Introduction
The liver is important for metabolic homeostasis,
detoxification, immunity, and secretory functions (1). Sustained and progressive liver
disorders will result in severe liver injury due to increasing
cellular, tissue, and function disruption, and is associated with
high mortality (2). Management of
liver injury remains among the most challenging issues of
contemporary medicine (3) and the
development of new therapies for acute liver injury is needed.
Carbon tetrachloride (CCl4) is one of the
most potent hepatotoxic compounds (4). Acute liver injury models produced
using CCl4 are good models of acute chemical liver
injury in humans. CCl4 result in the generation of
trichloromethyl free radicals (•CCl3) by cytochrome
P450. The •CCl3 causes lipid peroxidation and
excessive production of reactive oxygen species (ROS), resulting in
liver injury (5,6). The imbalance between ROS production
and the antioxidant defense leads to oxidative stress, as shown by
increased lipid peroxidation and decreased activities of
antioxidant enzymes such as the superoxide dismutase (SOD),
catalase, and glutathione peroxidase (7).
The nucleotide-binding domain,
leucine-rich-containing family, pyrin domain-containing-3 (NLRP3)
inflammasome has been reported to be involved in the pathogenesis
of acute liver injury (8). It is
composed of three proteins: NLRP3, apoptosis-associated speck-like
protein (ASC), and caspase-1. Once activated by stimuli, the NLRP3
binds to the adaptor protein ASC, and in turn leads to
oligomerization of caspase-1, subsequently promoting the activation
of caspase-1. Active caspase-1 initiates the cleavage of
pro-interleukin (IL)-1β and pro-IL-18 and then promotes the
secretion of IL-1β, IL-18, and high mobility group protein B1
(HMGB1) (9). These inflammatory
cytokines are critical in various liver diseases such as hepatic
ischemia reperfusion injury, alcoholic steatohepatitis,
non-alcoholic steatohepatitis, and drug-induced liver injury
(10–13).
Many herbs exhibit anti-inflammatory and
anti-oxidative capacity, and are being used as adjunctive therapy
for liver injury. Some compounds extracted from traditional Chinese
medicine herbs possess anti-inflammatory and antioxidants effects
against CCl4-induced oxidative stress, such as
cardamomum, esculentoside A, Ocimum gratissimum, resveratrol, and
quercetin (14–18). Among these herbs, Radix bupleuri is
one of the most commonly used for many diseases such as viral
hepatitis and chronic hepatic inflammation (19). Saikosaponin-d (SSd) is the major
active component of Bupleurum falcatum L. and has been
reported to have anti-inflammatory, antiviral, immunomodulatory,
and antioxidant activity (20,21).
SSd inhibits the proliferation of rat hepatic stellate cells via
decreasing lipid peroxidation (22) and restrains the proliferation of
activated T lymphocyte via the NF-AT, NF-κB, and AP-1 pathways
(23). In addition, SSd alleviates
ventilator-induced lung injury via inhibiting inflammatory
responses and oxidative stress (24). Nevertheless, the hepatoprotective
effects of SSd against CCl4-induced acute hepatocellular
injury remain unclear.
Based on the known effects of SSd, we hypothesized
that SSd alleviates the effects of CCl4 on hepatocytes
by reducing inflammation and oxidative stress. Therefore, the aim
of this study was to investigate the protective effects of SSd on
CCl4-induced acute hepatocellular injury in the HL-7702
cell line and whether the effects were related to oxidative stress
and NLRP3 inflammasome activation.
Materials and methods
Cell culture
HL-7702 cells (the human normal liver cell line from
FuDan IBS Cell Center, Shanghai, China) were routinely cultured in
RPMI-1640 medium (Wuhan Boster Biological Technology, Ltd., Wuhan,
China) containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in 5% CO2 at 37°C.
The cells were treated with CCl4 (Beijing Chemical
Reagent Company, Beijing, China), SSd (batch no. 110778-201409;
China's Drug Supervision, Beijing, China), and N-acetyl-L-cysteine
(NAC; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). SSd,
CCl4, and NAC were dissolved in dimethyl sulphoxide
(DMSO) immediately before use (final concentration of DMSO:
<0.1%). DMSO (<0.1%) alone was included as a control in all
experiments and did not have any effect on the parameters
measured.
MTT assay
Cell proliferation was analyzed by the MTT assay.
HL-7702 cells were washed twice with phosphate-buffered saline
solution (PBS) and counted. Then, 1×104 cells were
seeded on 96-well plates and cultured for 24 h. The cells were
exposed to SSd at various concentrations for 24 h. Other cells were
seeded onto 96-well plates, incubated for 24 h, and preincubated
with NAC (100 µmol/l) or the indicated doses of SSd for 1 h. After
that, CCl4 was added at 10 mmol/l to induce acute injury
for 24 h (25,26). Then, 100 µl of MTT solution (0.5
mg/ml; Sigma-Aldrich; Merck KGaA) were added to each well and
incubated for 4 h. The medium was discarded and 150 µl of DMSO was
added for 24 h. The absorbance of each well was measured at 570 nm
with an ELx800 universal microplate reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The relative cell viability was
calculated as: (absorbance of drug treated group)/(absorbance of
untreated group) × 100 (%).
Alanine aminotransferase (ALT) and
aspartate transaminase (AST) in supernatants
HL-7702 cells were seeded onto 6-well plates at
8×105 cells/well and cultured in RPMI-1640 with 10% FBS
for 24 h. After HL-7702 cells were pretreated with NAC or the
indicated doses of SSd for 1 h, CCl4 was added at 10
mmol/l to induce acute hepatocellular injury for 24 h (25,26).
The supernatants were collected and stored at −20°C. ALT and AST
levels were measured using a Var10skan Flash fluorescence plate
reader (Thermo Fisher Scientific, Inc.) using the ALT and AST assay
kits (C009-1 and C010-1; Nanjing Jiancheng Institute of
Biotechnology, Nanjing, China).
Total-superoxide dismutase (T-SOD)
activity and malondialdehyde (MDA) levels
HL-7702 cells were seeded into 6-well plates and
cultured in RPMI-1640 with 10% FBS for 24 h. The cells were
pretreated with NAC or the indicated doses of SSd for 1 h, followed
by CCl4 treatment for another 24 h (25,26).
The supernatants were collected and stored at −20°C. MDA was
detected by the thiobarbituric acid (TBA) assay using a MDA assay
kit (A003-1; Nanjing Jiancheng Institute of Biotechnology). T-SOD
activity was determined using the hydroxylamine method with the
T-SOD assay kit (A001-1-1; Nanjing Jiancheng Institute of
Biotechnology).
Western blotting
NLRP3, caspase-1, ASC, and HMGB1 proteins were
measured by western blot. Whole cellular proteins were extracted
using the M-PER lysis buffer (Thermo Fisher Scientific, Inc.).
Lysates were centrifuged at 12,000 × g for 15 min at 4°C to remove
debris. Proteins (50 µg) were separated by 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to
polyvinylidene difluoride membranes (Immobilon-P; EMD Millipore,
Billerica, MA, USA). Membranes were blocked with 5% skimmed milk
and probed using NLRP3 (1:500; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), ASC (1:500; Santa Cruz Biotechnology, Inc.),
pro-caspase-1 (1:1,000; Abcam, Cambridge, MA, USA), caspase-1
(1:1,000; Abcam), HMGB1 (1:1,000; Abcam), or β-actin (1:2,000;
Santa Cruz Biotechnology, Inc.) antibodies overnight at 4°C.
Primary antibodies were detected with the corresponding horseradish
peroxidase-conjugated secondary antibodies (Cell Signaling
Technology, Inc., Danvers, MA, USA) and visualized using an
enhanced chemiluminescence (ECL) kit (EMD Millipore) western
blotting detection system (Amersham, GE Healthcare, Waukesha, WI,
USA). The band intensity was quantified using a Bio-Rad GS-690
Scanner (Bio-Rad Laboratories, Inc.). The relative expression level
of each protein was normalized to β-actin or pro-caspase-1.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.). RNA (3 µg) was reverse-transcribed
to cDNA with the ReverTra Ace-α-® RT kit (Toyobo, Inc.,
Tokyo, Japan), according to the manufacturer's protocols. RT-qPCR
was performed using the SYBR-Green PCR Master Mix (Toyobo, Inc.) in
a PCR detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The primers were: NLRP3 forward
5′-TTGACTTCCGCAAGGACCTCGG-3′ and reverse
5′-GCGCCCGGGTTATGCTGCTGGT-3′; ASC forward 5′-GGCTGCTGGATGCTCTGTA-3′
and reverse 5′-AGGCTGGTGTGAAACTGAAGA-3′; caspase-1 forward
5′-CAGACAAGGGTGCTGAACAA-3′ and reverse 5′-TCGGAATAACGGAGTCAATCA-3′;
IL-1β forward 5′-TGGCAATGAGGATGACTTGT-3′ and reverse
5′-TGGTGGTCGGAGATTCGTA-3′; IL-18 forward
5′-GACCTTCCAGATCGCTTCCTC-3′ and reverse
5′-GATGCAATTGTCTTCTACTGGTTC-3′; and HMGB1 forward
5′-TATCGCCCAAAAATCAAAGG-3′ and reverse 5′-TAAGGCTGCTTGTCATCTGC-3′.
The amplification process was the same for all genes and was 35
cycles of denaturation at 95°C for 45 sec, annealing at 50°C for 45
sec, and elongation at 72°C for 45 sec. β-actin was used as
internal control and the primers were: forward
5′-CTCCATCCTGGCCTCGCTGT-3′ and reverse 5′-GCTGTCACCTTCACCGTTCC-3′.
The 2−ΔΔCq method was used to represent the relative
mRNA expression of the target genes.
Enzyme-linked immunosorbent assay
(ELISA)
The levels of IL-18 and IL-1β in the supernatants of
HL-7702 cells were measured by ELISA (BMS224HS and BMS267-2;
eBioscience, San Diego, CA, USA), according to the manufacturer's
instructions.
Statistical analysis
Statistical analysis was conducted using SPSS 21.0
(IBM Corp., Armonk, NY, USA). Results were expressed as mean ±
standard deviation. The differences among groups were analyzed
using one way analysis of variance (ANOVA) with the
Student-Newman-Keuls test for post hoc analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of SSd on the viability of
HL-7702 cells
The MTT cell viability assay was performed to
examine whether SSd produced cytotoxic effects on HL-7702 cell
line. The treatment of HL-7702 cells with SSd at 0.5–2 µmol/l for
24 h did not affect cell viability (Fig. 1), while cell viability was
decreased using 2–24 µmol/l (Fig.
1). Therefore, SSd at 0.5, 1, and 2 µmol/l were selected as the
low-dose, moderate-dose, and high-dose groups in the subsequent
experiments.
SSd alleviates CCl4-induced
acute hepatocellular injury
The MTT assay was used to investigate the effects of
SSd on CCl4-induced acute hepatocellular injury. As
shown in Fig. 2A, CCl4
alone significantly decreased cell viability and SSd reversed the
phenomenon in a dose-dependent manner (all P<0.01; Fig. 2A). NAC, as a positive control, also
attenuated the decreased cell viability induced by CCl4
(P<0.01; Fig. 2A).
 | Figure 2.Effects of SSd on
CCl4-induced acute hepatocellular injury. HL-7702 cells
were pretreated with 100 µmol/l NAC or different doses of SSd for 1
h, and then treated with 10 mmol/l CCl4 for 24 h. (A)
Cell viability was examined by the MTT assay. The levels of (B) AST
and (C) ALT in the supernatants were determined using commercial
kits. Data are shown as mean ± standard deviation for at least
three independent experiments. *P<0.05, **P<0.01 vs. the
CCl4 only group. CCl4, carbon tetrachloride;
SSd, saikosaponin-d; NAC, N-acetyl-L-cysteine; L, low dose, 0.5
µmol/l; M, moderate dose, 1 µmol/l; H, high dose, 2 µmol/l; ALT,
alanine aminotransferase; AST, aspartate transaminase. |
The effects of SSd on CCl4-induced serum
AST and ALT levels were examined. CCl4 significantly
increased the levels of serum ALT and AST. SSd treatment prevented
CCl4-induced increase of AST level in a dose-dependent
manner (P<0.05 for low-dose and P<0.01 for the other doses,
Fig. 2B). The levels of ALT in the
SSd groups were decreased significantly at the moderate and high
doses compared to the CCl4 group (P>0.05 for low-dose
and P<0.01 for the other doses, Fig. 2C). NAC almost completely reversed
the increases of AST and ALT induced by CCl4 (P<0.01;
Fig. 2B-C).
SSd attenuates CCl4-induced oxidative
stress
To explore the mechanisms by which SSd alleviates
CCl4-induced acute hepatocellular injury, the
anti-oxidative effects of SSd on CCl4-induced acute
hepatocellular injury in HL-7702 cells were explored. We examined
the levels of T-SOD and MDA in the cell culture supernatants. The
decreased T-SOD induced by CCl4 was attenuated in a
dose-dependent manner by SSd (all P<0.01; Fig. 3A), while MDA showed opposite
changes (P<0.05 for low-dose and P<0.01 for the other doses,
Fig. 3B). CCl4-induced
oxidative stress was blocked by NAC (P<0.01; Fig. 3A and B). Thus, the suppressive
effect of SSd on oxidative stress could be related to the increase
of T-SOD and the reduction of MDA.
 | Figure 3.Effects of SSd on
CCl4-induced change of T-SOD and MDA in the culture
supernatants. HL-7702 cells were pretreated with 100 µmol/l NAC or
different doses of SSd for 1 h and then treated with 10 mmol/l
CCl4 for 24 h. The levels of (A) T-SOD and (B) MDA in
the supernatants were determined using commercial kits. Data are
shown as mean ± SD for at least three independent experiments.
*P<0.05, **P<0.01 vs. the CCl4 only group.
CCl4, carbon tetrachloride; SSd, saikosaponin-d; T-SOD,
total-superoxide dismutase; MDA, malondialdehyde; NAC,
N-acetyl-L-cysteine; L, low dose, 0.5 µmol/l; M, moderate dose, 1
µmol/l; H, high dose, 2 µmol/l. |
SSd inhibits CCl4-induced NLRP3
inflammasome activation
The NLRP3 inflammasome is composed of the NLRP3,
caspase-1, and ASC (27,28). To verify whether SSd may affect the
NLRP3 inflammasome, NLRP3, ASC, caspase-1, and HMGB1 were assessed
by RT-qPCR and western blotting. As shown in Fig. 4A, CCl4 significantly
increased NLRP3, caspase-1, ASC, and HMGB1 mRNA levels. Treatment
with SSd decreased CCl4-induced NLRP3, ASC, and HMGB1
mRNA expression in a dose-dependent manner (all P<0.01; Fig. 4A), and decreased the level of
caspase-1 mRNA at the moderate and high doses (P>0.05 for
low-dose, P<0.05 for the moderate dose, and P<0.01 for the
high dose; Fig. 4C). The effects
of CCl4 injury were blocked by the positive control drug
NAC (P<0.01; Fig. 4A).
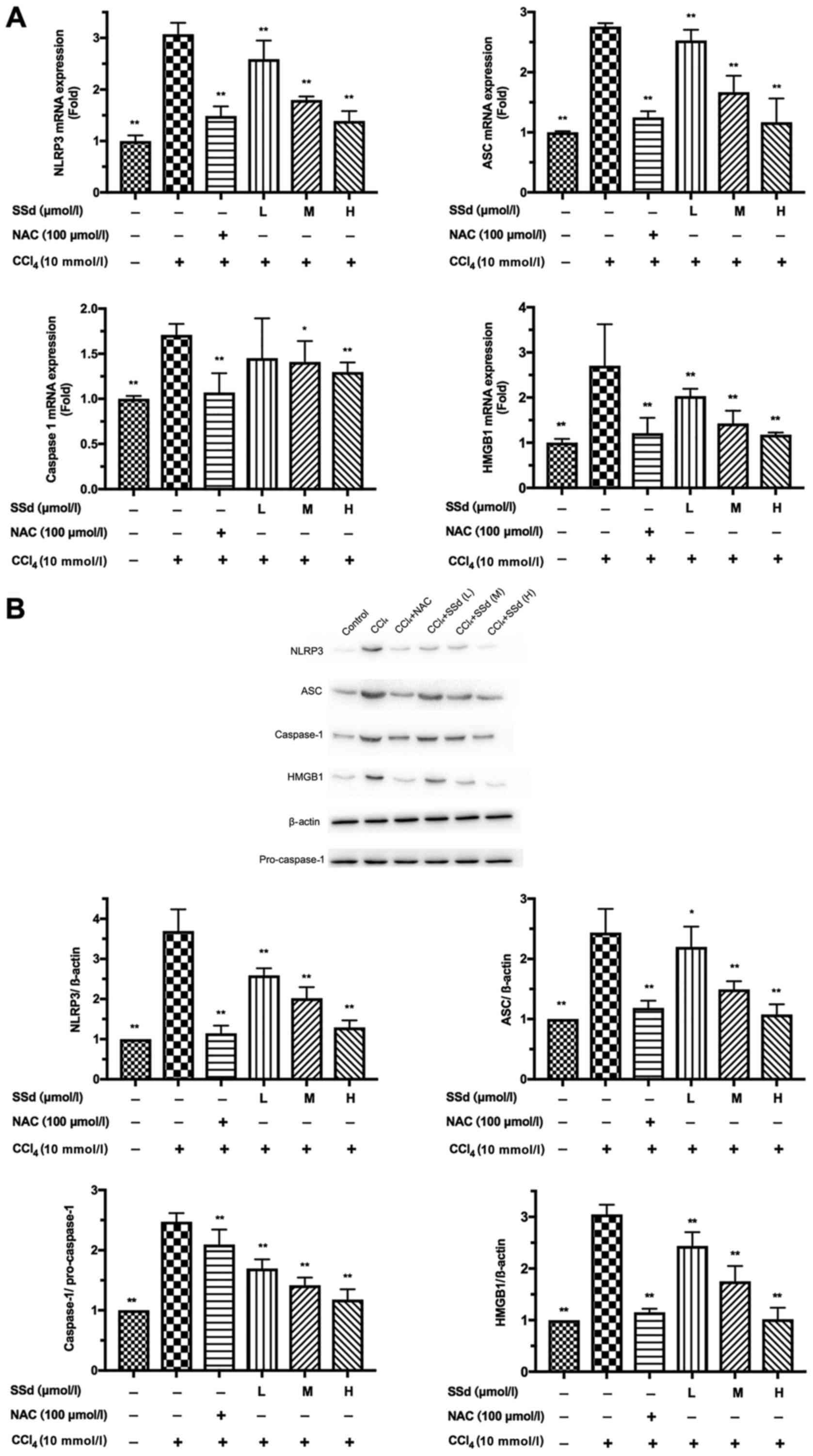 | Figure 4.Effects of SSd on
CCl4-induced NLRP3, ASC, caspase-1 and HMGB1 expression.
HL-7702 cells were pretreated with 100 µmol/l NAC or different
doses of SSd for 1 h and then treated with 10 mmol/l
CCl4 for 24 h. (A) The mRNA levels of NLRP3, ASC,
caspase-1, and HMGB1 were detected by reverse
transcription-quantitative polymerase chain reaction. (B) The
protein levels of NLRP3, ASC, caspase-1 and HMGB1 were determined
by western blotting. Data are shown as mean ± standard deviation
for at least three independent experiments. *P<0.05, **P<0.01
vs. the CCl4 only group. CCl4, carbon
tetrachloride; SSd, saikosaponin-d; NAC, N-acetyl-L-cysteine; L,
low dose, 0.5 µmol/l; M, moderate dose, 1 µmol/l; H, high dose, 2
µmol/l; NLRP3, nucleotide-binding domain, leucine-rich-containing
family, pyrin domain-containing-3; HMGB1, high mobility group
protein B1; ASC, apoptosis-associated speck-like protein. |
Similar to the qPCR results, western blotting showed
that the expressions of NLRP3, ASC, caspase-1 and HMGB1 were
increased by CCl4, but these effects were blocked by NAC
(P<0.01; Fig. 4B). The protein
expressions of NLRP3, ASC, caspase-1, and HMGB1 were gradually
reduced as the dosage of SSd increased (P<0.05 for the low-dose
group for ASC, and all other P<0.01; Fig. 4B). Therefore, the anti-inflammatory
effects of SSd could be associated with the inhibition of the NLRP3
inflammasome.
SSd inhibits the secretion of
proinflammatory cytokines
The production of inflammatory cytokines IL-1β and
IL-18 in culture supernatant was examined by qPCR and ELISA.
CCl4 significantly increased IL-1β and IL-18 expression,
which could be attenuated by NAC. More importantly, treatment with
SSd gradually attenuated the expressions of IL-1β and IL-18 as the
dosage of SSd increased (all P<0.01; Fig. 5).
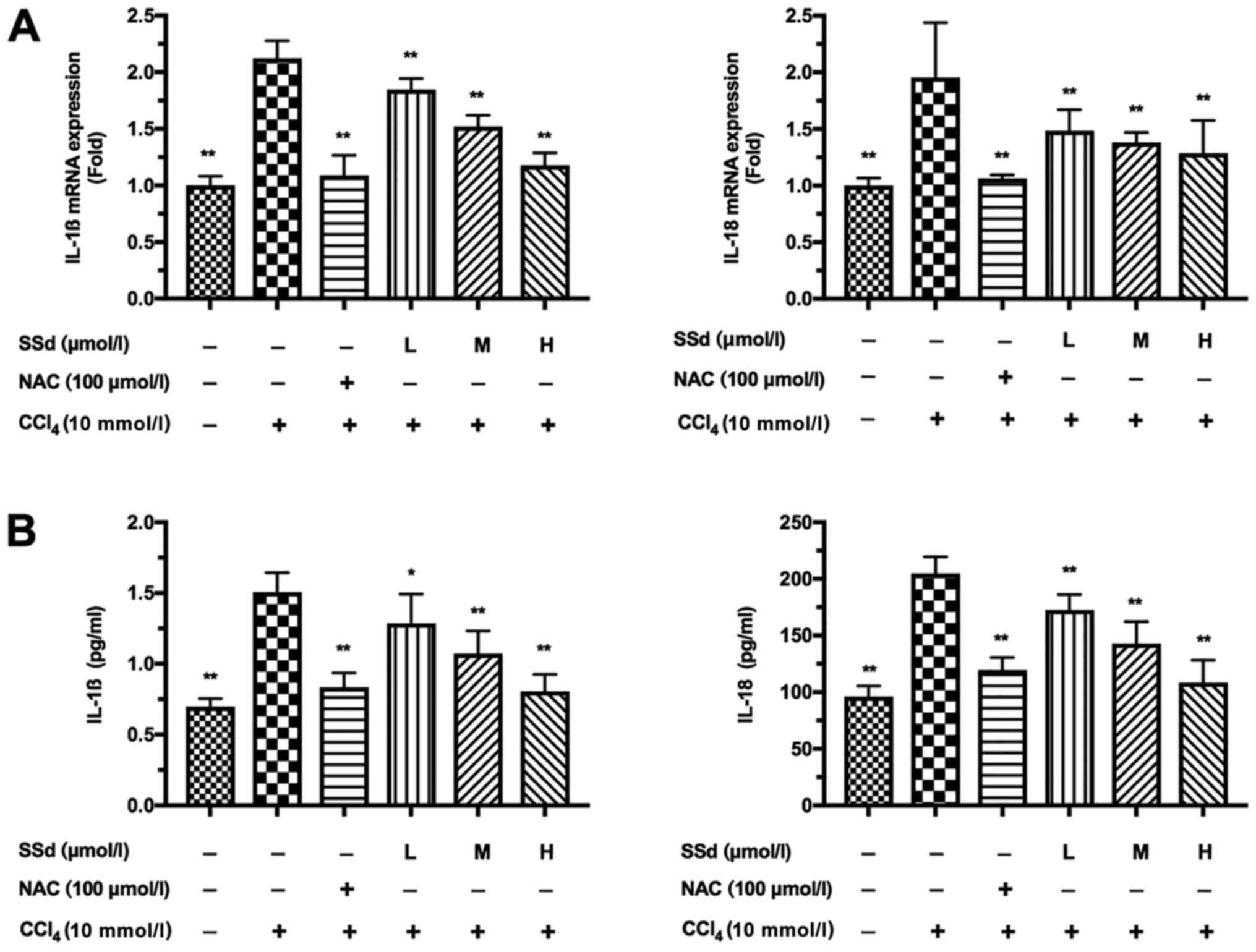 | Figure 5.Effects of SSd on IL-1β and IL-18
production in CCl4-induced acute hepatocellular injury.
HL-7702 cells were pretreated with 100 µmol/l NAC or different
doses of SSd for 1 h and then treated with 10 mmol/l
CCl4 for 24 h. (A) The mRNA expressions of IL-1β and
IL-18 were determined using reverse transcription-quantitative
polymerase chain reaction. (B) The protein levels of IL-1β and
IL-18 were detected by ELISA assay. Data are shown as mean ±
standard deviation for at least three independent experiments.
*P<0.05, **P<0.01 vs. the CCl4 only group.
CCl4, carbon tetrachloride; SSd, saikosaponin-d; NAC,
N-acetyl-L-cysteine; L, low dose, 0.5 µmol/l; M, moderate dose, 1
µmol/l; H, high dose, 2 µmol/l; IL, interleukin. |
Discussion
The CCl4-induced acute liver injury model
is frequently used to examine the efficacy of liver protective
agents. SSd has been found to attenuate CCl4-induced
hepatic injury in rats by inhibiting lipid peroxidation (22). Nevertheless, the downstream
molecular mechanism remains unclear. Therefore, this study aimed to
examine the effects of SSd on CCl4-induced acute injury
in HL-7702 cells and whether the mechanisms could be related to
oxidative stress and NLRP3 inflammasome activation. The results
suggest that SSd attenuated CCl4-induced acute injury by
inhibiting oxidative stress and NLRP3 inflammasome activation in
the HL-7702 cell line.
In the present study, SSd at 0.5–2 µmol/l was
protective against CCL4-induced injury, but SSd was
toxic at doses >2 µmol/l. Chen et al showed that
mitochondrial apoptosis was the main toxic effect of SSd at high
concentration (21). Li et
al found that saikosaponins at 200 µg/ml induced hepatotoxicity
through oxidative stress and lipid metabolism dysregulation
(29). On the other hand, Zhao
et al found that SSd at 45–60 µmol/l could protect renal
tubular epithelial cell against high-glucose-induced injury by
regulation of SIRT3 (30). Yao
et al found that SSd inhibited the proliferation of prostate
cancer cells at 50 µM (31). These
results suggest that SSd is toxic at high concentrations, but
protective at lower doses.
Oxidative stress is known to be related to the
pathogenesis of acute liver injury (32–34).
CCl4, is widely used to induce acute hepatic injury in
animals, has strong hepatotoxic effects that leads to the excessive
generation of free radicals associated with oxidative stress, and
ultimately results in acute liver injury with functional
impairment. Serum AST and ALT are widely used as makers of acute
hepatic injury. Decreased levels of AST and ALT associated with
fewer necrotic lesions and lipid peroxidation could imply
protection against CCl4-induced injury (35). Therefore, we first verified that
SSd could attenuate CCl4-induced AST and ALT increases.
MDA and T-SOD are indicators of CCl4-induced oxidative
stress (36). The increase of MDA,
a product of lipid peroxidation, is considered to be a direct
indicator of abnormal peroxidation and impaired antioxidant
defenses (36). As an antioxidant
enzyme, T-SOD catalyzes the dismutation of superoxide anions into
hydrogen peroxide and oxygen. Studies showed that CCl4
could lead to excessive generation of free radicals and oxidative
stress in the liver by increasing the levels of MDA and decreasing
the levels of T-SOD, ultimately leading to acute liver injury
(37–39). In the present study, SSd played an
antioxidant role by inhibiting the production of MDA and improving
T-SOD levels in CCl4-induced acute injury in liver
cells.
The activation of the NLRP3 inflammasome results in
the maturation of the inflammatory cytokines IL-1β and IL-18, and
also leads to the release of HMGB1 (9). These potent proinflammatory cytokines
further aggravate the inflammatory progress initiated by oxidative
stress. IL-1β and IL-18 are members of the IL-1 superfamily and
contribute to inflammation (40,41).
HMGB1 is a nuclear protein and proinflammatory mediator, and
promotes inflammation and necrotic cell death. IL-1β, IL-18, and
HMGB1 may be involved in acute hepatocellular injury (42–46).
The effect of the NLRP3 inflammasome has been explored in many
liver conditions such as drug-induced liver injury, non-alcoholic
steatohepatitis, alcoholic steatohepatitis, hepatic ischemia
reperfusion injury, and fibrosis (10–13,47,48).
Kim et al (49) showed that
NLRP3 inflammasome activation play a central role in
GalN/LPS-induced inflammatory responses and the development of
hepatic injury. Gong et al (50) showed that the activation of the
NLRP3 inflammasome contributes to the induction of inflammation,
which might be associated with BDL-induced fibrosis in
non-alcoholic and alcoholic steatohepatitis. In the present study,
SSd decreased the mRNA and protein expression of the NLRP3
inflammasome components after induction by CCl4, which
is consistent with the role of the NLRP3 inflammasome in liver
injury. In addition, the levels of IL-1β and IL-18 in the culture
supernatants were reduced.
Oxidative stress induced by the overproduction of
ROS is an important cause of organ and tissue injury in various
inflammatory diseases (33,34).
In addition, ROS play crucial roles in the activation of the NLRP3
inflammasome (51,52). Recent evidence also indicate that
some herb extracts have anti-inflammatory effects by suppressing
ROS production (53). Moreover, it
has been confirmed that oxidative stress and inflammatory cytokines
are frequently identified as the main factors contributing to acute
liver injury, and anti-inflammatory and antioxidant compounds are
thought to prevent acute hepatocellular injury (54–56).
Dang et al (57) showed
that SSd attenuated CCl4-induced liver fibrosis in rats
through the downregulation of TNF-α, IL-6, and NF-κBp65, and the
upregulation of I-κBα. Wu et al (55) also showed the beneficial effects of
SSd against liver fibrosis in CCl4 rats models. Taken
together, ROS-induced NLRP3 inflammasome activation may be involved
in CCl4-induced acute hepatocellular injury.
Furthermore, the anti-inflammatory effect of SSd may depend on the
modulation the inflammation through the NLRP3 inflammasome.
Nevertheless, ROS and factors such as TNF-α, IL-6, NF-κBp65, and
I-κBα were not evaluated in this study, and further experiments are
needed to confirm those mechanisms. In addition, NLRP3 inhibitors
should be used to confirm the role of the NLRP3 inflammasome in
CCl4-induced injury and the beneficial effects of
SSd.
In conclusion, this study suggests that the
suppressive effects of SSd on CCl4-induced acute
hepatocellular injury may depend on the inhibition of oxidative
stress and NLRP3 inflammasome activation. This study suggests new
evidence for the potential efficacy of SSd for the treatment of
acute hepatocellular injury.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 81573775), the
Shanghai Natural Science Foundation (grant no. 09ZR1429700) and the
Shanghai Outstanding Academic Leader of Health System (grant no.
XBR2013120).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL made substantial contributions to the acquisition
of the data, data analysis and interpretation of the data and
writing the manuscript. RQ and YL participated in the conception
and design of the study and the revision of the manuscript. LL, YS,
YC and NY performed the experiments. YL was primarily responsible
for the revision of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SSd
|
saikosaponin-d
|
|
CCl4
|
carbon tetrachloride
|
|
NLRP3
|
nucleotide-binding domain,
leucine-rich-containing family, pyrin domain-containing-3
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate transaminase
|
|
T-SOD
|
total-superoxide dismutase
|
|
MDA
|
malondialdehyde
|
|
HMGB1
|
high mobility group protein B1
|
|
IL
|
interleukin
|
References
|
1
|
Zhao P, Qi C, Wang G, Dai X and Hou X:
Enrichment and purification of total flavonoids from Cortex
Juglandis Mandshuricae extracts and their suppressive effect on
carbon tetrachloride-induced hepatic injury in Mice. J Chromatogr B
Analyt Technol Biomed Life Sci. 1007:8–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tuñón MJ, Miguel San B, Crespo I, Jorquera
F, Santamaria E, Alvarez M, Prieto J and González-Gallego J:
Melatonin attenuates apoptotic liver damage in fulminant hepatic
failure induced by the rabbit hemorrhagic disease virus. J Pineal
Res. 50:38–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Auzinger G and Wendon J: Intensive care
management of acute liver failure. Curr Opin Crit Care. 14:179–188.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Assayed ME, Khalaf AA and Salem HA:
Protective effects of garlic extract and vitamin C against in vivo
cypermethrin-induced teratogenic effects in rat offspring. Food
Chem Toxicol. 48:3153–3158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kodai S, Takemura S, Minamiyama Y, Hai S,
Yamamoto S, Kubo S, Yoshida Y, Niki E, Okada S, Hirohashi K and
Suehiro S: S-allyl cysteine prevents CCl(4)-induced acute liver
injury in rats. Free Radic Res. 41:489–497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Recknagel RO, Glende EA Jr, Dolak JA and
Waller RL: Mechanisms of carbon tetrachloride toxicity. Pharmacol
Ther. 43:139–154. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zelen I, Djurdjevic P, Popovic S,
Stojanovic M, Jakovljevic V, Radivojevic S, Baskic D and
Arsenijevic N: Antioxidant enzymes activities and plasma levels of
oxidative stress markers in B-chronic lymphocytic leukemia
patients. J BUON. 15:330–336. 2010.PubMed/NCBI
|
|
8
|
Pan CW, Pan ZZ, Hu JJ, Chen WL, Zhou GY,
Lin W, Jin LX and Xu CL: Mangiferin alleviates lipopolysaccharide
and D-galactosamine-induced acute liver injury by activating the
Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Eur J
Pharmacol. 770:85–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Keyel PA: How is inflammation initiated?
Individual influences of IL-1, IL-18 and HMGB1. Cytokine.
69:136–145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arteel G, Marsano L, Mendez C, Bentley F
and McClain CJ: Advances in alcoholic liver disease. Best Pract Res
Clin Gastroenterol. 17:625–647. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Neuman MG: Cytokines-central factors in
alcoholic liver disease. Alcohol Res Health. 27:307–316.
2003.PubMed/NCBI
|
|
12
|
Petrasek J, Bala S, Csak T, Lippai D,
Kodys K, Menashy V, Barrieau M, Min SY, Kurt-Jones EA and Szabo G:
IL-1 receptor antagonist ameliorates inflammasome-dependent
alcoholic steatohepatitis in mice. J Clin Invest. 122:3476–3489.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu P, Duan L, Chen J, Xiong A, Xu Q,
Zhang H, Zheng F, Tan Z, Gong F and Fang M: Gene silencing of NALP3
protects against liver ischemia-reperfusion injury in mice. Hum
Gene Ther. 22:853–864. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang F, Wang X, Qiu X, Wang J, Fang H,
Wang Z, Sun Y and Xia Z: The protective effect of Esculentoside A
on experimental acute liver injury in mice. PLoS One.
9:e1131072014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lim DW, Kim H, Park JY, Kim JE, Moon JY,
Park SD and Park WH: Amomum cardamomum L. ethyl acetate fraction
protects against carbon tetrachloride-induced liver injury via an
antioxidant mechanism in rats. BMC Complement Altern Med.
16:1552016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chiu YW, Chao PY, Tsai CC, Chiou HL, Liu
YC, Hung CC, Shih HC, Lai TJ and Liu JY: Ocimum gratissimum is
effective in prevention against liver fibrosis in vivo and in
vitro. Am J Chin Med. 42:833–852. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chan CC, Lee KC, Huang YH, Chou CK, Lin HC
and Lee FY: Regulation by resveratrol of the cellular factors
mediating liver damage and regeneration after acute toxic liver
injury. J Gastroenterol Hepatol. 29:603–613. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang JQ, Shi L, Xu XN, Huang SC, Lu B, Ji
LL and Wang ZT: Therapeutic detoxification of quercetin against
carbon tetrachloride-induced acute liver injury in mice and its
mechanism. J Zhejiang Univ Sci B. 15:1039–1047. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee CH, Wang JD and Chen PC: Risk of liver
injury associated with Chinese herbal products containing radix
bupleuri in 639,779 patients with hepatitis B virus infection. PLoS
One. 6:e160642011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wong VK, Zhang MM, Zhou H, Lam KY, Chan
PL, Law CK, Yue PY and Liu L: Saikosaponin-d enhances the
anticancer potency of TNF-α via overcoming its undesirable response
of activating NF-Kappa B signalling in cancer cells. Evid Based
Complement Alternat Med. 2013:7452952013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen L, Zhang F, Kong D, Zhu X, Chen W,
Wang A and Zheng S: Saikosaponin D disrupts platelet-derived growth
factor-β receptor/p38 pathway leading to mitochondrial apoptosis in
human LO2 hepatocyte cells: A potential mechanism of
hepatotoxicity. Chem Biol Interact. 206:76–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan J, Li X, Li P, Li N, Wang T, Shen H,
Siow Y, Choy P and Gong Y: Saikosaponin-d attenuates the
development of liver fibrosis by preventing hepatocyte injury.
Biochem Cell Biol. 85:189–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu CN, Yuan ZG, Zhang XL, Yan R, Zhao YQ,
Liao M and Chen JX: Saikosaponin a and its epimer saikosaponin d
exhibit anti-inflammatory activity by suppressing activation of
NF-κB signaling pathway. Int Immunopharmacol. 14:121–126. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang HW, Liu M, Zhong TD and Fang XM:
Saikosaponin-d attenuates ventilator-induced lung injury in rats.
Int J Clin Exp Med. 8:15137–4512. 2015.PubMed/NCBI
|
|
25
|
Lu Q, Yang L, Zhao HY, Jiang JG and Xu XL:
Protective effect of compounds from the flowers of Citrus aurantium
L. var. amara Engl against carbon tetrachloride-induced hepatocyte
injury. Food Chem Toxicol. 62:432–435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han WJ, Shi HB, Shi HL, Song JY, Ren F,
Duan ZP and Chen Y: Augmenter of liver regeneration promotes the
proliferation of HL-7702 cells in carbon tetrachloride-induced
acute liver injury via increasing autophag. Zhonghua Gan Zang Bing
Za Zhi. 24:761–766. 2016.(In Chinese). PubMed/NCBI
|
|
27
|
DeNicola GM, Karreth FA, Humpton TJ,
Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES,
et al: Oncogene-induced Nrf2 transcription promotes ROS
detoxification and tumorigenesis. Nature. 475:106–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schroder K and Tschopp J: The
inflammasomes. Cell. 140:821–832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li X, Li X, Lu J, Huang Y, Lv L, Luan Y,
Liu R and Sun R: Saikosaponins induced hepatotoxicity in mice via
lipid metabolism dysregulation and oxidative stress: A proteomic
study. BMC Complement Altern Med. 17:2192017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao L, Zhang H, Bao J, Liu J and Ji Z:
Saikosaponin-d protects renal tubular epithelial cell against high
glucose induced injury through modulation of SIRT3. Int J Clin Exp
Med. 8:6472–6481. 2015.PubMed/NCBI
|
|
31
|
Yao M, Yang J, Cao L, Zhang L, Qu S and
Gao H: Saikosaponind inhibits proliferation of DU145 human prostate
cancer cells by inducing apoptosis and arresting the cell cycle at
G0/G1 phase. Mol Med Rep. 10:365–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kadiiska MB, Gladen BC, Baird DD, Germolec
D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, et
al: Biomarkers of oxidative stress study II: Are oxidation products
of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic
Biol Med. 38:698–710. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alfadda AA and Sallam RM: Reactive oxygen
species in health and disease. J Biomed Biotechnol.
2012:9364862012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brüne B, Dehne N, Grossmann N, Jung M,
Namgaladze D, Schmid T, von Knethen A and Weigert A: Redox control
of inflammation in macrophages. Antioxid Redox Signal. 19:595–637.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim WR, Flamm SL, Di Bisceglie AM and
Bodenheimer HC: Public Policy Committee of the American Association
for the Study of Liver Disease: Serum activity of alanine
aminotransferase (ALT) as an indicator of health and disease.
Hepatology. 47:1363–1370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hoek JB and Pastorino JG: Ethanol,
oxidative stress, and cytokine-induced liver cell injury. Alcohol.
27:63–68. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Weber LW, Boll M and Stampfl A:
Hepatotoxicity and mechanism of action of haloalkanes: carbon
tetrachloride as a toxicological model. Crit Rev Toxicol.
33:105–136. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheng N, Ren N, Gao H, Lei X, Zheng J and
Cao W: Antioxidant and hepatoprotective effects of Schisandra
chinensis pollen extract on CCl4-induced acute liver damage in
mice. Food Chem Toxicol. 55:234–240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tirkey N, Pilkhwal S, Kuhad A and Chopra
K: Hesperidin, a citrus bioflavonoid, decreases the oxidative
stress produced by carbon tetrachloride in rat liver and kidney.
BMC Pharmacol. 5:22005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dinarello CA: Immunological and
inflammatory functions of the interleukin-1 family. Annu Rev
Immunol. 27:519–550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Davis BK, Wen H and Ting JP: The
inflammasome NLRs in immunity, inflammation, and associated
diseases. Annu Rev Immunol. 29:707–735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Petrilli V, Papin S, Dostert C, Mayor A,
Martinon F and Tschopp J: Activation of the NALP3 inflammasome is
triggered by low intracellular potassium concentration. Cell Death
Differ. 14:1583–1589. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Franchi L, Eigenbrod T, Muñoz-Planillo R
and Nuñez G: The inflammasome: A caspase-1-activation platform that
regulates immune responses and disease pathogenesis. Nat Immunol.
10:241–247. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang H, Antoine DJ, Andersson U and Tracey
KJ: The many faces of HMGB1: Molecular structure-functional
activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol.
93:865–873. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen Q, Yin YX, Wei J, Tong M, Shen F,
Zhao M and Chamley L: Increased expression of high mobility group
box 1 (HMGB1) in the cytoplasm of placental syncytiotrophoblast
from preeclamptic placentae. Cytokine. 85:30–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu L, Zhang Z, Zhang L, Shi Y, Qi J,
Chang A, Gao J, Feng Y and Yang X: HMGB1-RAGE signaling pathway in
severe preeclampsia. Placenta. 36:1148–1152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Imaeda AB, Watanabe A, Sohail MA, Mahmood
S, Mohamadnejad M, Sutterwala FS, Flavell RA and Mehal WZ:
Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9
and the Nalp3 inflammasome. J Clin Invest. 119:305–314.
2009.PubMed/NCBI
|
|
48
|
Artlett CM: The Role of the NLRP3
Inflammasome in Fibrosis. Open Rheumatol J. 6:80–86. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim SJ and Lee SM: NLRP3 inflammasome
activation in D-galactosamine and lipopolysaccharide-induced acute
liver failure: Role of heme oxygenase-1. Free Radic Biol Med.
65:997–1004. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gong Z, Zhou J, Zhao S, Tian C, Wang P, Xu
C, Chen Y, Cai W and Wu J: Chenodeoxycholic acid activates NLRP3
inflammasome and contributes to cholestatic liver fibrosis.
Oncotarget. 7:83951–83963. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dostert C, Pétrilli V, Van Bruggen R,
Steele C, Mossman BT and Tschopp J: Innate immune activation
through Nalp3 inflammasome sensing of asbestos and silica. Science.
320:674–677. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sayan M and Mossman BT: The NLRP3
inflammasome in pathogenic particle and fibre-associated lung
inflammation and diseases. Part Fibre Toxicol. 13:512016.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shen YC, Chou CJ, Wang YH, Chen CF, Chou
YC and Lu MK: Anti-inflammatory activity of the extracts from
mycelia of Antrodia camphorata cultured with water-soluble
fractions from five different Cinnamomum species. FEMS Microbiol
Lett. 231:137–43. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jaeschke H: Reactive oxygen and mechanisms
of inflammatory liver injury. J Gastroenterol Hepatol. 15:718–724.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wu SJ, Lin YH, Chu CC, Tsai YH and Chao
JC: Curcumin or saikosaponin a improves hepatic antioxidant
capacity and protects against CCl4-induced liver injury in rats. J
Med Food. 11:224–229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yu H, Zheng L, Yin L, Xu L, Qi Y, Han X,
Xu Y, Liu K and Peng J: Protective effects of the total saponins
from Dioscorea nipponica Makino against carbon
tetrachloride-induced liver injury in mice through suppression of
apoptosis and inflammation. Int Immunopharmacol. 19:233–244. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dang SS, Wang BF, Cheng YA, Song P, Liu ZG
and Li ZF: Inhibitory effects of saikosaponin-d on CCl4-induced
hepatic fibrogenesis in rats. World J Gastroenterol. 13:557–563.
2007. View Article : Google Scholar : PubMed/NCBI
|



















