this
study.
|
| Sequence |
|---|
|
|
|
|---|
| Gene name | Reverse | Forward |
|---|
| P53 |
5′-TTAAGCTTTTTGCGTTCGGGCTGGGAGC-3′ |
5′-ATGGTGGCATGAACCTGTGG-3′ |
| Bcl-2 |
5′-CGTCATAACTAAAGACACCCC-3′ |
5′-TTCATCTCCAGTATCCGACTC-3′ |
| Caspase-3 |
5′-ATGGAGAACAACAAAACCTCAGT-3′ |
5′-TTGCTCCCATGTATGGTCTTTAC-3′ |
| Caspase-9 |
5′-GCCCTTGCCTCTGAGTAGTG-3′ |
5′-CCAACCAAATGAAGCCAAGT-3′ |
| Bax |
5′-CACCAATCACCTGCGGTACA-3′ |
5′-CAGATCACGTCATCGCAC-3′ |
| Bid |
5′-CGACGAGGTGAAGACATCCT-3′ |
3′-AGCAGAGATGGTGCATGACT-3′ |
| β-actin |
5′-CCTTCCTGGGCATGGAGTCCT-3′ |
5′-GGAGCAATGATCTTGATCTTC-3′ |
Western blotting
Hippocampal cells were homogenized in
radioimmunoprecipitation assay lysis buffer containing protease
inhibitor (Sigma-Aldrich; Merck KGaA) and centrifuged at 6,000 × g
at 4°C for 10 min. The supernatant was used for the analysis of
target proteins using a protein extraction kit (Qiagen Sciences,
Inc., Gaithersburg, MD, USA), according to manufacturer's protocol.
The protein concentration was measured in samples by a
bicinchoninic acid protein assay kit (Thermo Fisher Scientific,
Inc.). Protein samples (10 µg) were separated on 12.5% SDS-PAGE
gels and transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). SDS-PAGE was performed as
previously described (32). For
western blotting, primary antibodies: IL-1β (1:400; cat. no.
ab200478), IL-6, TNF-α (1:500; cat. no. ab1793), Foxp2 (1:400; cat.
no. ab16046), Src homology 2-containing inositol phosphatase (SxIP;
1:400; cat. no. ab45142), EB (1:400; cat. no. ab157217), P53
(1:400; cat. no. ab26), Bcl-2 (1:400; cat. no. ab32124), caspase-3
(1:500; cat. no. ab217), caspase-9 (1:500; cat. no. ab52298), Bid
(1:400; cat. no. ab62469), Bax (1:400; cat. no. ab32124),
Neprilysin (1:500; cat. no. ab79423), insulin (1:500; cat. no.
ab32216), insulin-like growth factor-binding protein 3 (IGFBP-3;
1:500; cat. no. ab75988), vascular endothelial growth factor β
(VEGF-β; 1:500; cat. no. ab53463), superoxide dismutase (SOD;
1:500; cat. no. ab13533), catalase (CAT; 1:500; cat. no. ab16731),
glutathione (GSH; 1:500; cat. no. ab26255), PI3K (1:1,000; cat. no.
ab182651; Thermo Fisher Scientific, Inc.), AKT (1:1,000; cat. no.
ab8933; Thermo Fisher Scientific, Inc.), pAKT (1:1,000; cat. no.
ab18260; Thermo Fisher Scientific, Inc.), ROS (1:1,000; cat. no.
PA5-14732; Thermo Fisher Scientific, Inc.) and β-actin (1:2,000,
cat. no. ab8226) were added following blocking with 5% skimmed milk
for 1 h at 37°C, then the membranes incubated with primary
antibodies overnight at 4°C and then incubated with secondary
antibodies (1:5,000; PV-6001; OriGene Technologies, Inc.,
Rockville, MD, USA)for 1 h at 37°C. Finally, protein bands were
visualized using WesternBright Enhanced Chemiluminescent substrate
(Advansta, Inc., Menlo Park, CA, USA).
Cognitive and behavioral tests
The cognitive competence of mice was determined by
measuring the open field activity levels of mice in black
plexiglass boxes (60×60×25 cm), to analyze the therapeutic effects
of nicergoline. Mice were placed in open black boxes for 10 min and
the behavior of the mice was monitored and evaluated using an
auto-tracking system (EthoVision 8.0; Noldus, Wageningen, the
Netherlands). A Morris water maze test was performed prior to and
post-treatment with nicergoline to measure cognitive function. The
Morris water maze experiment was performed three times in a
circular stainless steel tank (155 cm diameter, 60 cm depth) filled
with water to a depth of 40 cm (27.0±1.0°C) that was made opaque by
the addition of skimmed milk. Mice learned to find a hidden
circular platform (10 cm diameter, 1 cm below the surface of the
water) in a fixed area in one quadrant of the tank.
Thionin staining
Brain sections (30 µm thick) were prepared and
stained with thionin for 2 h at room temperature. Images of stained
tissues were digitally captured with an Olympus IX71 microscope
controlled by DP manager software (Olympus Corporation, Tokyo,
Japan) at a magnification of ×1.5.
Statistical analyses
All data are presented as the mean ± standard error
of the mean. Statistical significance was determined using a
two-tailed Student's t-test with SPSS 19.0 (IBM Corp., Armonk, NY,
USA). One-way analysis of variance followed by Tukey's post hoc
test, Kaplan-Meier analysis and log-rank statistical analysis were
performed using GraphPad software version 5.0 (GraphPad Software,
Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Nicergoline improves impaired
neurogenesis and cognitive competence in mice with Alzheimer's
disease
In order to analyze the efficacy of nicergolinein
the treatment of Alzheimer's disease, mice with Alzheimer's disease
received treatment with nicergoline (10 mg/kg body weight) or were
administered PBS as a control. It was determined that nicergoline
treatment markedly improved neurogenesis and cognitive competence
compared with the control (Fig. 1A and
B). In mice that received treatment with nicergoline, the
degree of dementia was decreased following a 60-day course of
treatment (Fig. 1C). Immunological
staining indicated that pathogenic Aβ-42 and −40 peptides and APP
were downregulated in the hippocampi of experimental mice (Fig. 1D). Levels of the neuroprotective
forkhead box protein P2 (Foxp2), Src homology 2-containing inositol
phosphatase (SxIP) and end-binding proteins (EB) were increased in
hippocampi of nicergoline-treated mice (Fig. 1E). Additionally, marked differences
in the dispersion of the pyramidal cell layer were observed between
the nicergoline-treated and control groups, as determined by
thionin staining (Fig. 1F). These
results suggested that nicergoline exerted beneficial effects on
neurogenesis and cognitive competence in mice with Alzheimer's
disease.
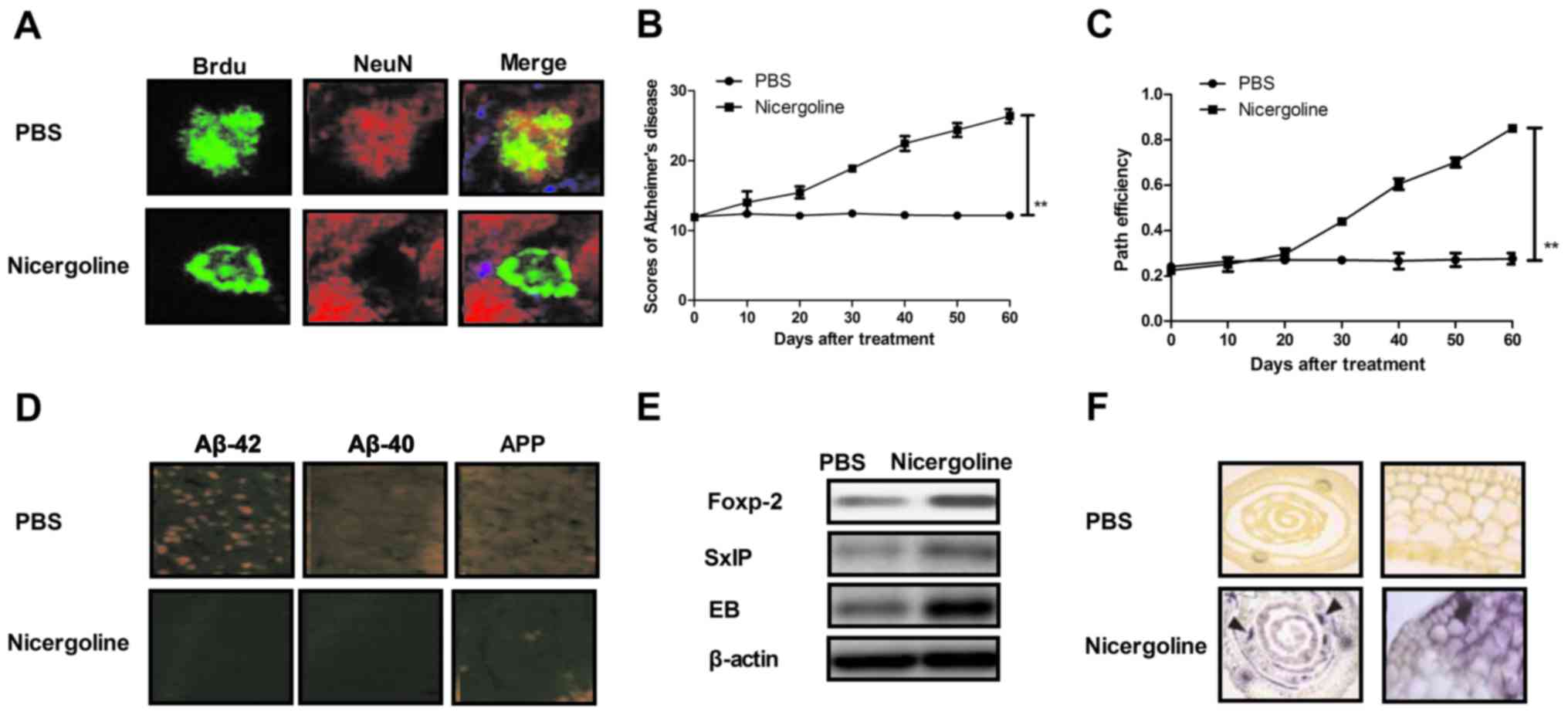 | Figure 1.In vivo efficacy of
nicergoline for impaired neurogenesis and cognitive competence in
mice with Alzheimer's disease. (A) Analysis of impaired
neurogenesis determined by immunofluorescence in mice treated with
nicergoline. (B) Evaluation of cognitive competence of mice with
Alzheimer's disease treated with nicergoline. (C) Degree of
dementia determined by path efficiency in mice receiving treatment
with nicergoline compared with the control. (D) Abundance of the
pathogenic factors Aβ-42, Aβ-40 and APP in the hippocampus of
experimental mice, determined by immunohistochemistry. (E) Levels
of neuroprotective proteins Foxp-2, SxIP and EB in hippocampus of
nicergoline-treated mice determined by western blotting. (F)
Hippocampus excitability following treatment with nicergoline
compared with the control group. Arrowheads indicate the dispersion
of the pyramidal cell layer. All data are presented as the mean ±
standard error of the mean. **P<0.01 vs. PBS. APP, amyloid
precursor protein; Foxp-2, forkhead box protein P2; Aβ, amyloid β;
APP, amyloid precursor protein; EB, end-binding proteins; SxIP, Src
homology 2-containing inositol phosphatase; Brdu,
bromodeoxyuridine. |
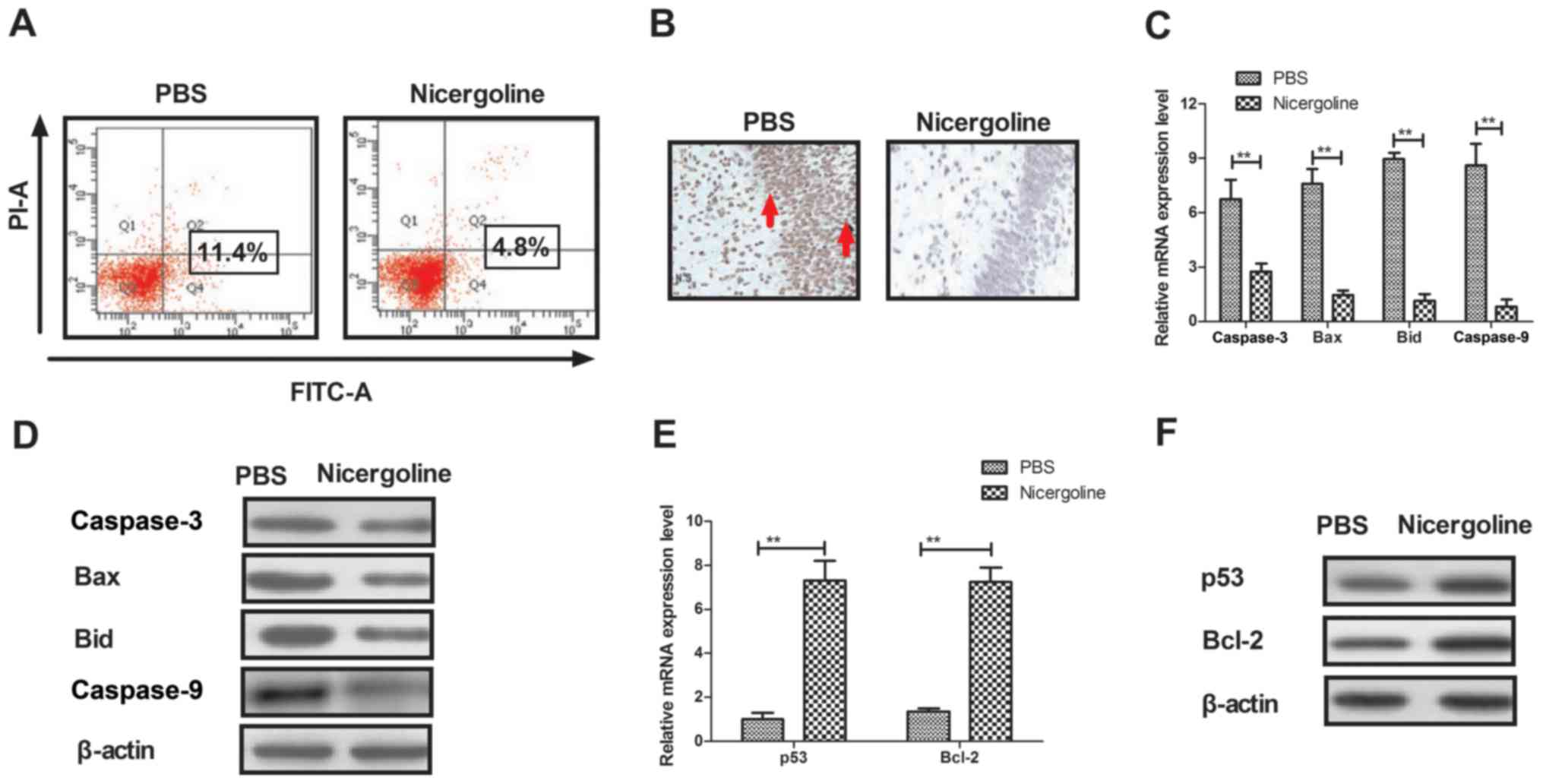
Nicergoline inhibits apoptosis in
hippocampal cells from mice with Alzheimer's disease
As previously indicated, apoptosis of hippocampal
cells is ubiquitous in patients with Alzheimer's disease (33). Therefore, the present study
examined the apoptosis of hippocampal cells in a mouse model of
Alzheimer's disease following treatment with nicergoline or PBS. It
was demonstrated that nicergoline inhibited apoptosis in
hippocampal cells isolated from mice with Alzheimer's disease and
analyzed by flow cytometry in vitro (Fig. 2A). The TUNEL assay demonstrated
that apoptotic hippocampal cells were significantly less prevalent
in nicergoline-treated mice in vivo (Fig. 2B). In addition, the expression
levels of apoptotic factors in hippocampal cells isolated from
nicergoline-treated mice were investigated. The mRNA and protein
levels of pro-apoptotic caspase-3, BCL2 associated X (Bax), BH3
interacting domain death agonist (Bid) and caspase-9 were
downregulated in the hippocampal cells of nicergoline-treated mice
(Fig. 2C and D). However, the mRNA
and protein levels of anti-apoptotic p53 and apoptosis regulator
Bcl-2 were upregulated in the hippocampal cells of
nicergoline-treated mice (Fig. 2E and
F). The results of the present study indicated that nicergoline
may inhibit apoptosis in hippocampal cells by regulating the
activation of apoptosis-associated genes.
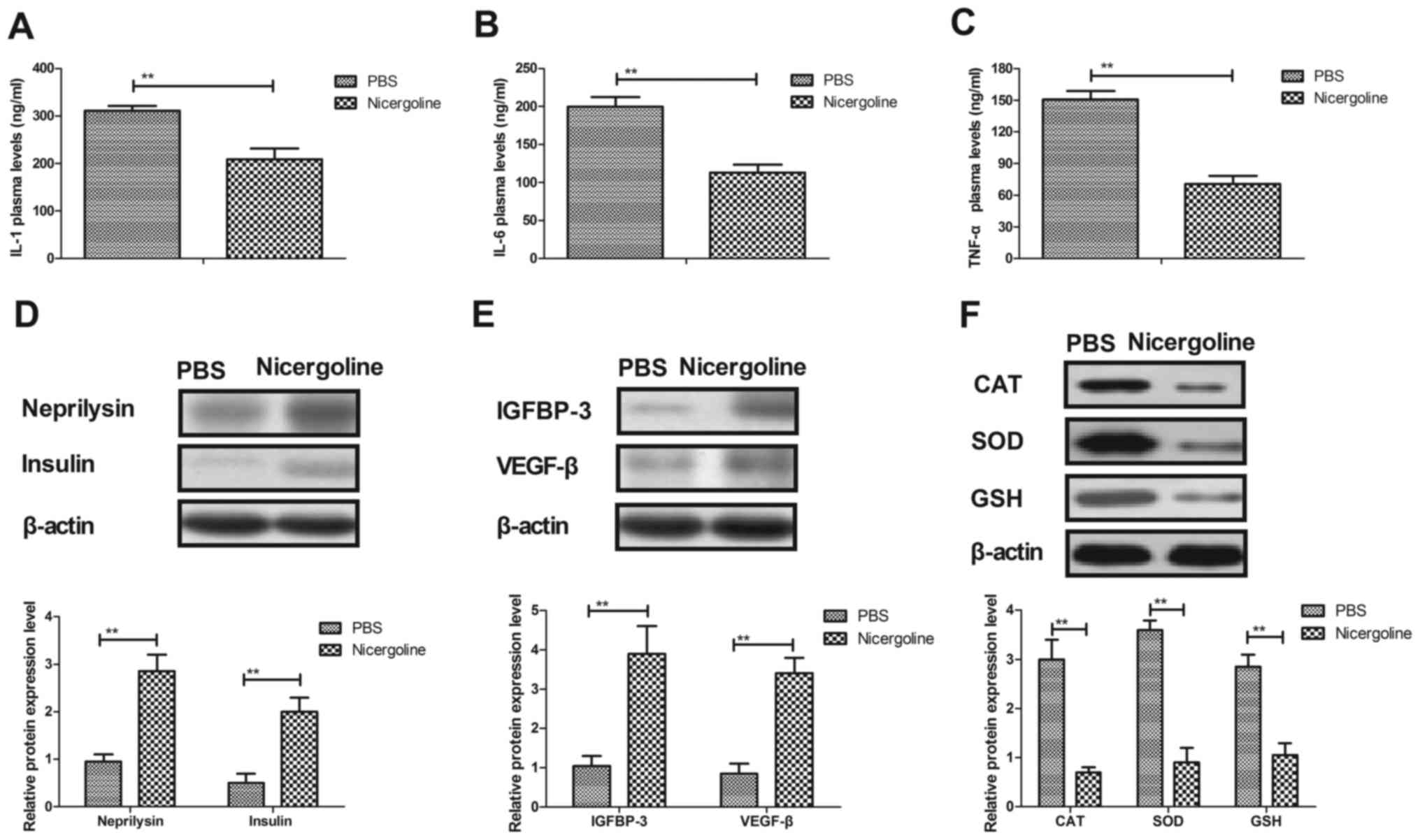
Nicergoline inhibits inflammation and
oxidative stress in hippocampal cells from mice with Alzheimer's
disease
The expression levels of inflammatory factors in
hippocampal cells from experimental mice were examined. It was
demonstrated that the plasma concentrations of IL-1, IL-6 and TNF-α
were decreased in hippocampal cells treated with nicergoline
(Fig. 3A-C). Neprilysin and
insulin expression levels were upregulated in hippocampal cells in
the mice with Alzheimer's disease treated with nicergoline for 60
days (Fig. 3D). In addition,
insulin-like growth factor-binding protein 3 and vascular
endothelial growth factor β protein levels were increased in
hippocampal cells in the experimental mice treated with nicergoline
(Fig. 3E). Oxidative stress
differences between experimental mice we analyzed following
treatment with nicergoline. As presented in Fig. 3F, the expression levels of reactive
oxygen species, superoxide dismutase and glutathione were
downregulated in hippocampal cells in the experimental mice treated
with nicergoline. The results of the present study indicated that
nicergoline mitigated Alzheimer's disease in mice via inhibition of
inflammation and oxidative stress in hippocampal cells.
Nicergoline regulates the activity of
hippocampal cells through the PI3K/AKT signaling pathway
In order to elucidate the nicergoline-mediated
mitigation of Alzheimer's disease in mice, molecular alterations in
the PI3K/AKT signaling pathway in hippocampal cells were studied.
As presented in Fig. 4A, treatment
with nicergoline significantly increased the expression of PI3K and
AKT in hippocampal cells isolated from experimental mice. In
addition, nicergoline significantly promoted the phosphorylation of
AKT compared with the negative control (Fig. 4B). It was observed that inhibition
of PI3K activity (PI3KI) by LY294002 obviated the AKT expression
and phosphorylation stimulated by treatment with nicergoline
(Nicergoline + PI3KI; NiPI3KI) in hippocampal cells (Fig. 4C). In addition, the expression
levels of IL-1, IL-6 and TNF-α were upregulated in
PI3K-inhibitedhippocampal cells in vitro (Fig. 4D). Additionally, oxidative stress
was increased in PI3K-inhibited hippocampal cells in vitro
(Fig. 4E). Notably, the apoptosis
rate was increased in PI3K inhibitor-treated hippocampal cells
in vitro (Fig. 4F). The
aforementioned results demonstrated that nicergoline regulated the
activity of hippocampal cells through stimulation of the PI3K/AKT
signaling pathway.
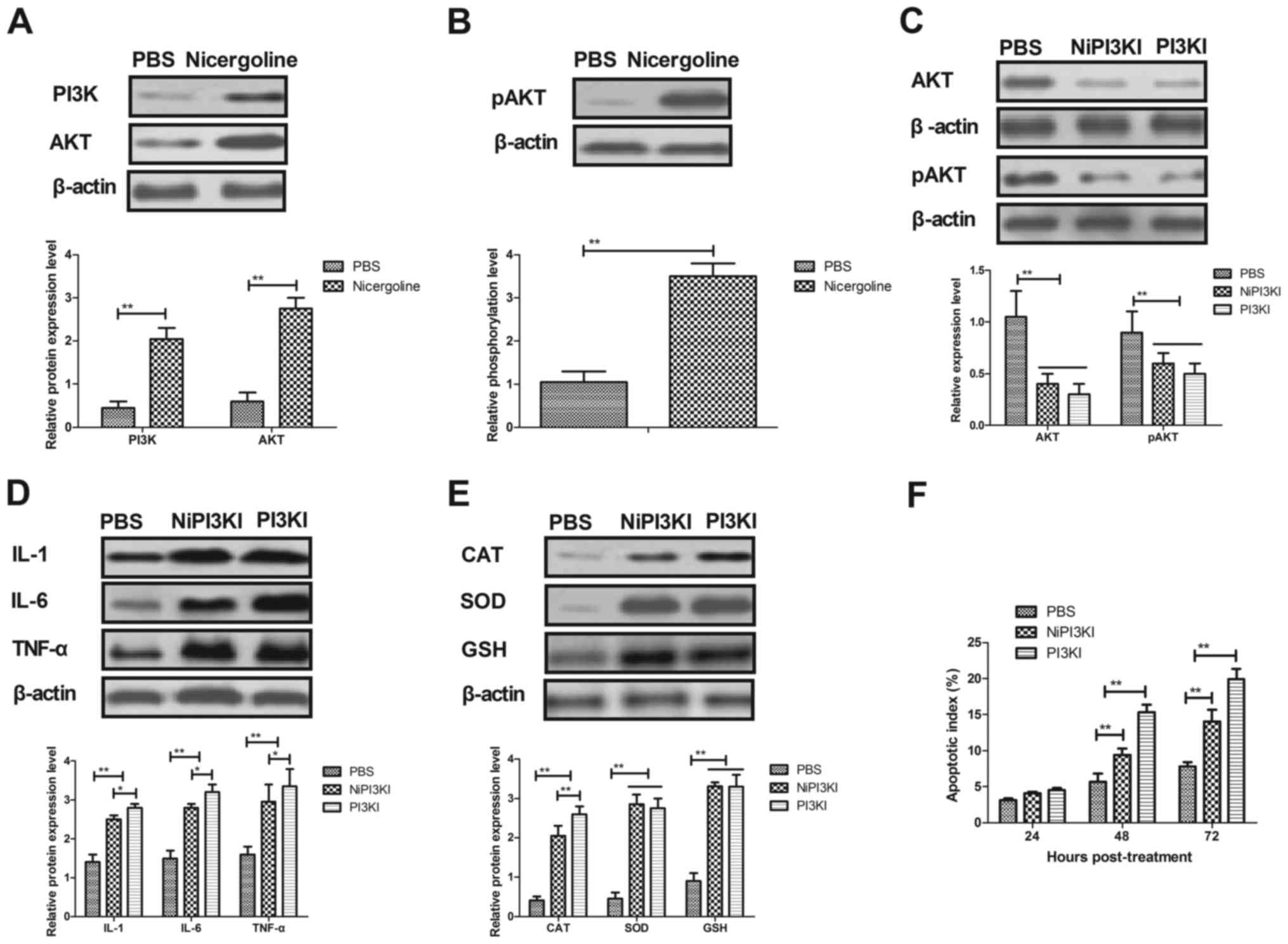 | Figure 4.Nicergoline-regulated activity of
hippocampal cells through the PI3K/AKT signaling pathway. (A) The
effect of treatment with nicergoline on the protein expression of
PI3K and AKT in hippocampal cells isolated from experimental mice.
(B) The phosphorylation of AKT in the hippocampus in
nicergoline-treated mice with Alzheimer's disease in vivo.
(C) The effect of inhibition of PI3K activity on AKT expression and
phosphorylation. (D) The effect of inhibition of PI3K activity on
the expression of inflammatory factors in hippocampal cells in
vitro. (E) The effect of inhibition of PI3K activity on
oxidative stress in the hippocampus in vitro. (F) The effect
of inhibition of PI3K activity on the apoptosis rate in hippocampal
cells in vitro. All data are presented as the mean ±
standard error of the mean. *P<0.05, **P<0.01 vs. PBS. PI3K,
phosphatidylinositol 3-kinase; AKT, RAC-α serine/threonine-protein
kinase; PI3KI, PI3K inhibitor; NiPI3KI, nicergoline + PI3KI; IL,
interleukin; TNF-α, tumor necrosis factor-α; CAT, catalase; SOD,
superoxide dismutase; GSH, glutathione. |
Discussion
In the present study, the effect of nicergoline on
Alzheimer's disease and the mechanism of action of nicergoline were
analyzed. The results of the present study contributed to the
efforts to develop an effective treatment for Alzheimer's disease.
Nicergoline is as an efficient drug for the treatment of the
cranial nerve symptoms associated with dementia, although there is
no available data on its molecular mechanism of action in
Alzheimer's disease. The PI3K/AKT pathway has been hypothesized to
mediate the nicergoline-induced therapeutic effect on Alzheimer's
disease in a mouse model. The results of the present study
confirmed that nicergoline regulated PI3K/AKT expression and
phosphorylation in the hippocampus of mice with Alzheimer's
disease. The findings of the present study indicated that
nicergoline inhibited inflammation, apoptosis and oxidative stress
through upregulation of the PI3K/AKT signaling pathway.
The PI3K/AKT signaling pathway serves an essential
role in cell growth, proliferation and survival under physiological
conditions (34). There is scarce
evidence of the association between the PI3K/AKT signaling pathway
and Alzheimer's disease. Kong et al (35) reported that nicorandil serves a
neuroprotective role in a cellular model of Alzheimer's disease
through activation of the PI3K/AKT signaling pathway. Kitagishi
et al (36) demonstrated
that the PI3K/AKT/glycogen synthase kinase-3 β pathway is involved
in cell signaling in neuronal cells affected by Alzheimer's
disease. In addition, ginseng induced a neuroprotective effect on
D-galactose/aluminum chloride via the PI3K/AKT signaling pathway in
the hippocampus, improved memory and reduced the content of Aβ-42
and MAPT in rats with Alzheimer's disease (37). Additionally, following treatment
with salidroside, the PI3K/AKT signaling pathway ameliorated
toxicity in Aβ-treated primary brain neural cultures (38). These previous reports suggested
that neuroprotection is associated with the activity of the
PI3K/AKT signaling pathway in hippocampal cells in cellular and
animals models of Alzheimer's disease. The results of the present
study indicated that nicergoline was able to improve neurogenesis
and cognitive competence through upregulation of the PI3K/AKT
signaling pathway and inhibition of inflammation, apoptosis and
oxidative stress in mice with Alzheimer's disease.
Alzheimer's disease is characterized by apoptosis of
hippocampal cells, senile plaques, oxidative stress,
neurofibrillary tangles and inflammation (39). Chronic inflammatory responses
stimulate the progression of Alzheimer's disease (40). Quinn et al (41) studied the association between
inflammation and cerebral amyloidosis in an animal model of
Alzheimer's disease. Balez et al (20) reported that apigenin may induce
neuroprotective effects in a pluripotent stem cell model of
Alzheimer's disease via inhibition of inflammation, improvement of
neuronal excitability and reduced apoptosis. The results of the
present study demonstrated that nicergoline was able to reduce
inflammation via downregulation of inflammatory factors in
hippocampal cells. Downregulation of inflammation contributes to
reduced apoptosis in hippocampal cells. In addition, chronic
inflammatory responses and oxidative stress are associated with the
expression of Aβ protein during the progression of Alzheimer's
disease (42). The results of the
present study suggested that nicergoline was able to improve Aβ
protein accumulation in the hippocampi of experimental mice.
Additionally, apoptosis, a ubiquitous biological phenomenon in
cells, is genetically controlled. Bcl-2 and caspase-3 are two key
molecular apoptosis regulators and their function in the regulation
of brain cell apoptosis was previously reported (43–46).
One study demonstrated that downregulation of Bcl-2 and
simultaneous upregulation of Bax and Bid activated p53 and
caspase-3, and consequently induced neuronal apoptosis in the rat
hippocampus (47). Bcl-2 and
caspase-3 are two important molecular regulators of apoptosis and
overexpression of Bcl-2 inhibited the activation of caspase-3 and
apoptosis (48). Therefore, the
data presented in the present study may contribute to the
understanding of the therapeutic properties of nicergoline for the
treatment of Alzheimer's disease.
In conclusion, the results of the present
preclinical study suggested that nicergoline may be an effective
drug for the treatment of impaired neurogenesis and cognitive
competence in Alzheimer's disease. Treatment with nicergoline
markedly improved visual attention, inhibitory control and the
neural cell count via inhibition of apoptosis in hippocampal cells
and prevention of the expression of inflammatory mediators in mice
with Alzheimer's disease. Notably, nicergoline-mediated activation
of the PI3K/AKT signaling pathway was investigated in mice with
Alzheimer's disease. The results suggested that nicergoline
regulated neurogenesis and cognitive competence through the
PI3K/AKT signaling pathway in the hippocampal cells of experimental
mice. In conclusion, the results of the present study suggested
that nicergoline may be a promising drug for the treatment of
Alzheimer's disease.
References
|
1
|
Armstrong RA: Survival in the pre-senile
dementia frontotemporal lobar degeneration with TDP-43
proteinopathy: Effects of genetic, demographic and
neuropathological variables. Folia Neuropathol. 54:137–148. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bando N and Nakamura Y: Preliminary
evidence that rivastigmine-induced inhibition of serum
butyrylcholinesterase activity improves behavioral symptoms in
Japanese patients with Alzheimer's disease. Geriatr Gerontol Int.
17:1306–1312. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Verkhratsky A, Rodriguez-Arellano JJ,
Parpura V and Zorec R: Astroglial calcium signalling in Alzheimer's
disease. Biochem Biophys Res Commun. 483:1005–1012. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cortese GP and Burger C: Neuroinflammatory
challenges compromise neuronal function in the aging brain:
Postoperative cognitive delirium and Alzheimer's disease. Behav
Brain Res. 322:269–279. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pettigrew C, Soldan A, Zhu Y, Wang MC,
Brown T, Miller M and Albert M: BIOCARD Research Team: Cognitive
reserve and cortical thickness in preclinical Alzheimer's disease.
Brain Imaging Behav. 11:357–367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fritz NE, Kegelmeyer DA, Kloos AD, Linder
S, Park A, Kataki M, Adeli A, Agrawal P, Scharre DW and Kostyk SK:
Motor performance differentiates individuals with Lewy body
dementia, Parkinson's and Alzheimer's disease. Gait Posture.
50:1–7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stella F, Laks J, Govone JS, de Medeiros K
and Forlenza OV: Association of neuropsychiatric syndromes with
global clinical deterioration in Alzheimer's disease patients. Int
Psychogeriatr. 28:779–786. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garcia KO, Ornellas FL, Martin PK, Patti
CL, Mello LE, Frussa-Filho R, Han SW and Longo BM: Therapeutic
effects of the transplantation of VEGF overexpressing bone marrow
mesenchymal stem cells in the hippocampus of murine model of
Alzheimer's disease. Front Aging Neurosci. 6:302014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ramos B, Baglietto-Vargas D, del Rio JC,
Moreno-Gonzalez I, Santa-Maria C, Jimenez S, Caballero C,
Lopez-Tellez JF, Khan ZU, Ruano D, et al: Early neuropathology of
somatostatin/NPY GABAergic cells in the hippocampus of a PS1×APP
transgenic model of Alzheimer's disease. Neurobiol Aging.
27:1658–1672. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Youn H, Ji I, Ji HP, Markesbery WR and Ji
TH: Under-expression of Kalirin-7 Increases iNOS activity in
cultured cells and correlates to elevated iNOS activity in
Alzheimer's disease hippocampus. J Alzheimers Dis. 12:271–281.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Namba T, Maekawa M, Yuasa S, Kohsaka S and
Uchino S: The Alzheimer's disease drug memantine increases the
number of radial glia-like progenitor cells in adult hippocampus.
Glia. 57:1082–1090. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen J, Shu H, Wang Z, Liu D, Shi Y, Xu L
and Zhang Z: Protective effect of APOE epsilon 2 on intrinsic
functional connectivity of the entorhinal cortex is associated with
better episodic memory in elderly individuals with risk factors for
Alzheimer's disease. Oncotarget. 7:58789–58801. 2016.PubMed/NCBI
|
|
13
|
Ando K, Oka M, Ohtake Y, Hayashishita M,
Shimizu S, Hisanaga S and Iijima KM: Tau phosphorylation at
Alzheimer's disease-related Ser356 contributes to tau stabilization
when PAR-1/MARK activity is elevated. Biochem Biophys Res Commun.
478:929–934. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu W, Zhao J and Lu G: miR-106b inhibits
tau phosphorylation at Tyr18 by targeting Fyn in a model of
Alzheimer's disease. Biochem Biophys Res Commun. 478:852–857. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Absalon S, Kochanek DM, Raghavan V and
Krichevsky AM: MiR-26b, upregulated in Alzheimer's disease,
activates cell cycle entry, tau-phosphorylation, and apoptosis in
postmitotic neurons. J Neurosci. 33:14645–14659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jahng GH, Oh J, Lee DW, Kim HG, Rhee HY,
Shin W, Paik JW, Lee KM, Park S, Choe BY and Ryu CW: Glutamine and
glutamate complex, as measured by functional magnetic resonance
spectroscopy, alters during face-name association task in patients
with mild cognitive impairment and Alzheimer's disease. J
Alzheimers Dis. 53:7452016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nesteruk T, Nesteruk M, Styczynska M,
Barcikowska-Kotowicz M and Walecki J: Radiological evaluation of
strategic structures in patients with mild cognitive impairment and
early Alzheimer's disease. Pol J Radiol. 81:288–294. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Minter MR, Zhang C, Leone V, Ringus DL,
Zhang X, Oyler-Castrillo P, Musch MW, Liao F, Ward JF, Holtzman DM,
et al: Antibiotic-induced perturbations in gut microbial diversity
influences neuro-inflammation and amyloidosis in a murine model of
Alzheimer's disease. Sci Rep. 6:300282016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang Y, Zhao Z, Wei X, Zheng Y, Yu J,
Zheng J and Wang L: Long-term trihexyphenidyl exposure alters
neuroimmune response and inflammation in aging rat: Relevance to
age and Alzheimer's disease. J Neuroinflammation. 13:1752016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Balez R, Steiner N, Engel M, Muñoz SS, Lum
JS, Wu Y, Wang D, Vallotton P, Sachdev P, O'Connor M, et al:
Neuroprotective effects of apigenin against inflammation, neuronal
excitability and apoptosis in an induced pluripotent stem cell
model of Alzheimer's disease. Sci Rep. 6:314502016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Justin Thenmozhi A, William Raja TR,
Manivasagam T, Janakiraman U and Essa M: Hesperidin ameliorates
cognitive dysfunction, oxidative stress and apoptosis against
aluminium chloride induced rat model of Alzheimer's disease. Nutr
Neurosci. 20:360–368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim SY, Yang J and Lee YC: The effects of
nicergoline on corneal nerve regeneration in rat corneas after
photorefractive keratectomy. Curr Eye Res. 36:29–33. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakashima T, Hattori N, Okimoto M,
Yanagida J and Kohno N: Nicergoline improves dysphagia by
upregulating substance P in the elderly. Medicine. 90:279–283.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pilkowska E, Jakubowska T, Witkowska K and
Kulczycki J: Nicergoline in the treatment of patients after a mild
ischemic stroke. Neurol Neurochir Pol. 36:1075–1085. 2002.(In
Polish). PubMed/NCBI
|
|
25
|
Felisati G, Pignataro O, Di Girolamo A,
Bruno E, Alessandrini M, Guidetti G, Monzani D, Beldi AM, Mira E,
Benazzo M, et al: Nicergoline in the treatment of dizziness in
elderly patients. A review. Arch Gerontol Geriatr Suppl. 163–170.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saletu B, Garg A and Shoeb A: Safety of
nicergoline as an agent for management of cognitive function
disorders. Biomed Res Int. 2014:6101032014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caraci F, Chisari M, Frasca G, Canonico
PL, Battaglia A, Calafiore M, Battaglia G, Bosco P, Nicoletti F,
Copani A and Sortino MA: Nicergoline, a drug used for age-dependent
cognitive impairment, protects cultured neurons against
beta-amyloid toxicity. Brain Res. 1047:30–37. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fioravanti M and Flicker L: Efficacy of
nicergoline in dementia and other age associated forms of cognitive
impairment. Cochrane Database Syst Rev: CD003159. 2001. View Article : Google Scholar
|
|
29
|
Dirani M, Nasreddine W, Abdulla F and
Beydoun A: Seizure control and improvement of neurological
dysfunction in Lafora disease with perampanel. Epilepsy Behav Case
Rep. 2:164–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sharma R, Ahmad G, Esteves SC and Agarwal
A: Terminal deoxynucleotidyl transferase dUTP nick end labeling
(TUNEL) assay using bench top flow cytometer for evaluation of
sperm DNA fragmentation in fertility laboratories: Protocol,
reference values, and quality control. J Assist Reprod Genet.
33:291–300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wai-Hoe L, Wing-Seng L, Ismail Z and
Lay-Harn G: SDS-PAGE-based quantitative assay for screening of
kidney stone disease. Biol Proced Online. 11:145–160. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thinnes FP: Apoptogenic interactions of
plasmalemmal type-1 VDAC and Abeta peptides via GxxxG motifs induce
Alzheimer's disease-a basic model of apoptosis? Wien Med
Wochenschr. 161:274–276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi LX, Wang JH and Shi XD: PI3K/AKT/mTOR
pathway and pediatric T acute lymphoblastic leukemia-review.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 24:1269–1274. 2016.(In
Chinese). PubMed/NCBI
|
|
35
|
Kong J, Ren G, Jia N, Wang Y, Zhang H,
Zhang W, Chen B and Cao Y: Effects of nicorandil in neuroprotective
activation of PI3K/AKT pathways in a cellular model of Alzheimer's
disease. Eur Neurol. 70:233–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kitagishi Y, Nakanishi A, Ogura Y and
Matsuda S: Dietary regulation of PI3K/AKT/GSK-3β pathway in
Alzheimer's disease. Alzheimers Res Ther. 6:352014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li H, Kang T, Qi B, Kong L, Jiao Y, Cao Y,
Zhang J and Yang J: Neuroprotective effects of ginseng protein on
PI3K/Akt signaling pathway in the hippocampus of D-galactose/AlCl3
inducing rats model of Alzheimer's disease. J Ethnopharmacol.
179:162–169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang B, Wang Y, Li H, Xiong R, Zhao Z,
Chu X, Li Q, Sun S and Chen S: Neuroprotective effects of
salidroside through PI3K/Akt pathway activation in Alzheimer's
disease models. Drug Des Devel Ther. 10:1335–1343. 2016.PubMed/NCBI
|
|
39
|
Ferencik M, Novak M, Rovensky J and Rybar
I: Alzheimer's disease, inflammation and non-steroidal
anti-inflammatory drugs. Bratisl Lek Listy. 102:123–132.
2001.PubMed/NCBI
|
|
40
|
McGeer EG and McGeer PL: Chronic
inflammation in Alzheimer's disease offers therapeutic
opportunities. Expert Rev Neurother. 1:53–60. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Quinn J, Montine T, Morrow J, Woodward WR,
Kulhanek D and Eckenstein F: Inflammation and cerebral amyloidosis
are disconnected in an animal model of Alzheimer's disease. J
Neuroimmunol. 137:32–41. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Verdile G, Keane KN, Cruzat VF, Medic S,
Sabale M, Rowles J, Wijesekara N, Martins RN, Fraser PE and
Newsholme P: Inflammation and oxidative stress: The molecular
connectivity between insulin resistance, obesity, and alzheimer's
disease. Mediators Inflamm. 2015:1058282015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jiang L, Tang Y and Huang X: Brain cell
apoptosis after cerebral hypoxia-ischemia in neonatal rat. Zhonghua
YI XUE ZA ZHI. 78:567–569. 1998.(In Chinese). PubMed/NCBI
|
|
44
|
Zhang XQ, Zhang ZM, Yin XL, Zhang K, Cai H
and Ling F: Exploring the optimal operation time for patients with
hypertensive intracerebral hemorrhage: Tracking the expression and
progress of cell apoptosis of prehematomal brain tissues. Chin Med
J (Engl). 123:1246–1250. 2010.PubMed/NCBI
|
|
45
|
Johnson S, Tazik S, Lu D, Johnson C,
Youdim MB, Wang J, Rajkowska G and Ou XM: The new inhibitor of
monoamine oxidase, M30, has a Neuroprotective effect against
dexamethasone-induced brain cell apoptosis. Front Neurosci.
4:1802010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang S, Zhou G, Liu H, Zhang B, Li J, Cui
R and Du Y: Protective effects of p38 MAPK inhibitor SB202190
against hippocampal apoptosis and spatial learning and memory
deficits in a rat model of vascular dementia. Biomed Res Int.
2013:2157982013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yürüker V, Nazıroğlu M and Şenol N:
Reduction in traumatic brain injury-induced oxidative stress,
apoptosis, and calcium entry in rat hippocampus by melatonin:
Possible involvement of TRPM2 channels. Metab Brain Dis.
30:223–231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chatterjee A, Chattopadhyay D and
Chakrabarti G: MiR-16 targets Bcl-2 in paclitaxel-resistant lung
cancer cells and overexpression of miR-16 along with miR-17 causes
unprecedented sensitivity by simultaneously modulating autophagy
and apoptosis. Cell Signal. 27:189–203. 2015. View Article : Google Scholar : PubMed/NCBI
|