Introduction
Hepatocellular carcinoma (HCC) is the second most
common type of cancer, which is characterized by high morbidity and
mortality rates, and accounts for >90% of primary liver cancer
cases (1,2). A previous systematic review and
meta-analysis indicated that HCC is associated with a high level of
recurrence and has the second highest cancer-associated mortality
rate worldwide, even following radiotherapy, chemotherapy and/or
surgery (3,4). At present, the available therapeutic
strategies remain limited, particularly for patients with advanced
HCC, which exhibit poor survival, according to a 5-year survival
study (5–7). Previous studies have revealed that
HCC is a genetically complex, multifactorial and heterogeneous
tumor (8,9). Therefore, various anticancer
therapies that target different signaling pathways have been
investigated for HCC therapy (10,11).
The common clinical therapeutic strategies used to
treat patients with cancer are currently surgery, chemotherapy and
radiotherapy; however, efficacies remain poor and these treatments
are associated with severe side effects during and after treatment
(12). Numerous studies have
focused on inhibition of the growth and aggressiveness of HCC,
which may be associated with targeting proliferative signaling
cascades (13,14). The anti-invasive and anti-migratory
effects of drugs contribute to limit regional migration and
long-distance tumor metastasis of HCC cells by targeting cellular
signaling pathways, which are involved in key signal transduction
for various extracellular growth factors, and receptors of HCC
cells (15,16). In addition, the induction of human
HCC cell apoptosis has been reported to stimulate anticancer
activity via mitochondria-mediated activation of caspase-9 and
caspase-8-mediated proteolysis of BH3 interacting-domain death
agonist, which may warrant further investigation for application in
cancer treatment (17,18).
Tumor necrosis factor (TNF)-α-induced protein
8-like-2 (TIPE-2) is a negative regulator of innate and adaptive
immunity (19,20). TIPE-2 is downregulated in the
majority of human cancer cells, including lung cancer, colon
cancer, HCC and gastric cancer cells. Recently, Li et al
(21) reported that TIPE-2 is a
novel inflammatory regulator that may inhibit Toll-like receptor 4
(TLR4)-mediated development of colon cancer via TLR4-mediated
upregulation of caspase-8; this may be considered a novel
therapeutic target for clinical treatment. Zhao et al
(22) also indicated that TIPE-2
is associated with the pathogenesis of gastric cancer and acts as a
novel negative regulator of the immune system, which has been
systematically investigated in murine and human cancer.
Furthermore, a previous study demonstrated that regulating T-cell
apoptosis by directly targeting the tumor suppressor gene TIPE-2
enhances the apoptotic sensitivity of tumor cells (23).
In the present study, TIPE-2-mediated
phosphoinositide 3-kinase (PI3K)/protein kinas B (AKT) signaling
was investigated in HCC cells. In addition, the inhibitory effects
of TIPE-2 were analyzed on HCC cells; the results demonstrated that
treatment with TIPE-2 significantly suppressed the growth and
proliferation of HCC cells in vitro, and inhibited tumor
formation in vivo. These findings suggested that TIPE-2
treatment may markedly inhibit tumor growth in HepG2-bearing mice,
which further prolonged survival time for experimental mice.
Mechanistic analysis indicated that TIPE-2 may induce apoptosis of
HCC cells via the PI3K/AKT signaling pathway, which provides a
potential therapeutic target for patients with HCC.
Materials and methods
Ethics statement
The present study was conducted in strict accordance
with the recommendations of the Guide for the Care and Use of
Laboratory Animals of Tianjin Medical University General Hospital
(Tianjin, China). This study was approved by the Ethics Committee
of Tianjin Medical University General Hospital. All surgery and
euthanasia were performed under sodium pentobarbital anesthesia,
and all efforts were made to minimize suffering.
Cell culture
HepG2 cells were purchased from the American Type
Culture Collection (Manassas, VA, USA). HepG2 cells were cultured
in Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10%
heat-inactivated fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in an atmosphere containing 5%
CO2.
Endogenous overexpression of PI3K or
TIPE-2
HepG2 cells were cultured in 6-well plates until
they reached 90% confluence and the medium was then removed.
Sequences were cloned into vectors as previously described
(24). Subsequently, HepG2 cells
(1×106) were transfected with pedue12.4-PI3K (100 nM),
pedue12.4-TIPE-2 (100 nM) or pedue12.4-vector (100 nM) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 48 h at 37°C. Cells that exhibited stable
PI3K or TIPE-2 overexpression were selected according to the
dihydrofolate reductase/glutamine synthetase screening system
(Invitrogen; Thermo Fisher Scientific, Inc.).
MTT cytotoxicity assays
HepG2 cells (5×103) were incubated with
TIPE-2 (0.5–2.5 mg/ml; SinoGenoMax Co., Ltd., Beijing, China) in
96-well plates for 24, 48 and 72 h at 37°C in triplicate for each
condition. In the control group, cells were treated with 2 ml PBS
instead of TIPE-2. Cell proliferation was determined using a MTT
assay kit (Roche Diagnostics, Indianapolis, IN, USA). At each time
point, 20 µl MTT (5 mg/ml) in PBS was added to each well and the
plate was incubated for 4 h at 37°C. Most of the medium was then
removed and 100 µl dimethyl sulfoxide was added to the wells to
solubilize the crystals. Optical density was measured using a
Bio-Rad ELISA reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) at a wavelength of 450 nm.
Colony formation assay
Culture medium supplemented with TIPE-2 (2 mg/ml) or
with PBS was added to 6-well plates (2 ml per well). HepG2 cells
(1×105 cells/well) were seeded into 6-well plates.
Medium was replaced at 48 h following cell attachment and
subsequently every 48 h. Cells were trypsinized (1% trypsin),
suspended in a 500 µl volume of medium and a hemacytometer was used
to determine the total number of cells per well on day 4. The
colony formation assays were performed in triplicate.
mRNA expression analysis by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total cellular RNA was extracted from
PI3K-overexpressed (PI3KOR), TIPE-2-overexpressed (TIPE-2OR) or
control HepG2 cells using the RNeasy mini kit (Qiagen Sciences,
Inc., Gaithersburg, MD, USA) according to the manufacturer's
protocol. A total of 1 µg total RNA was reverse transcribed into
cDNA for 2 h at 42°C. cDNA (0.1 µg) was subjected to qPCR using an
iQ SYBR-Green system (Bio-Rad Laboratories, Inc.). All primers were
synthesized by Invitrogen (Thermo Fisher Scientific, Inc.) and the
sequences used were as follows: PI3K forward,
5′-CTCATGCCAGGCACTGTGCTA-3′ and reverse,
5′-GAATCAAGGCACACCTGTGGAA-3′; TIPE-2 forward,
5′-GGAACATCCAAGGCAAGACTG-3′ and reverse,
5′-AGCACCTCACTGCTTGTCTCATC-3′; and β-actin forward,
5′-GACTACCTCATGAAGATCCTCACC-3′ and reverse,
5′-TCTCCTTAATGTCACGCACGATT-3′. PCR was performed with the following
thermocycling conditions: 45 amplification cycles of 96°C for 30
sec, annealing at 63°C for 20 sec, with touchdown to 54°C for 20
sec and extension at 72°C for 5 min. Results were expressed as a
fold change by comparing levels of target mRNA expression to that
of the reference gene using the 2−ΔΔCq method (25).
Western blot analysis
HepG2 cells were treated with TIPE-2 (2.0 mg/ml) for
24 h and were then homogenized with lysis buffer containing
protease-inhibitor (cat. no. P3480; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). Protein concentration was measured with a
bicinchoninic acid protein assay kit (Thermo Fisher Scientific,
Inc.), protein (10 µg/lane) was separated by 12.5% SDS-PAGE and
subsequently transferred to polyvinylidene fluoride membranes.
Membranes were blocked with 5% milk for 2 h at 37°C prior to
incubation with the following primary antibodies overnight at 4°C:
PI3K (1:1,000; cat. no. ab191606), AKT (1:1,000; cat. no. ab8805),
neuroblastoma Ras viral oncogene (N-ras; 1:1,000; cat. no.
ab206969), P27 (1:1,000; cat. no. ab191606), phosphorylated (p)AKT
(1:1,000; cat. no. ab81283), binding immunoglobulin protein (BIP;
1:1,000; cat. no. ab108615), P53 (1:1,000; cat. no. ab1431), B-cell
lymphoma 2 (Bcl-2; 1:1,000; cat. no. ab59348), cyclin D1 (1:1,000;
cat. no. ab134175), cyclin-dependent kinase (CDK)1 (1:1,000; cat.
no. ab131450), CDK2 (1:1,000; cat. no. ab32147), vimentin (VIM;
1:1,000; cat. no. ab92547), collagen type I (CT-I; 1:1,000; cat.
no. ab34710), Slug (1:1,000; cat. no. ab27568), c-Jun N-terminal
kinase (JNK; 1:1,000; cat. no. ab124956), nuclear factor (NF)-κB
(1:1,000; cat. no. ab28849), nuclear factor (erythroid-derived
2)-like 2 (NRF2) (1:1,000; cat. no. ab62352), caspase-3 (1:1,000;
cat. no. ab13847), caspase-8 (1:1,000; cat. no. ab25901),
glucose-regulated protein 78 (GRP78; 1:1,000; cat. no. ab21685),
CCAAT-enhancer-binding protein homologous protein (CHOP; 1:1,000;
cat. no. ab179823), eukaryotic initiation factor 2 (eIF2α; 1:1,000;
cat. no. ab32713), peIF2α (1:1,000; cat. no. ab214434) and β-actin
(1:1,000; cat. no. ab827; Abcam, Shanghai, China) for 12 h at 4°C.
Membranes were then incubated with goat anti-rabbit horseradish
peroxidase (HRP)-conjugated immunoglobulin G secondary antibody
(1:2,000; cat. no. PV-6001; OriGene Technologies, Inc., Beijing,
China) for 24 h at 4°C. The blots were visualized using a
chemiluminescence detection system (GE Healthcare, Chicago, IL,
USA). Relative protein expression levels were calculated using
Quantity-One software (version 3.0; Bio-Rad Laboratories, Inc.) and
are presented as n-fold of β-actin expression levels.
Cell invasion and migration
assays
HepG2 cells were treated with TIPE-2 (2.0 mg/ml) for
24 h; untreated cells were used as a control. Migration and
invasion assays of HepG2 cells were conducted in 6-well culture
plates with chamber inserts (BD Biosciences, San Diego, CA, USA).
For migration assays, 1×106/well HepG2 cells were placed
into the upper chamber with DMEM medium. The lower chamber
contained DMEM medium with 0.1% FBS. For invasion assays, cells
(1×106/well) were placed into the upper chamber with a
Matrigel-coated insert. After 24 h culture at 37°C, migratory and
invasive cells were fixed with 1.4% paraformaldehyde for 30 min at
37°C and were stained for 30 min in 0.1% crystal violet solution in
PBS. Migration and invasion of HepG2 cells was counted in at least
three randomly selected fields per membrane using a light
microscope (Olympus BX51; Olympus Corporation, Tokyo, Japan).
Flow cytometric analysis
HepG2 cells were cultured until they reached 85%
confluence. Apoptotic rate was assessed following incubation with
TIPE-2 (2.0 mg/ml) for 48 h. Briefly, HepG2 cells were trypsinized
and collected, the cells were then washed in cold PBS, adjusted to
1×108 cells/ml with PBS, and labeled with Annexin
V-fluorescein isothiocyanate (FITC) and propidium iodide (PI;
Annexin V-FITC kit; BD Biosciences) for 2 h at 4°C. Apoptotic rate
was analyzed using FCS Express™ IVD software (version 4;
De Novo Software, Glendale, CA, USA).
Viability assay
HepG2 cells were cultured in 6-well plates until
they reached 85% confluence and the medium was then removed.
Subsequently, HepG2 cells were washed three times and were
incubated with DMEM supplemented with 10% FBS and TIPE-2 (2.0
mg/ml) for 24 h. Subsequently, the supernatant was removed and
cells were incubated with Triton X-100 (1%) for 30 min. Lactate
dehydrogenase activity in cell lysates was used to analyze cell
viability using the Promega CytoTox 96 assay kit (Promega
Corporation, Madison, WI, USA), according to the manufacturer's
protocol.
Analysis of the cell cycle
To analyze the effects of TIPE-2 (2 mg/ml) on the
HepG2 cell cycle, flow cytometry was performed. HepG2 cells in the
exponential phase of growth were treated with TIPE-2 (2 mg/ml) for
48 h at 37°C. Cells were subsequently washed with PBS, trypsinized
(1% trypsin) for 5 min at 37°C and rinsed with PBS. Cells were
fixed in 75% ice-cold ethanol for 15 min and washed with PBS three
times. RNaseA (20 µg ml/l; Fermentas; Thermo Fisher Scientific,
Inc.) was added to fixed cells, which were subsequently stained
with PI (20 µg ml/l; Sigma-Aldrich; Merck KGaA) for 10 min at 37°C.
The percentage of cells in the G1, G2 and S phase were analyzed
using a cell cycle analysis kit (cat. no. C34568; Invitrogen;
Thermo Fisher Scientific, Inc.), a BD FACSCalibur flow cytometer
(BD Biosciences) and FCS Express IVD software (version 4; De Novo
Software).
Animal experiments
A total of 80 specific pathogen-free (SPF) female
BALB/c nude mice (age, 6–8 weeks) were purchased from Harbin
Veterinary Research Institute (Harbin, China). All mice were feed
under pathogen-free conditions. Mice were maintained at 22–24°C in
a 12 h light/dark cycle with ad libitum access to food and
water. A total of 5×107 HepG2 cells were injected into
the right flank of female BALB/c nude mice at a total volume of 200
µl. Tumor-bearing mice then underwent intratumoral injection with
TIPE-2 (6.0 mg/ml) or PBS (n=40/group), once tumor diameters
reached 5–8 mm on day 6 after tumor inoculation. The treatment was
continued 15 times at intervals of every two days for a total of 30
days. Tumor diameters were recorded once every 2 days and tumor
volume was calculated using the following formula: 0.52 × smallest
diameter2 × largest diameter. Survival analysis was
conducted over 120 days to analyze the therapeutic effects of
TIPE-2 in tumor-bearing mice.
Immunohistochemistry
Immunohistochemical staining was performed according
to the avidin-biotin-peroxidase technique. HCC tissues were
isolated from experimental mice and paraffin-embedded tissue
sections (4 µm) were prepared and epitope retrieval was performed
by heating the tissue sections at 100°C for 30 min in a citrate
solution (10 mmol/l; pH 6.0) followed by dewaxing in xylene and
rehydrating in a graded ethanol series for further analysis.
Subsequently, paraffin-embedded sections were treated with hydrogen
peroxide (3%) for 10–15 min and were blocked in 5% bovine serum
albumin (Sigma-Aldrich; Merck KGaA) for 10–15 min at 37°C. Finally,
the sections were incubated with biotinylated goat anti-mouse
caspase-3 (1:1,000; cat. no. ab13847), caspase-9 (1:1,000; cat. no.
ab32539), PI3K (1:1,000; cat. no. ab191606), AKT (1:1,000; cat. no.
ab8805), GRP78 (1:1,000; cat. no. ab21685) and CHOP (1:1,000; cat.
no. ab179823) antibodies (Abcam) at 4°C for 12 h. Samples were
washed three times with PBS and then incubated with HRP-conjugated
goat anti-rabbit secondary antibody (1:2,000, cat. no. PV-6001;
OriGene Technologies, Inc.) for 2 h at 37°C. 3,3′-diaminobenzidene
(0.05%) was used as the chromogen for 30 min at 37°C and 1%
hematoxylin as the nuclear counterstain for 30 min at 37°C. The
relative protein expression levels were analyzed using a
chemiluminescence detection system (GE Healthcare). Tumor tissue
images were captured with a ZEISS LSM 510 confocal microscope
(magnification, ×40; Zeiss AG, Oberkochen, Germany). Relative
protein expression levels were determined using Quantity-One
software 3.0 (Bio-Rad Laboratories, Inc.) and are presented as the
n-fold of β-actin expression levels.
Immunocytochemistry
HepG2 cells were treated with TIPE-2 (2 mg/ml) for
12 h at 37°C. Following this, cells were washed with PBS at room
temperature and fixed with 4% paraformaldehyde for 1 h at 37°C. The
cells were washed again with PBS three times, blocked with 5%
bovine serum albumin (Sigma-Aldrich; Merck KGaA) for 2 h at 37°C
and subsequently stained with the following antibodies for 12 h at
4°C: Ki67 (1:1,000; cat. no. ab15580; Abcam), PCNA (1:1,000; cat.
no. ab18197; Abcam), E-cadherin (1:1,000; cat. no. ab40772; Abcam)
and fibronectin (1:1,000; cat. no. ab2413; Abcam). Cells were
washed with PBS and subsequently incubated with an Oregon
Green® 488-conjugated IgG (1:1,000; cat. no. O-6382;
Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h in a
light-protected chamber at 37°C. Immunofluorescence signals were
detected using a laser scanning confocal microscope (magnification,
×40; Zeiss AG).
Statistical analysis
All data are presented as the mean ± standard error
of the mean of triplicate experiments. SPSS 19.0 software (IBM
Corp., Armonk, NY, USA) was used for statistical analysis. Unpaired
data were analyzed by Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Analysis of the inhibitory effects of
TIPE-2 on HCC cell growth and proliferation in vitro
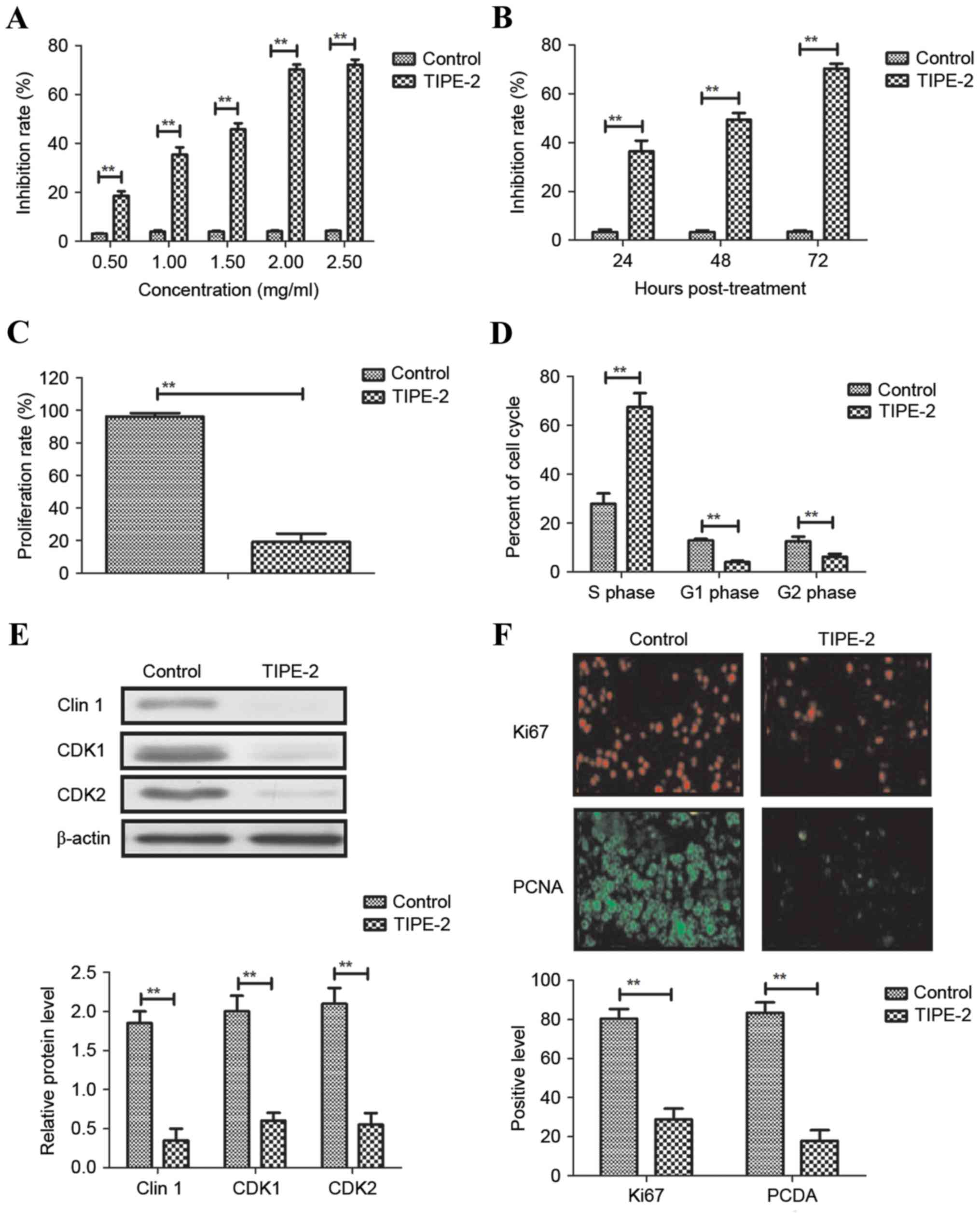
As shown in Fig. 1A and
B, TIPE-2 administration (0.5, 1, 1.5, 2.0 and 2.5 mg/ml)
significantly inhibited HepG2 cell growth in a dose- and
time-dependent manner. The results indicated that 2.0 mg/ml TIPE-2
markedly suppressed HCC cell proliferation and increased the number
of cells in S phase of the cell cycle (Fig. 1C and D). Western blot analysis
demonstrated that TIPE-2 administration (2.0 mg/ml) decreased the
expression levels of cyclin D1, CDK1 and CDK2 in HepG2 cells
(Fig. 1E). Immunofluorescence
indicated that TIPE-2 administration (2.0 mg/ml) decreased the
expression levels of Ki67 and proliferating cell nuclear antigen in
HepG2 cells (Fig. 1F). These
results suggested that TIPE-2 may significantly inhibit HCC cell
growth and proliferation in vitro.
Analysis of the inhibitory effects of
TIPE-2 on HCC cell migration and invasion in vitro
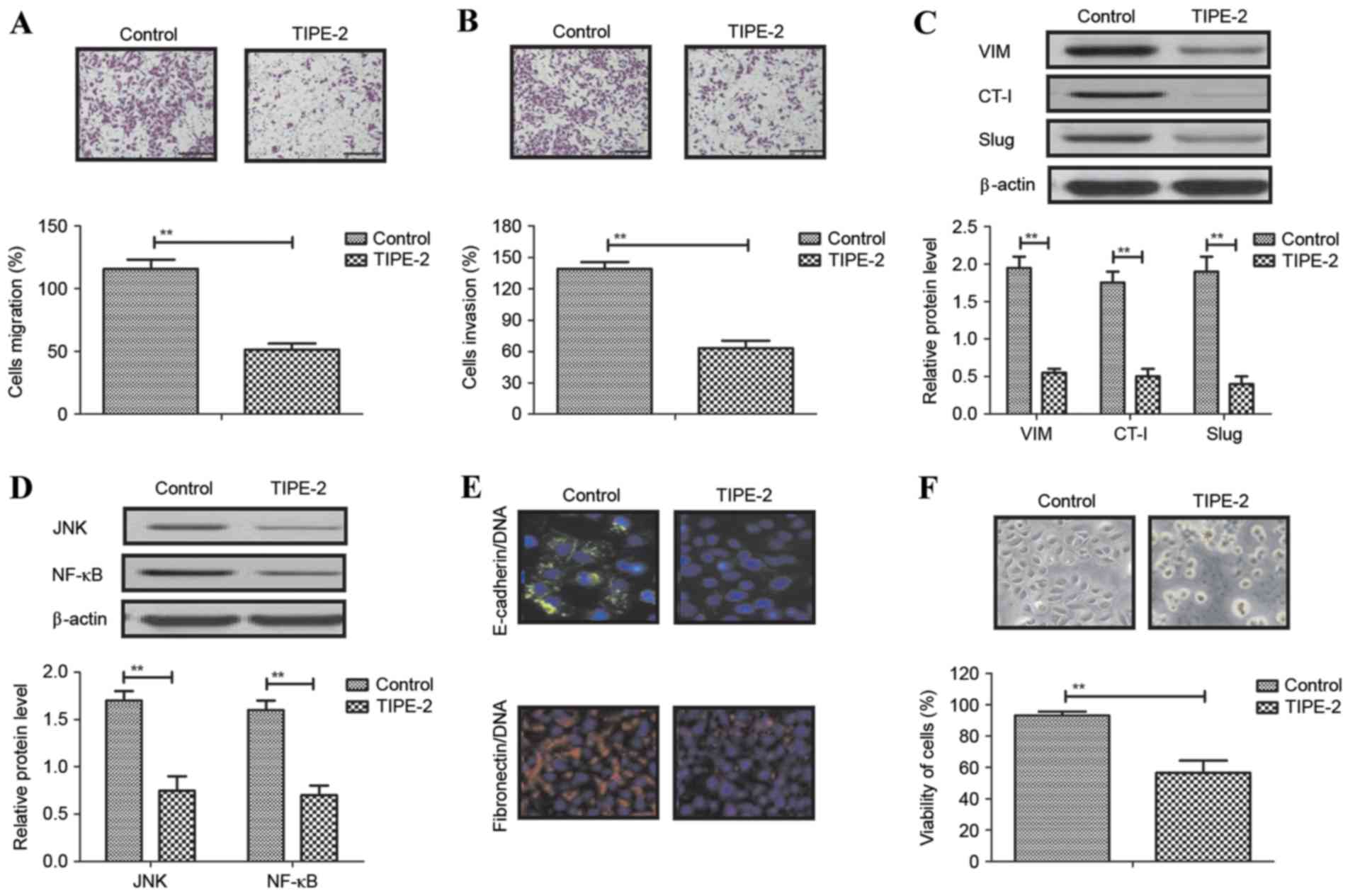
Local migration and long-distance invasion are the
most common characteristics of HCC. The present study investigated
migration and invasion of HepG2 cells following treatment with
TIPE-2 (2.0 mg/ml). Migration and invasion assays demonstrated that
TIPE-2 significantly suppressed HepG2 cell migration and invasion
in vitro (Fig. 2A and B).
Western blot analysis demonstrated that TIPE-2 treatment decreased
the expression levels of VIM, CT-I and Slug in HepG2 cells
(Fig. 2C). In addition, TIPE-2
decreased JNK and NF-κB expression in HepG2 cells (Fig. 2D). Immunofluorescence demonstrated
that E-cadherin/DNA and fibronectin/DNA expression levels were
decreased in HepG2 cells following TIPE-2 treatment (Fig. 2E). HepG2 cell viability was also
decreased by TIPE-2 treatment (Fig.
2F). These results indicated that TIPE-2 may significantly
inhibit migration and invasion of HepG2 cells via inhibiting the
expression levels of cancer cell migration-associated proteins.
Analysis of the effects of TIPE-2 on
HCC cell apoptosis in vitro
As shown in Fig.
3A, TIPE-2 administration (2.0 mg/ml) promoted apoptosis of
HepG2 cells after 48 h. Western blotting demonstrated that the
expression levels of caspase-3 and caspase-8 were increased by
TIPE-2 administration (Fig. 3B).
In addition, TIPE-2 administration downregulated P53 and Bcl-2
expression levels in HepG2 cells (Fig.
3C). Results also indicated that the expression and levels of
eIF2α and peIF2α were upregulated in HepG2 cells following TIPE-2
treatment (Fig. 3D). Furthermore,
the expression levels of NRF2 and BIP were increased in HepG2 cells
following TIPE-2 treatment (Fig.
3E). The expression levels of GRP78 and CHOP were also
increased in HepG2 cells following TIPE-2 treatment (Fig. 3F). These results suggested that
TIPE-2 may promote HCC apoptosis in vitro.
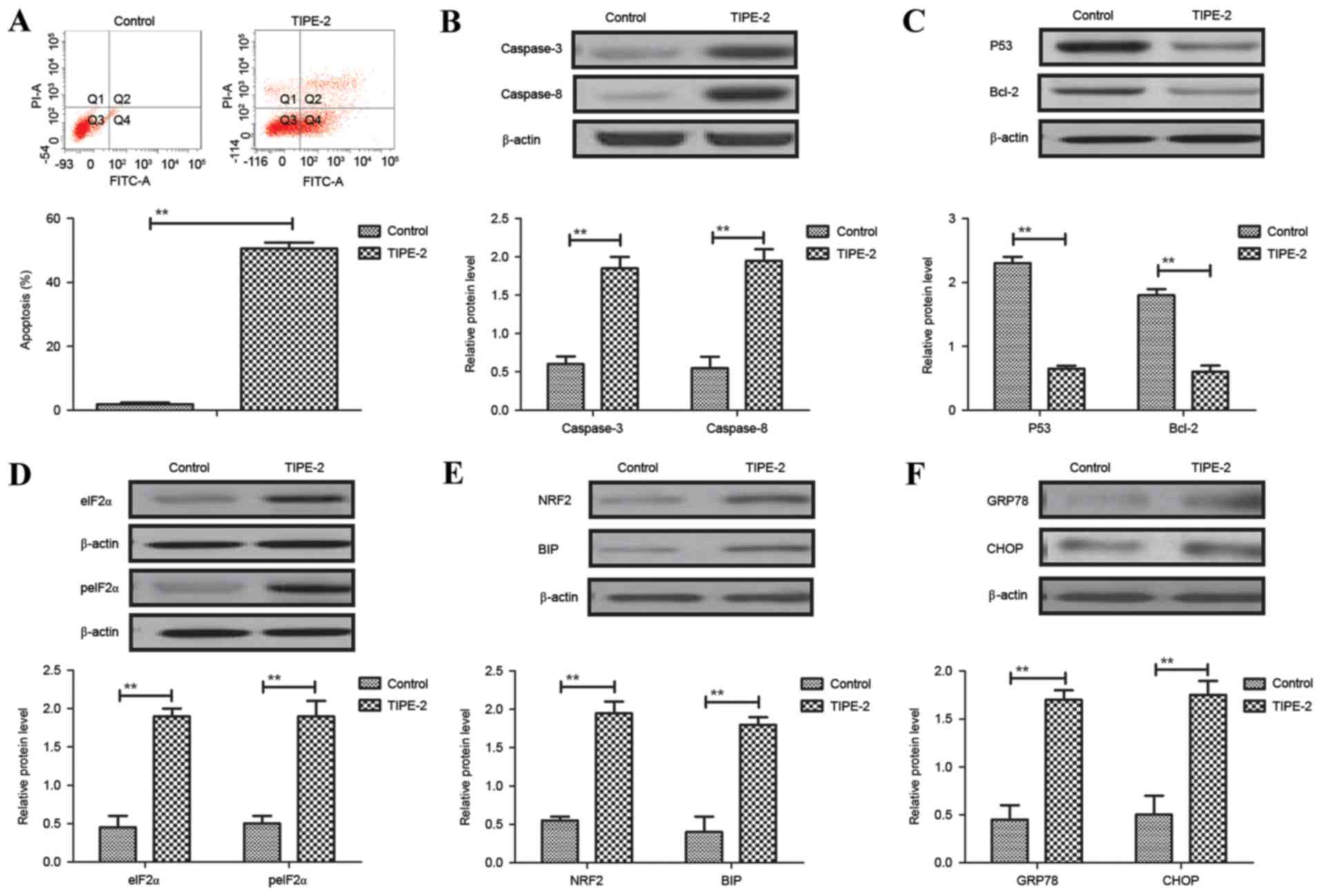 | Figure 3.Effects of TIPE-2 (2.0 mg/ml) on
hepatocellular carcinoma cell apoptosis in vitro. (A)
Effects of TIPE-2 on apoptosis of HepG2 cells after 48 h, as
determined by flow cytometry. Effects of TIPE-2 treatment on the
expression levels of (B) caspase-3 and caspase-8, (C) P53 and
Bcl-2, (D) peIF2α and eIF2α, (E) NRF2 and BIP, and (F) GRP78 and
CHOP in HepG2 cells, as determined by western blotting. **P<0.01
vs. control group. Bcl-2, B-cell lymphoma 2; BIP, binding
immunoglobulin protein; CHOP, CCAAT-enhancer-binding protein
homologous protein; eIF2α, eukaryotic initiation factor; FITC,
fluorescein isothiocyanate; GRP78, glucose-regulated protein 78;
peIF2α, phosphorylated-eIF2α; PI, propidium iodide; TIPE-2, tumor
necrosis factor-α-induced protein-8 like-2. |
TIPE-2 regulates HCC cell apoptosis
via regulation of the PI3K/AKT signaling pathway
As shown in Fig.
4A, the present study demonstrated that TIPE-2 significantly
inhibited PI3K and AKT expression in HepG2 cells. In addition,
N-ras and P27 expression were decreased by TIPE-2 treatment in
HepG2 cells (Fig. 4B). pAKT levels
were also decreased by TIPE-2 treatment in HepG2 cells when
compared with pAKT levels in the control group (Fig. 4C). We observed that TIPE-2
decreased ratio of pAkt:Akt in HepG2 cells (Fig. 4D). As shown in Fig. 4E and F, pedue12.4-PI3K or
pedue12.4-TIPE-2 upregulated PI3K and TIPE-2 mRNA expression,
respectively, compared with pedue12.4-vector in HepG2 cells.
Mechanistic analysis indicated that PI3K overexpression alone
(PI3KOR) inhibited the apoptosis of HepG2 cells, whereas TIPE-2
treatment abolished this effect (Fig.
4G). In addition, N-ras and BIP expression was increased by
PI3KOR in HepG2 cells (Fig. 4H).
In addition, PI3KOR increased the expression levels of P53 and
Bcl-2 in HepG2 cells when compared to the control group (Fig. 4I). These results suggested that
TIPE-2 may regulate HCC cell apoptosis through regulation of the
PI3K/AKT signaling pathway.
 | Figure 4.TIPE-2 regulates hepatocellular
carcinoma cell apoptosis through regulation of the PI3K/AKT
signaling pathway. Effects of TIPE-2 treatment on the expression
levels of (A) PI3K and AKT, (B) N-ras and P27, and (C) pAKT in
HepG2 cells. (D) TIPE-2 decreased the ratio of pAkt:Akt in HepG2
cells. Transfection with (E) pedue12.4-PI3K or (F) TIPE-2OR
upregulates PI3K or TIPE-2 mRNA expression in HepG2 cells,
respectively. **P<0.01 vs. control group. (G) PI3KOR prevented
the apoptosis promoted by TIPE-2 treatment in HepG2 cells
**P<0.01, PI3KOR-TIPE-2 vs. PI3KOR or control group. Effects of
PI3KOR on (H) N-ras and BIP, and (I) P53 and Bcl-2 expression in
HepG2 cells. **P<0.01, PI3KOR-TIPE-2 or PI3KOR vs. control
group. AKT, protein kinase B; Bcl-2, B-cell lymphoma 2; FITC,
fluorescein isothiocyanate; N-ras, neuroblastoma Ras viral
oncogene; OR, overexpression; pAKT, phosphorylated-AKT; PI,
propidium iodide; PI3K, phosphoinositide 3-kinase; TIPE-2, tumor
necrosis factor-α-induced protein-8 like-2. |
In vivo anticancer effects of TIPE-2
treatment on HepG2-bearing mice
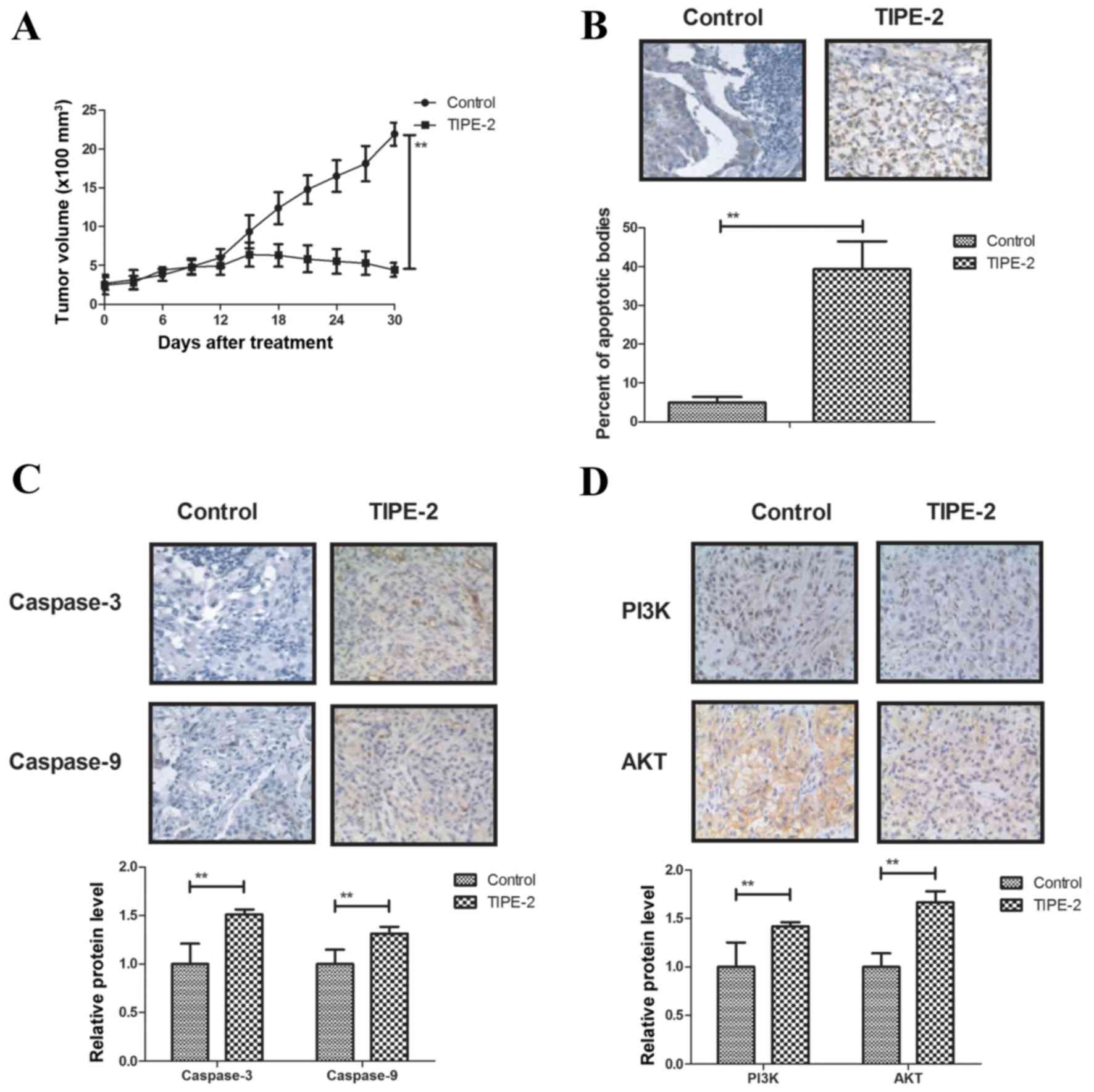
The present study further investigated the antitumor
effects of TIPE-2 treatment on HepG2-bearing nude mice. In order to
avoid the interference of pathogenic microorganisms, SPF female
BALB/c mice (body weight, 30–35 g) were implanted with HepG2 cells
and treated with TIPE-2. As shown in Fig. 5A, TIPE-2 treatment (6 mg/kg)
inhibited tumor growth during the 30-day observation period
compared with in the control group. TUNEL assay demonstrated that
TIPE-2 treatment increased the number of apoptotic cells in tumor
tissues compared with in the PBS group (Fig. 5B). In addition, TIPE-2 treatment
increased the expression levels of caspase-3 and caspase-8
(Fig. 5C), and upregulated PI3K
and AKT in tumor tissues (Fig.
5D). Furthermore, TIPE-2 treatment increased GRP78 and CHOP
expression in tumor tissues (Fig.
5E). Notably, survival of HepG2-bearing mice was prolonged by
TIPE-2 treatment during the 120-day experiment (Fig. 5F). These results suggested that
TIPE-2 may be a potential anticancer agent for the treatment of
HCC.
Discussion
Advanced stage HCC possesses aggressive potential
for migration to adjacent and distant cells, and/or organs
(26,27). Clinical therapies are required that
inhibit the migration and invasion of HCC cells, in order to
prolong the survival of patients with HCC (28,29).
It has previously been suggested that TIPE-2 serves an important
role in tumorigenesis, and tumor growth, proliferation,
aggressiveness and apoptosis. Although a previous study indicated
the relevance of targeting the tumor suppressor gene TIPE-2, the
molecular mechanism underlying TIPE-2-mediated apoptosis remains to
be fully elucidated (30). The
present study evaluated the inhibitory effects of TIPE-2 on growth,
aggressiveness and apoptosis of HCC cells in vitro, as well
as the underlying mechanism of TIPE-2-mediated apoptosis of HepG2
cells. The results demonstrated that TIPE-2 administration
significantly inhibited HCC cell growth, proliferation and
aggressiveness. In addition, TIPE-2 administration promoted HCC
cell apoptosis through regulation of the PI3K/AKT signaling
pathway. Notably, the present findings suggested that TIPE-2 may be
a potential anticancer agent for the treatment of HCC, as it was
able to inhibit tumor growth and prolong survival. However, it must
be noted that the HepG2 cell line is potentially misidentified, and
may be derived from hepatoblastoma, rather than HCC (31). Therefore, the present study may
identify the inhibitory effects of TIPE-2 on hepatic cancer cells
in general, rather than on HCC cells specifically.
Induction of apoptosis and tumor cell death, thus
resulting in the inhibition of growth and aggressiveness in
patients, is the ultimate goal in neoplastic therapy (32). TIPE-2 is able to inhibit
TLR4-mediated development of colon cancer via the downregulation of
caspase-8 activity. In the present study, TIPE-2 increased
caspase-8 expression in HCC cells, thus promoting apoptosis of
HepG2 cells. A previous study demonstrated that TIPE-2 may inhibit
TNF-α-mediated HCC cell metastasis through the downregulation of
extracellular signal-regulated kinase and the NF-κB signaling
pathway (33). In addition, it has
previously been indicated that regulation of P53-, Bcl-2- and
caspase-dependent signaling pathways in drug-induced apoptosis of
HepG2 cells contributes to anti-proliferative effects (34). Notably, enhancement of P53 protein
nuclear export may inhibit cisplatin-induced HepG2 cell apoptosis,
which may hinder anticancer drug-induced HepG2 cell apoptosis
(35). The present study
demonstrated that TIPE-2 not only downregulated the expression of
anti-apoptotic proteins (P53 and Bcl-2), but also indicated that
TIPE-2 regulated apoptosis of HCC cells via the PI3K/AKT signaling
pathway. In addition, another study revealed that bortezomib may
arrest the proliferation of HepG2 HCC cells by increasing P27
(kip1) (36). Furthermore, Lin and
Chiang (37) suggested that
increasing P27 levels is conducive to anticancer drug-induced
inhibition of human HCC HepG2 cells proliferation. The present data
indicated that TIPE-2 treatment downregulated p27 expression, which
may lead to inhibition of HepG2 cell proliferation.
Apoptosis induction and G2/M cell cycle
arrest in human cancer cells via the Ras/Raf/mitogen-activated
protein kinase and PI3K/AKT signaling pathways has been reported in
previous studies (38,39). The present results indicated that
TIPE-2 decreased PI3K and AKT expression in HCC cells, which may
have promoted apoptosis, and inhibited HepG2 cell growth and
aggressiveness. Yue et al (40) reported that PI3K/AKT activation
underlies human prostate cancer aggressiveness, and may be a target
signaling pathway for human cancer therapy. The present findings
indicated that TIPE-2 inhibited growth and aggressiveness of HCC
cells through downregulation of the expression levels of VIM, CT-I
and Slug. Notably, the present findings suggested that TIPE-2
promoted apoptosis in HCC in vitro and in vivo.
Endoplasmic reticulum stress is implicated in the
progression of human carcinoma via the regulation of apoptotic
signaling pathways in tumor cells (41). Edagawa et al (42) indicated that endoplasmic reticulum
stress induced sensitization of P53-deficient human colon cancer
cells to TNF-related apoptosis-inducing ligand-mediated apoptosis
via the upregulation of death receptor 5, which may further lead to
tumor growth inhibition. In the present study, the results
demonstrated that TIPE-2 could promote HCC cell apoptosis through
endoplasmic reticulum stress in vitro. Although research has
indicated that TIPE-2 overexpression suppresses the proliferation,
migration and invasion of prostate cancer cells by downregulation
of the PI3K/AKT signaling pathway, to the best of our knowledge,
TIPE-2-mediated apoptosis has been not reported in a previous study
in HCC cells (43). In addition,
TIPE-2 has been reported to suppress angiogenesis and invasiveness
of non-small cell lung cancer via inhibiting Rac1 activation and
vascular endothelial growth factor expression (44). The present study indicated that
TIPE-2 treatment may regulate HCC cell apoptosis via regulation of
the PI3K/AKT signaling pathway, which may result in activation of
endoplasmic reticulum stress in HCC cells through promoting NRF2
and BIP expression (45).
In conclusion, the present study identified that
TIPE-2 administration may efficiently inhibit HCC cell growth and
aggressiveness via downregulation of the expression levels of VIM,
CT-I and Slug. In addition, the results indicated that TIPE-2
administration promoted apoptosis of HCC cells through the PI3K/AKT
signaling pathway, which may further contribute to the inhibition
of tumor growth and may prolong survival of HepG2-bearing mice.
These findings suggested that TIPE-2 may be a promising anticancer
agent for the treatment of HCC.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Tianjin, China (grant no. 11JCYBJC28300), the
Science and Technology Foundation of Tianjin Health Bureau (grant
no. 2014KZ119), and the National Clinical Key Subject Construction
Project of NHFPC Fund.
References
|
1
|
Menon KV, Hakeem AR and Heaton ND: Review
article: Liver transplantation for hepatocellular carcinoma-a
critical appraisal of the current worldwide listing criteria.
Aliment Pharmacol Ther. 40:893–902. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shariff MI, Cox IJ, Gomaa AI, Khan SA,
Gedroyc W and Taylor-Robinson SD: Hepatocellular carcinoma: Current
trends in worldwide epidemiology, risk factors, diagnosis and
therapeutics. Expert Rev Gastroenterol Hepatol. 3:353–367. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fung SK and Lok AS: Management of patients
with hepatitis B virus-induced cirrhosis. J Hepatol. 42
Suppl:S54–S64. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chinnaratha MA, Chuang MY, Fraser RJ,
Woodman RJ and Wigg AJ: Percutaneous thermal ablation for primary
hepatocellular carcinoma: A systematic review and meta-analysis. J
Gastroenterol Hepatol. 31:294–301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang YH, Wu JC, Chen SC, Chen CH, Chiang
JH, Huo TI, Lee PC, Chang FY and Lee SD: Survival benefit of
transcatheter arterial chemoembolization in patients with
hepatocellular carcinoma larger than 10 cm in diameter. Aliment
Pharmacol Ther. 23:129–135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim SS, Cho HJ, Lee HY, Park JH, Noh CK,
Shin SJ, Lee KM, Yoo BM, Lee KJ, Cho SW and Cheong JY: Genetic
polymorphisms in the Wnt/β-catenin pathway genes as predictors of
tumor development and survival in patients with hepatitis B
virus-associated hepatocellular carcinoma. Clin Biochem.
49:792–801. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dhir M, Melin AA, Douaiher J, Lin C, Zhen
WK, Hussain SM, Geschwind JF, Doyle MB, Abou-Alfa GK and Are C: A
review and update of treatment options and controversies in the
management of hepatocellular carcinoma. Ann Surg. 263:1112–1125.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Simonetti RG, Cammà C, Fiorello F, Politi
F, D'Amico G and Pagliaro L: Hepatocellular carcinoma. A worldwide
problem and the major risk factors. Dig Dis Sci. 36:962–972. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zidan A, Scheuerlein H, Schüle S,
Settmacher U and Rauchfuss F: Epidemiological pattern of hepatitis
B and hepatitis C as etiological agents for hepatocellular
carcinoma in iran and worldwide. Hepat Mon. 12:e68942012.PubMed/NCBI
|
|
10
|
Guo Z, Yu H, Liu C, Si T, Yang X, Zhang W,
Xu Y and Li Y: Advances in endovascular therapy to treat primary
hepatocellular carcinoma. Drug Discov Ther. 9:342–351. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang J, Yu C, Chen M, Tian S and Sun C:
Over-expression of TRIM37 promotes cell migration and metastasis in
hepatocellular carcinoma by activating Wnt/beta-catenin signaling.
Biochem Biophys Res Commun. 464:1120–1127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Forner A, Reig M, Varela M, Burrel M,
Feliu J, Briceño J, Sastre J, Martí-Bonmati L, Llovet JM, Bilbao
JI, et al: Diagnosis and treatment of hepatocellular carcinoma.
Update consensus document from the AEEH, SEOM, SERAM, SERVEI and
SETH. Med Clin (Barc). 146:511.e1–511.e22. 2016.(In Spanish).
View Article : Google Scholar
|
|
13
|
Kim JH, Badawi M, Park JK, Jiang J, Mo X,
Roberts LR and Schmittgen TD: Anti-invasion and anti-migration
effects of miR-199a-3p in hepatocellular carcinoma are due in part
to targeting CD151. Int J Oncol. 49:2037–2045. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu M, Liu Q, Jia Y, Tu K, Yao Y and Guo C:
BCAT1 promotes tumor cell migration and invasion in hepatocellular
carcinoma. Oncol Lett. 12:2648–2656. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng XS, Sun SB, Zhong F, He K and Zhou
J: Knockdown of histone methyltransferase hSETD1A inhibits
progression, migration, and invasion in human hepatocellular
carcinoma. Oncol Res. 24:239–245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu L, Zhang M, Zheng X, Yi P, Lan C and Xu
M: The circular RNA ciRS-7 (Cdr1as) acts as a risk factor of
hepatic microvascular invasion in hepatocellular carcinoma. J
Cancer Res Clin Oncol. 143:17–27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen YJ, Chen CC and Huang HL: Induction
of apoptosis by Armillaria mellea constituent armillarikin in human
hepatocellular carcinoma. Onco Targets Ther. 9:4773–4783. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Banjerdpongchai R, Wudtiwai B and Khawon
P: Induction of human hepatocellular carcinoma HepG2 cell apoptosis
by naringin. Asian Pac J Cancer Prev. 17:3289–3294. 2016.PubMed/NCBI
|
|
19
|
Wang K, Ren Y, Liu Y, Zhang J and He JJ:
Tumor necrosis factor (TNF)-α-induced protein 8-like-2 (TIPE2)
inhibits proliferation and tumorigenesis in breast cancer cells.
Oncol Res. 25:55–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao X, Zhang L, Shi Y, Sun Y, Dai S, Guo
C, Zhu F, Wang Q, Wang J, Wang X, et al: Human tumor necrosis
factor (TNF)-alpha-induced protein 8-like 2 suppresses
hepatocellular carcinoma metastasis through inhibiting Rac1. Mol
Cancer. 12:1492013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li XM, Su JR, Yan SP, Cheng ZL, Yang TT
and Zhu Q: A novel inflammatory regulator TIPE2 inhibits
TLR4-mediated development of colon cancer via caspase-8. Cancer
Biomark. 14:233–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao Q, Zhao M, Dong T, Zhou C, Peng Y,
Zhou X, Fan B, Ma W, Han M and Liu S: Tumor necrosis
factor-α-induced protein-8 like-2 (TIPE2) upregulates p27 to
decrease gastic cancer cell proliferation. J Cell Biochem.
116:1121–1129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ruan Q, Wang P, Wang T, Qi J, Wei M, Wang
S, Fan T, Johnson D, Wan X, Shi W, et al: MicroRNA-21 regulates
T-cell apoptosis by directly targeting the tumor suppressor gene
Tipe2. Cell Death Dis. 5:e10952014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Renshaw A and Elsheikh TM: A validation
study of the Focalpoint GS imaging system for gynecologic cytology
screening. Cancer Cytopathol. 121:737–738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Poon RT, Fan ST, O'Suilleabhain CB and
Wong J: Aggressive management of patients with extrahepatic and
intrahepatic recurrences of hepatocellular carcinoma by combined
resection and locoregional therapy. J Am Coll Surg. 195:311–318.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Au WY, Lie AK, Liang R, Liu CL, Shek TW
and Lau GK: Aggressive hepatocellular carcinoma complicating
pregnancy after autologous bone marrow transplantation for
non-Hodgkin's lymphoma. Bone Marrow Transplant. 29:177–179. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lubienski A, Bitsch RG, Schemmer P,
Grenacher L, Dux M and Kauffmann GW: Long-term results of
interventional treatment of large unresectable hepatocellular
carcinoma (HCC): Significant survival benefit from combined
transcatheter arterial chemoembolization (TACE) and percutaneous
ethanol injection (PEI) compared to TACE monotherapy. Rofo.
176:1794–1802. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yeh ML, Huang CI, Huang CF, Hsieh MY,
Huang JF, Dai CY, Lin ZY, Chen SC, Yu ML and Chuang WL: Neoadjuvant
transcatheter arterial chemoembolization does not provide survival
benefit compared to curative therapy alone in single hepatocellular
carcinoma. Kaohsiung J Med Sci. 31:77–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu QQ, Zhang FF, Wang F, Qiu JH, Luo CH,
Zhu GY and Liu YF: TIPE2 inhibits lung cancer growth attributing to
promotion of apoptosis by regulating some apoptotic molecules
expression. PLoS One. 10:e01261762015. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
López-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: Hep G2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar
|
|
32
|
Huang CS, Lee YR, Chen CS, Tu SH, Wang YJ,
Lee CH, Chen LC, Chang HW, Chang CH, Chih-Ming S, et al: Long-term
ethanol exposure causes human liver cancer cells to become
resistant to mitomycin C treatment through the inactivation of
bad-mediated apoptosis. Mol Carcinog. 49:728–738. 2010.PubMed/NCBI
|
|
33
|
Zhang YH, Yan HQ, Wang F, Wang YY, Jiang
YN, Wang YN and Gao FG: TIPE2 inhibits TNF-α-induced hepatocellular
carcinoma cell metastasis via Erk1/2 downregulation and NF-κB
activation. Int J Oncol. 46:254–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Handayani T, Sakinah S, Nallappan M and
Pihie AH: Regulation of p53-, Bcl-2- and caspase-dependent
signaling pathway in xanthorrhizol-induced apoptosis of HepG2
hepatoma cells. Anticancer Res. 27:965–971. 2007.PubMed/NCBI
|
|
35
|
Zhang LJ, Li ZQ, Yang YP, Li XW and Ji JF:
Tunicamycin suppresses cisplatin-induced HepG2 cell apoptosis via
enhancing p53 protein nuclear export. Mol Cell Biochem.
327:171–182. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baiz D, Pozzato G, Dapas B, Farra R,
Scaggiante B, Grassi M, Uxa L, Giansante C, Zennaro C, Guarnieri G
and Grassi G: Bortezomib arrests the proliferation of
hepatocellular carcinoma cells HepG2 and JHH6 by differentially
affecting E2F1, p21 and p27 levels. Biochimie. 91:373–382. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin YW and Chiang BH:
4-acetylantroquinonol B isolated from Antrodia cinnamomea arrests
proliferation of human hepatocellular carcinoma HepG2 cell by
affecting p53, p21 and p27 levels. J Agric Food Chem. 59:8625–8631.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shin DY, Kim GY, Hwang HJ, Kim WJ and Choi
YH: Diallyl trisulfide-induced apoptosis of bladder cancer cells is
caspase-dependent and regulated by PI3K/Akt and JNK pathways.
Environ Toxicol Pharmacol. 37:74–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hatashita M, Taniguchi M, Baba K, Koshiba
K, Sato T, Jujo Y, Suzuki R and Hayashi S: Sinodielide A exerts
thermosensitizing effects and induces apoptosis and G2/M cell cycle
arrest in DU145 human prostate cancer cells via the Ras/Raf/MAPK
and PI3K/Akt signaling pathways. Int J Mol Med. 33:406–414. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yue S, Li J, Lee SY, Lee HJ, Shao T, Song
B, Cheng L, Masterson TA, Liu X, Ratliff TL and Cheng JX:
Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT
activation underlies human prostate cancer aggressiveness. Cell
Metab. 19:393–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
de SàBacelar T, da Silva AJ, Costa PR and
Rumjanek VM: The pterocarpanquinone LQB 118 induces apoptosis in
tumor cells through the intrinsic pathway and the endoplasmic
reticulum stress pathway. Anticancer Drugs. 24:73–83. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Edagawa M, Kawauchi J, Hirata M, Goshima
H, Inoue M, Okamoto T, Murakami A, Maehara Y and Kitajima S: Role
of activating transcription factor 3 (ATF3) in endoplasmic
reticulum (ER) stress-induced sensitization of p53-deficient human
colon cancer cells to tumor necrosis factor (TNF)-related
apoptosis-inducing ligand (TRAIL)-mediated apoptosis through
up-regulation of death receptor 5 (DR5) by zerumbone and celecoxib.
J Biol Chem. 289:21544–21561. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lu Q, Liu Z, Li Z, Chen J, Liao Z, Wu WR
and Li YW: TIPE2 overexpression suppresses the proliferation,
migration and invasion in prostate cancer cells by inhibiting
PI3K/Akt signaling pathway. Oncol Res. 24:305–313. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li Z, Guo C, Liu X, Zhou C, Zhu F, Wang X,
Wang Q, Shi Y, Wang J, Zhao W and Zhang L: TIPE2 suppresses
angiogenesis and non-small cell lung cancer (NSCLC) invasiveness
via inhibiting Rac1 activation and VEGF expression. Oncotarget.
7:62224–62239. 2016.PubMed/NCBI
|
|
45
|
Tang J, Guo YS, Zhang Y, Yu XL, Li L,
Huang W, Li Y, Chen B, Jiang JL and Chen ZN: CD147 induces UPR to
inhibit apoptosis and chemosensitivity by increasing the
transcription of Bip in hepatocellular carcinoma. Cell Death
Differ. 19:1779–1790. 2012. View Article : Google Scholar : PubMed/NCBI
|