Introduction
To date, approximately three billion people have
received Bacille Calmette-Guerin (BCG) vaccination (1). BCG may prevent up to 80% of
tuberculosis (TB) infections, and the period of efficacy is 15
years; however, its protective effect varies according to
geographical variations (2,3). BCG
is one of the world's most widely-used and safest vaccines;
however, if vaccinating an immunodeficient infant with BCG may
cause disseminated or fatal infection (4). The World Health Organization (WHO)
recommends that in TB-prevalent countries, all newborn children be
vaccinated against tuberculosis (5,6). BCG
is a non-toxic cultured bacterium, which is used to prevent
tuberculosis infection. A minor reaction may follow inoculation,
including aseptic abscesses. The majority of the side effects of
BCG immunotherapy appear to be self-limiting (7). The most common visceral involvement
is the formation of asymptomatic liver granulomas, termed
granulomatous hepatitis (8,9).
Plasmodium merozoites primarily invade the red blood
cells of the host, which is where development and reproduction
occurs (10). Plasmodium infected
erythrocytes are able to adhere to the capillary endothelia of the
host and escape from the spleen (11,12).
Studies have demonstrated that pre-inoculation treatment with BCG
improves the strength of the host immune response to malaria
(13,14), although the effects of Plasmodium
following malaria infection in BCG-vaccinated patients remains
unclear (15). Therefore, the
present study examined the effects of Plasmodium infection in
BCG-vaccinated patients and the underlying mechanism. BCG-infected
BALB/c mice subsequently infected with Plasmodium were used to
observe the occurrence of hepatic granulomas. The secretion of
pro-inflammatory and anti-inflammatory cytokines was analyzed at
different time points following infection.
Materials and methods
Bacterial strain, parasites, mice and
infections
Mycobacterium bovis BCG, Pasteur strain were
grown in Middlebrook 7H9 medium (Difco Laboratories; BD
Biosciences, Franklin Lakes, NJ, USA) with 10% albumin dextrose
catalase (ADC) (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and
0.05% Tween 80. Cryopreserved blood pre-treated with Plasmodium
yoelii (P. yoelii 17XNL) was defrosted and used to
infect BALB/c mice. A total of 60 female BALB/c mice (6-week-old,
18–23 g) were purchased from Vital River Laboratory Technology
Animal Co., Ltd. (Beijing, China) and maintained in specific
pathogen-free grade experimental animal facilities at 25°C with
free access to acidified water and food and maintained at 45–70%
humidity with a 12-h light/dark cycle. All mice were divided into
four groups. The uninfected mice were set as the control group. BCG
group mice were intravenously injected with 1×107
colony-forming units (CFU) BCG per mouse. Plasmodium group
mice were injected intraperitoneally with 1×105 blood
cells infected with P. yoelii 17XNL. BCG + Plasmodium
group mice were intravenously injected with 1×107 CFU
BCG and 1×105 blood cells infected with P. yoelii
17XNL. The kinetics of the infections was followed over 8 weeks.
Following treatment mice had free access to water and food and were
kept at 45–70% humidity and 25°C; the housing room was sterilized
by UV lights for 12 h and had a 12-h light/dark cycle. Mice were
sacrificed at weeks 1, 2, 4, 6 and 8. The present study was
approved by the Institutional Animal Care and Use Committee of
Pearl Animal Sci. & Tech. Co., Ltd. (Dongguan, China).
Histopathological analysis
Following sacrifice, histological examinations were
performed. The livers were removed, fixed in 10% formaldehyde
solution at 4°C for 1 week, and then dehydrated. The paraffin
sections (5 µm) were cut and hematoxylin and eosin (H&E)
staining was performed at room temperature for 2 h. The results of
the staining (magnification, ×200) were analyzed with an optical
inverted microscope (Olympus Corporation, Tokyo, Japan).
Simultaneously, the number of granulomas in the liver was
determined in the H&E-stained sections (5 sections per mouse, 3
mice per group).
Determination of liver bacterial
load
To assess the bacterial load in the liver following
co-infection, the mice were sacrificed and the entire liver was
homogenized using PBS supplemented with 0.05% Tween 80, and serial
dilutions of homogenized liver tissues were plated on 7H11 agar
with oleic ADC. The plates were cultured in an incubator at 37°C
with 5% CO2, and the CFU (copies/mg) were determined at
1, 2, 4, 6 and 8 weeks respectively.
Immunohistochemical analysis
Inflammatory activity in the liver in BCG and
Plasmodium coinfected mice were evaluated by
immunohistochemistry. The liver tissues were cut into 4-µm sections
using a cryostat. Inflammatory activity was evaluated via iNOS
immunostaining. Briefly, sections were deparaffinized and hydrated,
and heated in citrate buffer (0.01 M, Ph 6.0) for 1 min at 100°C
and were then treated with endogenous peroxidase (3% hydrogen
peroxide solution) for 20 min at room temperature. Following
blocking with 10% goat serum (Beyotime Institute of Biotechnology,
Shanghai, China) for another 30 min at room temperature, sections
were immunostained with primary antibodies with anti-iNOS (Rabbit
monoclonal to iNOS; cat. no. ab178945; 1:500; Abcam, Cambridge, UK)
antibody overnight at 4°C, and subsequently incubated with the
secondary antibody (Goat Anti-Rabbit; cat. no. ab6720; 1:1,000;
Abcam) for 30 min at room temperature. Sections were then incubated
with avidin-biotin-peroxidase complex for 30 min and DAB reagent
for 5 min at room temperature. Subsequently, all sections were
double stained with hematoxylin and visualized under the microscope
(magnification, ×10; BX51, Olympus Corporation, Tokyo, Japan), and
six fields were selected for statistical analysis.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
RT-PCR was performed as previously described
(16). mRNA was extracted from
small pieces of liver tissues. First strand cDNA was synthesized
from the 1 µg total RNA. Sequences for primers were as follows: For
iNOS, forward TCACTGGGACAGCACAGAAT, and reverse
TGTGTCTGCAGATGTGCTGA; and for β-actin, forward ACCACACCTTCTACAATGA,
and reverse ATAGCACAGCCTGGATAG, and were designed using Primer
Premier version 6.0 (Premier Biosoft International, Palo Alto, CA,
USA). RT-PCR was carried out in the Applied Biosystems 7300 PCR
system (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Light
Cycler Software version 3 (Roche Applied Science, Penzberg,
Germany) was used for the analysis of the results.
Statistical analysis
Data were analyzed using GraphPad version 6.0
(GraphPad Software, Inc., La Jolla, CA, USA). One-way analysis of
variance with least significant difference post hoc tests were used
to indicate the significant differences among the different groups.
A total of three replicates were performed for each experiment.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Liver index analysis demonstrates that
liver granulomas slowly return to normal in co-infected mice
As presented in Fig.
1, from the liver index analysis it was apparent that, due to
the co-infection of Plasmodium and BCG, the return to normal
levels was notably delayed. Treatment with BCG or Plasmodium
causes swelling of the liver, although this process is transient
and the swelling is rapidly reversed (17). In the present study, following
co-infection with Plasmodium and BCG, the swelling level of
the liver returned to normal slowly (P<0.05).
Histopathological analysis
demonstrates that Plasmodium causes the number of granulomas to
slowly decrease in BCG-infected mice
As presented in Fig.
2, from a histopathological perspective, it was observed that
Plasmodium infection did not lead to the formation of
granulomas. BCG infection caused the formation of TB-specific
granulomas; the number of granulomas reached a peak at week 4 and
subsequently subsided. However, following co-infection with
Plasmodium and BCG, due to the induction of
Plasmodium, the peak formation of granulomas was delayed
until week 6 and subsequently subsided (P<0.05).
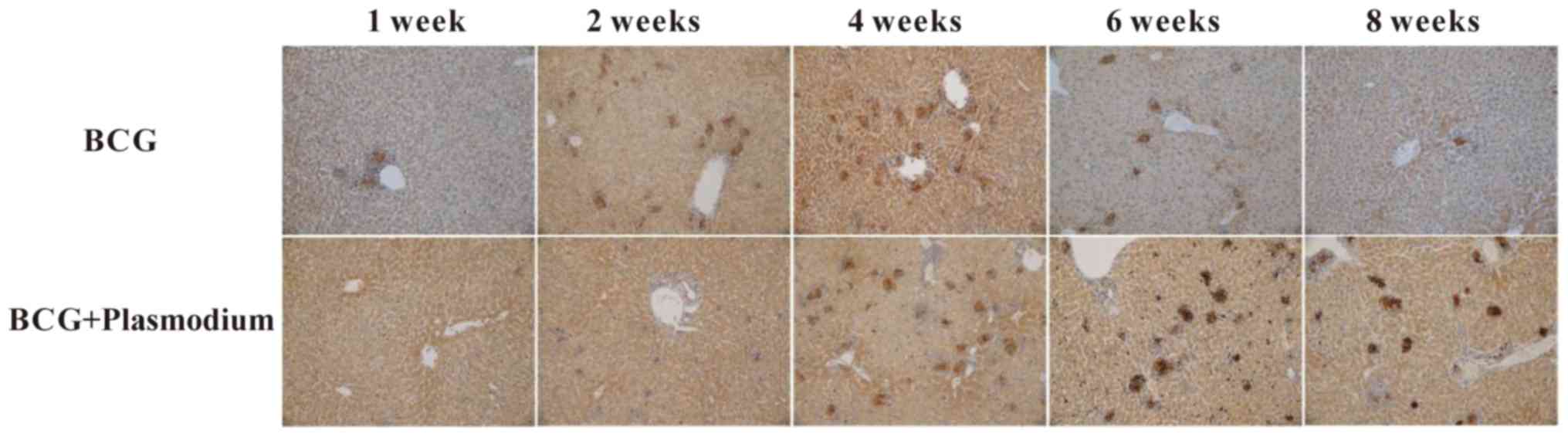
Expression of iNOS increases and
decreases with the formation and disappearance of granulomas in the
liver
As demonstrated in Fig.
3, iNOS was highly expressed in the granulomas of the BCG group
and BCG + Plasmodium co-infected group. In order to further
examine whether the expression of iNOS is different among different
groups, immunohistochemical staining was performed. As presented in
Fig. 4A, it was observed that the
Plasmodium group had decreased expression of iNOS; the
expression of iNOS in the BCG group reached a peak at week 4, while
in the BCG + Plasmodium co-infection group, it reached a
peak at week 6 (P<0.05).
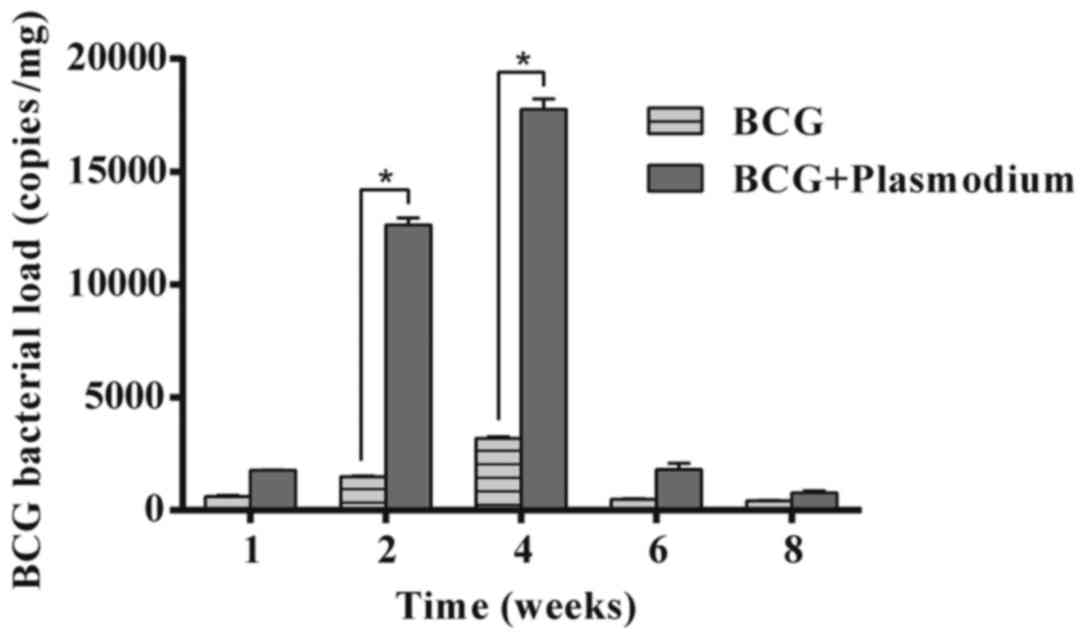
Expression of iNOS and IL-10 exhibits
reverse trends
The present study aimed to assess why a difference
in the expression of iNOS was observed. As exhibited in Figs. 4B and 5, the expression of IL-10 and BCG
bacterial load in liver tissues was examined. IL-10 is considered
to have immunosuppressive effects. The experimental results
demonstrated that the expression of IL-10 was initially high and
gradually decreased (*P<0.05), in the Plasmodium and BCG
+ Plasmodium groups. There was a higher bacterial load in
the coinfected group compared with the BCG group at 2 weeks. A
similar significant increase in bacterial load in the liver was
observed in the BCG + Plasmodium group when compared with
the BCG group at 4 weeks.
Discussion
BCG is a mutant strain of M. bovis. During the 20 th
Century, BCG has been used to vaccinate newborns in order to
prevent TB (18). As its safety
has been determined, BCG has been used widely around the world; it
has officially been recognized as the most safe and effective
vaccine against tuberculosis, and it has become one of the
vaccinations at birth recommended by the WHO (19,20).
BCG is an important approach in the prevention of severe TB
infection. The majority of BCG-vaccinated infants do contract an
infection, although there remain a small number of infected infants
(21,22). BCG infection occurs only in some
children; those whose host immune defense ability against BCG may
be low (23). The infection may
lead to the occurrence of granulomatous hepatitis in the liver
(24).
Malaria, an insect-borne disease caused by
Plasmodium infection, is one of the most serious communicable
diseases (25,26). The prevention and treatment of
malaria faces serious challenges and understanding of the
biological characteristics of the malaria parasite and its
association with the host immune system remains limited (27). The present study demonstrated that
the use of Plasmodium in BCG-infected mice generated a high level
of iNOS and that granuloma formation was delayed, although
sustained.
In order to confirm the findings in the liver
tissues of co-infected mice, histopathological analysis was
performed (28). The H&E
staining images demonstrated that the livers of co-infected mice
exhibited extensive granulomas, which were decreased in number in
the Plasmodium group. Granuloma formation in liver was delayed in
BCG-infected mice co-infected with Plasmodium, indicating that
Plasmodium was the primary cause of this phenomenon. The present
study suggested that the reason for this was that Plasmodium may
exert a direct impact on the delayed-type allergic immune response,
although the mechanism is not yet clear.
To illustrate the mechanism of action of Plasmodium
in BCG-infected mice, immunohistochemical and RT-PCR analyses were
performed. The results demonstrated that with the formation and
reversal of hepatic granulomas, the expression of iNOS increased
and decreased, while the expression of IL-10 exhibited the opposite
trend. These results demonstrated that iNOS and IL-10 were involved
in the pathological process.
In conclusion, the present study provided an
innovative examination of the role of Plasmodium in BCG granuloma
formation. The results of the present study demonstrated that,
following co-infection with Plasmodium and BCG, the formation of
granulomas in liver was relatively delayed, although sustained, in
mice.
Acknowledgements
Not applicable.
Funding
The present study was supported by Provincial
Science and Technology Project of Guangdong Province (grant nos.
2016A030303056).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC and LQ designed the present study and approved
this submission. BN and JY performed the experiments and wrote the
manuscript. MJ performed the immunohistochemical staining. SZ
helped to collect data and revised the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Pearl Animal Sci. & Tech. Co.,
Ltd. (Dongguan, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Painter JA, Graviss EA, Hai HH, Nhung DT,
Nga TT, Ha NP, Wall K, le Loan TH, Parker M, Manangan L, et al:
Tuberculosis screening by tuberculosis skin test or QuantiFERON-TB
Gold in-tube assay among an immigrant population with a high
prevalence of tuberculosis and BCG vaccination. Plos One.
8:e827272013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
James PM, Ganaie FA and Kadahalli RL: The
performance of quantiferon-TB gold in-tube (QFT-IT) test compared
to tuberculin skin test (TST) in detecting latent tuberculosis
infection (LTBI) in the presence of HIV coinfection in a high
TB-burden area with BCG-vaccinated population. J Int Assoc Provid
AIDS Care. 13:47–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Favorov M, Ali M, Tursunbayeva A,
Aitmagambetova I, Kilgore P, Ismailov S and Chorba T: Comparative
tuberculosis (TB) prevention effectiveness in children of Bacillus
Calmette-Guerin (BCG) vaccines from different sources, Kazakhstan.
Plos One. 7:e325672012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nkurunungi G, Lutangira JE, Lule SA,
Akurut H, Kizindo R, Fitchett JR, Kizito D, Sebina I, Muhangi L,
Webb EL, et al: Determining mycobacterium tuberculosis infection
among BCG-immunised Ugandan children by T-SPOT.TB and tuberculin
skin testing. Plos One. 7:e473402012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Urzua CA, Liberman P, Abuauad S, Sabat P,
Castiglione E, Beltran-Videla MA and Aguilera R: Evaluation of the
accuracy of T-SPOT.TB for the diagnosis of ocular tuberculosis in a
BCG-vaccinated, non-endemic population. Ocul Immunol Inflamm.
25:455–459. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Minassian AM, Ronan EO, Poyntz H, Hill AV
and McShane H: Preclinical development of an in vivo BCG challenge
model for testing candidate TB vaccine efficacy. Plos One.
6:e198402011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bishai W, Sullivan Z, Bloom BR and
Andersen P: Bettering BCG: A tough task for a TB vaccine? Nat Med.
19:410–411. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saubi N, Gea-Mallorquí E, Ferrer P,
Hurtado C, Sánchez-Úbeda S, Eto Y, Gatell JM, Hanke T and Joseph J:
Engineering new mycobacterial vaccine design for HIV-TB pediatric
vaccine vectored by lysine auxotroph of BCG. Mol Ther Methods Clin
Dev. 1:140172014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang G, Zhang L, Zhang M, Pan L, Wang F,
Huang J, Li G, Yu J and Hu S: Screening and assessing 11
mycobacterium tuberculosis proteins as potential serodiagnostical
markers for discriminating TB patients from BCG vaccinees. Genomics
Proteomics Bioinformatics. 7:107–115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rapeah S, Dhaniah M, Nurul AA and Norazmi
MN: Phagocytic activity and pro-inflammatory cytokines production
by the murine macrophage cell line J774A.1 stimulated by a
recombinant BCG (rBCG) expressing the MSP1-C of Plasmodium
falciparum. Trop Biomed. 27:461–469. 2010.PubMed/NCBI
|
|
11
|
Wammes LJ, Hamid F, Wiria AE, de Gier B,
Sartono E, Maizels RM, Luty AJ, Fillié Y, Brice GT, Supali T, et
al: Regulatory T cells in human geohelminth infection suppress
immune responses to BCG and Plasmodium falciparum. Eur J Immunol.
40:437–442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng C, Xie P and Chen Y: Recombinant
mycobacterium bovis BCG producing the circumsporozoite protein of
Plasmodium falciparum FCC-1/HN strain induces strong immune
responses in BALB/c mice. Parasitol Int. 51:1–7. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mohamad D, Suppian R and Nor Mohd N:
Immunomodulatory effects of recombinant BCG expressing MSP-1C of
Plasmodium falciparum on LPS- or LPS+IFN-gamma-stimulated J774A.1
cells. Hum Vaccin Immunother. 10:1880–1886. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Teo WH, Nurul AA and Norazmi MN:
Immunogenicity of recombinant BCG-based vaccine expressing the 22
kDa of serine repeat antigen (SE22) of Plasmodium falciparum. Trop
Biomed. 29:239–253. 2012.PubMed/NCBI
|
|
15
|
Nurul AA, Rapeah S and Norazmi MN:
Plasmodium falciparum 19 kDa of merozoite surface protein-1
(MSP-1(19)) expressed in Mycobacterium bovis bacille Calmette
Guerin (BCG) is reactive with an inhibitory but not a blocking
monoclonal antibody. Trop Biomed. 27:60–67. 2010.PubMed/NCBI
|
|
16
|
Zibara K, Awada Z, Dib L, El-Saghir J,
Al-Ghadban S, Ibrik A, El-Zein N and El-Sabban M: Anti-angiogenesis
therapy and gap junction inhibition reduce MDA-MB-231 breast cancer
cell invasion and metastasis in vitro and in vivo. Sci Rep.
5:125982015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Frevert U, Nacer A, Cabrera M, Movila A
and Leberl M: Imaging Plasmodium immunobiology in the liver, brain,
and lung. Parasitol Int. 63:171–186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Villarreal DO, Walters J, Laddy DJ, Yan J
and Weiner DB: Multivalent TB vaccines targeting the esx gene
family generate potent and broad cell-mediated immune responses
superior to BCG. Hum Vaccin Immunother. 10:2188–2198. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garza-Cuartero L, McCarthy E, Brady J,
Cassidy J, Hamilton C, Sekiya M, NcNair J and Mulcahy G:
Development of an in vitro model of the early-stage bovine
tuberculous granuloma using mycobacterium bovis-BCG. Vet Immunol
Immunopathol. 168:249–257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jallad S, Goubet S, Symes A, Larner T and
Thomas P: Prognostic value of inflammation or granuloma after
intravesival BCG in non-muscle-invasive bladder cancer. BJU Int.
113:E22–E27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arkhipov SA, Shkurupy VA, Akhramenko ES,
Solomatina MV and Iljine DA: In vitro study of phenotypical
characteristics of BCG granuloma macrophages over the course of
granuloma development. Bull Exp Biol Med. 155:655–658. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nagase K, Koba S, Okawa T, Inoue T, Misago
N and Narisawa Y: Generalized granuloma annulare following BCG
vaccination, mimicking papular tuberculid. Eur J Dermatol.
21:1001–1002. 2011.PubMed/NCBI
|
|
23
|
Williams SB, Viani KL and Loughlin KR: A
72-year-old male with left testicular pain. Diagnosis: BCG
granuloma of the epididymis. Urology. 78:505–507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fernando A and Montgomery B: Penile
granuloma following intravesical bacillus calmette-guerin (BCG)
therapy. Ann R Coll Surg Engl. 2010.PubMed/NCBI
|
|
25
|
Fairhurst RM and Dondorp AM:
Artemisinin-resistant Plasmodium falciparum Malaria. Microbiol
Spectr. 4:2016.PubMed/NCBI
|
|
26
|
Stevenson JC, Simubali L, Mbambara S,
Musonda M, Mweetwa S, Mudenda T, Pringle JC, Jones CM and Norris
DE: Detection of Plasmodium falciparum Infection in Anopheles
squamosus (Diptera: Culicidae) in an Area Targeted for Malaria
Elimination, Southern Zambia. J Med Entomol. 53:1482–1487. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Palinauskas V, Žiegytė R, Iezhova TA,
Ilgūnas M, Bernotienė R and Valkiūnas G: Description, molecular
characterisation, diagnostics and life cycle of Plasmodium
elongatum (lineage pERIRUB01), the virulent avian malaria parasite.
Int J Parasitol. 46:697–707. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Annabi A, Said K and Messaoudi I:
Monitoring and assessment of environmental disturbance on natural
Gambusia affinis populations-histopathological analysis. Environ
Monit Assess. 187:3182015. View Article : Google Scholar : PubMed/NCBI
|