Introduction
Gastric cancer (GC) is a common digestive system
tumor worldwide, with high incidence and mortality rates,
particularly in eastern Asia (1).
In China, GC ranks as the second most prevalent malignancy, with
679,100 new cases and 498,000 mortalities in 2015 (2). Chemotherapy is an efficient treatment
for patients with GC, however, the majority of chemotherapeutic
agents cause significant side effects in patients. Thus,
development of more effective therapies for the treatment of GC is
required.
Cinobufacini, which is extracted from the skin and
parotid venom glands of the toad Bufo gargarizans, is
extensively used in the treatment of patients with advanced cancer
in China (3,4). Several studies have demonstrated the
anti-neoplastic activities of cinobufacini, including inhibiting
cancer cell proliferation (4),
inducting cell apoptosis (5),
causing cell cycle arrest and cytoskeleton function (6), exerting genotoxic effects (7) and others. A meta-analysis of GC
treatments concluded that cinobufacini combined with chemotherapy
provide benefits for advanced GC (8). Yang et al (9) reported that cinobufacini improved
leukopenia and exhibited benefit for adverse events in the
digestive system caused by chemotherapy. However, the detailed
molecular mechanism of action of cinobufacini in the treatment of
GC is not fully elucidated.
MicroRNAs (miRNAs/miRs) are a class of
post-transcriptional regulators that participate in the regulation
of a wide variety of cellular functions (10). Dysregulated miRNAs expression
patterns have been commonly found in various cancer types (11,12).
miR-494 is located at chromosome 14q32.31 (13). It has been reported that miR-494
can act as an oncogene or tumor suppressor gene in different cancer
types, and influences various stages of tumorigenesis (11,14,15).
Our previous study revealed that miR-494 was upregulated in
cinobufacini-treated GC cells (16). Accordingly, whether the anti-tumor
effects of cinobufacini are mediated by miR-494 as examined in the
current study.
In the present study it is reported that
cinobufacini inhibited proliferation and promoted apoptosis of GC
cells. Additionally, miR-494 was downregulated in the plasma of
patients with GC, and knockdown of miR-494 in cinobufacini-treated
cells promoted cell proliferation and inhibited apoptosis.
Furthermore, the effects of miR-494 on cinobufacini-induced cell
proliferation and apoptosis were mediated by BCL2 associated
athanogene 1 (BAG-1).
Materials and methods
Blood samples and plasma
preparation
This study was approved by the Ethical Committee of
Nanjing Jiangning Hospital (Nanjing, China). Written informed
consent was obtained from all of the participants. Blood samples
from 50 cases of patients with GC (31 males and 19 females; aged
51–81 years old) and 50 healthy control subjects (30 males and 20
females; aged 54–82 years old) were collected in Nanjing Jiangning
Hospital during December 2015 and October 2016. Blood samples of
patients with GC were collected who were first diagnosed with
cancer via histopathological examination, and negative control
blood samples were obtained from healthy control subjects (all of
whom did not suffer from cancer) visited the same hospital for
physical examinations in the same period. Blood samples were
centrifuged at 1,800 × g for 5 min to separate the plasma. All the
plasma samples were stored at −80°C prior to RNA extraction.
Cell lines
The human GC cell lines (BGC-823 and SGC-7901) were
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). Cells were cultured in RPMI-1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% FBS (Invitrogen; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and streptomycin (Invitrogen; Thermo
Fisher Scientific, Inc.), and incubated at 37°C with 5%
CO2.
Cell transfection
Cells (3×105) were seeded into 6-well
plates and transiently transfected with 50 nM miRNA inhibitor,
miRNA negative control (NC) or 50 nM small interfering RNA (siRNA)
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) when the cell confluence reached 70–90%. The
miR-494 inhibitor and negative control were purchased from
Guangzhou RiboBio Co., Ltd. [Guangzhou, China; cat. nos.
miR20002816-1 and miR02201-1-5 (sequences not available)] and the
BAG-1 siRNA was synthesized by Genepharma (Shanghai, China). The
BAG-1 siRNA sequence was 5′-GGGAAAAUCUCUGAAGGAAtt-3′ (17). The medium was replaced with fresh
medium 6 h post-transfection. The expression level of miR-494 or
BAG-1 was measured after 48 or 72 h, respectively.
Cell proliferation assay
Cells were seeded, at a density of 3,000 cells/well,
in triplicate into the 96-well plate. At various time-points (24,
48 and 72 h), the optical density value was measured by Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) on an iMark microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at 450 nm after 2 h
incubation with the CCK-8 reagent.
Flow cytometry
Cell apoptosis was analyzed with an Annexin
V-propidium iodide apoptosis detection kit (Nanjing KeyGen Biotech
Co., Ltd., Nanjing, China) on a flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA). Briefly, A total of 48 h
post-transfection, 5×105 cells were harvested and washed
twice in cold PBS, and suspended in binding buffer. Following
incubation with Annexin V-fluorescein isothiocyanate and propidium
iodide for 10 min in the dark, at room temperature, cells were
subjected to flow cytometry assay analysis.
Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR). A total of 48 h post-transfection, total RNA
from 1×106 cells was isolated using TRIzol reagent
(Thermo Fisher Scientific, Inc.) and RT was performed using
PrimeScript™ RT reagent kit with gDNA Eraser (Takara Bio, Inc.,
Otsu, Japan) following to the manufacturer's instructions. The
incubation conditions of RT were 42°C for 2 min, 37°C for 15 min
and 85°C for 5 sec. The qPCR reaction was performed on an ABI
StepOne Plus System (Thermo Fisher Scientific, Inc.), and the
cycling conditions were 95°C for 30 sec, followed by 40 cycles of
95°C for 5 sec and 60°C for 30 sec. The β-actin or U6 were used as
the internal control. The primer sequences were as follows:
miR-494, forward 5′-TGACCTGAAACATACACGGGA-3′ and reverse
5′-TATCGTTGTACTCCACTCCTTGAC-3′; U6, forward
5′-ATTGGAACGATACAGAGAAGATT-3′ and reverse
5′-GGAACGCTTCACGAATTTG-3′; The expression of the target genes were
calculated using the 2−ΔΔCq method (18).
Western blot analysis
A total of 72 h post-transfection, 1×106
cells were trypsinized and lysed in radioimmunoprecipitation assay
lysis buffer (Beyotime Institute of Biotechnology, Haimen, China)
for 10 min on ice. Proteins were isolated by centrifuging at 12,000
× g for 5 min at 4°C. The protein concentration was determined by
the bicinchoninic acid (BCA) method using the BCA Protein Assay kit
(Beyotime Institute of Biotechnology). Equal amounts of proteins
(30 µg) were separated on ١٠٪ SDS-PAGE and transferred to
polyvinylidene difluoride membranes, and then incubated in blocking
buffer (5% non-fat milk in tris-buffered saline with 0.05%
Tween-20) at room temperature for 1 h. The membranes were incubated
with the primary antibodies of β-actin (cat. no. A5441; mouse
monoclonal; 1:8,000; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
and BAG-1 (cat. no. ab32109; rabbit monoclonal; 1:500; Abcam,
Cambridge, UK) overnight at 4°C. Following washing in TBS-Tween,
the membrane was incubated in secondary antibodies (cat. no.
ZB-2301, goat anti-rabbit; cat. no. ZB-2305, goat anti-mouse;
1:10,000; OriGene Technologies, Inc., Beijing, China) for 2 h.
Protein bands were detected using an enhanced chemiluminescence kit
(Pierce; Thermo Fisher Scientific, Inc.) on a FluorChem E System
(ProteinSimple, San Jose, CA, USA).
Statistical analysis
The data are presented as the mean ± standard
deviation from at least three independent experiments. Data was
analyzed using SPSS 17 (SPSS, Inc., Chicago, IL, USA). Student's
t-test was used to determine statistical significance between two
groups. Differences multiple groups were evaluated using one-way
analysis of variance followed by a Dunnett multiple-range test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cinobufacini suppresses proliferation
and promotes apoptosis of GC cells
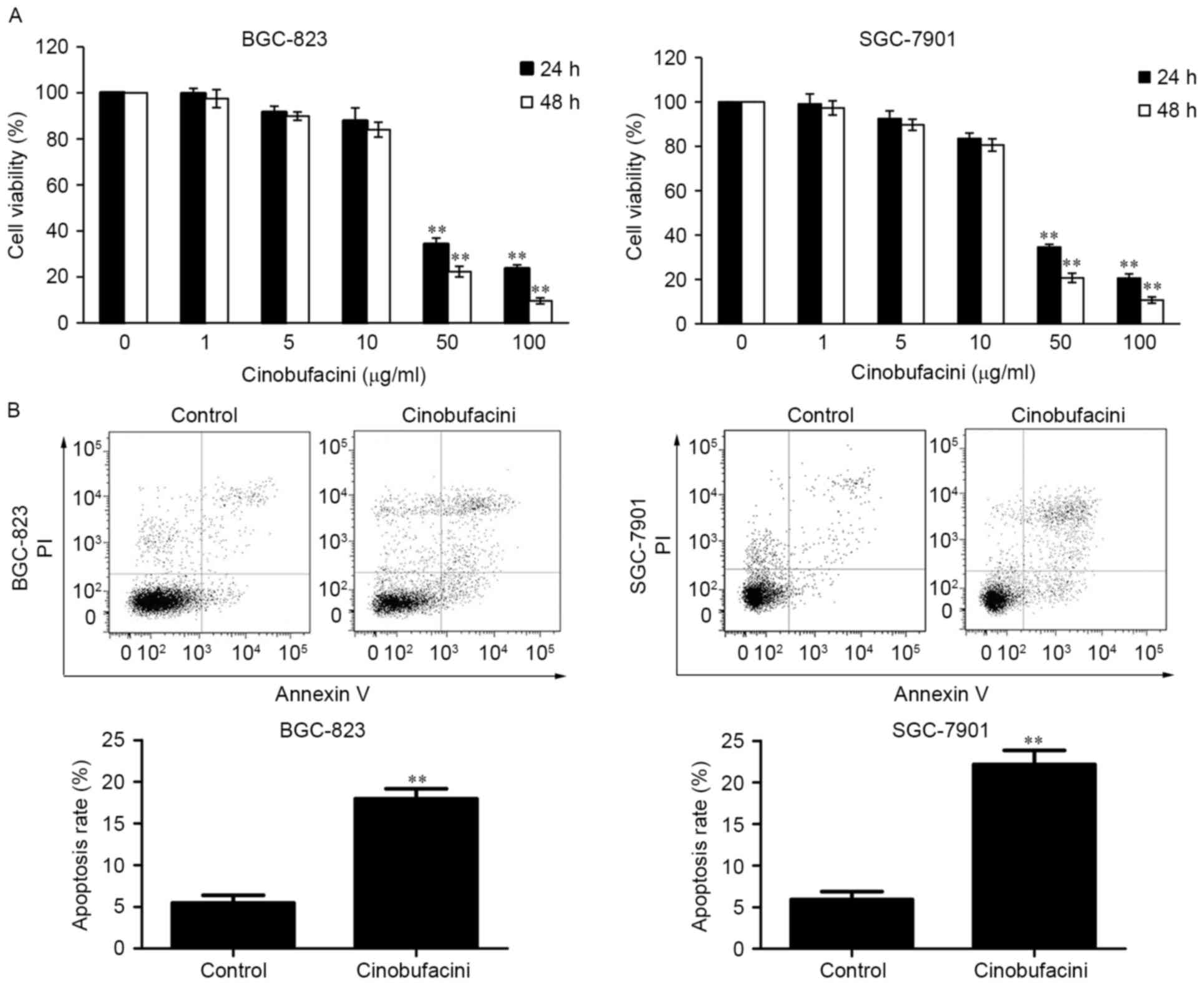
BGC-823 and SGC-7901 cells were treated with
different concentrations of cinobufacini (0, 1, 5, 10, 50 and 100
µg/ml) for 24 h and 48 h. Subsequently, cell viability was measured
using a CCK-8 assay kit. The results demonstrated that the growth
of GC cells was inhibited by treatment with cinobufacini in a
concentration-dependent manner (Fig.
1A). As cinobufacini at the concentrations of 50 and 100 µg/ml
exhibited significant toxic effects on the BGC-823 and SGC-7901
cells, the 10 µg/ml concentration of cinobufacini was selected to
be used in the subsequent experiments. After treatment with 10
µg/ml cinobufacini for 24 h, the cells were analyzed to detect
apoptosis. The results indicated that cinobufacini promoted the
apoptosis of BGC-823 cells (control vs. cinobufacini, 5.49 vs.
17.96%) and SGC-7901 cells (control vs. cinobufacini, 5.93 vs.
22.15%; Fig. 1B).
miR-494 is a tumor suppressor gene and
exhibits an opposing expression trend compared with BAG-1 in
cinobufacini-treated cells
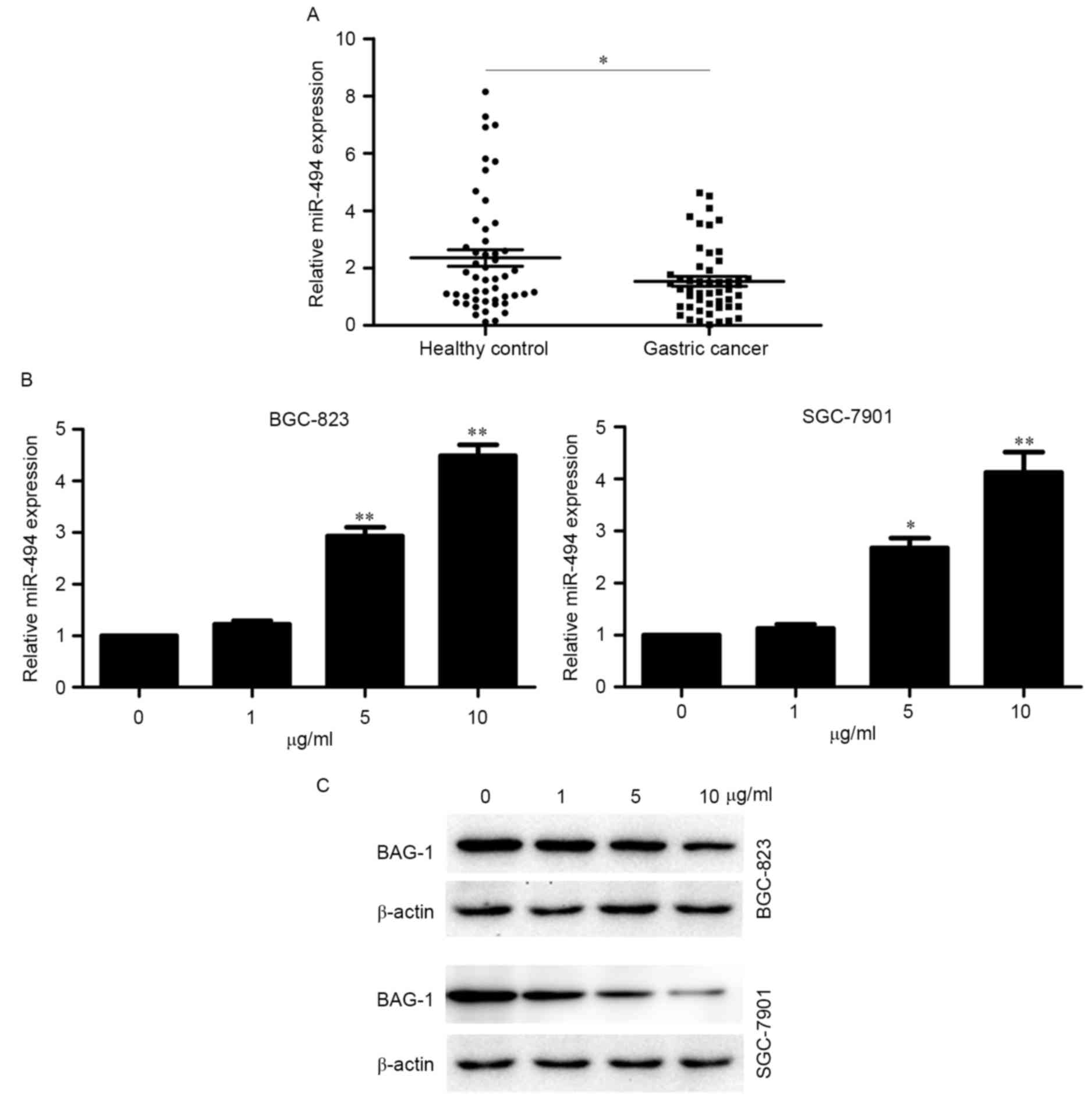
The expression levels of miR-494 were determined in
plasma samples from 50 patients with GC and 50 healthy control
subjects. The RT-qPCR analysis indicated that the expression level
of miR-494 was lower in patients with GC than in healthy control
subjects (Fig. 2A). The results
also suggested that miR-494 may be a tumor suppressor. Thus, we
hypothesized that the antitumor activity of cinobufacini may
partially be mediated by miR-494. As a previous study demonstrated
that BAG-1 is a direct target of miR-494 (16), the expression of miR-494 and BAG-1
was examined in cinobufacini-treated BGC-823 and SGC-7901 cells.
The results indicated that expression of miR-494 and BAG-1
exhibited opposing trends of in cinobufacini-treated cells, with
miR-494 was upregulated and BAG-1 downregulated by cinobufacini
(Fig. 2B and C). The results
suggested that miR-494 and BAG-1 are involved in the activity of
cinobufacini.
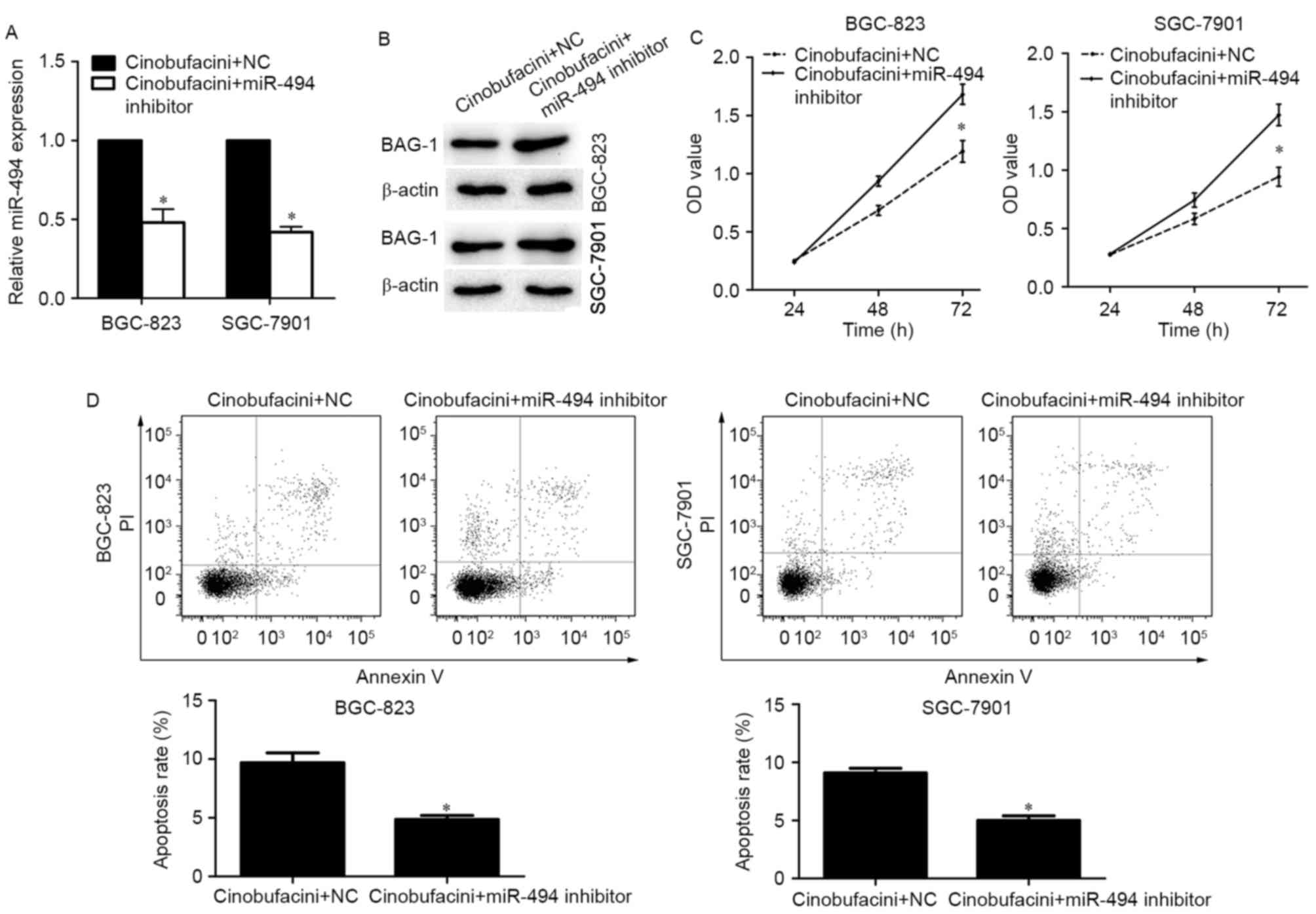
miR-494 reduces the expression of BAG-1 following
cinobufacini treatment. To investigate whether miR-494 mediates the
decreased expression of BAG-1 and alters cell behaviors in
cinobufacini-treated cells, BGC-823 and SGC-7901 cells were treated
with cinobufacini for 24 h, and then transfected with a miR-494
inhibitor or a scramble sequence. The transfection efficacy was
determined by RT-qPCR (Fig. 3A).
The miR-494 inhibitor partially reversed the cinobufacini-induced
decreased expression of BAG-1 (Fig.
3B). Additionally, knockdown of miR-494 promoted the growth of
GC cells compared with the control cinobufacini-treated cells
(Fig. 3C). The results of the
apoptosis analysis suggested that the miR-494 inhibitor attenuated
cinobufacini-induced apoptosis of BGC-823 cells (NC vs. miR-494
inhibitor, 9.69 vs. 4.79%) and SGC-7901 cells (NC vs. miR-494
inhibitor, 8.94 vs. 4.36%; Fig.
3D).
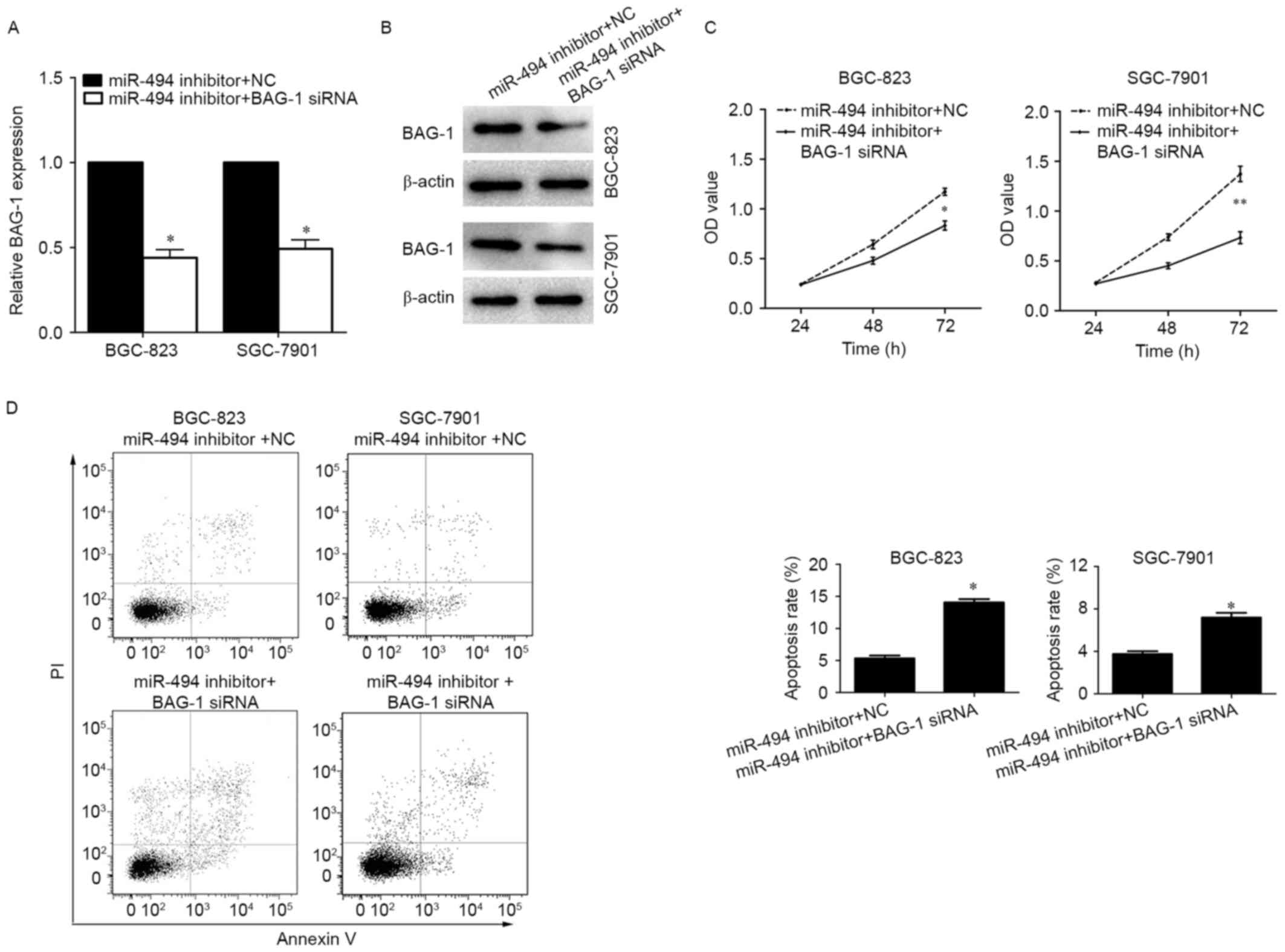
Effect of miR-494 on
cinobufacini-induced cell proliferation and apoptosis is mediated
by BAG-1
To investigate whether the regulatory role of
miR-494 on cinobufacini-induced cell behavior changes are dependent
on BAG-1 inhibition, the BGC-823 and SGC-7901 cells were treated
with cinobufacini for 24 h and transfected with the miR-494
inhibitor plus BAG-1 siRNA or miR-494 inhibitor plus a scramble
sequence (Fig. 4A and B). The data
indicated that inhibition of BAG-1 partially abrogated the effects
of miR-494 inhibitor on cell proliferation and apoptosis. Knockdown
of BAG-1 reduced the growth rates and promoted the apoptotic
ability of miR-494-silenced BGC-823 cells (NC vs. BAG-1 siRNA, 5.33
vs. 14.04%) and SGC-7901 cells (NC vs. BAG-1 siRNA, 3.75 vs. 7.57%;
Fig. 4C and D).
Discussion
Cinobufacini is a traditional Chinese medicine that
has been used for many years. The biological activities of
cinobufacini include anesthetic, cardiotonic, antimicrobial and
antineoplastic effects (19),
thus, it is widely used in the treatment of malignant tumors
(20,21). Cinobufacini exhibited great
clinical efficacy in the treatment of hepatocellular carcinoma
(22,23), non-small-cell lung cancer (24) and advanced gallbladder carcinoma
(25). Thus, in the current study
the biological function and underlying mechanisms of cinobufacini
were investigated in GC cells.
In the present study, the anti-proliferative and
pro-apoptotic activities of cinobufacini were evaluated in GC
cells, and the results were consistent with previous studies
(26,27). Our previous microarray study on
cinobufacini-treated BGC-823 cells demonstrated that miR-494 was
upregulated by cinobufacini (16).
Furthermore, miR-494 was reported to be involved in regulation of
the tumorigenesis of various cancer types (14,15,28).
Accordingly, the expression profile of miR-494 was evaluated in the
plasma of patients with GC and healthy control subjects. The result
indicated miR-494 was downregulated in blood samples from patients
with GC, which was in accordance with previous studies on GC
tissues (11,14,29).
We previously reported that miR-494 inhibited BGC-823 cell
proliferation and promoted apoptosis, thus, we hypothesize that
cinobufacini-induced cancer cell apoptosis may be partially
mediated by miR-494.
miRNAs exert their functional effects by regulating
target genes, and the results of the current study demonstrated
that BAG-1 is a direct target of miR-494 (16), thus the expression of BAG-1 was
analyzed in cinobufacini-treated GC cells. The results demonstrated
that BAG-1 exhibited an opposing expression level trend compared
with miR-494, and was decreased by cinobufacini in a
concentration-dependent manner, suggesting miR-494 and BAG-1 are
involved in the antitumor activity of cinobufacini.
BAG-1 is a multifunctional protein that is
over-expressed in various cancer types and regulated in a wide
variety of cellular processes, including proliferation, apoptosis,
metastasis and cell survival (30–33).
The most important function of BAG-1 is the enhancement of the
anti-apoptotic ability of Bcl-2; thus, we hypothesized that miR-494
may regulate cinobufacini-induced cell proliferation and apoptosis
by targeting BAG-1. Notably, following silencing of miR-494 in
cinobufacini-treated GC cells, the expression of BAG-1 was
decreased, and the inhibitory effects of cinobufacini on cell
proliferation and apoptosis were attenuated. Knockdown BAG-1 in the
miR-494-silenced and cinobufacini-treated GC cells reduced cell
viability and enhanced apoptosis. Our previous study demonstrated
that miR-494 may participate in the anti-tumor activity of
cinobufacini (16). In the present
study, the data demonstrated that cinobufacini inhibited cell
proliferation and promoted apoptosis via the upregulation of
miR-494 and subsequent downregulation of its target, BAG-1. The
present study focused on the role of miR-494 and BAG-1 in
cinobufacini-induced proliferation and apoptosis of GC cells;
however, cinobufacini exhibits a variety of clinical effects. Thus,
future studies are required to investigate the underlying molecular
mechanism of cinobufacini in the treatment of GC.
In conclusion, the results of the present study
demonstrated that cinobufacini suppresses GC cells proliferation
and promotes apoptosis, partially via the miR-494-BAG-1 axis. These
findings provide a new insight into the mechanism of the effects of
cinobufacini in GC. Futures studies should investigate the
molecular mechanisms underlying the effect of miR-494-BAG-1 axis
resulting in the suppression of GC cell proliferation and promote
apoptosis.
Acknowledgments
Not applicable.
Funding
This study was supported by the Science and
Technology Development Plan of the Jiangning District (grant no.
2015Eg06) and the Nanjing Health Bureau Research Project (grant no.
YKK14194).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RPZ and ZLS conceived and designed the experiments.
GC and YL collected the blood samples and evaluated the data from
the patients. CCZ and FW performed the experiments. ZLS and GC
wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethical Committee of
Jiangning Hospital (Nanjing, China). Written informed consent was
obtained from all of the participants.
Consent for publication
Consent forms for the publication of associated data
were obtained from all participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA:
CA Cancer J Clin. 65:87–108. 2015.
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qi F, Li A, Zhao L, Xu H, Inagaki Y, Wang
D, Cui X, Gao B, Kokudo N, Nakata M, et al: Cinobufacini, an
aqueous extract from Bufo bufo gargarizans Cantor, induces
apoptosis through a mitochondria-mediated pathway in human
hepatocellular carcinoma cells. J Ethnopharmacol. 128:654–661.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang D and Bi Z: Bufalin inhibited the
growth of human osteosarcoma MG-63 cells via down-regulation of
Bcl-2/Bax and triggering of the mitochondrial pathway. Tumour Biol.
35:4885–4890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qi F, Li A, Inagaki Y, Xu H, Wang D, Cui
X, Zhang L, Kokudo N, Du G and Tang W: Induction of apoptosis by
cinobufacini preparation through mitochondria- and Fas-mediated
caspase-dependent pathways in human hepatocellular carcinoma cells.
Food Chem Toxicol. 50:295–302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma L, Song B, Jin H, Pi J, Liu L, Jiang J
and Cai J: Cinobufacini induced MDA-MB-231 cell
apoptosis-associated cell cycle arrest and cytoskeleton function.
Bioorg Med Chem Lett. 22:1459–1463. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee S, Lee Y, Choi YJ, Han KS and Chung
HW: Cyto-/genotoxic effects of the ethanol extract of Chan Su, a
traditional Chinese medicine, in human cancer cell lines. J
Ethnopharmacol. 152:372–376. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie X, Huang X, Li J, Lv X, Huang J, Tang
S and Sun Y: Efficacy and safety of Huachansu combined with
chemotherapy in advanced gastric cancer: A meta-analysis. Med
Hypotheses. 81:243–250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang J, Zhu L, Wu Z and Wang Y: Chinese
herbal medicines for induction of remission in advanced or late
gastric cancer. Cochrane Database Syst Rev (Issue 4).
CD0050962013.
|
|
10
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He W, Li Y, Chen X, Lu L, Tang B, Wang Z,
Pan Y, Cai S, He Y and Ke Z: MiR-494 acts as an anti-oncogene in
gastric carcinoma by targeting c-myc. J Gastroenterol Hepatol.
29:1427–1434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nip H, Dar AA, Saini S, Colden M, Varahram
S, Chowdhary H, Yamamura S, Mitsui Y, Tanaka Y, Kato T, et al:
Oncogenic microRNA-4534 regulates PTEN pathway in prostate cancer.
Oncotarget. 7:68371–68384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song L, Liu D, Wang B, He J, Zhang S, Dai
Z, Ma X and Wang X: MiR-494 suppresses the progression of breast
cancer in vitro by targeting CXCR4 through the Wnt/beta-catenin
signaling pathway. Oncol Rep. 34:525–531. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li N, Zhao X, Wang L, Zhang S, Cui M and
He J: MiR-494 suppresses tumor growth of epithelial ovarian
carcinoma by targeting IGF1R. Tumour Biol. 37:7767–7776. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen B, Hou Z, Li C and Tong Y: MiRNA-494
inhibits metastasis of cervical cancer through Pttg1. Tumour Biol.
36:7143–7149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou RP, Chen G, Shen ZL and Pan LQ:
Cinobufacin suppresses cell proliferation via miR-494 in BGC-823
gastric cancer cells. Asian Pac J Cancer Prev. 15:1241–1245. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clemo NK, Collard TJ, Southern SL, Edwards
KD, Moorghen M, Packham G, Hague A, Paraskeva C and Williams AC:
BAG-1 is up-regulated in colorectal tumour progression and promotes
colorectal tumour cell survival through increased NF-kappaB
activity. Carcinogenesis. 29:849–857. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qi F, Inagaki Y, Gao B, Cui X, Xu H,
Kokudo N, Li A and Tang W: Bufalin and cinobufagin induce apoptosis
of human hepatocellular carcinoma cells via Fas- and
mitochondria-mediated pathways. Cancer Sci. 102:951–958. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhai XF, Chen Z, Li B, Shen F, Fan J, Zhou
WP, Yang YK, Xu J, Qin X, Li LQ, et al: Traditional herbal medicine
in preventing recurrence after resection of small hepatocellular
carcinoma: A multicenter randomized controlled trial. J Integr Med.
11:90–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meng Z, Garrett CR, Shen Y, Liu L, Yang P,
Huo Y, Zhao Q, Spelman AR, Ng CS, Chang DZ, et al: Prospective
randomised evaluation of traditional Chinese medicine combined with
chemotherapy: A randomised phase II study of wild toad extract plus
gemcitabine in patients with advanced pancreatic adenocarcinomas.
Br J Cancer. 107:411–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dong J, Zhai X, Chen Z, Liu Q, Ye H, Chen
W and Ling C: Treatment of huge hepatocellular carcinoma using
Cinobufacini injection in transarterial chemoembolization: A
retrospective study. Evid Based Complement Alternat Med: 2754542.
2016. View Article : Google Scholar
|
|
23
|
Chen Z, Chen HY, Lang QB, Li B, Zhai XF,
Guo YY, Yue XQ and Ling CQ: Preventive effects of jiedu granules
combined with cinobufacini injection versus transcatheter arterial
chemoembolization in post-surgical patients with hepatocellular
carcinoma: A case-control trial. Chin J Integr Med. 18:339–344.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang Y, Liu LS, Shen LP, Han ZF, Jian H,
Liu JX, Xu L, Li HG, Tian JH and Mao ZJ: Traditional Chinese
Medicine treatment as maintenance therapy in advanced
non-small-cell lung cancer: A randomized controlled trial.
Complement Ther Med. 24:55–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qin TJ, Zhao XH, Yun J, Zhang LX, Ruan ZP
and Pan BR: Efficacy and safety of gemcitabine-oxaliplatin combined
with huachansu in patients with advanced gallbladder carcinoma.
World J Gastroenterol. 14:5210–5216. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu Q, Lin WD, Liao GQ, Zhang LG, Wen SQ
and Lin JY: Antiproliferative effects of cinobufacini on human
hepatocellular carcinoma HepG2 cells detected by atomic force
microscopy. World J Gastroenterol. 21:854–861. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yin JQ, Wen L, Wu LC, Gao ZH, Huang G,
Wang J, Zou CY, Tan PX, Yong BC, Jia Q, et al: The glycogen
synthase kinase-3beta/nuclear factor-kappa B pathway is involved in
cinobufagin-induced apoptosis in cultured osteosarcoma cells.
Toxicol Lett. 218:129–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J, Wang L, Liu Z, Zu C, Xing F, Yang P,
Yang Y, Dang X and Wang K: MicroRNA-494 inhibits cell proliferation
and invasion of chondrosarcoma cells in vivo and in vitro by
directly targeting SOX9. Oncotarget. 6:26216–26229. 2015.PubMed/NCBI
|
|
29
|
Shrestha S, Hsu SD, Huang WY, Huang HY,
Chen W, Weng SL and Huang HD: A systematic review of microRNA
expression profiling studies in human gastric cancer. Cancer Med.
3:878–888. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ni W, Chen B, Zhou G, Lu C, Xiao M, Guan
C, Zhang Y, He S, Shen A and Ni R: Overexpressed nuclear BAG-1 in
human hepatocellular carcinoma is associated with poor prognosis
and resistance to doxorubicin. J Cell Biochem. 114:2120–2130. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun NF, Meng QY, Hu SY, Tian AL, Wang RH,
Liu ZX and Xu L: Correlation between the expression of the BAG-1
gene and clinicopathologic factors in colorectal cancer. J Cancer
Res Clin Oncol. 137:1419–1424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheng HC, Xu XY, Xing YN, Wei ZL,
Takahashi H, Masuda S and Takano Y: Nuclear or cytoplasmic
localization of Bag-1 distinctly correlates with pathologic
behavior and outcome of gastric carcinomas. Hum Pathol. 41:724–736.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Krajewska M, Turner BC, Shabaik A,
Krajewski S and Reed JC: Expression of BAG-1 protein correlates
with aggressive behavior of prostate cancers. Prostate. 66:801–810.
2006. View Article : Google Scholar : PubMed/NCBI
|