Introduction
As one of the most common malignancies and the
second leading cause of cancer related deaths worldwide, gastric
cancer is a heterogeneous disease with a variety of pathological
entities and varied clinical behavior (1–3).
There are about three hundred thousand newly diagnosed cases per
year in China (4). Gastric cancer
carries a poor prognosis that is largely attributable to early and
frequent lymphatic, hematogenous metastasis and peritoneal
dissemination, with a 5-year overall survival (OS) rate of less
than 24% (5). Despite research
endeavors and resources dedicated to elucidating the molecular
mechanisms, and numerous genetic variants and genes with irregular
expression discovered over the past decades, the precise molecular
mechanisms of metastasis focused on gastric cancer is unclear and
molecular markers for gastric cancer metastasis and tumor
progression remain elusive. This ambiguity hampers the design of
efficient and personalized chemotherapy and biotherapy strategies.
Thus, finding metastasis-related genes and elucidating their
function and clinical implication in gastric cancer are urgently
demanded.
MicroRNAs (miRNAs) are a family of endogenous small
non-coding RNAs (~22 nucleotides in length) that negatively
regulate the expression of multiple genes either by inducing
translational silencing or by causing the degradation of messenger
RNAs (mRNAs) of the targeted gene, via incomplete base-pairing to a
complementary sequence in the 3′-untranslated region (3′-UTR)
(6). In some settings, miRNAs also
interact with amino acid coding regions of their mRNA targets
(7). Increasing evidence shows
that dysregulated miRNAs expression is involved in cancer
progression and metastasis, and they might be the novel biomarkers
or therapeutic targets in disease treatment (8–11).
During our efforts to discover new novel targets
significantly associated with gastric cancer metastasis by
integrative analysis of existing public data, we found that
miRNA-29 (miR-29) family (miR-29a, miR-29b and miR-29c) can
critically affect cancer progression by functioning as tumor
suppressors (12). It has been
found that the transcriptional levels of miRNA-29 are dramatically
reduced in multiple cancer types (13–19),
and are significantly correlated with patient survival (15–19).
miRNA-29 acts as an integrator and an indispensable node of major
signaling pathways, such as nuclear factor-κB signaling, and can
mediates downstream signaling events that are involved in cancer
cell motility and invasion, cell cycle and apoptosis, epithelial
mesenchymal transition (EMT) and chemoradiotherapy efficiency
through multiple layers of mechanisms (14,16,20–26).
Recent evidence has revealed that the expression of
miR-29 family members was significantly reduced in gastric cancer
tissues compared with adjacent controls (14). miR-29b/c has been noted to form a
cross-talk regulation with DNA methyltransferase 3A (DNMT3A),
leading to the epigenetic silencing of CDH1 and subsequent
metastasis phenotypes of gastric cancer (27). Also, it has been suggested that
miR-29c activation may contribute to the
chemotherapeutic-suppressed gastric cancer cell invasion (28). Nevertheless, to the best of our
knowledge, the role of miR-29a in gastric cancer metastasis remains
to be elucidated. Therefore, in this study we identified the
expression profiles of miR-29a-3p, one transcripts of miR-29a
(14), in gastric cancer cell
lines with different metastatic potential. We then analyzed the
biological functions of miR-29a-3p on gastric cancer cells and
further verified its potential targets.
Materials and methods
Cell culture
Three pairs of high/low metastatic gastric cancer
cell lines used in this study, denoted GC9811-p/GC9811,
MKN28M/MKN-28NM, SGC7901M/SGC7901NM, were routinely cultured in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10%
heat-inactivated fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 10 mmol/l glutamine, 100 units/ml penicillin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and 100 µg/ml
streptomycin (Sigma-Aldrich; Merck KGaA) at 37°C in a humidified
atmosphere containing 5% CO2. The MKN28M and SGC7901
cell lines were amended to make them low metastatic and high
metastatic as previously described (29,30).
RNA extraction and quantitative
polymerase chain reaction (qPCR)
Total RNA from cells in logarithmic phase was
isolated using an E.Z.N.A.™ Total RNA Kit (Omega Bio-tek Inc,
Norcross, GA, USA) according to the manufacturer's instructions.
Quantified RNA (1 µg for miRNA 20 µl system and 500 ng for mRNA per
10 µl-systerm) was reverse transcribed using a PrimeScrip miRNA
qPCR Starter kit for miRNA and a PrimeScript RT Master Mix reagent
kit (Takara Biotechnology Co., Ltd., Dalian, China). Glyceraldehyde
3-phosphate dehydrogenase (GAPDH) and U6 snRNA were used as an
endogenous control. The primers for different PCR products are
listed in Table I. qPCR was
performed using a TaqMan Universal PCR Master Mix kit (Invitrogen;
Thermo Fisher Scientific, Inc.) in an Applied Biosystems 7500
Real-time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The 40 cycles of amplification reaction were allowed, each
consisting of 95°C 30 sec, 95°C 5 sec, 60°C 20 sec, 65°C 5 sec for
miRNA and 95°C 30 sec, 95°C 5 sec, 55°C 30 sec, 72°C 1 min, 72°C 5
min, 65°C 5 sec for mRNA. The formula RQ=2−ΔΔCq was used
to calculate the relative expression levels.
 | Table I.PCR primer sequences. |
Table I.
PCR primer sequences.
| Primer | Sequence
(5′-3′) |
|---|
| miR-29a-3p |
UAGCACCAUCUGAAAUCGGUUA |
| HAS3-forward |
CAGCACTAAGGTGGACAGCA |
| HAS3-reverse |
GGAGATGAAGGAAAGCACCA |
| GAPDH-forward |
GCACCGTCAAGGCTGAGAAC |
| GAPDH-reverse |
TGGTGAAGACGCCAGTGGA |
| U6-stem-loop |
AGCGGGAAATCGTGCGTGACA |
| U6-reverse |
GTGGACTFGGGAGAGGACTGG |
| Universal
downstream |
CGCCGCCCAGTGTTCAGA |
Lentiviral infection and
oligonucleotide transfection
The lentiviral GV369/GV280 vector was purchased from
GeneChem Inc. (Shanghai, China), and the up and downstream flanking
sequences of has-miR-29a-3p was insert into the vectors to
overexpress its transcript. Lentiviral production was performed
according to the manufacturer's instructions and puromycin (0.5
µg/ml) was used for selection. To RNA interference, pre-designed
validated siRNA targeting miR-29a-3p and HAS3 were obtained from
RiboBio (Guangzhou, China) and transfected into cells using a ribo
FECT™ CP Transfection kit (RiboBio) following the manufacturer's
protocol.
Cell proliferation and colony
formation assays
The cell proliferative and cologenic capacities were
determined using a
3-(4,5-dimethylthiazol-2-yl)-2′5-diphenyl-2H-tetrazolium bromide
(MTT) colorimetric assay and a colony formation assay respectively,
according to standard methods described before (31,32).
Wound healing and transwell migration
assays
The cell motility capacities were determined using a
wound healing assay and a transwell chamber assay respectively,
according to standard methods described before (33). All the experiments were performed
in triplicate wells and repeated three times.
Western blot analysis
Total protein was extracted by RIPA buffer
(Biyuntian, Beijing, China) mixed with PMSF (Roche Applied Science,
Rotkreuz, Switzerland) in the rate 10:1. 30 µg of proteins were
electrophoresed through 12% SDS polyacrylamide gels and transferred
to a nitrocellulose filter membrane (EMD Millipore, Billerica, MA,
USA). The membranes were blocked with 5% non-fat milk for 1 h and
then incubated with a rabbit PTEN antibody (catalog no. 9559;
dilution 1:1,000; Cell Signaling Technology, Inc., Beverly, MA,
USA) and a rabbit HAS3 antibody (catalog no. 15609-1-AP; dilution,
1;1,000; Proteintech Group Inc., Rosemont, IL, USA) in 4°C
overnight. A secondary HRP-conjugated goat anti-rabbit
immunoglobulin G (IgG; catalog no. sc-2004; dilution, 1:2,000;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) incubated the
membranes for 1 h and then the ECL western blotting analysis system
(Bio-Rad installed with Quantity One; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used to detect the signals. The blots were
re-probed with β-actin monoclonal antibody (catalog no. bs-0061R;
dilution, 1:1,000; Bioss, Beijing, China) to confirm equal loading
of the different samples.
Statistical analysis
All experiments were repeated at least three times
in order to insure the repeatability. Measurement data were shown
with the method of means ± standard deviation. A paired t-test was
used for the comparison of two sample means. One-way analysis of
variance (ANOVA), followed by the Student-Newman-Keuls post-test,
was used to compare the means among multiple groups. A Chi-square
test was used for the comparison of rate after the standardization.
All statistical analyses were performed using the SPSS 15.0
statistical software package (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-29a-3p expression is
down-regulated in high metastatic cell lines
Given that evidence that miR-29a-3p may act as a
tumor suppressor, we first set out to examine the expression levels
of miR-29a-3p in three pairs of gastric cell lines, each having
different metastatic potentials. As shown in Fig. 1, miR-29a-3p expression levels
appeared higher in cell lines with low metastatic potentials
(GC9811, MKN-28NM and SGC7901-NM) than in their corresponding
derived cell lines with high metastatic potentials (GC9811-p,
MKN28-M and SGC7901-M). These data suggests that down-regulation of
miR-29a-3p might associate with malignant properties mainly
relevant to metastasis in gastric cancer cells.
miR-29a-3p regulates proliferation and
migration of gastric cancer cells
Encouraged by the above data, we next performed
gain- and loss-of-function analyses to clarify the biological
functions of miR-29a-3p in terms of cancer cell proliferation and
migration. Knockdown of miR-29a-3p and overexpression of miR-29a-3p
were achieved in GC9811-p and GC9811 cells by lentiviral
transduction, respectively, and confirmed by RT-qPCR analyses
(Fig. 2A). As evidenced by MTT
assays, we found that the proliferation rate of GC9811 cells with
miR-29a-3p knockdown were significantly increased in comparison to
their control cells. By contrast, GC9811-p cells with forced
expression of miR-29a-3p grew slower than their control cells
(Fig. 2B). Similarly, GC9811 cells
with miR-29a-3p knockdown formed more colonies than their control,
while GC9811-p cells with miR-29a-3p over-expression exhibited
reduced colonies compared with their own control cells (Fig. 2C).
We further investigated the effect of miR-29a-3p on
cell migration using a transwell migration assay. GC9811 cells with
miR-29a-3p knockdown showed a significant increase in cell
migration through the membrane of tranwell chambers than the
control. As expected, GC9811-p cells with forced expression of
miR-29a-3p achieved the opposite effects (Fig. 2D). The inhibitory effect of
miR-29a-3p on cell migration was confirmed using a wound-healing
assay. GC9811 cells with miR-29a-3p knockdown statistically
significantly accelerated the closure of wound area, while GC9811-p
cells with over-expression of miR-29a-3p delayed the wound closure,
as compared with their controls respectively (Fig. 2E).
Bioinformatic target prediction of
miR-29a-3p
In attempts to explore the molecular mechanisms
through which miR-29a-3p might regulate gastric cancer progression,
we searched for its putative target genes using three online
prediction tools including Targetscan (http://www.targetscan.org), PicTar (http://pictar.mdc-berlin.de/) and Miranda (http://www.microrna.org/microrna/home.do). The
intersectional potential target genes were selected as shown in
Fig. 3A. Among them, we screened
out the hyaluronan synthase 3 (HAS3) gene which may be directly
regulated by miR-29a. In this regard, the 3′UTR sequence UGGUGCUA
upstream of both genes had perfect complimentarity with bases 2
through 8 counting from the 5′end of miR-29a-3p. We then examined
whether miR-29a-3p expression was correlated with HAS3 by qRT-PCR
analysis. By doing so, we observed that knockdown of miR-29a-3p in
GC9811 cells obviously increased HAS3 mRNA levels, whereas
overexpression of miR-29a-3p in GC9811-p cells decreased HAS3
expression (Fig. 3B). Altogether,
these findings indicate that miR-29a-3p may negatively associate
with HAS3 expression.
Knockdown of HAS3 suppresses gastric
cancer cell proliferation and migration
To validate the biological functions of HAS3 in
gastric cancer, we transfected a pre-designed and validated siRNA
targeting HAS3 into GC9811-p cells, in which HAS3 was relatively
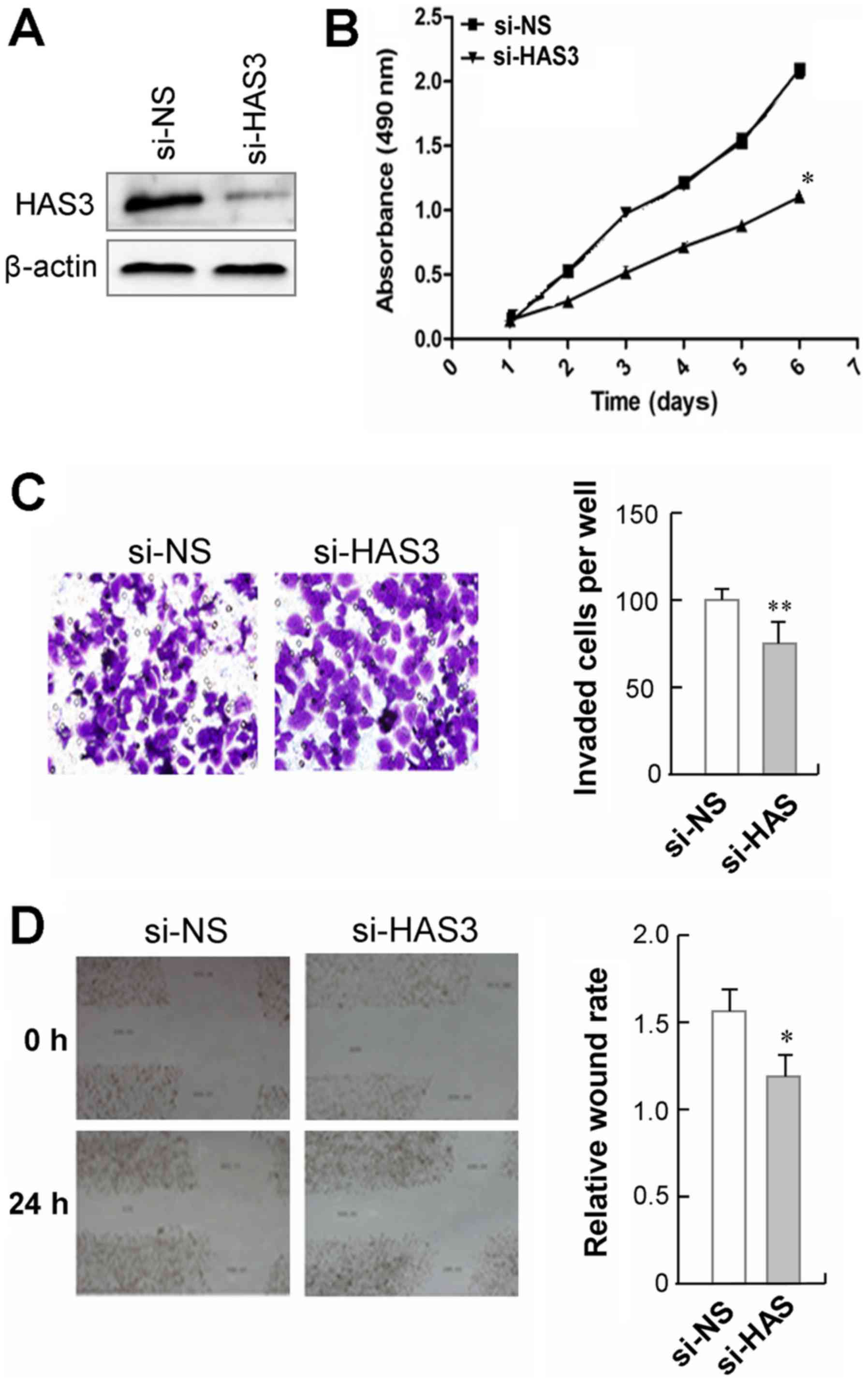
highly expressed (Fig. 4A). We
observed that GC9811-p cells with HAS3 knockdown showed a
significant decrease in cell proliferation, as compared to those
cells transfected with non-specific control siRNA (Fig. 4B). The effect of HAS3 on cell
mobility was analyzed using a Transwell migration assay. The
results showed that GC9811-p cells with HAS3 knockdown showed a
significant decrease in cell migration compare to their control
cells (Fig. 4C). Moreover, the
wound healing assay revealed that silencing endogenous HAS3 in
GC9811-p cells led to slower closure of the wound area than their
control (Fig. 4D). Together, these
findings clearly demonstrate that knockdown of CDK10 suppresses the
proliferation and the migration GC9811-p cells.
HAS3 is crucial for
miR-29a-3p-associated phenotypes in gastric cancer cells
Having observed that miR-29a-3p expression was
negatively associated HAS3, we then carried out knockdown analysis
was to confirm that HAS3 is required for miR-29a-3p-associated
phenotypes in gastric cancer cells. GC9811 cells with miR-29a-3p
knockdown were transfected with a HAS3-targeting siRNA (Fig. 5A), and the cell proliferation and
metastasis abilities were measured. We found that silencing HAS3
expression led to a decreased proliferation rate in GC9811 cells
with miR-29a-3p knockdown (Fig.
5B). Similarly, silencing HAS3 expression dramatically reduced
the invasiveness and migration of GC9811 cells with miR-29a-3p
knockdown, as determined by migration and would healing assays
respectively (Fig. 5C and D).
These data confirm that HAS3 is required for miR-29a-3p-associated
phenotypes induced in gastric cancer cells.
Discussion
MiR-29a has been reported to have a close relation
to many cancer types, such as malignant bile duct carcinoma,
nasopharyngeal carcinoma and breast cancer. MiR-29 family members
usually function as tumor suppressors by direct targeting oncogenic
genes (13–19). In the present study, gastric cancer
cell line GC9811 and its derived gastric line GC9811-p with high
peritoneum metastasis potential were used in to analyze the
biological functions of miR-29a-3p, one transcripts of miR-29a. We
found that miR-29a-3p expression level was lower in GC9811-p cells
than in GC9811 cells, indicating that miR-29a-3p may serve as a
tumor-suppressive miRNA for gastric cancer metastasis. The
observation was confirmed in other two pairs of high/low metastatic
gastric cancer cell lines. Based on these observations, we further
carried out the gain- and loss-of-function analyses to clarify the
biological functions of miR-29a-3p in terms of cancer cell
proliferation and migration. The results indicate that miR-29a-3p
can suppress gastric cancer cell growth and metastasis.
To understand the molecular mechanisms of miR-29a as
a tumor suppressor in gastric cancer, we searched for putative
miR-29a targets by online target gene prediction and HAS3 were
selected out for subsequent research in this study. All family
members of HSA proteins including HSA1, HSA2 and HSA3 have been
closely linked to cancer progression and metastasis, in which HAS3
has the highest activity (34,35).
It has been found that that the expression of HAS3 is conspicuous
up-regulated in metastatic colon carcinoma cells and metastatic
prostate cancer cells, in which the content of hyaluronic acid
dramatically rise (36,37). Moreover, elevated HAS3 expression
is found to be correlated with poor outcome for oral cancer and
breast cancer (38,39). Overexpression of HAS3 could promote
the proliferation of prostate cancer and melanoma cells, while
knockdown of HAS3 inhibits the growth of colon cancer cells
(40–42). Although its anti-cancer effect
remains controversial (43), HAS3
has been postulated as a candidate tumor suppressor.
To validate the biological functions of HAS3 in
gastric cancer, we silenced HAS3 by siRNA interference in GC9811-p
cells with highly expressed HAS3 levels. As expected, knockdown of
HAS3 could inhibit gastric cancer proliferation and metastasis. We
proceeded to address the biological significance of the
miR-29a-HAS3 connection in the regulation of gastric cancer cell
penotypes. GC9811 cells with miR-29a-3p knockdown were transfected
with a HAS3-targeting siRNA and then examined for cell growth,
migration and invasion. Silencing of HAS3 significantly attenuated
both proliferation and metastasis abilities of GC9811 cells with
miR-29a-3p knockdown, suggesting that miR-29a-HAS3 axis is an
important pathway in regulating growth and mobility
anchorage-independent in gastric cancers and that miR-29a exerts
its function, at least in part, through regulating HAS3
expression.
In summary, we demonstrate that miR-29a-3p can
inhibit gastric cancer cells proliferation and metastasis by
regulation HAS3 expression. These findings reveal a new mechanism
by which, at least partially, miR-29a-3p may act as a candidate
tumor suppressor. To the best of our knowledge, this is the first
report that miR-29a-3p could regulate HAS3 expression, which
broadens our understanding of miR-29a function. Our data suggest
that miR-29a may have great potential in controlling tumorigenesis
and metastasis, and further confirm that miR-29a is a promising
target for gastric cancer prevention and therapy.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant nos. 81760440 and 81360323) and
the Regional Science and Technology Development Program Conducted
by the Central Government of China (YDZX20176400004650).
Availability of data and materials
The dataset used and/or analyzed in the current
study is available from the corresponding authors on reasonable
request.
Authors' contributions
MJ, RX, XL YF and LM performed the experiments,
analyzed the data and participated in the experiment design and
manuscript writing. YN contributed to the analysis and
interpretation of data. FB and YY conceived the study, designed the
experiments and wrote the manuscript. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Crew KD and Neugut AI: Epidemiology of
gastric cancer. World J Gastroenterol. 12:354–362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Terry MB, Gaudet MM and Gammon MD: The
epidemiology of gastric cancer. Semin Radiat Oncol. 12:111–127.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoo CH, Noh SH, Shin DW, Choi SH and Min
JS: Recurrence following curative resection for gastric carcinoma.
Br J Surg. 87:236–242. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qin X, Xu H, Gong W and Deng W: The tumor
cytosol miRNAs, fluid miRNAs, and exosome miRNAs in Lung Cancer.
Front Oncol. 4:3572015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rigoutsos I: New tricks for animal
microRNAS: Targeting of amino acid coding regions at conserved and
nonconserved sites. Cancer Res. 69:3245–3248. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang H, Li M, Zhang R, Wang Y, Zang W, Ma
Y, Zhao G and Zhang G: Effect of miR-335 upregulation on the
apoptosis and invasion of lung cancer cell A549 and H1299. Tumour
Biol. 34:3101–3109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He H, Di Y, Liang M, Yang F, Yao L, Hao S,
Li J, Jiang Y, Jin C and Fu D: The microRNA-218 and ROBO-1
signaling axis correlates with the lymphatic metastasis of
pancreatic cancer. Oncol Rep. 30:651–658. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu YY, Wu HJ, Ma HD, Xu LP, Huo Y and Yin
LR: MicroRNA-503 suppresses proliferation and cell cycle
progression of endometrioid endometrial cancer via negatively
regulating cyclin D1. FEBS J. 280:3768–3779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao S, Yao DS, Chen JY and Ding N:
Aberrant expression of miR-20a and miR-203 in cervical cancer.
Asian Pac J Cancer Prev. 14:2289–2293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang H, Zhang G, Wu JH and Jiang CP:
Diverse roles of miR-29 in cancer. Oncol Rep. 31:1509–1516. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sengupta S, den Boon JA, Chen IH, Newton
MA, Stanhope SA, Cheng YJ, Chen CJ, Hildesheim A, Sugden B and
Ahlquist P: MicroRNA 29c is down-regulated in nasopharyngeal
carcinomas, up-regulating mRNAs encoding extracellular matrix
proteins. Proc Natl Acad Sci USA. 105:5874–5878. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gong J, Li J, Wang Y, Liu C, Jia H, Jiang
C, Wang Y, Luo M, Zhao H, Dong L, et al: Characterization of
microRNA-29 family expression and investigation of their
mechanistic roles in gastric cancer. Carcinogenesis. 35:497–506.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oliveira LH, Schiavinato JL, Fráguas MS,
Lucena-Araujo AR, Haddad R, Araújo AG, Dalmazzo LF, Rego EM, Covas
DT, Zago MA and Panepucci RA: Potential roles of microRNA-29a in
the molecular pathophysiology of T-cell acute lymphoblastic
leukemia. Cancer Sci. 106:1264–1277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bae HJ, Noh JH, Kim JK, Eun JW, Jung KH,
Kim MG, Chang YG, Shen Q, Kim SJ, Park WS, et al: MicroRNA-29c
functions as a tumor suppressor by direct targeting oncogenic SIRT1
in hepatocellular carcinoma. Oncogene. 33:2557–2567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu DW, Hsu NY, Wang YC, Lee MC, Cheng YW,
Chen CY and Lee H: c-Myc suppresses microRNA-29b to promote tumor
aggressiveness and poor outcomes in non-small cell lung cancer by
targeting FHIT. Oncogene. 34:2072–2082. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Inoue A, Yamamoto H, Uemura M, Nishimura
J, Hata T, Takemasa I, Ikenaga M, Ikeda M, Murata K, Mizushima T,
et al: MicroRNA-29b is a novel prognostic marker in colorectal
cancer. Ann Surg Oncol. 22 Suppl 3:S1410–S1418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qi Y, Huang Y, Pang L, Gu W, Wang N, Hu J,
Cui X, Zhang J, Zhao J, Liu C, et al: Prognostic value of the
MicroRNA-29 family in multiple human cancers: A meta-analysis and
systematic review. Clin Exp Pharmacol Physiol. 44:441–454. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Q, Costinean S, Croce CM, Brasier AR,
Merwat S, Larson SA, Basra S and Verne GN: MicroRNA 29 targets
nuclear factor-κB-repressing factor and Claudin 1 to increase
intestinal permeability. Gastroenterology. 148:158–169.e8. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishikawa R, Chiyomaru T, Enokida H,
Inoguchi S, Ishihara T, Matsushita R, Goto Y, Fukumoto I, Nakagawa
M and Seki N: Tumour-suppressive microRNA-29s directly regulate
LOXL2 expression and inhibit cancer cell migration and invasion in
renal cell carcinoma. FEBS Lett. 589:2136–2145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mizuno K, Seki N, Mataki H, Matsushita R,
Kamikawaji K, Kumamoto T, Takagi K, Goto Y, Nishikawa R, Kato M, et
al: Tumor-suppressive microRNA-29 family inhibits cancer cell
migration and invasion directly targeting LOXL2 in lung squamous
cell carcinoma. Int J Oncol. 48:450–460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiong Y, Fang JH, Yun JP, Yang J, Zhang Y,
Jia WH and Zhuang SM: Effects of microRNA-29 on apoptosis,
tumorigenicity, and prognosis of hepatocellular carcinoma.
Hepatology. 51:836–845. 2010.PubMed/NCBI
|
|
24
|
Xu XD, Wu XH, Fan YR, Tan B, Quan Z and
Luo CL: Exosome-derived microRNA-29c induces apoptosis of BIU-87
cells by down regulating BCL-2 and MCL-1. Asian Pac J Cancer Prev.
15:3471–3476. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu PN, Yan MD, Lai HC, Huang RL, Chou YC,
Lin WC, Yeh LT and Lin YW: Downregulation of miR-29 contributes to
cisplatin resistance of ovarian cancer cells. Int J Cancer.
134:542–551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rostas JW III, Pruitt HC, Metge BJ, Mitra
A, Bailey SK, Bae S, Singh KP, Devine DJ, Dyess DL, Richards WO, et
al: microRNA-29 negatively regulates EMT regulator N-myc interactor
in breast cancer. Mol Cancer. 13:2002014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cui H, Wang L, Gong P, Zhao C, Zhang S,
Zhang K, Zhou R, Zhao Z and Fan H: Deregulation between miR-29b/c
and DNMT3A is associated with epigenetic silencing of the CDH1
gene, affecting cell migration and invasion in gastric cancer. PLoS
One. 10:e01239262015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Liu C, Luo M, Zhang Z, Gong J, Li
J, You L, Dong L, Su R, Lin H, et al: Chemotherapy-induced
miRNA-29c/Catenin-δ signaling suppresses metastasis in gastric
cancer. Cancer Res. 75:1332–1344. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bai F, Guo X, Yang L, Wang J, Shi Y, Zhang
F, Zhai H, Lu Y, Xie H, Wu K and Fan D: Establishment and
characterization of a high metastatic potential in the peritoneum
for human gastric cancer by orthotopic tumor cell implantation. Dig
Dis Sci. 52:1571–1578. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xin R, Bai F, Feng Y, Jiu M, Liu X, Bai F,
Nie Y and Fan D: MicroRNA-214 promotes peritoneal metastasis
through regulating PTEN negatively in gastric cancer. Clin Res
Hepatol Gastroenterol. 40:748–754. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
You Y, Yang W, Wang Z, Zhu H, Li H, Lin C
and Ran Y: Promoter hypermethylation contributes to the frequent
suppression of the CDK10 gene in human nasopharyngeal carcinomas.
Cell Oncol. 36:323–331. 2013. View Article : Google Scholar
|
|
32
|
You Y, Yang W, Qin X, Wang F, Li H, Lin C,
Li W, Gu C, Zhang Y and Ran Y: ECRG4 acts as a tumor suppressor and
as a determinant of chemotherapy resistance in human nasopharyngeal
carcinoma. Cell Oncol (Dordr). 38:205–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin C, Xin S, Qin X, Li H, Lin L and You
Y: Zoledronic acid suppresses metastasis of esophageal squamous
cell carcinoma cells through upregulating the tight junction
protein occludin. Cytotechnology. 68:1233–1241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Adamia S, Maxwell CA and Pilarski LM:
Hyaluronan and hyaluronan synthases: Potential therapeutic targets
incancer. Curr Drug Targets Cardiovasc Haematol Disord. 5:3–14.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng XL, Wang FS and Ling PX: Research
status of hyaluronan synthases. Pharmaceutical Biotechnol.
11:413–416. 2004.
|
|
36
|
Bullard KM, Kim HR, Wheeler MA, Wilson CM,
Neudauer CL, Simpson MA and McCarthy JB: Hyaluronan synthase-3 is
upregulated in metastatic colon carcinoma cells and manipulation of
expression alters matrix retention and cellular growth. Int J
Cancer. 107:739–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Simpson MA, Reiland J, Burger SR, Furcht
LT, Spicer AP, Oegema TR Jr and McCarthy JB: Hyaluronan synthase
elevation in metastatic prostate carcinoma cells correlates with
hyaluronan surface retention, a prerequisite for rapid adhesion to
bone marrow endothelial cells. J Biol Chem. 276:17949–17957. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kuo YZ, Fang WY, Huang CC, Tsai ST, Wang
YC, Yang CL and Wu LW: Hyaluronan synthase 3 mediated oncogenic
action through forming inter-regulation loop with tumor necrosis
factor alpha in oral cancer. Oncotarget. 8:15563–15583. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Auvinen P, Rilla K, Tumelius R, Tammi M,
Sironen R, Soini Y, Kosma VM, Mannermaa A, Viikari J and Tammi R:
Hyaluronan synthases (HAS1-3) in stromal and malignant cells
correlate with breast cancer grade and predict patient survival.
Breast Cancer Res Treat. 143:277–286. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu N, Gao F, Han Z, Xu X, Underhill CB
and Zhang L: Hyaluronan synthase 3 overexpression promotes the
growth of TSU prostate cancer cells. Cancer Res. 61:5207–5214.
2001.PubMed/NCBI
|
|
41
|
Takabe P, Bart G, Ropponen A, Rilla K,
Tammi M, Tammi R and Pasonen-Seppänen S: Hyaluronan synthase 3
(HAS3) overexpression downregulates MV3 melanoma cell
proliferation, migration and adhesion. Exp Cell Res. 337:1–15.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Teng BP, Heffler MD, Lai EC, Zhao YL,
LeVea CM, Golubovskaya VM and Bullarddunn KM: Inhibition of
hyaluronan synthase-3 decreases subcutaneous colon cancer growth by
increasing apoptosis. Anticancer Agents Med Chem. 11:620–628. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chang IW, Liang PI, Li CC, Wu WJ, Huang
CN, Lin VC, Hsu CT, He HL, Wu TF, Hung CH and Li CF: HAS3
underexpression as an indicator of poor prognosis in patients with
urothelial carcinoma of the upper urinary tract and urinary
bladder. Tumour Biol. 36:5441–5450. 2015. View Article : Google Scholar : PubMed/NCBI
|