Introduction
Prostate cancer (PCa) is the second most prevalent
type of cancer affecting the health of men in Western countries.
According to Surveillance, Epidemiology, and End Results Program
(National Institutes of Health, Bethesda, MD, USA) statistics,
there were ~220,800 novel cases of PCa in the USA in 2015 and
86,380 patients succumbed to PCa (1). Due to the inefficiency of
chemotherapy and radiotherapy, therapeutic strategies for this
disease are limited and metastatic disease frequently develops,
even following potentially curative surgery (2–4).
Therefore, it is of great importance to develop novel therapeutics
for the treatment of prostate cancer.
Overexpression of epidermal growth factor receptor
(EGFR) has been reported in a number of types of solid tumor,
including prostate cancer (5).
Activation of EGFR induces the phosphorylation and activation of
its downstream signaling pathways, including the
phosphatidylinositol 3-kinase (PI3K)/RAC-α serine/threonine protein
kinase (AKT) and RAF proto-oncogene serine/threonine-protein kinase
(Raf)/extracellular signal-regulated kinase (Erk) pathways, and
finally leads to cell proliferation (6). Activated AKT disturbs the balance of
apoptosis and cell viability by promoting nuclear factor (NF)-κB
and inhibiting the pro-apoptotic transcription factors forkhead box
proteins (FOXs) (7–9). Overexpression of FOXs (including
FOXO1 in prostate cancer cells) triggers cell cycle arrest and
induces cellular apoptosis by increasing the levels of
Fas-ligand(Fas-L), tumor necrosis factor ligand superfamily member
10 (TRAIL), and Bcl-2-like protein 11 (Bim) in cells of various
tissue types (10). As reported,
the PI3K/AKT/serine/threonine-protein kinase mTOR (mTOR)/ribosomal
protein S6 kinase β-1 (p70S6K) signaling pathway is the primary
pathway that regulates autophagy when cells are exposed to certain
conditions, including starvation, oxidative stress and tumor
suppression (11). Beclin-1 serves
an important role in autophagosome formation and in the crosstalk
between autophagy and apoptosis, and the expression of Beclin-1 is
regulated by the PI3K/Raf/Erk pathway and Bcl-2 (12).
Previous studies have suggested that the occurrence
of certain diseases (including cancer) is associated with immune
suppression, and a number of traditional Chinese medicines (TCMs)
are able to correct the state of immune suppression by improving
the immune system and the disease resistance of the human body,
there by achieving the purpose of treating diseases (13,14).
In the clinical treatment of cancer in a number of Hospitals of
TCM, the results demonstrate that TCM has a holistic and a local
therapeutic effect, in addition to the synergistic effect of the
combination of single ingredients and monomers. Although TCM has
the unique advantages of the whole-body and complementary effects
that single medicine (Western medicine) does not have in
anti-cancer treatment (15), the
molecular mechanism of the bio-functions of TCM in the treatment of
various cancers remains to be completely elucidated.
The present study investigated the effects of TCM-1
(water-extract from a type of anti-PCa TCM) on prostate cancer
cells (including androgen-dependent LNCaP and androgen-independent
PC3 cell lines) in vitro and in vivo. The results
indicated that TCM-1 was able to decrease cell viability and arrest
the cell cycle at the G1 phase, finally resulting in marked
autophagy and apoptosis in prostate cancer cells. From the results
of the present study, TCM-1 exerteda dose-dependent downregulation
of the activity of the EGFR signaling pathway by inhibiting
EGF-induced autophosphorylation of EGFR. Inhibition of EGFR
activity resulted in restraining its downstream signaling pathways
(including the PI3K/AKT and Erk pathways) and then increased the
translocation (from cytoplasm to nucleus) and the transcriptional
activity of FOXO1; in addition, it upregulated the expression of
apoptosis-associated and autophagy-associated proteins and
down-regulated cell cycle-associated proteins. Therefore, the
Chinese medicine TCM-1 markedly inhibited cell growth and promoted
autophagy and apoptosis in prostate cancer cells, suggesting that
TCM-1 hada potential clinical value for treatment of prostate
cancer in clinic.
Materials and methods
Cell culture and TCM-1
Prostate cancer cell lines (including LNCaP,
CWR22Rv1, PC3 and DU145) and the normal prostate cell line WPMY-1
were cultured in RPMI 1640 medium (Wisent Biotechnology Co., Ltd.,
Nanjing, China), supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), penicillin (100
U/ml) and streptomycin (100 mg/ml) (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), in a humidified 5% CO2 atmosphere
at 37°C.
TCM-1, the water-extract from a compound TCM, was
provided by Jiangsu Province Hospital of TCM (Nanjing, China) and
contained 1 g/ml pharmaceutical raw materials, which were composed
of Astragalusmembranaceus, radix Rehmanniaepreparata, Curcuma
zedoaria, Rhizoma wenyujin concisum, Rhizoma paridis chinensis,
Polygonum perfoliatum L., Radix Glycyrrhizae, and Ficus
pumila L. For the in vivo experiments, TCM-1 was diluted
in 1XPBS for intragastric administration.
Antibodies and reagents
Antibodies against poly (ADP-ribose) polymerase 1
(PARP-1; cat. no. sc-56196), caspase3 (cat. no. sc-271028), Cyclin
D1 (cat. no. sc-246), E3 ubiquitin-protein ligase XIAP (cat. no.
sc-55552), AKT (cat. no. sc-55523), prostate specific antigen (PSA;
cat. no. sc-7316), phospho (p)-AKT (Ser-473; cat. no. sc-271964),
Histone H3 (cat. no. sc-10809), FOXO1 (cat. no. sc-514610), Bim
(cat. no. sc-11425), cyclin-dependent kinase inhibitor 1 (p21; cat.
no sc-817), p53 (cat. no. sc-126), p27 (cat. no. sc-53906) EGFR
(cat. no. sc-101), p-EGFR (Tyr1173; cat. no. sc-57545) and β-actin
(cat. no. sc-47778) were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Antibodies against Erk (cat. no. 5013),
p-Erk1/2 (T202/Y204; cat. no. 9106), p-AKT (Thr308; cat. no. 4050)
and GAPDH (cat. no. 2118) were purchased from Biogot Technology
Co., Ltd. (Nanjing, China). Antibodies against p-FOXO1
(Ser256/Thr24/Ser319; cat nos. 84192, 9464 and 2486, respectively),
p-PI3K p85 (Tyr458)/p55 (Tyr199) (cat. no. 4228) were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). Antibodies
against caspase8/p18 (cat. no. 66093-1), caspase9/p35/p10 (cat. no.
10380-1), Bcl-2 (cat. no. 60178-1), Bax (cat. no. 50599-2), Raf-1
(cat. no. 51140-1), microtubule-associated proteins 1A/1B light
chain 3B (LC3)-I/II (cat. no. 18725-1), Beclin-1 (cat. no.
11306-1), mTOR (cat. no. 20657-1), p70S6K (cat. no. 14485-1), PI3K
p85 (cat. no. 21739-1) and proliferation marker protein Ki-67 (cat.
no. 27309-1) were purchased from ProteinTech Group, Inc. (Chicago,
IL, USA). Antibodies for Fas ligand (FasL; cat. no. AF0157),
p-Raf-1 (Ser338; cat. no. AF3065), p-transcription factor p65 (p65;
Ser536; cat. no. AF2006), p65 (cat. no. BF0382), p-dual specificity
mitogen-activated protein kinase kinase (MEK)1/2(Ser217/Ser221)
(cat. no. AF8035), MEK1/2 (cat. no. AF6385), p-mTOR (Ser2448; cat.
no. AF3308) and p-p70S6K (Thr389/Thr412; cat. no. AF3228) were
purchased from Affinity Biosciences (Cambridge, UK). Horseradish
peroxidase conjugated secondary antibodies, including rabbit IgG
(cat. no. HAF008) and mouse IgG (HAF007), were purchased from
Bio-Techne China Co. Ltd., R & D Systems (Shanghai, China).
Assay kits for radioimmunoprecipitation assay (RIPA), MTT and Cell
Counting Kit-8 (CCK-8) were purchased from Beyotime Institute of
Biotechnology (Haimen, China). The Nuclear/Cytosol Fractionation
kit was purchased from AmyJet Scientific (Wuhan, China). DAPI was
purchased from Beijing Solarbio Science & Technology Co., Ltd.
(Beijing, China), dissolved in 1X PBS and used at a concentration
of 20 µg/ml. hEGF was purchased from Merck KGaA (Darmstadt,
Germany). hEGF was directly added to cell culture medium at the
concentration of 0, 20 and 30 ng/ml for 30 min in cell incubator
with 5% CO2 at 37°C. Other chemicals were all purchased
from Sigma-Aldrich (Merck KGaA).
DAPI staining assay for nuclear
condensation rupture
For the DAPI staining assay, LNCaP and PC3 cells
were cultured in 12-well plates and incubated with increasing doses
of TCM-1 (0, 2, 5 and 10 mg/ml) for 24 h. LNCaP and PC3 cells were
washed with 1X PBS briefly and fixed in 4% formaldehyde for 15 min,
and washed three times with 1X PBS and then permeabilized in 0.2%
Triton X-100 for 15 min. Cells were then stained with 6 µl DAPI (1
mg/ml) adding 300 µl 1X PBS at room temperature for 8 min and were
photographed with a fluorescence microscope (Nikon IX-71; Nikon
Corporation, Tokyo, Japan).
Flow cytometry assay for cellular
apoptosis and the cell cycle
LNCaP and PC3 cells were seeded in 6-well plates
(0.5×105-1×105/well) and treated with
different concentrations of TCM-1 (0, 2, 5 and 10 mg/ml) for 24 h,
and cells were collected for flow cytometric analysis (cellular
apoptosis assay) using an annexin V-fluorescein isothiocyanate
(FITC) apoptosis detection kit (Beyotime Institute of
Biotechnology, Haimen, China). In detail, cells were harvested and
resuspended in 1X binding buffer (contained in the kit). Annexin
V-FITC and propidium iodide (PI) were added to the cells, according
to manufacturer's protocol, for 10–20 min at room temperature while
protected from light. Flow cytometry analysis for cell apoptosis
was performed using a flow cytometer and analyzed by ModFit LT
(version 2.0; Verity Software House, Inc., Topsham, ME, USA), and a
minimum of 30,000 events was collected for each sample.
For the cell cycle assay, LNCaP and PC3 cells were
seeded in 6-well plates (0.5×105-1×105/well)
and treated with the concentrations of TCM-1 (0, 2, 5 and 10 mg/ml)
for 24 h. LNCaP and PC3 cells were collected and fixed with 70%
ethanol overnight at 4°C. Subsequently, the samples were treated
with 100 µl RNase A (10 mg/ml) for 30 min at 37°C, stained with 200
µl PI (10 mg/ml) for 0.5 h at 4°C, and 800 µl1X PBS was added. Flow
cytometry analysis was performed using a flow cytometer and a
minimum of 20,000 events was collected for each sample. The raw
collected data were analyzed by ModFit LT (version 2.0; Verity
Software House, Inc.) to determine cell cycle distribution.
MTT assay and CCK-8 assay for cell
viability
MTT and CCK-8 assays were performed to assess the
cell viability. Cells were seeded in a 96-well plate at a density
of 1×104 cells/well overnight and treated with different
concentrations of TCM-1 (0, 2, 5 and 10 mg/ml) for 24 h.
For the MTT assay, the culture medium was removed
and fresh medium (100 µl) was added with 10 µl MTT (5 mg/ml). The
plate was incubated at 37°C for 4 h in the dark. The medium was
removed again, and 100 µl dimethyl sulfoxide was added to each
well. The absorbance at 570 nm was measured using a microplate
reader (Thermo Fisher Scientific, Inc.).
For the CCK-8 assay, the culture medium was removed
and fresh medium (100 µl) was added with CCK-8 solution (5 µl). The
plate was incubated for 4 h at 37°C in the dark. Absorbance was
measured using a microplate reader (Thermo Fisher Scientific, Inc.)
at 450 nm.
The measured optical density values were converted
into cell viability according to the manufacturer's protocol.
Western blot analysis and chromatin
immunoprecipitation (ChIP) assay
Following 24 h of treatment with TCM-1 (0, 2, 5 and
10 mg/ml), LNCaP and PC3 cells were harvested and lysed by the
addition of 120 µl RIPA buffer [Tris-HCl, Ph 7.6;1% NP-40; 0.1%
sodium deoxycholate; 0.1% SDS; 150 mM NaCl; 1 mM EGTA; 1 mM
phenylmethylsulfonyl fluoride; 1% Triton X-100; and Roche complete
protease inhibitor cocktail (Roche Diagnostics, Basel,
Switzerland)]. Tumor tissues were cut in to small fragments. RIPA
buffer was added and the fragments were ground with a manual tissue
grinder. Following centrifugation (12,000 × g, 4°C, 10 min) of cell
lysates and tumor tissue lysates, the supernatants were collected
and the total protein concentration was quantified using Bradford
reagent (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Equal
amounts of total protein (15 µg) were separated by 10% SDS-PAGE and
transferred to a polyvinylidene difluoride membrane (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Following 1 h blocking with
5% skimmed milk at room temperature, the transferred membranes were
blotted using primary antibodies (all 1:1,000 dilution) overnight
at 4°C, and corresponding peroxidase-labeled secondary antibodies
(all 1:10,000 dilution) at room temperature for 1 h. Bands were
detected using an Enhanced Chemiluminescence Detection kit (GE
Healthcare, Chicago, IL, USA).
For the ChIP assay, LNCaP cells were treated with
TCM-1 (0 and 5 mg/ml). At 12 h, cells were cross-linked with 1%
formaldehyde for 10 min at 25°C. Cells were harvested, sonicated
(300W, sonication 2 sec, interval 20 sec, 20 cycles in an ice bath)
and the soluble chromatin fragments (200–500 bp) were incubated
with 2 µg rabbit IgG or FOXO1 antibodies. DNA-protein immune
complexes were eluted and reverse cross-linked, and DNA was
extracted using spin filter columns. The presence of the p21
promoter domain in immunoprecipitated DNA was identified using the
following primers: Forward primer, 5′-GGTGTCTAGGTGCTCCAGGT-3′; and
reverse primer, 5′-GCACTCTCCAGGAGGACACA-3′. The amplified p21
promoter region was analyzed via 1% agarose/ethidium bromide gel
electrophoresis. In the control samples, the primary antibodies
were replaced with non-immune rabbit IgG.
In vivo efficacy of treatment with
TCM-1 in the prostate tumor xenograft mouse model
A total of 32 nude mice (male, 5-week old) were
purchased from the Model Animal Research Center of Nanjing
University, Nanjing, China and raised in SPF level animal room at
room temperature with normal diet and drinking water, 40–70%
humidity, 0.03% CO2 and 12 h ligh/dark cycle. PC3 cells
(1×106) were suspended in PBS (100 µl) and injected
subcutaneously into the flanks of each animal. Mice were randomly
divided into four groups (8 mice/group), including a negative
control group (1X PBS), positive control group [5-fluorouracil
(FU); 30 mg/kg], a low-dose TCM-1 group (0.5 g/kg), and a high-dose
TCM-1 group (2.0 g/kg). When the tumors grew to 24–30
mm3, mice were intragastrically administered
negative/positive control reagents and low-/high-doses of TCM-1
every 2 days for 6 weeks. The tumor length/width and body weight
were measured at the end of each treatment week. The tumor volume
was calculated as: Volume (mm3)=(length ×
width2)/2. At the end of the experiments, the mice were
sacrificed on the 42nd day and tumors were dissected, weighed and
snap-frozen with liquid nitrogen for further western blot (as
described above) and immunohistochemical analyses. All animal
experiments in the present study were approved (permit no.
NL-129-02) by the Ethics Committee of Jiangsu Province Hospital of
TCM, Nanjing, China.
Immunohistochemistry (IHC) assay
Tumors were fixed in 10% buffered formalin (4°C,
overnight), embedded in paraffin and sectioned to 5 µm in size.
Each tissue section was deparaffinized and rehydrated with xylene
and gradient concentrations of ethanol. Tissue sections were boiled
in EDTA for 15 min, quenched with 0.3% hydrogen peroxide solution
for 10 min at room temperature and blocked with bovine serum
albumin (Gibco; Thermo Fisher Scientific Inc.) in PBS for 60 min at
30°C. Slides were subsequently incubated with specific primary
antibodies (all 1:50 dilution) overnight at 4°C. Sections were
counterstained with hematoxylin (10 min, at room temperature).
Antibody binding was detected with an EnVision Detection kit,
Peroxidase/Diaminobenzidine, Rabbit/Mouse (Gene Tech Biotechnology
Co., Ltd., Shanghai, China). The expression levels of specific
proteins were observed and photographed under a microscope at a
magnification of ×400 (CTR 6000; Leica Microsystems GmbH, Wetzlar,
Germany), and the proliferation index was expressed as the
percentage of positive cells relative to the total number of cells
in a given area.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Comparisons between groups were made by one-way analysis
of variance followed by Dunnett's test, using SPSS software
(version 19.0; IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference. All
experiments were replicated three times.
Results
TCM-1 induces morphological changes
and inhibits cell viability in prostate cancer cells
To assess the effect of TCM-1 on prostate cell
lines, WPMY-1 and PCa cells (including androgen-dependent LNCaP and
CWR22Rv1, and androgen-independent PC3 and DU145) were cultured and
treated with different concentrations of TCM-1 (0, 2, 5 and 10
mg/ml) for 24 h. Cellular morphological alterations and cell
viability were photographed using a microscope and determined by
MTT and CCK-8 assays. From the data, it was observed that TCM-1
induced morphological alterations in PCa cells in a
concentration-dependent manner; cells appeared shrunken and
rounded, and certain cells were lysed, whereas no distinct
alterations were noted in the normal prostate cell WPMY-1 even when
treated with 10 mg/ml of TCM-1 (Fig.
1A). In addition, the MTT and CCK-8 assays demonstrated that
the proliferation and viability of PCa cells were significantly
decreased by treatment with TCM-1 in a concentration-dependent
manner, whereas the proliferation and viability of WPMY-1 cells
were almost unaffected by treatment TCM-1 (Fig. 1B and C). These results indicated
that TCM-1 suppressed cell growth and proliferation, in addition to
decreasing cell viability, particularly in prostate cancer
cells.
 | Figure 1.TCM-1 induces cellular morphological
alterations and inhibits cell viability in prostate cancer cells.
(A) WPMY-1, LNCaP, CWR22Rv1, PC3 and DU145 cells were seeded in a
12-well plate and incubated for 24 h with different concentrations
of TCM-1 (0, 2, 5 and 10 mg/ml). Morphological alterations in the
cells were observed and photographed with a microscope at
×40magnification. In order to study the effects of TCM-1 on
proliferation and viability, cells were treated with different
concentrations of TCM-1 (0, 2, 5 and 10 mg/ml) for 24 h. Cell
viability was measured via (B) MTT and (C) CCK-8 assays.
Experiments were performed three times. The results are expressed
as the mean ± standard deviation (n=6) and as a percentage of the
vehicle-treated control.*P<0.05 vs. respective 0 mg/ml group.
TCM-1, traditional Chinese medicine 1; CCK-8, Cell Counting
Kit-8. |
In order to address the molecular mechanism of the
effect of TCM-1 on PCa cells, the androgen-dependent LNCaP cell
line and the androgen-independent PC3 cell line were selected as
the cellular models for subsequent experiments.
TCM-1 induces cell cycle arrest and
apoptosis in PCa cells
To identify TCM-1-induced cell cycle arrest and
apoptosis in PCa cells, LNCaP and PC3 cells were cultured and
treated with different concentrations of TCM-1 (0, 2, 5 and 10
mg/ml) for 24 h. Cells were treated for flow cytometry analysis to
assess cell cycle arrest and apoptosis and for the DAPI staining
assay to determine PCa cellular apoptosis (Fig. 2). As presented in Fig. 2A, apoptosis in PCa cells (including
LNCaP and PC3 cells) was markedly induced by treatment with TCM-1.
The degree of cellular apoptosis (including early apoptosis and
late apoptosis) was dependent on the concentration of TCM-1. As
presented in Fig. 2C, the cell
cycle of LNCaP and PC3 cells was arrested at the G1 phase following
treatment with TCM-1, and the degree of G1 phase arrest was
dependent on the concentration of TCM-1. The number of cells in the
G1 phase was increased, while cells in the S and G2/M phases were
decreased following treatment with TCM-1. In addition, the DAPI
staining assay demonstrated that TCM-1 induced the formation of
apoptotic bodies and nuclear shriveling in LNCaP and PC3 cells. The
number of cells exhibiting shriveled nuclei and apoptotic bodies
was increased with the increasing concentration of TCM-1 (Fig. 2B).
 | Figure 2.TCM-1 induces cellular apoptosis and
cell cycle arrest in LNCaP and PC3cells. (A) LNCaP and PC3 cells
were cultured and treated with different concentrations of TCM-1
(0, 2, 5 and 10 mg/ml) for 24 h. Cells were harvested and subjected
to a cellular apoptosis assay using annexin V-FITC and PI
double-staining flow cytometry analysis. Percentages of cellular
apoptosis (including early and late apoptosis) were quantified. (B)
Cells were treated with TCM-1 (0, 2, 5 and 10 mg/ml) for 24 h, and
the nuclear morphology was observed and photographed following DAPI
staining under a microscope, using a blue filter with ×40
magnification. Arrows indicate fragmented nuclei. (C) To assess the
effect of TCM-1 on the cell cycle, LNCaP and PC3 cells were
cultured in 12-well plates overnight and treated with different
concentrations of TCM-1 (0, 2, 5 and 10 mg/ml) for 24 h. The cells
were harvested and stained with PI to analyze the cell cycle by
flow cytometric analysis. Percentages of cell cycle phases were
quantified. TCM-1, traditional Chinese medicine 1; FITC,
fluorescein isothiocyanate; PI, propidium iodide. |
TCM-1 inhibits the PI3K/AKT signaling
pathway and activates FOXO1 by downregulating the phosphorylation
levels of PI3K, AKT and FOXO1 in PCa cells
LNCaP and PC3 cells were seeded in 6-well plates and
treated with different concentrations of TCM-1, as indicated in
Fig. 3. At 24 h, cells were
harvested for western-blot analysis. The results demonstrated that
treatment with TCM-1 in PCa cells decreased p-AKT (including Ser473
and Thr308) and p-PI3K expression levels, while no apparent
alterations in the total AKT and PI3K protein expression levels
were noted. The decreasing p-AKT and p-PI3K levels presented a
TCM-1 concentration-dependent manner (Fig. 3A and B). In addition, the
phosphorylation level of P-65 (p-p65Ser536), a subunit of NF-κB
which as a downstream target of PI3K/AKT pathway, was also
decreased by treated TCM-1 in a concentration dependent manner
(Fig. 3A and B). These results
indicated that TCM-1 inhibited the activity of NF-κB by inhibiting
the PI3K/AKT signaling pathway.
 | Figure 3.TCM-1 inhibits the PI3K/AKT signaling
pathway and activates FOXO1 by downregulating the phosphorylation
levels of PI3K, AKT and FOXO1 in LNCaP and PC3cells. (A) LNCaP
cells and (B) PC3 cells were cultured in 6-well plates and treated
with different concentrations of TCM-1 (0, 2, 5 and 10 mg/ml). At
24 h, cells were harvested and lysed for western blot analysis to
assess the protein expression levels of p-PI3K/p85(Y458), PI3K/p85,
p-AKT(S473), p-AKT(T308), AKT, p-p65(S536), p65 and β-actin
(loading control). (C) LNCaP cells and (D) PC3 cells were cultured
and treated as above. At 24 h, cells were harvested and lysed for
western blot analysis to assess the protein expression levels of
p-FOXO1 (Ser256/Thr24/Ser319), FOXO1, and β-actin (loading
control). (E) LNCaP cells and (F) PC3 cells were harvested for the
isolation of subcellular fractions of the nucleus and cytoplasm
using a Nuclear/Cytosol Fractionation kit. Protein expression
levels of FOXO1 in the nucleus and cytoplasm were separately
analyzed by western-blot. (G) LNCaP cells were treated with 0 and 5
mg/ml TCM-1 for 12 h and cells were treated for the ChIP assay. The
polymerase chain reaction products were assayed by agarose gel
electrophoresis. (H) LNCaP and PC3 cells were cultured in 6-well
plates and treated with different concentrations of TCM-1 (0, 2, 5
and 10 mg/ml). At 24 h, cells were harvested and lysed for western
blot analysis to assess the protein expression levels of PSA and
β-actin (loading control). p, phosphorylated; PI3K,
phosphatidylinositol 3-kinase; AKT, RAC-αserine/threonine-protein
kinase; FOXO1, forkhead box protein O1; p65, transcription factor
p65; PSA, puromycin-sensitive aminopeptidase; IgG, immunoglobulin
G; IB, immunoblotting; TCM-1, traditional Chinese medicine 1; ChIP,
chromatin immunoprecipitation; p21, cyclin-dependent kinase
inhibitor 1; C, cytoplasmic; N, nuclear; Ab, antibody. |
FOXO1 serves an important role in a number of
cellular physiological processes, including cell growth, cell
proliferation and apoptosis in prostate cancer cells; in addition,
FOXO1 is an important downstream target of the PI3K/AKT pathway
(16). Phosphorylation of FOXO1 at
Ser256/Thr24/Ser319 by the PI3K/AKT pathway induces the
translocation of FOXO1 from the nucleus to the cytoplasm (17). The results of the present study
demonstrated that the phosphorylation levels at
Ser256/Thr24/Ser319, and not the total protein level of FOXO1, were
decreased in LNCaP and PC3 cells following treatment with TCM-1;
this decrease was dependent on the treatment concentration of TCM-1
(Fig. 3C and D). In addition,
these data illustrated that the nuclear FOXO1 levels increased and
the cytoplasmic FOXO1 levels decreased following treatment with
TCM-1, and these trends were dependent on the treatment
concentration of TCM-1 (Fig. 3E and
F). The ChIP assay data demonstrated that TCM-1 increased the
levels of FOXO1 binding to the promoter of the p21 gene (Fig. 3G). In addition, treatment with
TCM-1 in LNCaP cells downregulated the levels of PSA, which is a
downstream target gene of the androgen receptor (AR) (Fig. 3H), suggesting that AR maybe
involved in TCM-1-induced cell growth inhibition and apoptosis in
androgen-dependent PCa cell lines.
These results indicated that TCM-1 inhibited the
activity of the PI3K/AKT signaling pathway by decreasing the
phosphorylation levels of PI3K and AKT, and additionally decreased
the phosphorylation level of FOXO1 (Ser256/Thr24/Ser319), resulting
in the translocation of FOXO1 proteins into the nucleus from the
cytoplasm and increasing the transcriptional activity of FOXO1.
TCM-1 induces the activation of
intrinsic and extrinsic apoptotic pathways in a cellular tumor
antigen p53 (p53)-independent manner in PCa cells
It has been reported that FOXO1 is a tumor
suppressor, and that overactivation of FOXO1 may induce cancer cell
apoptosis by stimulating the expression of death receptor ligands,
including FasL and TRAIL, in addition to inducing the expression of
a number of pro-apoptotic members of the apoptosis regulator Bcl-2
family of mitochondria-targeting proteins (10). From the present experimental data,
it was observed that the expression of the FOXO1-targeted
pro-apoptotic Bim and FasL proteins was markedly increased
following treatment with TCM-1, in a concentration-dependent manner
(Fig. 4A and B). It is known that
FasL is a death ligand that is able to trigger the extrinsic
apoptotic pathway through binding to its receptor Fas, expressed on
the majority of cancer cells; Bim is able to trigger the intrinsic
apoptotic pathway (10). In the
present study, the experimental data demonstrated that TCM-1
decreased the expression levels of anti-apoptotic proteins
(including Bcl-2, XIAP and survivin) (Fig. 4C and D), and increased the
expression levels of pro-apoptotic proteins [including Bax, cleaved
(c)-PARP-1, c-caspase 9, c-caspase 8 and c-caspase 3] (Fig. 4E and F) in LNCaP and PC3 cells.
These results indicated that treatment with TCM-1 in PCa cells
activated the intrinsic and extrinsic apoptotic pathways
simultaneously by increasing the expression of Bim and FasL via the
activation of FOXO1. In addition, p53 is a well-known tumor
suppressor, which is known to cause cell cycle arrest, autophagy
and apoptosis in a number of types of cancer cells (18). From the experimental results, it
was observed that the protein level of p53 was almost unaltered
following treatment with TCM-1 in LNCaP cells (no expression of p53
was detected in PC3 cells) (Fig. 4E
and F).
 | Figure 4.TCM-1 induces the activation of the
intrinsic and extrinsic apoptotic pathways in a p53-independent
manner in LNCaP and PC3 cells. (A) LNCaP and (B) PC3 cells were
incubated overnight and treated with different concentrations of
TCM-1 (0, 2, 5 and 10 mg/ml) for 24 h. The cells were harvested for
western blot analysis to assess the protein levels of Bim, Fas-L
and β-actin (loading control). (C) LNCaP and (D) PC3 cells, treated
as described above, were lysed for western blot analysis to detect
the protein levels of Bcl-2, XIAP, Survivin and β-actin (loading
control). Whole cell lysates of (E) LNCaP and (F) PC3 cells,
treated as above, were subjected to western blot analysis to
determine the protein levels of Bax, caspase-9/-8/-3, PARP-1, p53
and β-actin (loading control). TCM-1, traditional Chinese medicine
1; Bim, Bcl-2-like protein 11; Fas-L, Fas ligand; Bcl-2, apoptosis
regulator Bcl-2; XIAP, E3 ubiquitin-protein ligase XIAP; PARP-1,
poly (ADP ribose) polymerase 1; c, cleaved; Bax, apoptosis
regulator BAX; p53, cellular tumor antigen p53; IB,
immunoblotting. |
These data suggested that TCM-1 inhibited the
activity of the PI3K/AKT pathway and induced the translocation of
FOXO1 into the nucleus from the cytoplasm, activating the intrinsic
and extrinsic apoptosis pathways (caspase-dependent) in prostate
cancer cells. TCM-1-induced PCa apoptosis was observed to be
p53-independent.
TCM-1 inhibits the activity of the
Raf/MEK/Erk signaling pathway, resulting in downregulation of
Cyclin D1, and upregulation of p21 and cyclin-dependent kinase
inhibitor 1B (p27), in PCa cells
As previously reported, inhibition of the
Raf/MEK/Erk signaling pathway downregulates Cyclin D1 and
upregulates p21/p27 by activating the transcription factor FOXO1,
resulting in cell cycle arrest at the G1 phase (10,19).
In order to determine whether the Raf/MEK/Erk signaling pathway was
involved in cell cycle arrest at the G1 phase in TCM-1-treated PCa
cells, LNCaP and PC3 cells were cultured and treated with different
concentrations of TCM-1, as indicated in Fig. 5, for 24 h. Cells were harvested for
western blot analysis to assess the expression levels of cell
cycle-associated proteins, including Cyclin D1, p27 and p21. As
presented in Fig. 5A and B,
treatment with TCM-1 in LNCaP and PC3 cells markedly decreased the
protein level of Cyclin D1, whereas it increased the protein
expression levels of p21 and p27. These results indicated that
TCM-1 induced cell cycle arrest at the G1 phase in LNCaP and PC3
cells, and were consistent with the results of the flow cytometry
analysis presented in Fig. 2B. The
Raf/MEK/Erk signaling pathway has been demonstrated to serve an
important role in promoting the cell cycle, cell growth and cell
proliferation (6). In order to
identify that TCM-1 induced PCa cell cycle arrest and growth
inhibition by inhibiting the activity of the Raf/MEK/Erk signaling
pathway, LNCaP and PC3 cells were cultured and treated with
different concentrations of TCM-1, as indicated in Fig. 5C and D, for 24 h. Cells were
subsequently harvested and lysed for western blot analysis. The
experimental data demonstrated that TCM-1 decreased the
phosphorylation level of Raf-1 (Ser338) in a
concentration-dependent manner, while no apparent effect on the
total protein expression level of Raf-1 in LNCaP and PC3 cells was
observed (Fig. 5C and D).
Inactivation of Raf-1 further resulted in a decrease in the
phosphorylation levels of MEK1/2 (Ser217/Ser221) and Erk1/2
(Thr202/Tyr204), while no effect on the total protein expression
levels of MEK1/2 and Erk1/2 in LNCaP and PC3 cells was observed
(Fig. 5C and D). These results
suggested that TCM-1 induced cell cycle arrest and cell growth
inhibition by inactivating the Raf/MEK/Erk signaling pathway.
 | Figure 5.TCM-1 inhibits the activity of the
Raf/MEK/Erk signaling pathway, resulting in downregulation of
Cyclin D1, and upregulation of p21 and p27, in prostate cancer
cells. (A) LNCaP and (B) PC3 cells were cultured overnight and
treated with different concentrations of TCM-1 (0, 2, 5 and 10
mg/ml) for 24 h. Cells were harvested for western blot analysis to
assess the protein levels of Cyclin D1, p27, p21 and β-actin
(loading control). (C) LNCaP and (D) PC3 cells were treated as
described above for 24 h. Cells were harvested and lysed for
western blot analysis to determine the protein expression levels of
p-Raf (Ser338), Raf, p-MEK1/2(S217/S221), MEK1/2,
p-Erk1/2(T202/Y204), Erk1/2 and β-actin (loading control). TCM-1,
traditional Chinese medicine 1; Raf, RAF proto-oncogene
serine/threonine-protein kinase; p21, cyclin-dependent kinase
inhibitor 1; p27, cyclin-dependent kinase inhibitor 1B; MEK, dual
specificity mitogen-activated protein kinase kinase; Erk,
extracellular signal-regulated kinase; IB, immunoblotting; p,
phosphorylated. |
TCM-1 induces cellular autophagy by
dysregulating the mTOR/p70S6K pathway in PCa cells
mTOR, an important factor involved in cellular
autophagy, is a downstream target of and is regulated by the
PI3K/AKT pathway, originating from starvation, growth factors and
cellular suppressors (20). In
order to examine whether mTOR-associated cellular autophagy was
involved in TCM-1-induced cell growth inhibition and cellular
apoptosis, LNCaP and PC3 cells were cultured in 6-well plates and
treated with different concentrations of TCM-1, as indicated in
Fig. 6, for 24 h. Cells were
harvested and lysed for western blot analysis. The experimental
data demonstratedthat treatment with TCM-1 in PCa cells decreased
the phosphorylation level of mTOR (Ser2448) and resulted in
inhibition of mTOR activity, with no marked effect on the total
protein expression level of mTOR (Fig.
6A and B). Inhibition of mTOR activity further resulted in
activity inhibition of p70S6K by decreasing the phosphorylation
level of p70S6K (Thr389/Thr412) in LNCaP and PC3 cells. The
expression level of LC3-II was increased with the inhibition of
p70S6K, while the level of LC3-I was decreased (Fig. 6A and B). All the alterations were
TCM-1 concentration-dependent, as presented in Fig. 6. Additionally, Beclin-1, a protein
which is negatively correlated with Bcl-2, was significantly
increased by treatment with TCM-1 in a concentration-dependent
manner, via the TCM-1-mediated decrease in the protein expression
level of Bcl-2 (Figs. 4C and D,
6A and B).
 | Figure 6.Expression of autophagy-associated
proteins in LNCaP and PC3 cells following treatment with different
doses of TCM-1. (A) LNCaP and (B) PC3 cells were incubated and
treated with increasing doses of TCM-1 (0, 2, 5 and 10 mg/ml) for
24 h. Cells were harvested for western blot analysis to assess the
protein expression levels of p-mTOR(S2448), mTOR,
p-p70S6K(T389/412), p70S6K, Beclin-1, LC3-I, LC3-II and β-actin
(loading control). TCM-1, traditional Chinese medicine 1; p,
phosphorylated; mTOR, serine/threonine-protein kinase mTOR; p70S6K,
ribosomal protein S6 kinase β-1; LC3, microtubule-associated
proteins 1A/1B light chain 3B. |
Considering the inhibition of the PI3K/AKT signaling
pathway by TCM-1 (Fig. 3A and B),
the results of the present study demonstrated that the
TCM-1-induced cellular autophagy via inhibition of them TOR/p70S6K
signaling pathway in LNCaP and PC3 cells was mediated via
inhibition of the activity of the PI3K/AKT signaling pathway,
suggesting that cellular autophagy may be involved in TCM-1-induced
cell growth inhibition and apoptosis in prostate cancer cells.
TCM-1 targets EGFR and competitively
acts on EGFR with EGF, resulting in inhibition of the
auto-phosphorylation of EGFR
It has been reported that the PI3K/AKT and
Raf/MEK/Erk signaling pathways are two important downstream
signaling pathways of the EGF/EGFR signaling pathway (21). Over-activation of the EGF/EGFR
pathway frequently occurs in a number of types of cancer, and has
been demonstrated to contribute to cancer cell proliferation, tumor
vascularization and metastasis (22,23).
In order to determine whether the TCM-1-induced inhibition of the
PI3K/AKT and Raf/MEK/Erk signaling pathways occurred via the
TCM-1-induced inhibition of the EGF/EGFR signaling pathway, LNCaP
and PC3 cells were cultured and treated with different
concentration of EGF and/or TCM-1 (5 mg/ml), or different
concentrations of TCM-1 and/or EGF (30 ng/ml), as indicated in
Fig. 7. From the experimental
data, it was revealed that treatment with TCM-1 markedly decreased
the auto-phosphorylation levels of EGFR (p-EGFR Tyr1173) and did
not alter the total protein expression level of EGFR in LNCaP and
PC3 cells. The decrease in p-EGFR (Tyr1173) expression levels was
dependent on the treatment concentration of TCM-1 (Fig. 7A and B).
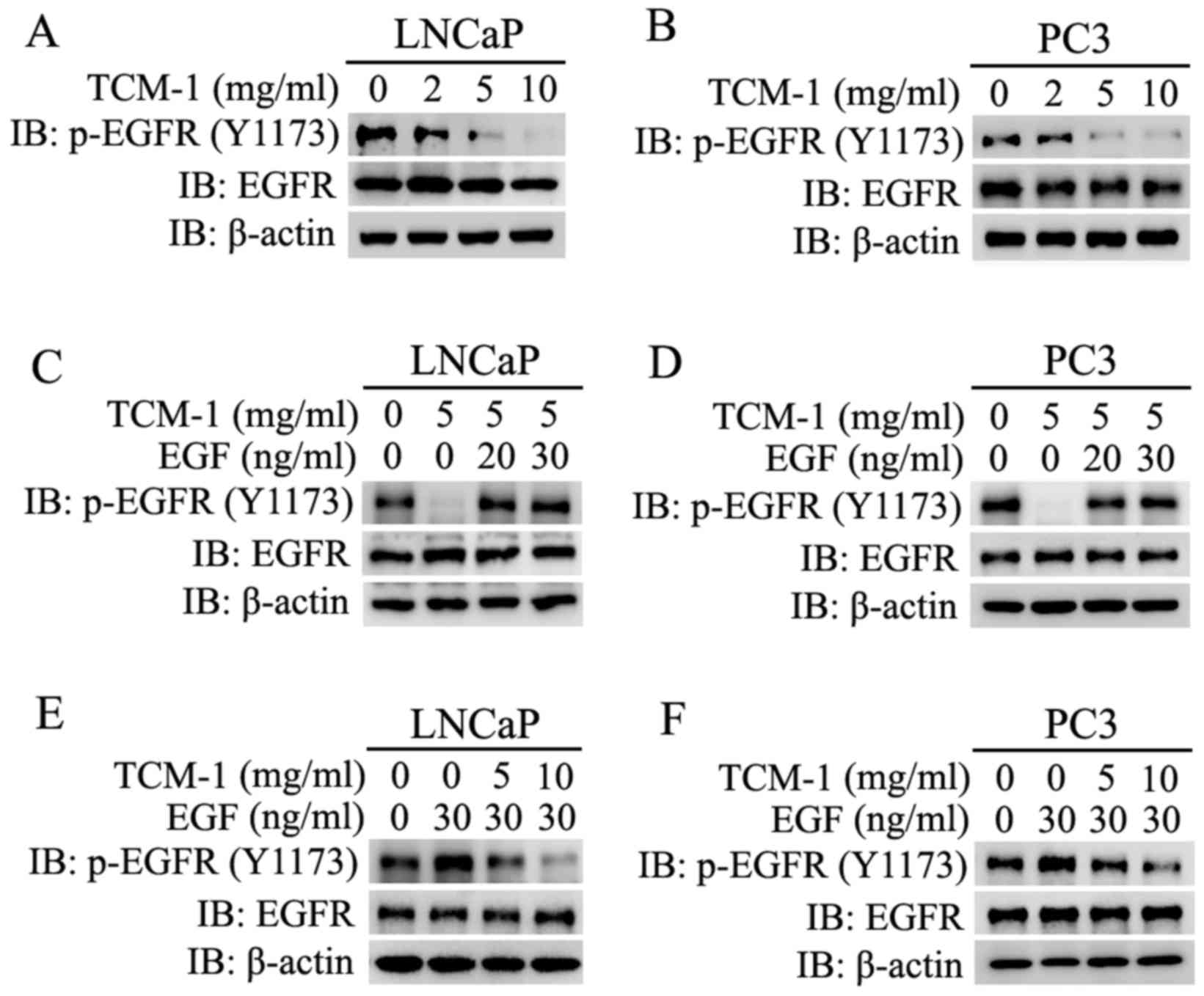 | Figure 7.Expression levels of p-EGFR and EGFR
in LNCaP and PC3 cells treated with TCM-1 or EGF, and co-treated
with TCM-1 and EGF. (A) LNCaP and (B) PC3 cells were incubated and
treated with increasing doses of TCM-1 (0, 2, 5 and 10 mg/ml) for
24 h. Cells were harvested for western blot analysis to assess the
protein expression levels of p-EGFR, EGFR and β-actin (loading
control). (C) LNCaP and (D) PC3 cells were incubated and
pre-treated with 5 mg/ml TCM-1, and stimulated with different
concentrations of EGF (20 or 30 µg/ml) for 10 min. Cells were
harvested and lysed for western blotting to assessthe protein
expression levels of p-EGFR (Y1173), EGFR and β-actin (loading
control). (E) LNCaP and (F) PC3 cells were pre-treated with the
indicated concentrations (0, 5 and 10 mg/ml) of TCM-1 for 24 h and
stimulated with EGF (30 µg/ml) for 10 min. Cells were harvested to
determine the expression of p-EGFR (Y1173) and EGFR by western blot
analysis. EGFR, epidermal growth factor receptor; TCM-1,
traditional Chinese medicine 1; p, phosphorylated. |
When LNCaP and PC3 cells were treated or co-treated
with TCM-1 (5 mg/ml) and EGF (0, 20 and 30 ng/ml), it was observed
that the TCM-1-induced inhibition of the auto-phosphorylation of
EGFR was rescued by co-treatment with EGF (Fig. 7C and D). It was additionally
demonstrated that the EGF-induced enhancement of the
auto-phosphorylation of EGFR was impaired by co-treatment with
TCM-1 in PCa cells (LNCaP and PC3) (Fig. 7E and F).
These results demonstrated that TCM-1 downregulated
the auto-phosphorylation and activation of the membrane receptor
EGFR, and additionally impaired the EGF-induced activation of EGFR.
These results indicated that the TCM-1-induced activity inhibition
of EGFR and its downstream signaling pathways, including the
EGFR/PI3K/AKT and EGFR/PI3K/Erk pathways, maybe due to TCM-1
competitively acting on EGFR with EGF.
TCM-1 suppresses the growth of human
PCa cells in vivo by inhibiting EGFR-associated signaling pathways
and inducing cellular autophagy and apoptosis
In order to validate the findings from the in
vitro studies and to test the efficacy of TCM-1 for prostate
cancer therapy, an in vivo study was performed using a PC3
cell tumor xenograft model in nude mice (Fig. 8). PC3 cells were implanted
subcutaneously into the right or left flank of nude mice and tumors
were allowed to grow. When the tumor volume reached 20–24
mm3, mice were treated with 1× PBS (as a negative
control), TCM-1 (0.5 g/kg as a low dose and 2 g/kg as a high dose)
and 5-FU (as a positive control; 30 mg/kg) by oral gavage every 2
days for 6 weeks. Tumor growth and mouse weights were monitored at
the end of each treatment week. Following treatment for 6 weeks,
mice were sacrificed and the subcutaneous tumors were resected,
weighed, photographed and measured immediately. The tumor samples
were sectioned and subjected to an IHC assay and western blotting.
Compared with the negative control group, tumor growth in
vivo was inhibited by low-dose TCM-1, high-dose TCM-1 and the
5-FU positive control (Fig. 8B).
Dunnett's test analysis demonstrated that significant differences
existed between the high-dose group or positive control group and
the negative control group (P=0.0353; P=0.0414), although a
significant difference was not observed between the low-dose group
and the negative control group (P=0.1230). Treatment with TCM-1
revealed a time-dependent inhibition of prostate tumor growth in
vivo (tumors in the high-dose group were markedly smaller
compared with those of the low-dose group), and an apparent
reduction in tumor volume was observed during the 2nd week in the
TCM-1-treated group (Fig. 8B),
while no signs of toxicity were observed during the treatment
period, as indicated by a stable bodyweight (Fig. 8A). The data in Fig. 8B and C demonstrated that the
inhibition of tumor growth by high-dose TCM-1 was almost equal to
that induced by 5-FU (positive control). At the end of experiment,
the tumor size in the mice was markedly reduced by the treatment
with TCM-1 in a dose-dependent manner (Fig. 8C). In addition, the protein
expression levels of Ki-67 (marker of cell proliferation), p-EGFR
(Tyr1173) and p-AKT (Ser473) were assessed via an IHC assay in PC3
tumor tissues from different groups. The results demonstrated that
the treatment with TCM-1 markedly decreased the protein expression
levels of Ki-67, and the phosphorylation levels of EGFR (Tyr1173)
and AKT (Ser473) in tumor tissues (Fig. 8E). In addition, the western
blotting data from the PC3 tumor tissues demonstrated that the
expression levels of p-Erk1/2, p-FOXO1 (Ser256) and CyclinD1 were
all downregulated by treatment with TCM-1, whereas the protein
expression levels of c-caspase 3, c-PARP-1 and LC3-II were
upregulated by treatment with TCM-1 (Fig. 8D). Therefore, the in vivo
experiments suggested that treatment with TCM-1 induced PCa
cellular autophagy and apoptosis by inhibiting the EGFR/PI3K/AKT
and EGFR/PI3K/Erk signaling pathways, resulting in PCa cell growth
inhibition. The experimental data demonstrated that TCM-1 inhibited
EGF/EGFR signaling pathway, and subsequently inhibited both
PI3K/AKT and Raf/Erk pathways and their downstream gene expression.
Finally, cell growth inhibition and apoptosis were induced by TCM-1
treatment in PCa cells (Fig.
8F).
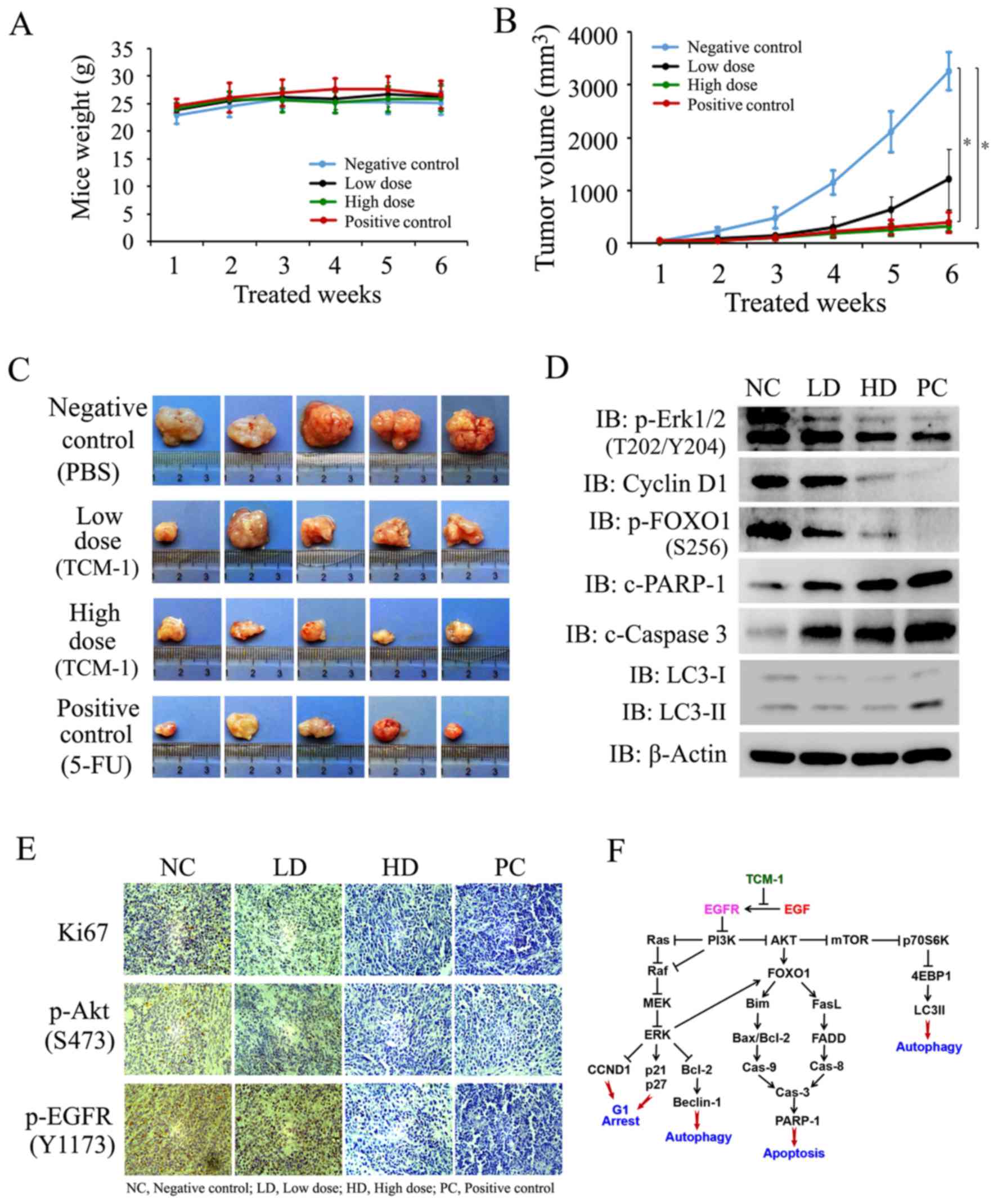 | Figure 8.TCM-1 suppresses tumor growth by
inhibiting cell growth and inducing cellular autophagy and
apoptosis in human PC3 cell xenograft mouse models. PC3 cell tumor
xenograft nude mice were treated by oral gavage with TCM-1 and
control reagents every 2 days for 6 weeks, and tumor size and mouse
weight were monitored at the end of each treatment week. At the end
of experiments, the subcutaneous tumors were resected, weighed,
photographed and measured immediately. The tumor samples were
divided and subjected to an immunohistochemistry assay and western
blotting. (A) Mouse weights in the four groups were measured at the
end of each treatment week. (B) The average tumor volume of
PBS-treated (n=8), TCM-1-treated (including low-dose and high-dose
groups; 8 mice/group) and 5-FU-treated (n=8) nude mice at the end
of each treatment week. (C) Tumor images from each group of mice at
the end of the 6 weeks (five tumors/group are presented). (D) The
expression of proteins, including p-AKT Ser473, p-Erk1/2, Cyclin
D1, p-FOXO1, c-PARP-1, c-Caspase 3 and LC3-I/II were assayed by
western blotting in tumor tissue samples. (E) Immunohistochemistry
analysis of the proliferation markerKi-67, p-EGFR (Y1173) and
p-AKT(S473) in tumor tissues. (F) Schematic diagram of the
apoptotic and autophagy pathways induced by TCM-1 in LNCaP and PC3
cells. *P<0.05. TCM-1, traditional Chinese medicine 1; AKT,
RAC-α serine/threonine-protein kinase; Erk, extracellular
signal-regulated kinase; FOXO1, forkhead box protein O1; PARP-1,
poly(ADP ribose) polymerase 1; c, cleaved; LC3,
microtubule-associated proteins 1A/1B light chain 3B; EGFR,
epidermal growth factor receptor; p, phosphorylated; 5-FU,
5-fluorouracil. |
Discussion
PCa is one of the most common malignancies
worldwide, and is associated with substantial mortality and
morbidity (24). In primary PCa,
the level of PSA is frequently an important factor in the clinical
detection and evaluation of prostate cancer (25). With the further researches on
cancer therapy, scientific consensus is that immunotherapy is a
promising treatment avenue for cancer (26). Thousands of years of clinical
practice in Chinese medicine has suggested that compound Chinese
traditional medicines may have unique advantages in treating cancer
in the clinic by increasing the ability of the body to inhibit and
kill cancer cells in vivo, although the underlying molecular
mechanisms remain to be elucidated. TCM-1 is a classical compound
Chinese traditional medicine which has been used in the clinic to
treat patients with prostate cancer in the Jiangsu Hospital of TCM
for a number of years. In the present study, it was demonstrated
that TCM-1 targeted EGFR and competitively acted on EGFR with EGF,
and inhibited the autophosphorylation and activity of EGFR, thereby
inhibiting the PI3K/AKT and PI3K/Erk signaling pathways by
decreasing the phosphorylation levels of PI3K, AKT and Erk in
androgen-dependent and -independent prostate cancer cells (LNCaP
and PC3 cells). Inhibition of the PI3K/Erk signaling pathway by
TCM-1 markedly induced anti-proliferative effects through the
induction of cell cycle arrest at the G1 phase in the two cell
lines, accompanied by marked alterations in the expression of key
cell cycle regulators (Cyclin D1, p21 and p27). Inhibition of the
PI3K/AKT signaling pathway by TCM-1 increased FOXO1 transcriptional
activity (by decreasing the phosphorylation levels of FOXO1
Ser256/Thr24/Ser319) and induced growth inhibition and initiated
cellular apoptosis through the mitochondrial and death receptor
pathways in LNCaP and PC3 cells. Additionally, TCM-1-induced
inhibition of the PI3K/AKT/mTOR signaling pathway was able to
induce cellular autophagy by inhibiting p70S6K and activating
LC3-II.
It is known that EGFR and its downstream signaling
pathways, including PI3K/AKT/mTOR and Raf/MEK/Erk, serve important
roles in a number of types of tissue cell tumorigenesis, and tumor
progress and metastasis, and are important in regulating bodily
immunity by suppressing T cell-induced tumor necrosis by decreasing
the expression of programmed cell death ligand 1 (PD-L1) (22,23,27).
To the best of our knowledge, the present study was the first to
demonstrate that TCM-1 targeted EGFR and competitively acted on
EGFR with EGF, resulting in auto-phosphorylation and activity
inhibition of EGFR, and decreased the activities of the PI3K/AKT
and Raf/MEK/Erk pathways. These blockades of EGFR and its
downstream signaling pathways may further inhibit the expression of
PD-L1 on cancer cell membranes by inhibiting the activity of the
mTOR signaling pathway. Therefore, TCM-1 treatment may be
associated with the regulation of immunity of patients with
prostate cancer. In addition, a number of ligand-receptors on the
cell membrane are associated with activation of the PI3K/AKT signal
pathway, and promote cancer call growth, proliferation and
metastasis (28). Therefore,
further studies are required to clearly demonstrate whether
EGF/EGFR is the only ligand-receptor involved in the TCM-1-mediated
inhibition of the PI3K/AKT signaling pathway, and induction of cell
growth inhibition and apoptosis in PCa cells.
The phosphorylation of FOXO1 by AKT inhibits the
transcriptional functions of FOXO1, and contributes to cell
survival, growth and proliferation (29,30).
Conversely, FOXO1 activation has been proposed to be important for
regulating apoptosis by stimulating the expression of death
receptor ligands, including FasL and TRAIL, in addition to inducing
the expression of multiple pro-apoptotic members of the Bcl-2
family (Bim, Bcl-2, BAX). FOXO1 is additionally important for
inducing cell cycle arrest via the upregulation of the cell cycle
inhibitor p21 and p27 (31,32).
The results of the present study demonstrated that TCM-1 decreased
the phosphorylation levels of FOXO1 (Ser256/Thr24/Ser319) by
inhibiting the PI3K/AKT and Ras/Erk signaling pathways, and induced
cell cycle arrest at the G1 phase and cellular apoptosis by
inhibiting the expression of Bcl-2/XIAP/survivin, activating
Fas-L/Bim/Bax, and increasing the expression of p27. In addition to
FOXO1, FOXO3a is an important member of the FOX family in terms of
the regulation of cell growth and apoptosis (30). Therefore, further studies are
required to establish whether FOXO3a was additionally involved in
TCM-1-induced cell growth inhibition and apoptosis in PCa cells. In
a previous study, p53 was indicated to be a key protein in cell
growth inhibition and cell apoptosis promotion (33). In the present study, it was
observed that the protein level of p53 was unaltered following
treatment with TCM-1. It was demonstrated that p53 was not an
important factor in TCM-1-induced PCa cell growth inhibition and
cell apoptosis.
mTOR is a rapamycin-sensitive serine/threonine
protein kinase and serves an important role in regulating cell
growth, motility and survival. Dysregulation of the mTOR signaling
pathway maybe observed in a number of types of cancer, with PI3K
and AKT being upstream regulators of the mTOR signaling pathway
(34). Activation of the AKT-mTOR
pathway increases the expression of PD-L1 and results in the
inactivation of anti-tumor T cells (27). The activation of mTOR complex 1, a
principal rapamycin-sensitive mTOR complex, promotes protein
synthesis in response to growth factors by increasing the
phosphorylation of p70S6K and eukaryotic translation initiation
factor 4E-binding protein 1 and exhibits an important role in
cellular autophagy (35).
According to the present results, TCM-1-induced inhibition of the
PI3K/AKT/mTOR signaling pathway impaired the phosphorylation and
activity of p70S6K, and resulting in increasing the levels of
LC3-II and Beclin-1, which serve essential roles in autophagosome
formation (36,37), finally promoting cell autophagy. In
the treatment of cancer cell with most anticancer drugs, the
cellular processes of autophagy and apoptosis frequently occur
simultaneously. Following treatment with TCM-1, it was observed
that TCM-1 induced PCa cellular autophagy and apoptosis by
inhibiting EGFR and its downstream PI3K/AKT and Raf/Erk pathways.
Further studies are required to ascertain whether associations
exist between TCM-1-induced PCa cellular autophagy and
apoptosis.
TCM has systemic and local therapeutic effects and
primarily affects the whole function of the organism. The active
components and functional mechanisms are complex; the functional
effects of TCM are associated with the active ingredients in each
herbal extract. In the study, even though we cannot ensure exactly
the same concentration of each component of TCM-1 each time
extracting from Chinese herbs, but it's for sure that concentration
of each component of TCM-1 in each extraction is almost the same as
long as we control the raw herbs.
In conclusion, the present study identified for the
first time, to the best of our knowledge, that TCM-1 had the
potential to exert a specific anti-cancer effect, in addition to
inducing PCa cell growth inhibition, autophagy and apoptosis in
vitro and in vivo, by targeting EGFR and further
suppressing the activity of the EGFR/PI3K/AKT and EGFR/PI3K/Erk
signaling pathways. The present results may partially provide a
molecular basis for the application of TCM-1 in the clinic to treat
patients with prostate cancer.
Acknowledgements
The authors would like to thank Professor Fusong Xu
(Jiangsu Province Hospital of Traditional Chinese Medicine,
Nanjing, China) for providing the classical prescription of TCM and
certain suggestions on the preparation of a water-extract from
pharmaceutical raw materials. The present study was supported by
grants from the National Natural Science Foundation of China (grant
nos. 81272850 and 81472415).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Petrylak DP: Chemotherapy for advanced
hormone refractory prostate cancer. Urology. 54 Suppl 6A:S30–S35.
1999. View Article : Google Scholar
|
|
3
|
Pisters LL: The challenge of locally
advanced prostate cancer. Semin Oncol. 26:202–216. 1999.PubMed/NCBI
|
|
4
|
Richie JP: Anti-androgens and other
hormonal therapies for prostate cancer. Urology. 54 6A Suppl:15–18.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mathew MP, Tan E, Saeui CT, Bovonratwet P,
Sklar S, Bhattacharya R and Yarema KJ: Metabolic flx-driven
sialylation alters internalization, recycling and drug sensitivity
of the epidermal growth factor receptor (EGFR) in SW1990 pancreatic
cancer cells. Oncotarget. 7:66491–66511. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Luca A, Maiello MR, D'Alessio A,
Pergameno M and Normanno N: The RAS/RAF/MEK/ERK and the PI3K/AKT
signalling pathways: Role in cancer pathogenesis and implications
for therapeutic approaches. Expert Opin Ther Targets. 16 Suppl
2:S17–S27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brunet A, Datta SR and Greenberg ME:
Transcription-dependent and -independent control of neuronal
survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol.
11:297–305. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang JY and Hung MC: A new fork for
clinical application: Targeting forkhead transcription factorsin
cancer. Clin Cancer Res. 15:752–757. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo JP, Tian W, Shu S, Xin Y, Shou C and
Cheng JQ: IKBKE phosphorylation and inhibition of FOXO3a: A
mechanism of IKBKE oncogenic function. PLoS One. 8:e636362013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Brachène Coomans A and Demoulin JB:
FOXO transcription factors in cancer development and therapy. Cell
Mol Life Sci. 73:1159–1172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marino G, Niso-Santano M, Baehrecke EH and
Kroemer G: Self-consumption: The interplay of autophagy and
apoptosis. Nat Rev Mol Cell Biol. 15:81–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang Y, Ting W, Xiao L, Shufei F, Wangxiao
T, Xiaoying W, Xiumei G and Boli Z: Immunoregulation of Shenqi
fuzheng injection combined with chemotherapy in cancer patients: A
systematic review and meta-analysis. Evid Based Complement Alternat
Med. 2017:51215382017.PubMed/NCBI
|
|
14
|
Youns M, Hoheisel JD and Efferth T:
Traditional Chinese medicines (TCMs) for molecular targeted
therapies of tumours. Curr Drug Discov Technol. 7:37–45. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun Y: Application of traditional Chinese
medicine in the comprehensive treatment of cancer. Chin J Integr
Trad West Med. 17:323–324. 1997.(In Chinese).
|
|
16
|
Li P, Lee H, Guo S, Unterman TG, Jenster G
and Bai W: AKT-independent protection of prostate cancer cells from
apoptosis mediated through complex formation between the androgen
receptor and FKHR. Mol Cell Biol. 23:104–118. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, Tang N, Hadden TJ and Rishi AK:
Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta.
1813:1978–1986. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Crighton D, Wilkinson S and Ryan KM: DRAM
links autophagy to p53 and programmed cell death. Autophagy.
3:72–74. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu J, Zhao H, Xu J, Zhang L, Yan L and
Shen Z: Elevated cyclin D1 expression is governed by plasma IGF-1
through Ras/Raf/MEK/ERK pathway in rumen epithelium of goats
supplying a high metabolizable energy diet. J Anim Physiol Anim
Nutr (Berl). 97:1170–1178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heras-Sandoval D, Pérez-Rojas JM,
Hernández-Damián J and Pedraza-Chaverri J: The role of
PI3K/AKT/mTOR pathway in the modulation of autophagy and the
clearance of protein aggregates in neurodegeneration. Cell Signal.
26:2694–2701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roskoski R Jr: The ErbB/HER family of
protein-tyrosine kinases and cancer. Pharmacol Res. 79:34–74. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
De Luca A, Carotenuto A, Rachiglio A,
Gallo M, Maiello MR, Aldinucci D, Pinto A and Normanno N: The role
of the EGFR signaling in tumor microenvironment. J Cell Physiol.
214:559–567. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Howe LR and Brown PH: Targeting the
HER/EGFR/ErbB family to prevent breast cancer. Cancer Prev Res
(Phila). 4:1149–1157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaplan AL, Hu JC, Morgentaler A, Mulhall
JP, Schulman CC and Montorsi F: Testosterone therapy in men with
prostate cancer. European Urology. 69:894–903. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ferro M, Lucarelli G, Bruzzese D, Di
Lorenzo G, Perdonà S, Autorino R, Cantiello F, La Rocca R, Busetto
GM, Cimmino A, et al: Low serum total testosterone level as a
predictor of upstaging and upgrading in low-risk prostate cancer
patients meeting the inclusion criteria for active surveillance.
Oncotarget. 8:18424–18434. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burstein HJ, Krilov L, Aragon-Ching JB,
Baxter NN, Chiorean EG, Chow WA, De Groot JF, Devine SM, DuBois SG,
El-Deiry WS, et al: Clinical cancer advances 2017: Annual report on
progress against cancer from the american society of clinical
oncology. J Clin Oncol. 35:1341–1367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lastwika KJ, Wilson W III, Li QK, Norris
J, Xu H, Ghazarian SR, Kitagawa H, Kawabata S, Taube JM, Yao S, et
al: Control of PD-L1 expression by oncogenic activation of the
AKT-mTOR pathway in non-small cell lung cancer. Cancer Res.
76:227–238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Westin JR: Status of PI3K/Akt/mTOR pathway
inhibitors in lymphoma. Clin Lymphoma Myeloma Leuk. 14:335–342.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Burgering BM and Kops GJ: Cell cycle and
death control: Longlive forkheads. Trends Biochem Sci. 27:352–360.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang X, Tang N, Hadden TJ and Rishi AK:
Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta.
1813:1978–1986. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Essafi A, de Mattos Fernández S, Hassen
YA, Soeiro I, Mufti GJ, Thomas NS, Medema RH and Lam EW: Direct
transcriptional regulation of Bim by FoxO3a mediates STI571-induced
apoptosis in Bcr-Abl-expressing cells. Oncogene. 24:2317–2329.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Accili D and Arden KC: FoxOs at the
crossroads of cellular metabolism, differentiation and
transformation. Cell. 117:421–426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meek DW: Regulation of the p53 response
and its relationship to cancer. Biochem J. 469:325–346. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee H, Kim JS and Kim E: Fucoidan from
seaweed Fucusvesiculosus inhibits migration and invasion of human
lung cancer cell via PI3K-Akt-mTOR pathways. PLoS One.
7:e506242012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bracho-Valdés I, Moreno-Alvarez P,
Valencia-Martínez I, Robles-Molina E, Chávez-Vargas L and
Vázquez-Prado J: mTORC1-and mTORC2-interacting proteins keep their
multifunctional partners focused. IUBMB Life. 63:896–914. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mizushima N: Methods for monitoring
autophagy. Int J Biochem Cell Biol. 36:2491–2502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guo GF, Jiang WQ, Zhang B, Cai YC, Xu RH,
Chen XX, Wang F and Xia LP: Autophagy-related proteins beclin-1 and
lc3 predict cetuximab efficacy in advanced colorectal cancer. World
J Gastroenterol. 17:4779–4786. 2011. View Article : Google Scholar : PubMed/NCBI
|






















