Introduction
Lung carcinoma is a prevalent disease, which is
associated with a marked impact on global health and an increasing
rate of incidence (1). Tobacco
smoking and environmental tobacco smoke, genetic factors, air
pollution, and certain occupational exposures, including to
asbestos and radon, are considered high risk factors for lung
cancer (2–4). Accumulating evidence has suggested
that there is an association between diabetes and lung cancer
(5–7). In certain clinical trials, the
combination of diabetes and lung cancer has been associated with an
elevated mortality rate (8),
whereas antidiabetic medication can improve the survival rate among
such patients (9). In
vitro, a high concentration of glucose (HG) has been
demonstrated to promote the invasion and metastatic potential of
A549 cells (10). These data
indicate the potential association between diabetes and lung
cancer.
Receptor for advanced glycation end-products (RAGE,
also known as AGER), the ligands of which were initially identified
as advanced glycation end products, serves a role in diabetes
mellitus. RAGE has been reported to be downregulated in non-small
cell lung cancer (NSCLC) (11–13),
which differs from other solid tumors, including liver cancer,
which exhibit high levels of RAGE (14). In the present study, the potential
role of RAGE in the effects of HG on A549 cells was
investigated.
Nicotinamide adenine dinucleotide phosphate oxidase
(NOX)-4 is a member of the NOX family, which is associated with
generation of endogenous reactive oxygen species (ROS) and
mediation of inflammatory responses (15). The NOX-4 protein may also be an
intermediary agent in the association between RAGE and
inflammation, which may influence the tumor cell microenvironment
(16). Both RAGE and NOXs mediate
the chronic inflammatory response, which may potentially increase
the risk of carcinogenesis; however, whether there is an
association between the factors remains largely unknown. The
present study aimed to clarify the possible mechanism of how
glucose affects the growth and migration of cancer cells.
Therefore, affecting glucose metabolism may be a potential target
to suppress cancer cells, which would avoid the side effects that
occur with the use of hypoglycemic drugs such as melamine.
Materials and methods
Cell culture
Human lung adenocarcinoma A549 cells, purchased from
Shanghai Institute of Cell Biology of the Chinese Academy of
Sciences (Shanghai, China), were cultured in Dulbecco's modified
Eagle's medium (DMEM; Servicebio, Inc., Woburn, MA, USA), for 24 h,
supplemented with 10% fetal bovine serum (FBS; Servicebio, Inc.),
25 mmol/l glucose in the HG group or 5.5 mmol/l glucose in the
normal concentration of glucose (NG) group, and 100 U/ml penicillin
at 37°C in a humidified atmosphere containing 5% CO2.
The cells were used between passages 6 and 10.
NOX inhibitor and RAGE-blocking
antibody
The NOX inhibitor diphenyl iodonium chloride (DPI)
was purchased from Tocris Bioscience (Bristol, UK; no. 4673-26-1)
and was dissolved in dimethyl sulfoxide (DMSO), in order to provide
a final concentration of 5 µM, which was stored at 4°C. RAGE
affinity-purified antibody (5 µg/ml; cat. no. PB0530; Boster
Biological Technology, Pleasanton, CA, USA) was dissolved in PBS,
and used as a RAGE-blocking antibody.
Cell proliferation and viability
Cell proliferation and viability were assessed using
an MTT assay. The cells were seeded at a density of
2×103 cells/well (in 5% FBS) or 5×103
cells/well (in 0.2% FBS) onto 96-well plates in 100 µl DMEM
overnight, after which the medium was removed. Following treatment
with different concentrations of glucose (0, 5, 10 and 25 mmol/l),
with or without 5 µM DPI or 5 µg/ml RAGE-blocking antibody for 24
h, 20 µl MTT solution (5 mg/ml; Servicebio, Inc.) was added and the
cells were cultured for a further 4 h. Subsequently, the medium was
removed and 150 µl DMSO was added to dissolve the purple formazan
for 5–10 min. Absorbance was measured at a wavelength of 555 nm
using an ELISA plate reader.
Cell migration assay
Cell migration was examined by wound-healing assay.
Following treatment with HG or NG, with or without 5 µM DPI or 5
µg/ml RAGE-blocking antibody for 24 h, A549 cells were seeded on
6-well plates at a density of 5×103/well and a straight
scratch was made using a 200-µl sterile pipette tip. Subsequently,
the 6-well plates were washed with PBS three times and fresh medium
was added. After 18 h, migration was determined by comparing the
wound area between the NG group and the HG group under an inverse
fluorescent microscope. Distance change rate was calculated by
initial distance-final distance/initial distance.
RNA extraction, cDNA synthesis and
qPCR
The cells were plated at a density of
1.5×105 cells/well in 12-well plates and were cultured
for 24 h after treated with HG or NG. Total RNA was extracted using
RNA rapid extraction solution (Servicebio, Inc.), according to the
manufacturer's protocol. The quality and quantity of isolated total
RNA were assessed using a NanoDrop™ 2000 Spectrophotometer
(NanoDrop; Thermo Fisher Scientific, Inc., Wilmington, DE, USA).
RNA was reverse transcribed to cDNA using a RevertAid First Strand
cDNA Synthesis kit (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's protocol. FastStart Universal
SYBR-Green Master (Rox) (Roche Diagnostics, Basel, Switzerland) was
used for qPCR analysis, according to the manufacturer's protocol.
Fluorescence detection was conducted using an ABI StepOne Plus
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The 2−∆∆Cq method was used to
quantify the expression levels of target genes (17). The following primers were used in
the present study (all 5′→3′): β-actin forward,
CACCCAGCACAATGAAGATCAAGAT and reverse, CCAGTTTTTAAATCCTGAGTCAAGC;
RAGE forward, CACTGGTGCTGAAGTGTAAGGG and reverse,
CGGACTCGGTAGTTGGACTTG; NOX-4 forward, ATTTAGATACCCACCCTCCCG and
reverse, CACAGTACAGGCACAAAGGTCC; hypoxia-inducible factor-1α
(HIF-1α) forward, TGATTGCATCTCCATCTCCTACC and reverse,
GACTCAAAGCGACAGATAACACG; and vascular endothelial growth factor
(VEGF) forward, GGAGGGCAGAATCATCACGA and reverse,
GACTCAAAGCGACAGATAACACG. Actin RNA levels were used as an
endogenous control.
Western blot analysis
The cells were plated at a density of 1.5×105
cells/well in 6-well plates and cultured for 24 h. The media were
subsequently removed and fresh media containing NG or HG, with or
without DPI (5 µM) or RAGE-blocking antibody (5 µg/ml), were added
for 24 h. Total protein was extracted with a RIPA Lysis Buffer
(Servicebio, Inc.), according to the manufacturer's protocol.
Proteins (20 µg) were separated by 10% SDS-PAGE and transferred
onto polyvinylidene fluoride membranes. Following blocking by 5%
milk, made with skim milk powder (YIli, China) and Tris Buffered
saline Tween buffer (0.1% Tween), at normal temperature for 1 h,
monoclonal primary antibodies, namely anti-RAGE antibody (1:500;
cat. no. PB0530; Wuhan Boster Biological Technology, Ltd., Wuhan,
China), anti-NOX-4 antibody (1:1,000; cat. no. ab109225; Abcam),
anti-VEGF antibody (1:500; cat. no. GB11034), anti-HIF-1α antibody
(1:1,000, rabbit; cat. no. GB11031) and anti-β-actin antibody
(1:2,000; cat. no. GB13001-3; all Servicebio, Inc.), were added and
incubated overnight at 37°C. Subsequently, the membranes were
washed five times by Tris Buffered saline Tween buffer, and
incubated with a goat anti-rabbit immunoglobulin G horseradish
peroxidase-conjugated secondary antibody (1:3,000; cat. no.
GB23303; Servicebio, Inc.) for 55 min. The membranes were
subsequently washed five times by Tris Buffered saline Tween
buffer. Finally, the protein bands were detected using an EPSON
Perfection V300 Photo scanner (Seiko Epson Corporation, Suwa,
Japan) after processing with E Enhanced Chemiluminescence
visualization reagent (G2014; Servicebio, Inc.).
Statistical analysis
Data are presented as the means ± standard error of
the mean of three repeat. Unpaired t-test was selected to compare
two groups, and analyses of multiple groups were performed using
one-way analysis of variance, followed by
least-significant-difference post hoc test. Analysis was performed
using SPSS 22.0 software (IBM Corp., Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
HG promotes proliferation and
migration of A549 cells, and increases the mRNA and protein
expression levels of RAGE and NOX-4
The present study determined how various
concentrations of glucose affected the proliferation and migration
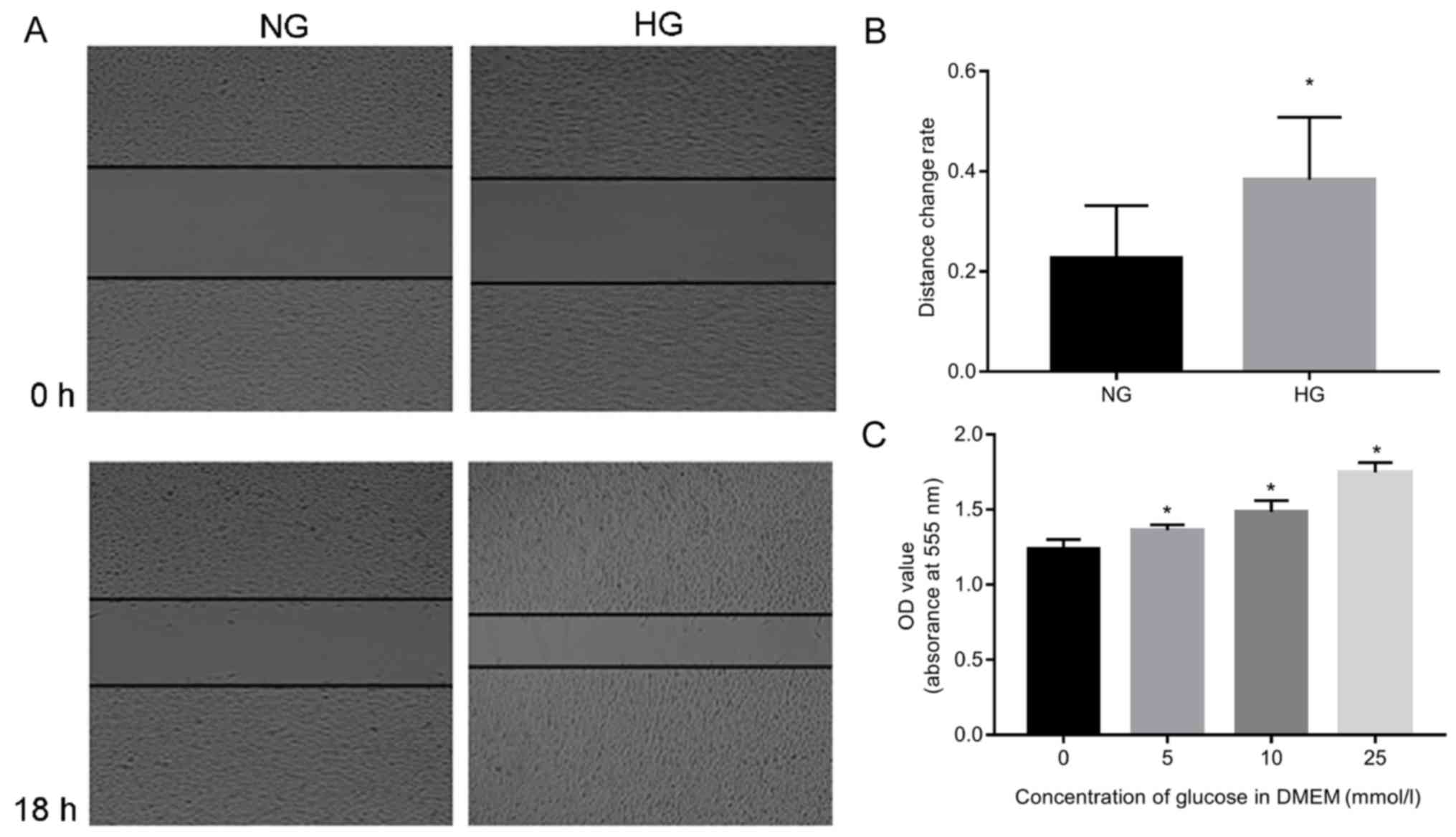
of A549 cells (Fig. 1). The
results demonstrated that A549 cells survived well in response to
glucose, and increased proliferation was detected when the
concentration of glucose was increased (P<0.05; Fig. 1A and B). The migration rate
increased significantly in the HG group compared to NC (P<0.05;
Fig. 1C).
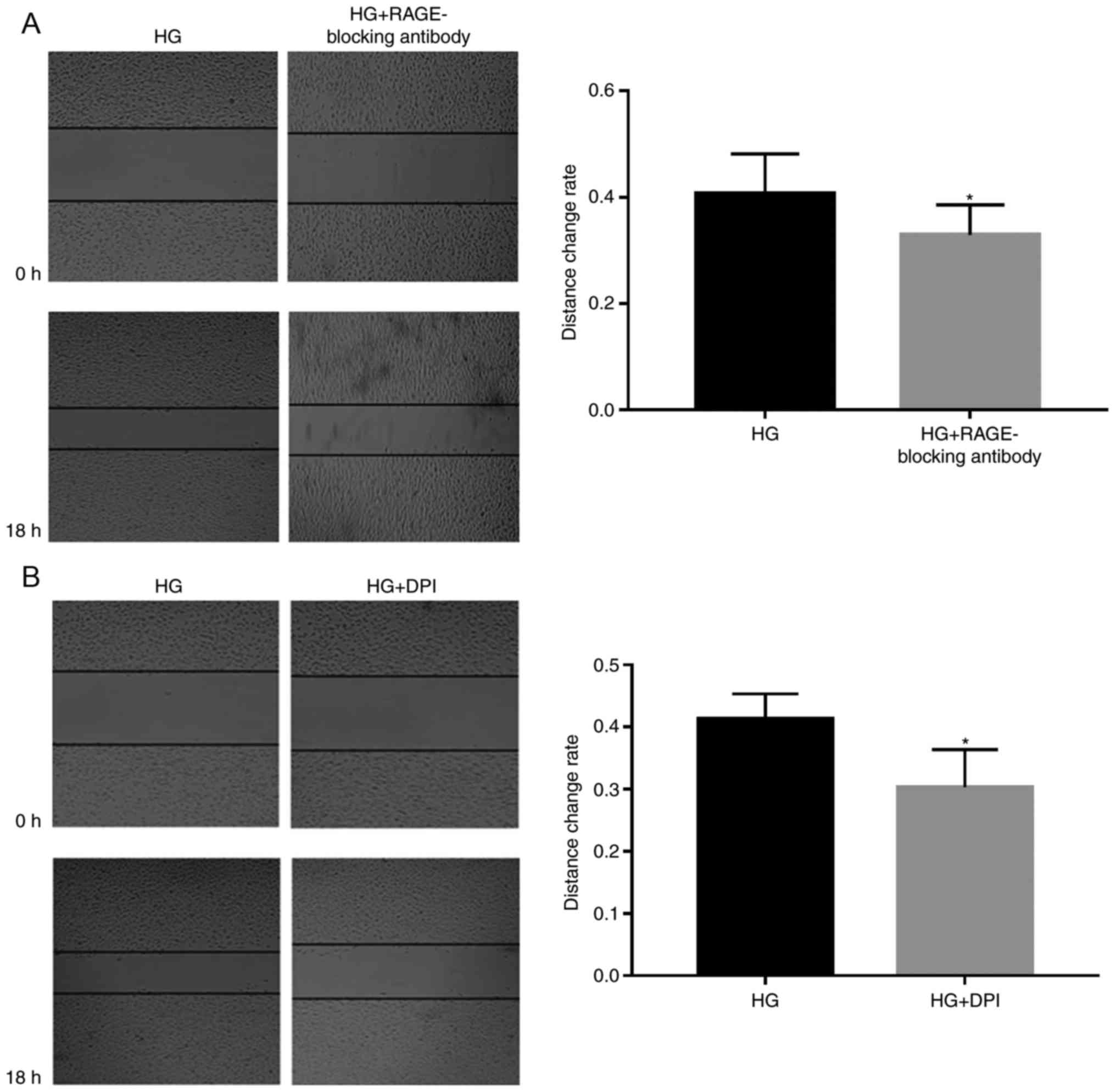
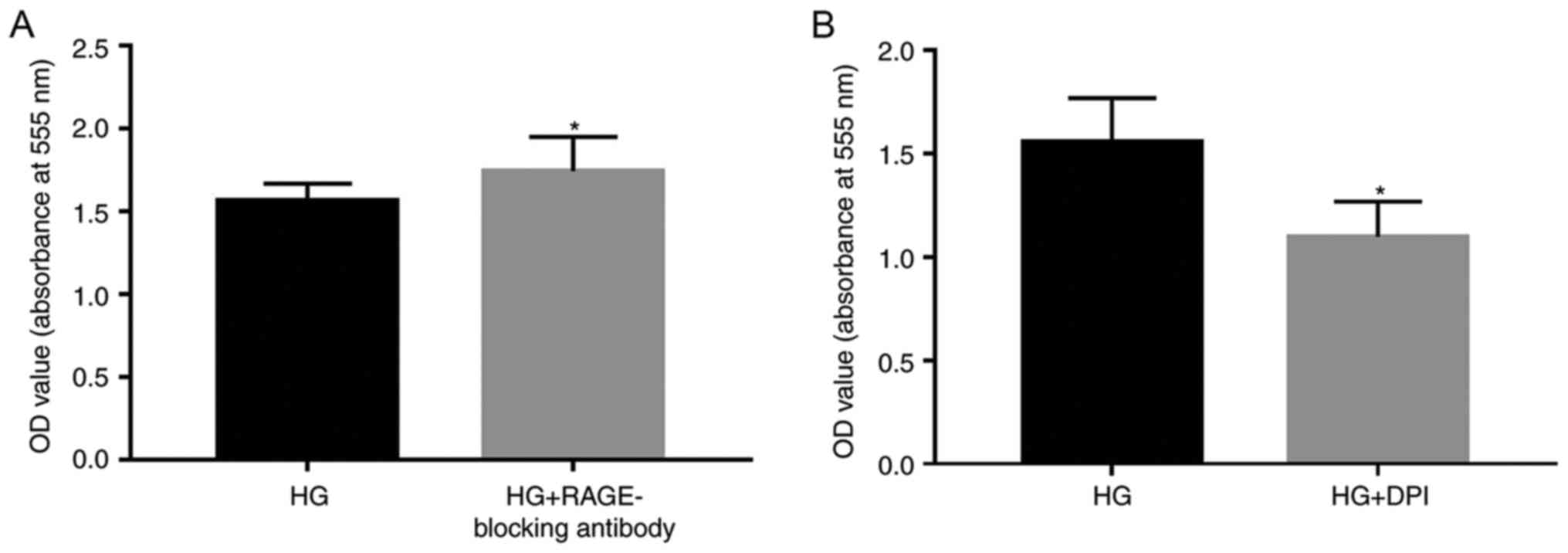
Subsequently, 5 µg/ml RAGE-blocking antibody and 5
µM DPI were added to the cells, in order to investigate whether
RAGE and NOXs serve roles in HG-induced effects (Figs. 2–4). RAGE-blocking antibody inhibited the
protein and mRNA expression levels of RAGE and NOX-4 under HG
condition, which has the ability to promote the expression of the
mRNA and protein of the RAGE and NOX-4 (Fig. 2A and B). Furthermore, treatment
with RAGE-blocking antibody accelerated the proliferation
(P<0.05; Fig. 3A) and
suppressed HG-induced migration (P<0.05; Fig. 4A) of A549 cells, thus indicating
that RAGE-blocking antibody may reverse HG-induced effects on cell
metastasis. Similar effects were detected in cells treated with the
NOX inhibitor DPI; briefly, HG-induced proliferation (P<0.05;
Fig. 3B) and migration (P<0.05;
Fig. 4B) of A549 cells were
inhibited by DPI, which also suppressed the protein expression
levels of NOX-4, (Fig. 2E) while
having no significant effect on the mRNA expression levels of RAGE
(Fig. 2A).
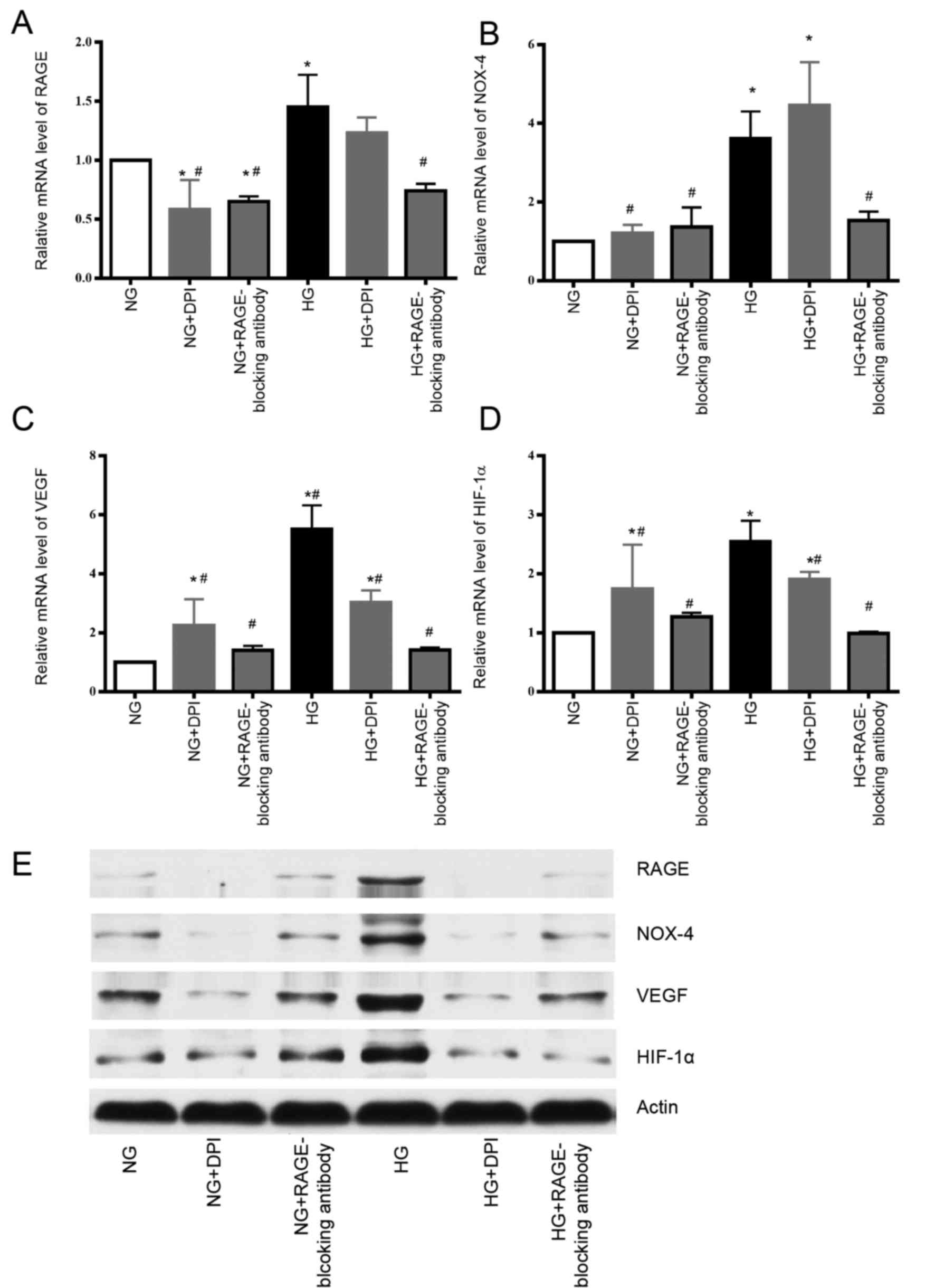 | Figure 2.HG increases the mRNA and protein
expression levels of RAGE and NOX-4, whereas RAGE-blocking antibody
inhibits these effects. DPI could suppress the protein expression
of NOX-4 and RAGE induced by HG compared with the HG group.
Individual treatment with DPI or the RAGE-blocking antibody
decreases the mRNA expression levels of VEGF and HIF-1α in HG
group. The protein expression levels of HIF-1α and VEGF were also
decreased following treatment with the RAGE-blocking antibody or
DPI in HG group. (A) RAGE mRNA, (B) NOX-4 mRNA, (C) VEGF mRNA and
(D) HIF-1α mRNA expression was detected by qPCR. (E) RAGE, NOX-4,
VEGF and HIF-1α protein expression was detected by western
blotting. *P<0.05 vs. NG group; #P<0.05 vs. HG
group. DPI, diphenyl iodonium chloride; HG, high glucose; NG,
normal glucose; NOX-4, nicotinamide adenine dinucleotide phosphate
oxidase-4; RAGE, receptor for advanced glycation end-products;
HIF-1α, hypoxia-inducible factor 1α; VEGF, vascular endothelial
growth factor; qPCR, quantitative polymerase chain reaction. |
RAGE-blocking antibody and DPI inhibit
the expression of HIF-1α and VEGF, which are involved in mediating
tumorigenesis, tumor growth and cancer metastasis
Finally, to determine how the RAGE-NOX-4 pathway
affects the biological functions of tumor cells, variations in the
expression levels of VEGF and HIF-1α were measured when RAGE and
NOX-4 were suppressed by their respective inhibitors. The mRNA and
protein expression levels of VEGF and HIF-1α were increased under
HG conditions compared with NG, whereas treatment with the
RAGE-blocking antibody and DPI reduced the expression levels of
VEGF and HIF-1α, which indicated that these inflammatory factors
may be downstream effectors in the glucose-associated cellular
pathways (Fig. 2C-E). However, the
mechanism by which the RAGE-blocking antibody and DPI act on VEGF
and HIF-1α in the NG groups, remain unclear.
Discussion
The constant increase in diabetes
mellitus-associated morbidity between 2002 and 2012 indicates that
the occurrence of diabetes is associated with a diverse range of
complications, which further increases the public health threat
posed by this disease (18).
Furthermore, recent studies have suggested that a pre-existing
hyperglycemic condition, together with the time of diagnosis and
insulin deficiency, may exert negative effects on patient prognosis
and contribute to the local recurrence of a pulmonary neoplasm by
impacting the signaling pathways of cancer cells (5,6).
This viewpoint has been verified by another study, which
demonstrated that metformin may improve the chemotherapy outcomes
and survival rate of patients presenting with both diabetes and
lung carcinoma in a dose-dependent manner (19). The present study confirmed that HG
may promote the proliferation and migration of the human NSCLC cell
line A549 in a dose-dependent manner, thus indicating that HG may
be a risk factor not only for metastasis, but also for the growth
of lung adenocarcinoma tumor mass.
RAGE, which is a member of the immunoglobulin
superfamily, is a pattern recognition receptor that can bind a
diverse range of ligands with similar three-dimensional structures
(12). Since the identification of
RAGE on endothelial cells, the role of RAGE has gradually been
established in certain pathological processes associated with
chronic inflammation, including asthma, lung cancer and chronic
obstructive pulmonary disease. It has previously been reported that
RAGE exhibits acceleration of the growth and metastasis of
pulmonary solid tumor tissue (20). Nevertheless, the signaling pathways
of RAGE are yet to be elucidated. The results of the present study
demonstrated that RAGE may be a protective factor in the growth of
lung adenocarcinoma tumor tissues, but a potential risk factor for
metastasis. Furthermore, its expression was increased in response
to HG exposure; therefore, it may be hypothesized that RAGE
potentially participates in HG-induced oxidative stress via
activating NOXs.
NOXs are multi-protein complexes that give rise to
the generation of ROS, which can in turn mediate oxidative stress
and inflammatory responses (21).
The effect of NOXs on ROS generation can also be initiated by HG,
thus suggesting that an association may exist between NOXs and HG.
In addition, NOXs may facilitate the proliferation and migration of
tumor cells by activating certain signaling pathways, including
nuclear factor-κB signaling (22).
In the present study, NOX-4, a member of the NOX family, was
induced in response to HG; it was suggested that this effect was
mediated by RAGE in lung adenocarcinoma cells. Furthermore, the NOX
inhibitor DPI served as a protective agent in HG-induced
proliferation and migration of A549 cells. These results revealed a
potential mechanism underlying HG-induced ROS production via the
RAGE-NOX-4 pathway.
Overexpression of HIF-1α has been observed in
various types of solid tumors, and through its corresponding
nuclear receptor, HIF-1α may regulate genes involved in cancer
progression, and subsequently promote the proliferation and
invasion of lung carcinoma cells (23). VEGF is a protein that mediates
angiogenesis and, in turn, sustains the growth of tumor tissue;
therefore, the abnormal expression of this factor may have a
stimulatory effect on cancer development (24). In addition, VEGF and HIF-1α are
associated with tumor growth and metastasis in various pathways,
and are likely associated with the poor prognosis of patients. The
present study unveiled a potential approach for controlling the
expression of these factors through obstructing the
glucose-RAGE-NOX-4 pathway, in order to ultimately inhibit tumor
progression.
In conclusion, abnormal glucose metabolism is a
pathological factor facilitating the oncogenic and biological
behavior of lung cancer cells, which likely occurs through
activation of the RAGE-NOX-4 pathway, and subsequent upregulation
of VEGF and HIF-1α. Through binding of these inflammatory factors
to their corresponding receptors to exert their specific biological
effects, cancer cells may grow faster and their malignancies may be
enhanced. Therefore, the significance of controlling blood glucose
levels in patients with lung cancer combined with diabetes is
evident, and RAGE and/or NOXs may be potential therapeutic targets
for the treatment of patients with lung adenocarcinoma and comorbid
diabetes. However, the method of administering antagonists rather
than performing gene silencing may not be the preferred plan, due
to its inconsistent effects during the present study. In addition,
why the RAGE-blocking antibody promoted HG-induced proliferation of
A549 cells, and whether RAGE-NOXs mediate other effects, as well as
inducing the expression of VEGF and HIF-1α, remain unknown;
therefore, we aim to address these issues in future research.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Wu Jieping
Medical Fund of China (grant no. 320.6750.17056).
Availability of data and materials
The data sets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
YFL and FL designed and conducted the experiment,
data analysis, and conclusion completed jointly by YFL, FL and BZ.
The manuscript was produced by YFL and then reviewed and revised by
FL and BZ. SY and ZQC participated in part of the experimental
process.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang Y, Ren JS, Huang HY, Shi JF, Li N,
Zhang Y and Dai M: International trends in lung cancer incidence
from 1973 to 2007. Cancer Med. Mar 14–2018.(Epub ahead of print).
View Article : Google Scholar
|
|
2
|
Cigarette smoking among adults-United
States, 2006. MMWR Morb Mortal Wkly Rep. 56:1157–1161.
2007.PubMed/NCBI
|
|
3
|
Morrison HI, Semenciw RM, Mao Y and Wigle
DT: Cancer mortality among a group of fluorspar miners exposed to
radon progeny. Am J Epidemiol. 128:1266–1275. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spitz MR, Hong WK, Amos CI, Wu X, Schabath
MB, Dong Q, Shete S and Etzel CJ: A risk model for prediction of
lung cancer. J Natl Cancer Inst. 99:715–726. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo J, Hendryx M, Qi L, Ho GY and Margolis
KL: Pre-existing diabetes and lung cancer prognosis. Br J Cancer.
115:76–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang X, Liu Y, Mani H, Olson J, Clawson G,
Caruso C, Bruggeman R, Varlotto JM, Zander DS and Rassaei N:
Biologic evaluation of diabetes and local recurrence in non-small
cell lung cancer. Pathol Oncol Res. 23:73–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu L, Cao H, Zhang T, Shen H, Dong W,
Wang L and Du J: The effect of diabetes mellitus on lung cancer
prognosis: A PRISMA-compliant meta-analysis of Cohort Studies.
Medicine (Baltimore). 95:e35282016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iachina M, Jakobsen E, Møller H,
Lüchtenborg M, Mellemgaard A, Krasnik M and Green A: The effect of
different comorbidities on survival of non-small cells lung cancer
patients. Lung. 193:291–297. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin JJ, Gallagher EJ, Sigel K, Mhango G,
Galsky MD, Smith CB, LeRoith D and Wisnivesky JP: Survival of
patients with stage IV lung cancer with diabetes treated with
metformin. Am J Respir Crit Care Med. 191:448–454. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang X, Kong F, Wu X, Ren Y, Wu S, Wu K,
Jiang Z and Zhang W: High glucose promotes tumor invasion and
increases metastasis-associated protein expression in human lung
epithelial cells by upregulating heme oxygenase-1 via reactive
oxygen species or the TGF-β1/PI3K/Akt signaling pathway. Cell
Physiol Biochem. 35:1008–1022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fritz G: RAGE: A single receptor fits
multiple ligands. Trends Biochem Sci. 36:625–632. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oczypok EA, Perkins TN and Oury TD: All
the ‘RAGE’ in lung disease: The receptor for advanced glycation
endproducts (RAGE) is a major mediator of pulmonary inflammatory
responses. Paediatr Respir Rev. 23:40–49. 2017.PubMed/NCBI
|
|
13
|
Wang H, Li Y, Yu W, Ma L, Ji X and Xiao W:
Expression of the receptor for advanced glycation end-products and
frequency of polymorphism in lung cancer. Oncol Lett. 10:51–60.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hollenbach M: The role of Glyoxalase-I
(Glo-I), Advanced Glycation Endproducts (AGEs), and their receptor
(RAGE) in chronic liver disease and Hepatocellular Carcinoma (HCC).
Int J Mol Sci. 18:pii: E2466. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang CT, Lin XL, Wu S, Liang Q, Yang L,
Gao YJ and Ge ZZ: NOX4-driven ROS formation regulates proliferation
and apoptosis of gastric cancer cells through the GLI1 pathway.
Cell Signal. 46:52–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim E, Kim W, Lee S, Chun J, Kang J, Park
G, Han I, Yang HJ, Youn H and Youn B: TRAF4 promotes lung cancer
aggressiveness by modulating tumor microenvironment in normal
fibroblasts. Sci Rep. 7:89232017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mayer-Davis EJ, Dabelea D and Lawrence JM:
Incidence trends of type 1 and type 2 diabetes among youths,
2002–2012. N Engl J Med. 377:3012017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mazzone PJ, Rai H, Beukemann M, Xu M, Jain
A and Sasidhar M: The effect of metformin and thiazolidinedione use
on lung cancer in diabetics. BMC Cancer. 12:4102012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu YX, Pan WC and Cheng YF: Silencing of
advanced glycosylation and glycosylation and product-specific
receptor (RAGE) inhibits the metastasis and growth of non-small
cell lung cancer. Am J Transl Res. 9:2760–2774. 2017.PubMed/NCBI
|
|
21
|
Souabni H, Ezzine A, Bizouarn T and Baciou
L: Functional assembly of soluble and membrane recombinant proteins
of mammalian NADPH oxidase complex. Methods Mol Biol. 1635:27–43.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Auer S, Rinnerthaler M, Bischof J,
Streubel MK, Breitenbach-Koller H, Geisberger R, Aigner E, Cadamuro
J, Richter K, Sopjani M, et al: The human NADPH oxidase, Nox4,
regulates cytoskeletal organization in two cancer cell lines, HepG2
and SH-SY5Y. Front Oncol. 7:1112017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang N, Liang Y, Yang P and Ji F: Propofol
suppresses LPS-induced nuclear accumulation of HIF-1α and tumor
aggressiveness in non-small cell lung cancer. Oncol Rep.
37:2611–2619. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Frezzetti D, Gallo M, Maiello MR,
D'Alessio A, Esposito C, Chicchinelli N, Normanno N and De Luca A:
VEGF as a potential target in lung cancer. Expert Opin Ther
Targets. 21:959–966. 2017. View Article : Google Scholar : PubMed/NCBI
|