Introduction
Stem cells, which are characterized by their
self-renewal and multilineage differentiation capacity, have
recently attracted attention for their potential medicinal
applications (1). The process of
osteogenic differentiation is known to involve many regulatory
transcriptional factors. Runt-related transcription factor 2
(Runx2) has been identified as a key transcription factor in the
early stage of osteogenesis (2,3).
Runx2 may regulate osteogenic differentiation by targeting several
bone-specific transcriptional factors (4,5).
Runx2 is also an important regulator of chondrocyte differentiation
and maturation. Evidence showing an alteration of chondrocyte
maturation in long bones of Runx2 null mutant mice, as well as in
cell culture studies, indicate that Runx2 is a positive regulator
of chondrocyte differentiation (6–8).
Under-expression of Runx2 in proliferating chondrocytes induces
chondrocyte hypertrophy and partially rescues the chondrocyte
phenotype of Runx2 null mutant mice (9,10).
Selective inactivation of Runx2 in chondrocytes results in severe
shortening of the limbs due to a disturbance in chondrocyte
differentiation (11). Fan et
al have shown that the CREB-Smad6-Runx2 axis is involved in
osteogenesis dysfunction of bone mesenchymal stem cells (BMSCs).
Smad6 can interact with Runx2 and enhance Smurf1-induced Runx2
degradation in a ubiquitin-proteasome-dependent manner (12,13),
suggesting that suppressed expression of Smad6 may promote
osteogenic differentiation in BMSCs.
How to effectively promote BMSC osteogenic
differentiation has become a core issue in bone repair or
regeneration. Bioinformatics analysis has found that miR-92a can
target Smad6 by direct integration with the 3′-UTR of Smad6 mRNA
(http://www.targetscan.org/), suggesting
that miR-92a expression of may promote the osteogenic
differentiation of BMSCs by targeting Smad6.
To define the function and regulatory mechanisms of
miR-92a in the osteogenic differentiation of BMSCs, BMSCs were
obtained from mice and miR-92a or Smad6 overexpression vectors were
constructed. Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and western blots were used to analyze miR-92a
and Smad6 expression, and a luciferase reporter assay was used to
examine the interaction between miR-92a and Smad6. BMSCs were
induced in osteogenic differentiation media for 21 days and then
analyzed by alkaline phosphatase (ALP) activity and Alizarin Red
histochemical staining. The results may improve our knowledge of
the epigenetic mechanisms governing BMSC osteoblastic lineage
differentiation, which would benefit the development of bone tissue
engineering or cell therapy based on BMSCs.
Materials and methods
Ethics statement
BALB/C mice (6–8 weeks old) were obtained from the
Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). All
animal studies were performed in accordance with the Guide for the
Care and Use of Laboratory Animals. All study protocols were
approved by the Scientific Research Projects Approval Determination
of the Ethics Committee of the 455th Hospital of PLA.
Isolation and characterization of
BMSCs
BMSCs were harvested from 6- to 8-week-old mice as
described previously (14). Mice
were euthanized and both femurs and tibiae were aseptically
removed. Then the ends of the femurs and tibiae were cut and the
bone marrow was flushed out and diluted 1:2 with phosphate buffer
solution and loaded in a 5 ml Percoll (density, 1.077; Pharmacia
Biotech, Uppsala, Sweden). Cells were harvested from the interface
after centrifugation at 2,000 × g for 20 min and washed with
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). They were then resuspended in
low-glucose DMEM containing 10% fetal bovine serum (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA), 100 U/ml penicillin, 100
g/ml streptomycin, and incubated at 37°C. Cells of the 2nd to 5th
passages were used in this study.
For BMSC characterization, cells were seeded again
at 1:4 density ratios and tested by immunofluorescence, with
positive results for CD29, CD44, CD90, and CD105, but negative
results for hematopoietic markers such as CD34 and CD45.
For multiple differentiation capacity assays, BMSCs
were cultured for 3 weeks in either adipogenic differentiation
media or osteogenic differentiation media (Cellular Engineering
Technologies Inc., Coralville, IA, USA). Oil-red O stain or ALP
were used to identify the multiple differentiation capacities of
BMSCs.
Transfection of cells with the miR-92a
mimics vector, Smad6 overexpression vector or treatment with
miR-92a inhibitor
For miR-92a overexpression, the miR-92a mimic or
corresponding negative control (miR-NC) were purchased from
GenePharma (Shanghai, China). BMSCs were transfected with either
the miR-92a mimic or miR-NC at a final concentration of 50 nM using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Cells
were used for miR-92a expression analysis or other experiments
after 48 h of transfection. For miR-92a inhibition, BMSCs were
treated with a miR-92a inhibitor (Invitrogen; Thermo Fisher
Scientific, Inc.) for 48 h, then BMSCs were collected for further
experiments.
For Smad6 overexpression, human Smad6 cDNA with the
3′-UTR was cloned into a pMSCV-hygro vector. The primers
corresponded to the NCBI reference sequence (AF202257.3) and were
as follows: Forward, 5′-CAGAGCTCATGTTCAGGTCTAAACG-3′ and reverse,
5′-GGTCTAGACTATCTGTGGTTGTTGAGT-3′. The Smad6 cDNA was inserted into
a pMD18-T Simple Vector (Takara Bio, Inc., Otsu, Japan) to form
pMD18-T-Smad6. After sequencing, the recombinant segment of the
correct clone was digested by BamHI and XbaI (Takara
Bio, Inc.). The recombinant segment was inserted into pMSCV-hygro
after digestion by the same two restriction endonucleases. The
pMSCV-hygro-Smad6 clones were sequenced and the correct clones were
amplified and identified by restriction enzyme digestion.
RT-qPCR for the detection of
miR-92a
Total RNA was isolated using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). RT was
performed using the RT-PCR system (Promega, Shanghai, China).
RT-qPCR was performed in a final reaction volume of 20 µl using
SYBR®-Green I Supermix (Takara Biotechnology Co., Ltd.,
Dalian, China) according to the manufacturer's protocol. All
reactions were run in triplicate on a iCycler IQ Multicolor
Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
with the following cycling parameters: 95°C for 10 sec followed by
40 cycles of 94°C for 15 sec, annealing at 55°C for 30 sec, and a
final extension at 70°C for 30 sec. All quantifications were
normalized to the level of human U6 snRNA in the same reaction. The
comparative quantification (Cq) cycle method (2−ΔΔCq),
which compares differences in Cq values between common reference
RNA and target gene RNA, was used to obtain the relative
fold-changes in gene expression. The miR-92a primers for PCR were
designed by GenePharma.
MTT assay
Cell toxicity was measured with an MTT assay to
detect NADH dependent dehydrogenase activity. Fifty microliters of
MTT solution (5 mg/ml) in 1X phosphate-buffered saline were
directly added to the cells, which were then incubated for 2 h to
allow MTT to metabolize to formazan. Absorbance was measured at a
wavelength of 540 nm using an ELISA reader (Beckman Coulter, Inc.,
Brea, CA, USA).
Osteoblastic differentiation
To identify the effect of miR-92a and Smad6 on
osteoblastic differentiation, BMSCs were transfected with miR-92a
or the Smad6 overexpression vector or treated with a miR-92a
inhibitor after BMSCs reached 70–80% confluence in basal medium.
Then the medium was changed to osteogenic differentiation media to
allow the cells to differentiate into osteoblasts and then cultured
for 21 days, with fresh culture medium changed every 3 days.
ALP activity assay and Alizarin red
staining
BMSCs were seeded into 96-well plates at a density
of 2×103 cells/well and cultured in routine medium or
mineralization-inducing medium (MM) containing α-MEM, 10% fetal
bovine serum, 100 U/ml penicillin, 100 µg/ml streptomycin, 100 µM
ascorbic acid, 2 mM 2-glycerophosphate, and 10 nM dexamethasone.
The ALP activity assay was performed using an ALP activity kit
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and the activity
was normalized to total protein content in the cell. Alizarin red
staining was performed after treatment on day 21. Calcified nodules
were stained using 10% cetylpyridinium chloride in 10 mM sodium
phosphate (pH 7.0). Calcium concentration was determined by
measuring the absorbance at 526 nm with a universal microplate
reader (BioTek Instruments, Inc., Winooski, VT, USA). This
experiment was performed in triplicate and the results are
presented as the mean ± standard deviation (SD).
Luciferase reporter assay
To construct luciferase reporter vectors, the 3′-UTR
of Smad6 cDNA fragments containing the predicted miR-92a binding
sites were amplified by PCR and subcloned downstream of the
luciferase gene in the PYr-MirTarget luciferase vector (Ambion;
Thermo Fisher Scientific, Inc.). The 3′-UTR of Smad6 (containing
the binding sites for miR-92a) was amplified from a cDNA library
with the following primers: Forward,
5′-CTCGAGCGTAATTATTTATATATGGTGCAATGTG-3′ and reverse,
5′-GCGGCCGCGGTCTGGGCATTAATATTT-3′. The mutant 3′-UTR of Smad6 (in
which six nucleotides were mutated in the binding sites) was
amplified using the following primer sequences: Forward,
5′-CTCGAGCGTAATTATTTATATATGCACGTTGTG-3′ and reverse,
5′-GCGGCCGCGGTCTGGGCATTAATATTT-3′.
For luciferase assays, HEK293T cells were cultured
in 24-well plates and co-transfected with 50 ng of the
corresponding vectors containing firefly luciferase together with
50 ng of miR-92a or the control. Transfection was performed using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). At 48 h post-transfection, the relative
luciferase activity was calculated by normalizing the firefly
luminescence to the Renilla luminescence using the Dual-Luciferase
Reporter Assay (Promega Corporation, Madison, WI, USA) according to
the manufacturer's instructions.
Western blot analysis
Western blot analysis was carried out using cell
lysates in urea buffer (8 M urea, 1 M thiourea, 0.5% CHAPS, 50 mM
dithiothreitol, 24 mM spermine). Protein fractions were prepared
using cytoplasmic extraction reagents (Pierce; Thermo Fisher
Scientific, Inc.) following manufacturer's protocols. GAPDH was
used as a loading control. Samples (40 µg total protein) were
separated on SDS-PAGE and transferred to nitrocellulose membranes
(EMD Millipore, Billerica, MA, USA). After blocking in 5% nonfat
milk for 1 h, the membranes were incubated with primary antibodies
against Smad6 (1:1,000), Runx2 (1:200), osteopontin (OPN, 1:200),
osteocalcin (OCN, 1:200), osterix (OSX, 1:200) and GAPDH (1:2,000),
respectively, at 4°C overnight. After washing, the membranes were
incubated with horseradish peroxidase-conjugated secondary
antibodies for 1 h at room temperature. Signals were detected using
an ECL detection system (GE Healthcare, Aurora, OH, USA) and
analyzed with ImageJ 1.42q software (National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
Results are expressed as the mean ± SD. Statistical
significance was evaluated by one-way analysis of variance followed
by the Tukey-Kramer multiple comparison test and by Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Isolation and identification of BMSCs
with stem cell markers
In order to identify if the expression of miR-92a
can promote osteogenic differentiation in BMSCs, BMSCs were
isolated from mice. The predominant cells after 9–10 days of
culture were morphologically homogeneous and spindle-shaped, and
most of them were mesenchymal stem cells (Fig. 1A). Isolated BMSCs were labeled with
different cell surface markers to verify that the isolated cells
were truly mesenchymal stem cells. The results show that BMSCs were
positive for the MSC markers CD29, CD90, CD44, and CD105 and
negative for the endothelial markers CD34 and CD45 (Fig. 1B-H), indicating that the isolated
cells were BMSCs. Oil-red O staining and ALP activity showed the
adipogenic (Fig. 1I) and
osteogenic differentiation (Fig.
1J) potential of BMSCs under adipogenic or osteogenic induction
conditions.
miR-92a expression promotes osteogenic
differentiation of BMSCs
To identify if miR-92a expression can promote the
osteogenic differentiation of BMSCs, miR-92a overexpression and
miR-92a mimic vectors were constructed and transfected into BMSCs
and cultured for 48 h. For the inhibition of miR-92a expression,
BMSCs were pretreated with a miR-92a inhibitor for 48 h. The
results show that miR-92a expression was significantly increased
compared with the negative control (NC), but decreased
significantly after treatment with a miR-92a inhibitor (Fig. 2A). The MTT assay showed that both
miR-92a overexpression and downregulation have not significantly
affected cell activity (Fig.
2B).
 | Figure 2.Expression of miR-92a promotes the
osteogenesis of bone mesenchymal stem cells (BMSCs). (A) RT-qPCR
shows miR-92a expression after transfection with miR-92a mimics or
treatment with miR-92a inhibitor for 48 h. (B) Cell viability was
determined with an MTT assay. Data are presented as the mean ± SD,
(n=5). (C and D) Alkaline phosphatase (ALP) histochemical staining
assay showing the effect of miR-92a on the osteogenic ability of
BMSCs after being induced in osteogenic differentiation media for
21 days. ALP activities were evaluated by spectrophotometry. Data
are presented as the mean ± SD. ***P<0.001 vs. the untreated
control (n=5). Magnification, ×400. (E and F) Mineralized bone
matrix formation was evaluated by Alizarin Red staining after BMSCs
were induced in osteogenic differentiation media for 21 days.
Mineralized nodule formation was assessed by Alizarin red staining.
The stains were eluted and measured at 590 nm. Data are presented
as the mean ± SD. ***P<0.001 vs. the untreated control (n=5).
Magnification, × 200. (G) Western blot assessment of Smad6, Runx2,
OPN, OCN, and OSX expression. (H-L) Quantification of relative
protein expression. Data are presented as the mean ± SD.
***P<0.001 vs. the untreated control (n=3). NC, negative
control; Runx2, runt-related transcription factor 2; OPN,
osteopontin; OCN, osteocalcin; OSX, osterix. |
To identify if miR-92a expression can promote the
osteogenic differentiation, BMSCs transfected with miR-92a mimic or
miR-92a-NC, or treated with a miR-92a inhibitor were induced in
osteogenic differentiation media for 21 days. Then ALP activity
(Fig. 2C and D) and Alizarin Red S
staining (Fig. 2E and F) were used
to detect osteogenic differentiation. The results show that miR-92a
overexpression significantly increased ALP activity and calcium
deposition compared with the control group. However, the ALP
activity and calcium deposition were decreased after treatment with
a miR-92a inhibitor.
Western blot detection showed that miR-92a
expression suppressed Smad6 expression, which increased Runx2
expression indirectly. Previous studies have found that Runx2 is
upstream of OSX in the osteogenic differentiation signaling pathway
(15,16). Our study also verified that miR-92a
overexpression promotes OCN, OPN, and OSX expression. However,
miR-92a-specific inhibitors suppressed Runx2 expression by
promoting Smad6, which then inhibited the relative protein
expression of osteogenic differentiation (Fig. 2G-L).
Smad6 overexpression reversed miR-92a
induced osteogenic differentiation of BMSCs
To identify if the promotion effect of miR-92a on
the osteogenic differentiation of BMSCs is by downregulating Smad6,
a Smad6 overexpression vector was constructed and transfected into
BMSCs. BMSCs were cultured for 48 h and then analyzed with western
blot (Fig. 3A) and RT-qPCR
(Fig. 3B). The results show that
the expression of Smad6 was significantly increased at both the
protein and mRNA level when Smad6 was overexpressed. After
osteogenic differentiation was induced in media for 21 days, ALP
activity (Fig. 3C and D) and
Alizarin Red S staining (Fig. 3E and
F) were used to detect osteogenic differentiation in BMSCs. The
results show that Smad6 overexpression reversed miR-92a induced ALP
activity and calcium deposition increased compared with the control
group. Western blot detection showed that the expression of Smad6
decreased Runx2 expression. Our study also verified that Smad6
overexpression decreased miR-92a induced OCN, OPN, and OSX
expression (Fig. 3G-L).
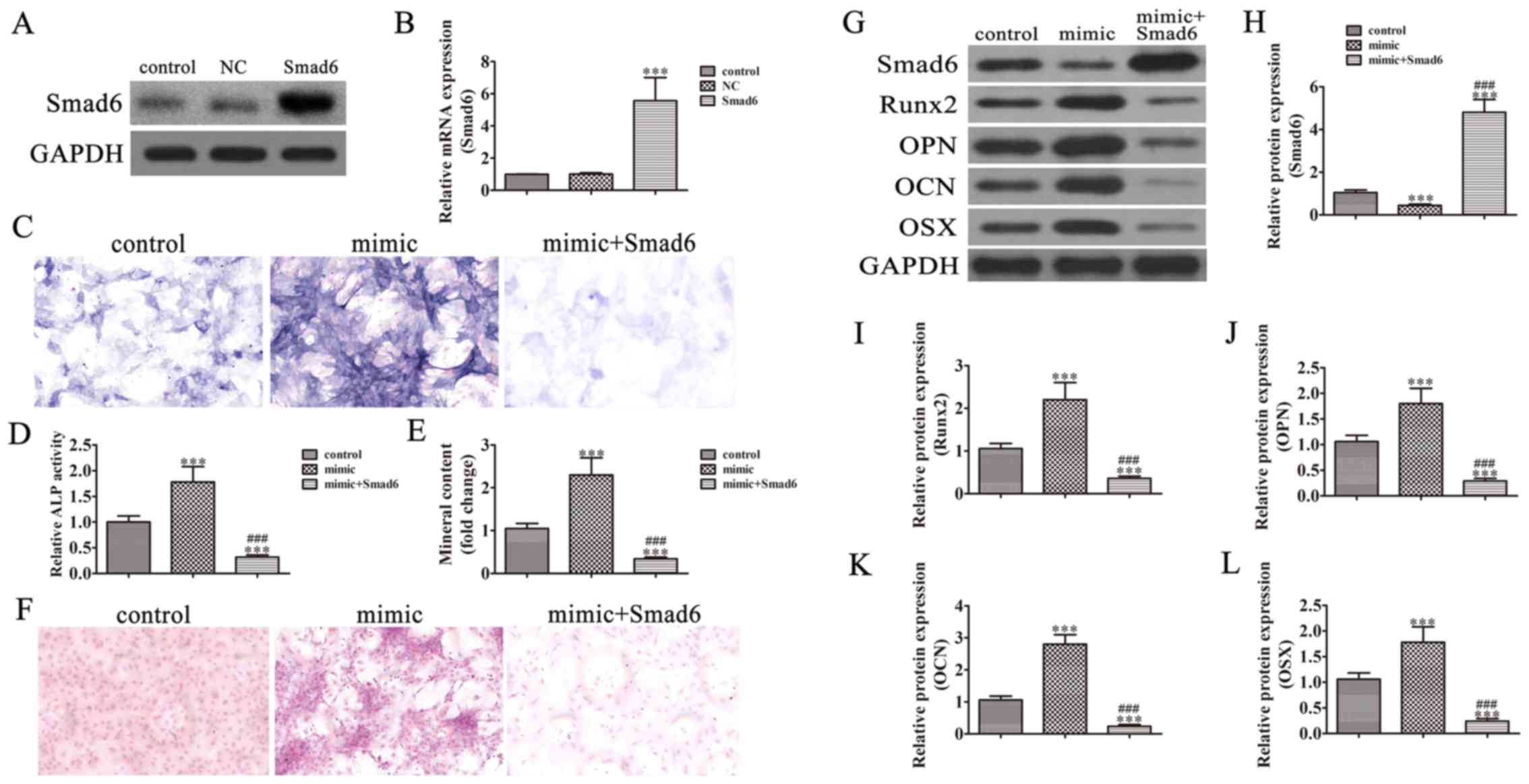 | Figure 3.Smad6 overexpression reversed miR-92a
induced osteogenesis of bone mesenchymal stem cells (BMSCs). (A)
Western blotting and (B) RT-qPCR analysis show the expression of
Smad6 in BMSCs cells after transfection with the Smad6
overexpression vector or the NC vector for 48 h. Data are presented
as the mean ± SD. ***P<0.001 vs. control (n=5). (C and D)
Alkaline phosphatase (ALP) histochemical staining assay showing the
effect of miR-92a on the osteogenic ability of BMSCs. ALP
activities were evaluated with spectrophotometry. Data are
presented as the mean ± SD. ***P<0.001 vs. the untreated
control. ###P<0.001 vs. the mimic (n=5).
Magnification, ×400. (E and F) Mineralized bone matrix formation
was evaluated with Alizarin Red staining after BMSCs were induced
in osteogenic differentiation media for 21 days. Mineralized nodule
formation was assessed with Alizarin red staining. The stains were
eluted and measured at 590 nm. Data are presented as the mean ± SD.
***P<0.001 vs. the untreated control. ###P<0.001
vs. the mimic (n=5). Magnification, ×200. (G) Western blot
assessment of the expression of Smad6, Runx2, OPN, OCN, and OSX.
(H-L) Quantification of relative protein expression. Data are
presented as the mean ± SD. ***P<0.001 vs. the untreated
control. ###P<0.001 vs. the mimic (n=3). NC, negative
control; Runx2, runt-related transcription factor 2; OPN,
osteopontin; OCN, osteocalcin; OSX, osterix. |
Smad6 is the direct target of
miR-92a
To determine the possible interaction between
miR-92a and Smad6, we first performed a bioinformatics screen for
its possible target genes using an online 3′-UTR binding site
prediction database (http://www.targetscan.org/). Overlap analyses showed
that miR-92a had a broadly conserved binding site. A mutated
version of the Smad6 3′-UTR was constructed in which six
complementary nucleotides in the binding site were altered
(Fig. 4A). This mutated construct
was fused to the luciferase coding region (PYr-Smad6 3′-UTR) and
co-transfected into HEK293T cells along with miR-92a mimics. The
relative luciferase activity showed that when the wild-type Smad6
3′-UTR was co-transfected with miR-92a mimics, Smad6 expression was
significantly decreased compared with co-transfection with the
control miRNA (Fig. 4B). However,
this effect was not observed after mutant 3′-UTR of Smad6,
indicating that miR-92a can specifically target and suppress the
3′-UTR of Smad6. Western blot analyses further confirmed that
miR-92a expression significantly inhibited Smad6 expression in
vitro (Fig. 4C).
Discussion
In this study, we investigated whether Smad6 could
be used as a potential target to promote osteogenic differentiation
of BMSCs. BMSCs were isolated from mice and a miR-92a
overexpression vector was constructed. Our data have provided the
evidence that overexpression of miR-92a promotes osteogenic
differentiation of BMSCs by targeting Smad6. Shen et al have
identified that Smad6 can interact with Runx2 and enhance
Smurf1-induced Runx2 degradation in a
ubiquitin-proteasome-dependent manner (12,17).
Runx2 expression was also increased with miR-92a overexpression,
which resulted in increased ALP activity and matrix mineralization
capacity in BMSCs.
Runx2 is an important osteoblast lineage-determining
transcription factor involved in directing osteoblastic
differentiation, and Runx2 appears to be the master gene in
osteogenesis as it induces the expression of OCN, OSX, and OPN,
which are required in terminal osteoblast differentiation (18,19).
Our data strongly suggest that miR-92a plays a major role in
osteogenic differentiation and functions in a Smad6-Runx2-dependent
manner, adding a new layer of control to the regulation of the
epigenetic program of osteogenesis. A previous report showed that
miR-92a may have the potential to promote osteoblast proliferation
and differentiation (20).
However, our results show that Runx2 expression was not affected by
Smad6overexpression in a miR-92a mimic treatment group in BMSCs
from mice, indicating that miR-92a can suppress Smad6 expression by
targeting the 3′-UTR of Smad6 at the mRNA level. This result was
confirmed with a bifluorescein reporting assay.
In conclusion, our in vitro study using
transgenic BMSCs is the first demonstration that miR-92a can induce
osteogenesis. Our findings are also consistent with the ability of
miRNA-modified BMSCs to promote osteoblast differentiation by
targeting Smad6. Although our results are very promising, further
experimentation will be needed to investigate whether
miRNA-modified BMSCs could also promote osteogenesis with
miR-92a-modified BMSCs in vivo. Despite these limitations,
our study supports the therapeutic promise of miR-92a in promoting
osteogenic differentiation.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY and HW designed the study and wrote the paper. YL
and WH analyzed the data and wrote the paper. YJ and QS performed
the experiments.
Ethics approval and consent to
participate
All study protocols were approved by the Scientific
Research Projects Approval Determination of the Ethics Committee of
the 455th Hospital of PLA.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zheng YH, Xiong W, Su K, Kuang SJ and
Zhang ZG: Multilineage differentiation of human bone marrow
mesenchymal stem cells in vitro and in vivo. Exp Ther Med.
5:1576–1580. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang L, Tang Y, Zhu X, Tu T, Sui L, Han
Q, Yu L, Meng S, Zheng L, Valverde P, et al: Overexpression of
MiR-335-5p promotes bone formation and regeneration in mice. J Bone
Miner Res. 32:2466–2475. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim MJ, Park JS, Kim S, Moon SH, Yang HN,
Park KH and Chung HM: Encapsulation of bone morphogenic protein-2
with Cbfa1-overexpressing osteogenic cells derived from human
embryonic stem cells in hydrogel accelerates bone tissue
regeneration. Stem Cells Dev. 20:1349–1358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kidwai F, Edwards J, Zou L and Kaufman DS:
Fibrinogen induces RUNX2 activity and osteogenic development from
human pluripotent stem cells. Stem Cells. 34:2079–2089. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang C, Liu D, Zhang C, Sun J, Feng W,
Liang XJ, Wang S and Zhang J: Defect-related luminescent
hydroxyapatite-enhanced osteogenic differentiation of bone
mesenchymal stem cells via an ATP-induced cAMP/PKA pathway. ACS
Appl Mater Interfaces. 8:11262–11271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Inada M, Yasui T, Nomura S, Miyake S,
Deguchi K, Himeno M, Sato M, Yamagiwa H, Kimura T, Yasui N, et al:
Maturational disturbance of chondrocytes in Cbfa1-deficient mice.
Dev Dyn. 214:279–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim IS, Otto F, Zabel B and Mundlos S:
Regulation of chondrocyte differentiation by Cbfa1. Mech Dev.
80:159–170. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Enomoto H, Enomoto-Iwamoto M, Iwamoto M,
Nomura S, Himeno M, Kitamura Y, Kishimoto T and Komori T: Cbfa1 is
a positive regulatory factor in chondrocyte maturation. J Biol
Chem. 275:8695–8702. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takeda S, Bonnamy JP, Owen MJ, Ducy P and
Karsenty G: Continuous expression of Cbfa1 in nonhypertrophic
chondrocytes uncovers its ability to induce hypertrophic
chondrocyte differentiation and partially rescues Cbfa1-deficient
mice. Genes Dev. 15:467–481. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ueta C, Iwamoto M, Kanatani N, Yoshida C,
Liu Y, Enomoto-Iwamoto M, Ohmori T, Enomoto H, Nakata K, Takada K,
et al: Skeletal malformations caused by overexpression of Cbfa1 or
its dominant negative form in chondrocytes. J Cell Biol.
153:87–100. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stricker S, Fundele R, Vortkamp A and
Mundlos S: Role of Runx genes in chondrocyte differentiation. Dev
Biol. 245:95–108. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen R, Chen M, Wang YJ, Kaneki H, Xing L,
O'keefe RJ and Chen D: Smad6 interacts with Runx2 and mediates Smad
ubiquitin regulatory factor 1-induced Runx2 degradation. J Biol
Chem. 281:3569–3576. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan QM, Yue B, Bian ZY, Xu WT, Tu B, Dai
KR, Li G and Tang TT: The CREB-Smad6-Runx2 axis contributes to the
impaired osteogenesis potential of bone marrow stromal cells in
fibrous dysplasia of bone. J Pathol. 228:45–55. 2012.PubMed/NCBI
|
|
14
|
Zhang L, Chen P, Chen L, Weng T, Zhang S,
Zhou X, Zhang B and Liu L: Inhibited Wnt signaling causes
age-dependent abnormalities in the bone matrix mineralization in
the Apert syndrome FGFR2(S252W/+) mice. PLoS One. 10:e1127162015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen S, Gluhak-Heinrich J, Wang YH, Wu YM,
Chuang HH, Chen L, Yuan GH, Dong J, Gay I and MacDougall M: Runx2,
osx, and dspp in tooth development. J Dent Res. 88:904–909. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du J, Wang Q, Yang P and Wang X: FHL2
mediates tooth development and human dental pulp cell
differentiation into odontoblasts, partially by interacting with
Runx2. J Mol Histol. 47:195–202. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee YS, Park JS, Kim JH, Jung SM, Lee JY,
Kim SJ and Park SH: Smad6-specific recruitment of Smurf E3 ligases
mediates TGF-β1-induced degradation of MyD88 in TLR4 signalling.
Nat Commun. 2:4602011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ducy P, Zhang R, Geoffroy V, Ridall AL and
Karsenty G: Osf2/Cbfa1: A transcriptional activator of osteoblast
differentiation. Cell. 89:747–754. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Komori T: Runx2, a multifunctional
transcription factor in skeletal development. J Cell Biochem.
87:1–8. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Kong D, Ahmad A, Bao B and Sarkar
FH: Targeting bone remodeling by isoflavone and
3,3′-diindolylmethane in the context of prostate cancer bone
metastasis. PLoS One. 7:e330112012. View Article : Google Scholar : PubMed/NCBI
|


















