Introduction
The role of the cerebellum was previously considered
to be confined to movement, along with the cortex and basal
ganglia; however, it has recently been redefined to represent a
broader area of functions, including cognitive functions (1,2). The
cerebellum receives and sends information from various brain
regions, including the hypothalamus, hippocampus and cortex
(3). The function of the
cerebellum is strongly associated with the hippocampus, and the
cerebellum has been revealed to affect hippocampal activity,
including spatial navigation and working memory (3–5).
Although it has not been fully determined, the cerebellum may
affect the hippocampus in two ways (1). The cerebellar brain region is
primarily associated with cognitive functioning, particularly
spatial navigation and executive tasks, and is located in the
posterior lobe of the cerebellum. A previous neuroimaging study has
demonstrated that cognitive functions are associated with the
posterior lobe of the cerebellum (6). Damage to the posterior lobe of the
cerebellum has been revealed to be associated with impairment of
spatial working memory, and transgenic mice with cerebellar
dysfunction have been demonstrated to exhibit spatial navigation
deficit (7,8).
Rats with Purkinje cells removed in the cerebellum
exhibited impaired spatial navigation performance following
analysis using the rotor-rod and Morris water maze tests (9). Purkinje cells are located the
Purkinje layer of the cerebellum, which exhibits the greatest
abundance of calcium-binding proteins in the cerebellum, such as
calbindin D28k and parvalbumin. Such proteins have important roles
in physiological processes, including cell cycle regulation, muscle
contraction and the regulation of intracellular Ca2+
concentration associated with apoptosis (10). Purkinje cells, which have important
roles in cerebellar function, are particularly vulnerable to damage
in ischemia (11). Purkinje cell
death induced by ischemia in the cerebellum has been reported to be
induced by excitotoxicity due to glutamate release and
intracellular calcium (12).
Chronic cerebral hypoperfusion (CCH) is a major
pathological feature of vascular dementia that results in
progressive cognitive impairment due to interference in the
circulatory system (13–15). CCH has been demonstrated to induce
hypoxia and oxidative stress, as well as cause inflammation of the
cerebral cortex, white matter, hippocampus and striatum, resulting
in impaired cognitive function (16–19).
However, the pathological mechanism of CCH has not been fully
determined. Research concerning the effects of CCH on the
cerebellum and determining novel therapeutic strategies to enhance
spatial navigation of patients suffering from CCH has been
limited.
Treadmill exercise has been demonstrated to
ameliorate neurological impairments following various brain
disorders, including ischemia and Alzheimer's disease (20,21).
Treadmill exercise has also been revealed to improve cognitive
function by reducing the apoptosis rate of neurons and the
reactivity of astrocytes in the cerebellum following transient
global ischemia (22), and also
protect against age-associated Purkinje cell loss (23). The aim of the present study was to
investigate whether CCH induces a loss of Purkinje cells, and to
investigate the effect of treadmill exercise on spatial navigation
impairment associated with CCH.
Materials and methods
Experimental animals
Male Wistar rats (n=30; weight 80±10 g, 3-weeks old,
Orient Bio, Inc., Seongnam, Korea) were used in the present study.
The rats were housed under controlled temperature (20±2°C),
humidity (60±5%), and lighting conditions (7:00 a.m. to 7:00 p.m.)
with food and water available ad libitum. All animal
experimental procedures conformed to the regulations stipulated by
the National Institutes of Health (Bethesda, MD, USA) (24) and the guidelines of the Korean
Academy of Medical Science (Seoul, Korea). The present study was
approved by the Kyung Hee University Institutional Animal Care and
Use Committee [Seoul, Korea; KHUASP (SE)-16-149]. The rats were
randomly divided into three groups: Sham group (n=10), bilateral
common carotid arteries occlusion (BCCAO) group (n=10) and a BCCAO
+ treadmill exercise (BCCAO + Ex) group (n=10).
Treadmill exercise protocol
The rats in the BCCAO + Ex group were made to run on
a treadmill for 30 min once a day for 8 weeks starting at 4 weeks
post-birth, according to a previously described method (21). The treadmill exercise load
consisted of running at 2 m/min for the first 5 min, 3 m/min for
the following 5 min and 5 m/min for the last 20 min, at 0 degrees
of inclination. The rats in the non-exercise groups were placed on
the treadmill without being made to run for the same time period as
the exercise group.
BCCAO
Rats were anesthetized via subcutaneous injection of
250 mg/kg tribromoethanol (Avertin®; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). BCCAO was performed to induce CCH
and was performed carefully to avoid damage to the vagus nerve and
surrounding tissues. Both carotid arteries were exposed by a
ventral midline incision and double ligated with 3-0 silk (Ailee,
Seoul, Korea) immediately below the carotid bifurcation. The rats
in the sham group underwent the same operation procedure without
vessel ligation.
Open field test
Activity was determined using the open field test.
As a previously described method (25), the rats were randomly assigned to
an order of testing and placed in a white square open-field arena
(100×100 cm) made of wood, enclosed by 40-cm-high walls and exposed
to strong illumination (200 lux). The arena was divided into 25
squares (20×20 cm), consisting of 9 central and 16 peripheral
squares. The rats were placed in the center of the arena and were
allowed to explore the environment for 1 min. Following this time,
the number of squares crossed was recorded for 5 min.
Morris water maze test
In order to investigate the spatial navigation in
rats, the latency time in the Morris water maze test was determined
according to a previously described method (1). The Morris water maze test consisted
of a circular pool (painted white, 200 cm diameter, 60 cm high)
filled with water (22±2°C, 37 cm deep) that was made opaque by the
addition 1 kg skim milk powder. A platform (15 cm diameter, 35 cm
high) was submersed 2 cm below the water surface in one of four
quadrants in the pool. A video recorder was hung from the ceiling
and was connected to a tracking device (SMART; Panlab, Barcelona,
Spain). The animals were subjected to three trials per session. In
each session, the rats were permitted to search for the platform
for 60 sec. If the rat located the platform, the animals were
permitted to remain on the platform for a further 10 sec. If the
rat did not locate the platform within 60 sec, the rat was guided
to the platform and permitted to remain for a further 10 sec. The
latency times taken to locate the submerged platform were recorded.
The animals were tested in this way for a total of 4 days with 3
trials per day.
Immunohistochemistry
A total of 6 animals per group were used for
immunohistochemistry staining. Serial sagittal sections (40 µm)
were obtained using a freezing microtome (−20 to −25°C; Leica
Microsystems GmbH, Wetzlar, Germany). Immunohistochemistry was
performed to investigate the expression of glial fibrillary acidic
protein (GFAP), ionized calcium-binding adaptor molecule 1 (Iba-1),
calbindin D28k and parvalbumin in each cerebellar vermis.
Free-floating sections were initially incubated in 3%
H2O2 for 30 min at room temperature.
Following this, the sections were incubated in blocking solution
(1% bovine serum albumin and 0.2% Triton X-100; Vector
Laboratories, Inc., Burlingame, CA, USA) for 2 h at room
temperature (27°C). The sections were subsequently incubated
overnight with the following primary antibodies: GFAP (1:500;
AB5804; Chemicon; Merck KGaA), Iba-1 (1:500; ab178846; Abcam,
Cambridge, UK), calbindin D28k (1:500; ab11426; Abcam), parvalbumin
(1:1,000; ab11427; Abcam), and caspase-3 (1:500; sc-56053; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C.
Following this, sections were incubated with biotinylated mouse
(BA-2000), goat (BA-9500) and rabbit (BA-1100) secondary antibodies
(1:200; Vector Laboratories, Inc., Burlingame, CA, USA) for 1 h at
room temperature (27°C). The sections were then incubated with an
avidin-biotin-peroxidase complex (1:1,000; Vector Laboratories,
Inc.) for 1 h at room temperature (27°C). For staining, the
sections were incubated in a solution consisting of 0.02% DAB and
0.03% H2O2 in 50 mM Tris-HCl (pH 7.6) for
approximately 5 min, following which they were washed with PBS and
mounted onto gelatin-coated slides. Cover slips were mounted using
Permount® (Fisher Scientific). Sections were assessed in
a quantitative fashion, according to a micro-densitometrical method
based on optical density using Image Pro® Plus software
version 6.0 (Media Cybernetics, Bethesda, MD, USA). The number of
positive cells per section was counted in 5 random fields from
every specimen with a Nikon Eclipse 80i microscope (magnification,
×40; Nikon Corporation, Tokyo, Japan).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) staining
To visualize DNA fragmentation, which is a marker of
apoptosis, TUNEL staining was performed using an in Situ
Cell Death Detection kit with fluorescein (Roche Diagnostics GmbH,
Mannheim, Germany). Serial sagittal brain sections (40 µm) were
fixed in 4% paraformaldehyde at room temperature for 15 min,
post-fixed in ethanol-acetic acid (2:1) for 5 min at −20°C and then
rinsed with PBS. Following this, sections were incubated with
proteinase K (100 µg/ml), rinsed with PBS and incubated in 3%
H2O2 for 30 min at room temperature (27°C).
Sections were subsequently permeabilized using 0.5% Triton X-100
for 1 h at room temperature (27°C), rinsed again and incubated in
the TUNEL reaction mixture for 1 h at 37°C. The sections were
rinsed and visualized using a Converter-POD for 1 h in a humidified
37°C chamber. The sections were incubated in a solution containing
in 50 mM Tris-HCl (pH 7.6) containing 0.03% DAB, 40 mg/ml nickel
chloride, and 0.03% hydrogen peroxide. The slides were
counterstained with Nissl staining and mounted onto a gelatin
coated slide. The slides were coverslipped with
Permount® (Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. SPSS software version 23.0 (IBM Corp., Armonk, NY, USA)
was used for statistical analysis. Statistical analysis was
performed using one-way analysis followed by Tukey's post-hoc test
or Pearson's correlation analysis. P<0.05 was considered to
indicate a statistically significant with 3–6 independent
experiments.
Results
Treadmill exercise alleviates spatial
navigation impairment induced by CCH
The Morris water maze test was performed to
investigate the effect of treadmill exercise on spatial navigation
impairment induced by CCH. The results indicated that the BCCAO
group demonstrated an increased escape latency time compared with
the sham group (P<0.05; Fig.
1). However, the BCCAO + Ex group demonstrated a significantly
shorter escape latency time compared with the BCCAO group on days 3
and 4 of testing (P<0.05; Fig.
1).
Treadmill exercise reduces levels of
reactive astrocytes and microglial activation in the
cerebellum
To investigate the effect of treadmill exercise on
glial cell activation, immunohistochemistry was performed.
Photomicrographs of GFAP- and Iba-1-positive cells in the posterior
regions of the cerebellar vermis are presented in Fig. 2. GFAP, a marker of reactive
astrocytes, was revealed to be significantly increased following
cerebral ischemia (26); however,
this effect was significantly attenuated following treadmill
exercise (P<0.001; Fig. 2A).
Iba-1 is a specific marker of microglia. Following transient focal
cerebral ischemia induced by BCCAO, Iba-1 expression was
demonstrated to be significantly increased in the BCCAO group
compared with the sham group. However, this effect was
significantly attenuated following treadmill exercise (P<0.001;
Fig. 2B).
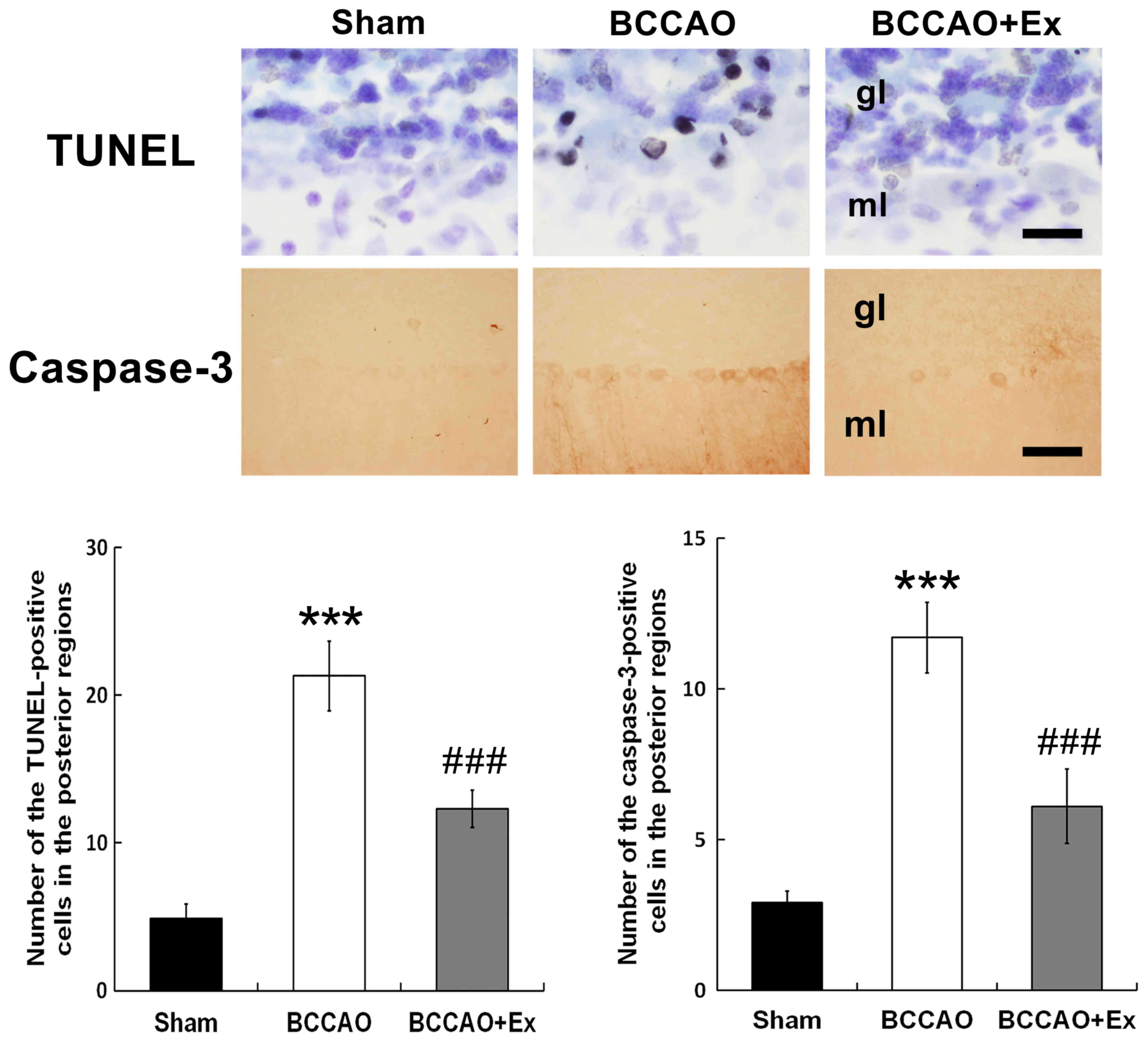
Treadmill exercise reduces apoptotic
cell death in the cerebellum
Apoptotic neuronal cell death was determined via
TUNEL analysis, which detects DNA fragmentation (27). The results demonstrated that the
BCCAO + Ex group exhibited a significantly decreased number of
TUNEL-positive cells in the posterior region compared with the
BCCAO group (P<0.001; Fig.
3).
Caspase-3, a member of the caspase family, has an
important function in apoptotic cell death (28). The BCCAO + Ex group demonstrated a
significantly decreased number of caspase-3-positive cells in the
posterior region compared with the BCCAO group (P<0.001;
Fig. 3). Furthermore, the BCCAO
group demonstrated increased TUNEL and caspase-3-positive cells in
the granular and molecular layers of the cerebellar vermis compared
with the sham group (Fig. 3).
Treadmill exercise attenuates the loss
of Purkinje cells in the cerebellum
Calbindin D28k has regulatory roles in motor
coordination, sensory integration and important functions of
Purkinje cells (29). The BCCAO +
Ex group demonstrated a significantly increased number of
calbindin-positive Purkinje cells in the posterior regions compared
with the BCCAO group (P<0.001; Fig.
4A). However, calbindin D28k-positive Purkinje cells did not
demonstrate a correlation with the latency time in the Morris water
maze test (r=−0.278, P=0.316; Fig.
4B).
Parvalbumin, a small protein that is involved in
Ca2+ signaling, protects neurons from cell death via
suppression of intracellular Ca2+ concentrations
(29). The BCCAO + Ex group
demonstrated a significantly higher number of parvalbumin-positive
Purkinje cells in the posterior region compared with the BCCAO
group (P<0.001; Fig. 4A).
Furthermore, the number of parvalbumin-positive Purkinje cells was
revealed to be negatively correlated with the latency time in the
Morris water maze test (r=−0.561, P=0.030; Fig. 4B).
Discussion
Physical exercise has numerous positive effects on
brain functions, and running exercises have been revealed to
improve various neurodegenerative disorders, such as ischemia,
Alzheimer's and Parkinson's disease (20,30,31).
In the present study, a low-intensity form of treadmill exercise
was employed. The low intensity exercise used in the present study
represents ~50–60% of maximum oxygen uptake (32). Previous studies have demonstrated
that low-intensity treadmill exercise induced positive effects on
neurogenesis in the hippocampal dentate gyrus as well as the
recovery of cognitive function in stroke models (33,34).
The present study was performed to investigate the effect of
treadmill exercise on spatial navigation impairment associated with
cerebellar Purkinje cell loss following CCH. The results revealed
that treadmill exercise significantly ameliorated the spatial
navigation performance of rats in the Morris water maze following
CCH. This result is consistent with previous studies, which
demonstrated that treadmill exercise alleviated spatial navigation
impairment in the radial 8 arm maze and Morris water maze (31,35).
Thus, the results of the present study indicate that treadmill
exercise may attenuate spatial navigation performance following
CCH.
Animal activity by open field test was investigated
in the present study (data not shown). The results demonstrated
that the activity score in the open field test was not
significantly different among the different experimental groups.
The present study did not include a sham + Ex group, so it is
difficult to expect the effect of exercise in normal group. Heo
et al (21) previously
revealed that treadmill exercise in normal mice did not exhibit a
significant effect on short-term memory. Furthermore, an additional
study demonstrated that the number of incorrect choices made in a
radial 8-arm maze test by rats in a sham + Ex group was not
decreased compared with the sham group (36). The present study investigated
whether involuntary exercise attenuates spatial navigation
impairment via inhibition of Purkinje cell loss in the cerebellum
following CCH. Therefore, a sham + Ex group was not deemed
necessary for inclusion in the study design.
Reactive astrocytes and microglia activation
following brain injury enhance neuronal death (37,38).
Reactive astrocytes result in neuroinflammation, neurotoxic
function and the formation of a glial scar (39). Activation of microglial cells, the
resident immune cells of the central nervous system, is an
important factor involved in central nervous system injury
responses and neuronal cell death (38). The results of the present study
revealed that treadmill exercise decreased the levels of reactive
astrocytes, and activation of microglial cells in the posterior
lobe of the cerebellum are consistent with previous studies
(40,41). Reactive astrocytes following brain
damage have previously been demonstrated to result in increased
GFAP expression in neurodegenerative diseases, such as Alzheimer's
disease and ischemia (41).
Activated microglial cells have been implicated in neuronal
apoptosis following ischemic stroke, and enhanced expression of
Iba-1 has previously been revealed to be upregulated following
transient middle cerebral artery occlusion (27). Therefore, the suppression of
reactive astrocytes and microglia activation may represent the
underlying neuroprotective effect of treadmill exercise against
neuronal damage in the posterior lobe of the cerebellum.
Several studies have demonstrated that cerebral
ischemia animal models exhibit increased apoptotic cell death in
the brain (28,42). TUNEL and caspase-3-positive cells
have also been revealed to be increased in ischemic brains
(38). In the present study,
treadmill exercise significantly decreased the levels of TUNEL- and
caspase-3-positive cells in the posterior region of the cerebellum.
Marques-Aleixo et al (43)
revealed that a rat treadmill endurance training group demonstrated
significantly increased expression levels of the anti-apoptotic
protein Bcl-2 in the cerebellum compared with the expression levels
exhibited by the sedentary group. A further study revealed that
treadmill exercise reduced the rate of apoptosis in the cerebellums
of autistic rats (44). The
results of the present study indicated that treadmill exercise
significantly decreased apoptosis in the cerebellum of rats
suffering from CCH and may have a role in attenuating the loss of
Purkinje cells.
Intracellular Ca2+ in neurons is
important for neural excitability, as calcium enters Purkinje cells
during action potentials following activation of voltage-gated
Ca2+ channels (45).
Cerebral ischemia is reported to induce an increase in
intracellular Ca2+ levels, which subsequently results in
a marked increase in the activation of proteases, thus resulting in
apoptotic cell death (46).
Calbindin D28k and parvalbumin regulate motor coordination and
sensory integration, which represent important functions of
Purkinje cells (47). Thus, the
regulation of intracellular Ca2+ levels by
calcium-binding proteins is important for the neuronal viability in
the brain (10). The results of
the present study demonstrated that treadmill exercise
significantly attenuated the loss of calbindin D28k- and
parvalbumin-positive Purkinje cells following CCH. These results
are consistent with a previous study that revealed that treadmill
exercise attenuated the loss of calbindin D28k-positive Purkinje
cells in the cerebellar vermis of rats suffering from amyloid
β25-35-induced Alzheimer's disease (41). Investigation into whether there was
a correlation between loss of Purkinje cells and cognitive
impairment revealed that there was a significant negative
correlation between the number of parvalbumin-positive Purkinje
cells and spatial navigation ability. Furthermore, a marginal
negative correlation was demonstrated between the number of
calbindin D28k-positive Purkinje cells and spatial navigation
ability, though this correlation was not significant. Consistent
with these observations, a previous study investigated loss of
Purkinje cells in the cerebellum by administration of OX7-saporin,
and the results revealed that rats exhibited an impaired spatial
navigation performance in the Morris water maze test compared with
the control group (48). Thus, the
results of the present study indicated that treadmill exercise
decreased the loss of the Purkinje cells in the cerebellum
following CCH in rats and that a negative correlation may exist
between loss of Purkinje cells and spatial navigation
performance.
In conclusion, the results of the present study
indicate that treadmill exercise may exert a neuroprotective effect
against the loss of Purkinje cells via the suppression of glial
cells and apoptosis. Therefore, treadmill exercise may have
potential as a therapeutic intervention strategy for the
attenuation of memory impairment in patients with CCH.
Acknowledgements
Not applicable.
Funding
This present study was supported by National
Research Foundation of Korea, which was funded by the Ministry of
Education, Science and Technology (Seoul, Korea; grant no.
NRF-2017R1A2B4012775).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
JL, JP and YK designed the experiments and the
study. JL and MS looked after the animals and performed the
experiments. CK participated in designing and discussing the study.
JL, JP and YK wrote the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Kyung Hee
University Institutional Animal Care and Use Committee [Seoul,
Korea; KHUASP (SE)-16-149].
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rochefort C, Lefort J and Rondi-Reig L:
The cerebellum: A new key structure in the navigation system. Front
Neural Circuits. 7:352013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taylor JA and Ivry RB: Cerebellar and
prefrontal cortex contributions to adaptation, strategies, and
reinforcement learning. Prog Brain Res. 210:217–253. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu W and Krook-Magnuson E: Cognitive
collaborations: Bidirectional functional connectivity between the
cerebellum and the hippocampus. Front Syst Neurosci. 9:1772015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Onuki Y, Van Someren EJ, De Zeeuw CI and
Van der Werf YD: Hippocampal-cerebellar interaction during
spatio-temporal prediction. Cereb Cortex. 25:313–321. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lalonde R and Botez MI: The cerebellum and
learning processes in animals. Brain Res Brain Res Rev. 15:325–332.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stoodley CJ and Schmahmann JD: Functional
topography in the human cerebellum: A meta-analysis of neuroimaging
studies. Neuroimage. 44:489–501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burguière E, Arleo A, Hojjati Mr, Elgersma
Y, De Zeeuw CI, Berthoz A and Rondi-Reig L: Spatial navigation
impairment in mice lacking cerebellar LTD: A motor adaptation
deficit? Nat Neurosci. 8:1292–1294. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tomlinson SP, Davis NJ, Morgan HM and
Bracewell RM: Cerebellar contributions to spatial memory. Neurosci
Lett. 578:182–186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martin LA, Goldowitz D and Mittleman G:
The cerebellum and spatial ability: Dissection of motor and
cognitive components with a mouse model system. Eur J Neurosci.
18:2002–2010. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao S, Chen N, Yang Z, Huang L, Zhu Y,
Guan S, Chen Q and Wang JH: Ischemia deteriorates the spike
encoding of rat cerebellar Purkinje cells by raising intracellular
Ca2+. Biochem Biophys Res Commun. 366:401–407. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kelley MH, Taguchi N, Ardeshiri A, Kuroiwa
M, Hurn PD, Traystman RJ and Herson PS: Ischemic insult to
cerebellar Purkinje cells causes diminished GABAA receptor function
and allopregnanolone neuroprotection is associated with GABAA
receptor stabilization. J Neurochem. 107:668–678. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Welsh JP, Yuen G, Placantonakis DG, Vu TQ,
Haiss F, O'Hearn E, Molliver ME and Aicher SA: Why do purkinje
cells die so easily after global brain ischemia? Aldolase C, EAAT4,
and the cerebellar contribution to posthypoxic myoclonus. Adv
Neurol. 89:331–359. 2002.PubMed/NCBI
|
|
13
|
Román GC, Erkinjuntti T, Wallin A, Pantoni
L and Chui HC: Subcortical ischaemic vascular dementia. Lancet
Neurol. 1:426–436. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
O'Brien JT, Erkinjuntti T, Reisberg B,
Roman G, Sawada T, Pantoni L, Bowler JV, Ballard C, DeCarli C,
Gorelick PB, et al: Vascular cognitive impairment. Lancet Neurol.
2:89–98. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kalaria RN, Maestre GE, Arizaga R,
Friedland RP, Galasko D, Hall K, Luchsinger JA, Ogunniyi A, Perry
EK, Potocnik F, et al: Alzheimer's disease and vascular dementia in
developing countries: Prevalence, management, and risk factors.
Lancet Neurol. 7:812–826. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmidt-Kastner R, Aguirre-Chen C, Saul I,
Yick L, Hamasaki D, Busto R and Ginsberg MD: Astrocytes react to
oligemia in the forebrain induced by chronic bilateral common
carotid artery occlusion in rats. Brain Res. 1052:28–39. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jing Z, Shi C, Zhu L, Xiang Y, Chen P,
Xiong Z, Li W, Ruan Y and Huang L: Chronic cerebral hypoperfusion
induces vascular plasticity and hemodynamics but also neuronal
degeneration and cognitive impairment. J Cereb Blood Flow Metab.
35:1249–1259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim SK, Cho KO and Kim SY: White matter
damage and hippocampal neurodegeneration induced by permanent
bilateral occlusion of common carotid artery in the rat: Comparison
between Wistar and Sprague-Dawley strain. Korean J Physiol
Pharmacol. 12:89–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hai J, Wan JF, Lin Q, Wang F, Zhang L, Li
H, Zhang L, Chen YY and Lu Y: Cognitive dysfunction induced by
chronic cerebral hypoperfusion in a rat model associated with
arteriovenous malformations. Brain Res. 1301:80–88. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baek SS and Kim SH: Treadmill exercise
ameliorates symptoms of Alzheimer disease through suppressing
microglial activation-induced apoptosis in rats. J Exerc Rehabil.
12:526–534. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heo YM, Shin MS, Kim SH, Kim TW, Baek SB
and Baek SS: Treadmill exercise ameliorates disturbance of spatial
learning ability in scopolamine-induced amnesia rats. J Exerc
Rehabil. 10:155–161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seo TB, Kim BK, Ko IG, Kim DH, Shin MS,
Kim CJ, Yoon JH and Kim H: Effect of treadmill exercise on Purkinje
cell loss and astrocytic reaction in the cerebellum after traumatic
brain injury. Neurosci Lett. 481:178–182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Larsen JO, Skalicky M and Viidik A: Does
long-term physical exercise counteract age-related Purkinje cell
loss? A stereological study of rat cerebellum. J Comp Neurol.
428:213–222. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
National Research Council: Guide for the
care and use of laboratory animals. National Academies Press;
Washington DC: 2010
|
|
25
|
Shin MS, Park SS, Lee JM, Kim TW and Kim
YP: Treadmill exercise improves depression-like symptoms by
enhancing serotonergic function through upregulation of 5-HT1A
expression in the olfactory bulbectomized rats. J Exerc Rehabil.
13:36–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Cao RY, Jia X, Li Q, Qiao L, Yan
G and Yang J: Treadmill exercise promotes neuroprotection against
cerebral ischemia-reperfusion injury via downregulation of
pro-inflammatory mediators. Neuropsychiatr Dis Treat. 12:3161–3173.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cho YS, Shin MS, Ko IG, Kim SE, Kim CJ,
Sung YH, Yoon HS and Lee BJ: Ulinastatin inhibits cerebral
ischemia-induced apoptosis in the hippocampus of gerbils. Mol Med
Rep. 12:1796–1802. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shin MS, Ko IG, Kim SE, Kim BK, Kim TS,
Lee SH, Hwang DS, Kim CJ, Park JK and Lim BV: Treadmill exercise
ameliorates symptoms of methimazole-induced hypothyroidism through
enhancing neurogenesis and suppressing apoptosis in the hippocampus
of rat pups. Int J Dev Neurosci. 31:214–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Celio MR: Calbindin D-28k and parvalbumin
in the rat nervous system. Neuroscience. 35:375–475. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cotman CW and Berchtold NC: Exercise: A
behavioral intervention to enhance brain health and plasticity.
Trends Neurosci. 25:295–301. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee JM, Park JM, Song MK, Oh YJ, Kim CJ
and Kim YJ: The ameliorative effects of exercise on cognitive
impairment and white matter injury from blood-brain barrier
disruption induced by chronic cerebral hypoperfusion in adolescent
rats. Neurosci Lett. 638:83–89. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schefer V and Talan MI: Oxygen consumption
in adult and Aged C57Bl/6J mice during acute treadmill exercise of
different intensity. Exp Gerontol. 31:387–392. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kwon SJ, Park JS, Park SY, Song KS, Jung
ST, Jung SB, Park IR, Choi WS and Kwon SO: Low-intensity treadmill
exercise and/or bright light promote neurogenesis in adult rat
brain. Neural Regen Res. 8:922–929. 2013.PubMed/NCBI
|
|
34
|
Shimada H, Hamakawa M, Ishida A, Tamakoshi
K, Nakashima H and Ishida K: Low-speed treadmill running exercise
improves memory function after transient middle cerebral artery
occlusion in rats. Behav Brain Res. 243:21–27. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Alaei H, Moloudi R and Sarkaki AR: Effects
of treadmill running on mid-term memory and swim speed in the rat
with Morris water maze test. J Bodyw Mov Ther. 12:72–75. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sim YJ: Treadmill exercise alleviates
impairment of spatial learning ability through enhancing cell
proliferation in the streptozotocin-induced Alzheimer's disease
rats. J Exerc Rehabil. 10:81–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liddelow SA, Guttenplan KA, Clarke LE,
Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS,
Peterson TC, et al: Neurotoxic reactive astrocytes are induced by
activated microglia. Nature. 541:481–487. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim M, Shin MS, Lee JM, Cho HS, Kim CJ,
Kim YJ, Choi HR and Jeon JW: Inhibitory effects of isoquinoline
alkaloid berberine on ischemia-induced apoptosis via activation of
phosphoinositide 3-kinase/protein kinase B signaling pathway. Int
Neurourol J. 18:115–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bruce AJ, Boling W, Kindy MS, Peschon J,
Kraemer PJ, Carpenter MK, Holtsberg FW and Mattson MP: Altered
neuronal and microglial responses to excitotoxic and ischemic brain
injury in mice lacking TNF receptors. Nat Med. 2:788–794. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vilhardt F: Microglia: Phagocyte and glia
cell. Int J Biochem Cell Biol. 37:17–21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cho HS, Kim TW, Ji ES, Park HS, Shin MS
and Baek SS: Treadmill exercise ameliorates motor dysfunction
through inhibition of Purkinje cell loss in cerebellum of valproic
acid-induced autistic rats. J Exerc Rehabil. 12:293–298. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee JM, Shin MS, Ji ES, Kim TW, Cho HS,
Kim CJ, Jang MS, Kim TW, Kim BK and Kim DH: Treadmill exercise
improves motor coordination through ameliorating Purkinje cell loss
in amyloid beta23-35-induced Alzheimer's disease rats. J Exerc
Rehabil. 10:258–264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Seo TB, Kim TW, Shin MS, Ji ES, Cho HS,
Lee JM, Kim TW and Kim CJ: Aerobic exercise alleviates
ischemia-induced memory impairment by enhancing cell proliferation
and suppressing neuronal apoptosis in hippocampus. Int Neurourol J.
18:187–197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Marques-Aleixo I, Santos-Alves E, Balça
MM, Rizo-Roca D, Moreira PI, Oliveira PJ, Magalhães J and Ascensão
A: Physical exercise improves brain cortex and cerebellum
mitochondrial bioenergetics and alters apoptotic, dynamic and
auto(mito)phagy markers. Neuroscience. 301:480–495. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim JE, Shin MS, Seo TB, Ji ES, Baek SS,
Lee SJ, Park JK and Kim CJ: Treadmill exercise ameliorates motor
disturbance through inhibition of apoptosis in the cerebellum of
valproic acid-induced autistic rat pups. Mol Med Rep. 8:327–334.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kántor O, Schmitz C, Feiser J, Brasnjevic
I, Korr H, Busto R, Ginsberg MD and Schmidt-Kastner R: Moderate
loss of cerebellar Purkinje cells after chronic bilateral common
carotid artery occlusion in rats. Acta Neuropathol. 113:549–558.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Meng Y, Li WZ, Shi YW, Zhou BF, Ma R and
Li WP: Danshensu protects against ischemia/reperfusion injury and
inhibits the apoptosis of H9c2 cells by reducing the calcium
overload through the p-JNK-NF-κB-TRPC6 pathway. Int J Mol Med.
37:258–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Barski JJ, Hartmann J, Rose CR, Hoebeek F,
Mörl K, Noll-Hussong M, De Zeeuw CI, Konnerth A and Meyer M:
Calbindin in cerebellar Purkinje cells is a critical determinant of
the precision of motor coordination. J Neurosci. 23:3469–3477.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gandhi CC, Kelly RM, Wiley RG and Walsh
TJ: Impaired acquisition of a Morris water maze task following
selective destruction of cerebellar purkinje cells with
OX7-saporin. Behav Brain Res. 109:37–47. 2000. View Article : Google Scholar : PubMed/NCBI
|