Introduction
The term reactive oxygen species (ROS) refers to
numerous reactive molecules and free radicals that are generated
from molecular oxygen (1). These
molecules are produced in all cell types as by-products of aerobic
respiration or by oxidoreductase enzymes and metal-catalysed
oxidation (1,2). In addition to this endogenous source,
ROS are also generated in response to exogenous stimuli, including
particles and their interactions with cellular components (3). ROS may lead to various detrimental
effects, but also function as important messengers for intra- and
intercellular communication (2).
The majority of eukaryotic cells possess an antioxidant defence
system, including glutathione (GSH) and thioredoxin systems, which
function alongside superoxide dismutase enzymes (SOD) (4–6). At
low levels, ROS are readily neutralised by antioxidant defences,
including GSH, SOD and antioxidant enzymes, which ensures a balance
between the production and removal of ROS; however, conditions that
lead to an imbalance, such as excessive ROS production, overwhelms
the endogenous defences and is associated with the development of
oxidative stress (2,3). A hierarchical oxidative stress
hypothesis associates low levels of oxidative stress with the
activation of antioxidant and detoxification enzymes, while genes
encoding phase II enzymes are reported to be regulated by
transcription factors, such as the nuclear factor erythroid
2-related factor 2 (3).
At higher levels of oxidative stress,
proinflammatory signalling cascades, including mitogen-activated
protein kinase and nuclear factor κB pathways, are activated, which
leads to inflammation and cytotoxicity; mitochondrial perturbation
and the release of proapoptotic factors subsequently occur to
induce apoptosis (1,3,7).
Several particles may also target the mitochondria directly
(7,8). Due to the high reactivity of ROS,
they damage the integrity of various biomolecules, including
nucleic acids, proteins and lipids (9). The biological impact of ROS is
dependent on the specific molecules involved, the microenvironment,
the physiological or pathological context in which it is generated
in, and the magnitude and duration of exposure (9–11).
In particle toxicology research, the induction of
ROS generation and oxidative stress is an important mechanism for
particle-induced cytotoxicity (12,13).
Particles are either able to generate oxidants themselves or may
stimulate the production of cellular oxidants (13). The hazards of granular and fibrous
dust is often associated with their physicochemical properties,
including size, surface properties, chemical composition,
crystalline structure, solubility and aggregation (3). Based on their length-to-diameter
ratio, particles may be divided into granular and fibrous dust. The
toxicity of biopersistent granular dust may be due to
biopersistence, which is also termed the particle effect, and not
due to substance-specific properties. In an animal model,
biopersistent granular dusts were reported to provoke lung cancer,
but did not appear to be primarily genotoxic (14). Nanosized particles, defined by a
diameter <100 nm, demonstrated a higher tendency to form
agglomerates compared with their larger counterparts (15). The size of nanoparticles is
comparable with the size of subcellular structures, including cell
organelles and macromolecules (16). The high surface/mass ratios of
these small particles accounts for their higher chemical reactivity
(17).
In the present study the ability of different
fibrous and granular dusts to generate ROS in a lung epithelial
adenocarcinoma cell model was investigated using A549 cells. Glass
fibres, and chrysotile and crocidolite asbestos, were selected as
representatives of fibrous dust. Glass fibres are non-crystalline,
synthetic, inorganic substances, that belong to the group of
so-called man-made vitreous fibres. Asbestos is an established
carcinogen associated with the promotion of lung cancer,
mesothelioma and lung fibrosis (18). DNA damage and apoptosis are
important downstream consequences of asbestos exposure that have
been reported in all major studies addressing lung cells, as
reported in a detailed review by Kamp (19). Exposure to asbestos fibres has been
reported to induce altered cell signalling (20) and the stimulation of various
proinflammatory molecules, including certain cytokines (21,22).
Crocidolite
[Na2(Fe3+)2(Fe2+)3Si8O22(OH)2]
has an iron content of ~26%, while the iron content of chrysotile
[Mg6Si4O10(OH)8] is
lower, ranging between 1 and 6%, the majority of which is
considered surface contamination (23). As biopersistent granular dust,
microsized titanium dioxide anatase (TiO2 ma), nanosized
titanium dioxide anatase (TiO2 na) and nanosized
titanium dioxide rutile (TiO2 nr) were selected to
compare variations in size and crystal structure modifications.
TiO2 is insoluble and rutile is chemically inert
(13,24), while, anatase is more reactive and
cytotoxic due to its crystalline structure (25). Finally, nanosized hematite
(α-Fe2O3) with an iron content of ~70% was
investigated due to its ability to induce ROS generation via the
Fenton reaction (26–28).
As the ability to generate oxidative stress may be
the crucial mechanism underlying particle-induced cytotoxicity, the
hazards of particles may be associated with their physicochemical
properties. Therefore, the particle properties that may be
responsible for oxidative stress were investigated in the present
study by analysing the ROS-generating potential of various
particles. In addition, the gene expression of oxidative
stress-associated genes involved in antioxidant defence processes
and mechanisms was investigated.
Materials and methods
Dust material and
characterisation
Chrysotile asbestos [Rhodesian NB #4173-11-2; Union
for International Cancer Control (UICC), Geneva, Switzerland] and
crocidolite asbestos (South African NB #4173-111-3; UICC) were used
as standard references for recognised toxic fibrous dust. Fibrous
glass (man-made vitreous fibres) representing biodurable fibres was
obtained from commercial glass wool fibres used for insulation.
TiO2 ma (www.sigmaaldrich.com/catalog/product/aldrich/232033?lang=de®ion=DE232033;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), TiO2 na
(cat. no. NO-0038-HP; IoLiTec-Ionic Liquids Technologies GmbH,
Heilbronn, Germany) and TiO2 nr (cat. no. NO-0046-HP;
IoLiTec-Ionic Liquids Technologies GmbH) represented biopersistent
granular dust. Hematite (α-Fe2O3; cat. no.
544884; Sigma-Aldrich; Merck KGaA) was investigated to represent
ultrafine particles.
Scanning electron microscopy (SEM; S-2700; Hitachi,
Ltd., Tokyo, Japan) was employed to identify particle geometry and
the microstructure of the samples. Element analysis was conducted
using energy dispersive X-ray spectroscopy (EDX). To optimise the
conductivity (electron beam), all samples were coated with a very
fine gold layer using the sputtering technique. Transmission
electron microscopy (TEM) analysis combined with EDX, as well as
electron diffraction (detection of crystallinity), was conducted
using a transmission electron microscope H-7100 (Hitachi, Ltd.). A
detailed description of the characterisation method is described in
our previous study (29).
Thermogravimetry (TG) measurements (corundum crucibles, heating
rate 5 K/min and synthetic air atmosphere) for controlling volatile
impurities, such as water, performed using a thermobalance TG 209
F1 Iris (Netzsch-Geräetebau GmbH, Selb, Germany) (30,31).
For all experiments, an autoclaved stock solution
with 5 mg particles/ml PBS was prepared. Prior to each application
the solutions were vortexed and sonicated.
Dosimetry
It is known that fibres and particles, depending on
their size and mass, behave differently when added to cell culture
medium, particularly with regards to the time it takes to settle
down on cells following addition to medium, which is termed the
sinking rate (32). To estimate
the sinking velocities of each particle and fibre under
experimental conditions, glass vessels were filled with 6 ml
H2O. Into each glass vessel particles of the highest
concentration (10 µg/cm2) were added. At 0.5, 1, 2, 5,
10, 30, 60, 120, 240 and 600 min time-points, an aliquot of 10 µl
was collected from the sample at a standardised position (1.5 cm
above the bottom). SEM was used to count the number of particles
within each aliquot, standardised to an area of 1 mm2.
The percentage of agglomeration of particles left in the suspension
was determined.
Cell culture and treatment
The A549 human lung epithelial adenocarcinoma cell
line, with characteristics of alveolar epithelial type II cells,
was employed in the present study. A549 cells (European Collection
of Cell Cultures) were purchased from Sigma-Aldrich (Merck KGaA).
Briefly, A549 cells were cultivated in complete growth medium at
37±1°C in an atmosphere of 5±0.5% CO2 and 95% humidity.
Complete growth medium consisted of RPMI-1640 cell culture medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 10%
foetal calf serum (Gibco; Thermo Fisher Scientific, Inc.) and 2 mM
L-glutamine (PAA Laboratories; GE Healthcare, Chicago, IL, USA).
Cells were cultured until ~75% confluent and subsequently rinsed
with PBS, followed by dislodging with TrypLE Express (Thermo Fisher
Scientific, Inc.). Cells were plated in 6-well plates at a density
of 100,000 cells/well. Culture medium was replaced with fresh
medium and cells were exposed to particles or fibres 24 h prior to
ROS-detection. Unexposed cells in one well served as internal
negative controls, while five other wells were exposed to 0.1, 0.5,
1, 5 or 10 µg/cm2, of the respective particle or fibre
for 24 h at 37°C.
Cell viability test with
PrestoBlue®
Viable cells maintain a reducing environment within
the cytosol. PrestoBlue uses the reducing ability of viable cells
and allows the quantification of cell proliferation to determine
the effects of various reagents on the viability of different cell
types. PrestoBlue reagent contains a nonfluorescent blue compound
that is cell-permeable; the reagent appears red and fluoresces
within the reducing environment of a viable cell, which is detected
via fluorescence or absorbance. A549 cells were plated in 96-well
plates at a density of 15,200 cells/well. At 24 h prior to
analysis, the culture medium was replaced with fresh medium and
cells were exposed to particles or fibres of varying concentrations
(0.1, 0.5,1, 2.5, 5 or 10 µg/cm2) at 37°C. All
experiments were replicated seven times (biological replicate);
unexposed cells in each 96-well plate served as internal negative
controls and each condition within one 96-well plate was repeated
three times (technical replicate). Fluorescence was analysed using
the Tecan i-control reader and software Magellan™ 6.0 (Tecan Group,
Ltd., Zurich, Switzerland).
Detection of ROS
Intracellular ROS generation was assessed using the
fluorescent probe 2′,7′-dichlorofluorescein (DCF). Cells were
plated in 6-well plates at a density of 100,000 cells/well. Culture
medium was replaced with fresh medium and cells were exposed to
particles or fibres 24 h prior to ROS-detection. Unexposed cells in
one well served as internal negative controls, while five other
wells were exposed to 0.1, 0.5, 1, 5 or 10 µg/cm2 of the
respective particle or fibre. The membrane-permeable diacetate
form, DCF-diacetate (H2-DCF-DA; EMD Millipore,
Billerica, MA USA), was added to the culture medium at a final
concentration of 10 µM and incubated for 30 min at 37°C. Following
penetration of the membrane, the acetate groups are removed and the
resulting H2-DCF becomes isolated intracellularly.
Intracellular ROS oxidises H2-DCF to yield the
fluorescent product, DCF. Fluorescence intensity was measured in
≥80 different cells per preparation and background was identified
as an area without cells and subtracted from the signal.
Fluorescence was analysed using a fluorescence microscope combined
with a video imaging system (TILL Photonics GmbH, Gräfelfing,
Germany). Fluorescence of the cells of each well was measured for 5
min. The increase in fluorescence intensity per min during the
stimulation time was compared to the alterations in fluorescence
intensity per min of the internal control well. Each preparation
was repeated six to eight times (biological replicate).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
For mRNA extraction, 3,000,000 cells were plated in
75 cm2 cell culture flasks (Greiner Bio-One
International GmbH, Kremsmünster, Austria) with 15 ml previously
aforementioned growth medium. Cells were exposed to 1
µg/cm2 chrysotile, crocidolite and glass fibres or 100
ng/cm2 TiO2 ma, TiO2 nr,
TiO2 na and hematite for 24 h at 37°C. Cells were
trypsinised for ~30 sec with 10 ml 0.05% trypsin and incubated for
10 min at 37°C following two washes with PBS at 37°C. Detached
cells were subsequently resuspended in 5 ml ice-cold PBS and
centrifuged at 400 × g (without brakes) for 10 min at 4°C. The
centrifugation step was repeated with 1 ml ice-cold PBS in 1.5 ml
Eppendorf tubes. RNA was extracted immediately with TRI
Reagent® (Sigma-Aldrich; Merck KGaA) according to the
manufacturer's protocol. Isolated RNA was resuspended in 10 µl
RNAse-free water. Each sample was treated twice with 2 µl
RNAse-free DNAse at 1 U/µl (Qiagen GmbH, Hilden, Germany) for 10
min at 37°C to eliminate remaining DNA. The prepared RNA underwent
RT as described previously (33).
For quantitative comparison of mRNA levels, qPCR was performed
using SYBR Green fluorescence in a LightCycler® System
(Roche Diagnostics GmbH, Mannenheim, Germany). Amplification
specificity was verified with melting curves. Negative and positive
controls were included in each PCR reaction. Gene expression was
compared with the mean of the expression of β-2-microglobulin,
β-actin and hypoxanthine phosphoribosyltransferase as the
housekeeping genes. Calculations of expression were performed with
the 2−ΔΔCq method, as previously described (34). All samples were analysed as
duplicates (technical replicate) and for each gene at least six
independent biological replicates were performed. Primer sequences
and specific primer annealing temperatures are presented in
Table I. PCR reactions were
conducted with a final volume of 20 µl using 1X ABsolute qPCR SYBR
Green Capillary Mixes (ABgene; Thermo Fisher Scientific, Inc.), 300
nM primers and 2 µl cDNA. The PCR conditions were as follows:
Initial activation of the Taq-DNA polymerase for 15 min at 95°C,
followed by 45 cycles of 10 sec denaturation at 95°C, annealing for
15 sec at the specific annealing temperature (Table I) and extension for 10 sec at 72°C.
All measurements were made without information regarding sample
origin.
 | Table I.Primer sequences and primer-specific
annealing temperature. |
Table I.
Primer sequences and primer-specific
annealing temperature.
| Gene | Sequence 5′-3′
(forward) | Sequence 5′-3′
(reverse) | Annealing
temperature (°C) |
|---|
| ACTBa |
CTGGAACGGTGAAGGTGACA |
AAGGGACTTCCTGTAACAACGCA | 56 |
| B2Ma |
ACTGAATTCACCCCCACTGA |
CCTCCATGATGCTGCTTACA | 63 |
| HPRTa |
ATGCTGAGGATTTGGAAAGGG |
GCACACAGAGGGCTACAATG | 61 |
| GPX2b |
TAAGTGGGCTCAGGCCTCTCT |
GGTCATAGAAGGACTTGGCAATG | 58 |
| GRb |
AGACCTATTCAACGAGCTTTACC |
CCTGCAGCATTTCATCACACC | 58 |
| GSTpib |
GGAGACCTCACCCTGTACCA |
GGGCAGTGCCTTCACATAGT | 55 |
| SOD1b |
AGGGCATCATCAATTTCGAG |
TGCCTCTCTTCATCCTTTGG | 55 |
| SOD2b |
AAGGGAGATGTTACAGCCCAGATA |
TCCAGAAAATGCTATGATTGATATGAC | 58 |
| TRX1b |
GGATGACTGTCAGGATGTTGC |
ATTCATTAATGGTGGCTTCAAGC | 58 |
| TRXR1b |
TGCCACTGGTGAAAGACCAC |
CAAGAAATCCAGCGCACTCC | 57 |
| TXNDC5b |
TCGATGACACCATTGCAGAAG |
TGCTGCAGATATTCCGTTCAG | 57 |
| XIAPb |
CCGTGCGGTGCTTTAGTTGT |
TTCCTCGGGTATATGGTGTCTGAT | 58 |
| NDRG1b |
TGGAGATTGAGCGACCAATG |
CACAGTCCGCCATCTTGAG | 55 |
Statistical analysis
Data are presented as the mean ± standard error from
n=8 different culture preparations. Statistical comparisons for ROS
production results were performed by one-way analysis of variance
followed by the Games-Howell post-hoc test. Statistical comparisons
for mRNA expression results were performed by the Kruskal-Wallis
test followed by Dunn-Bonferroni post-hoc tests. P<0.05 was
considered to indicate a statistically significant difference. All
data analyses were performed using SPSS 17 for Windows (SPSS, Inc.,
Chicago, IL, USA).
Results
Characterisation of particles
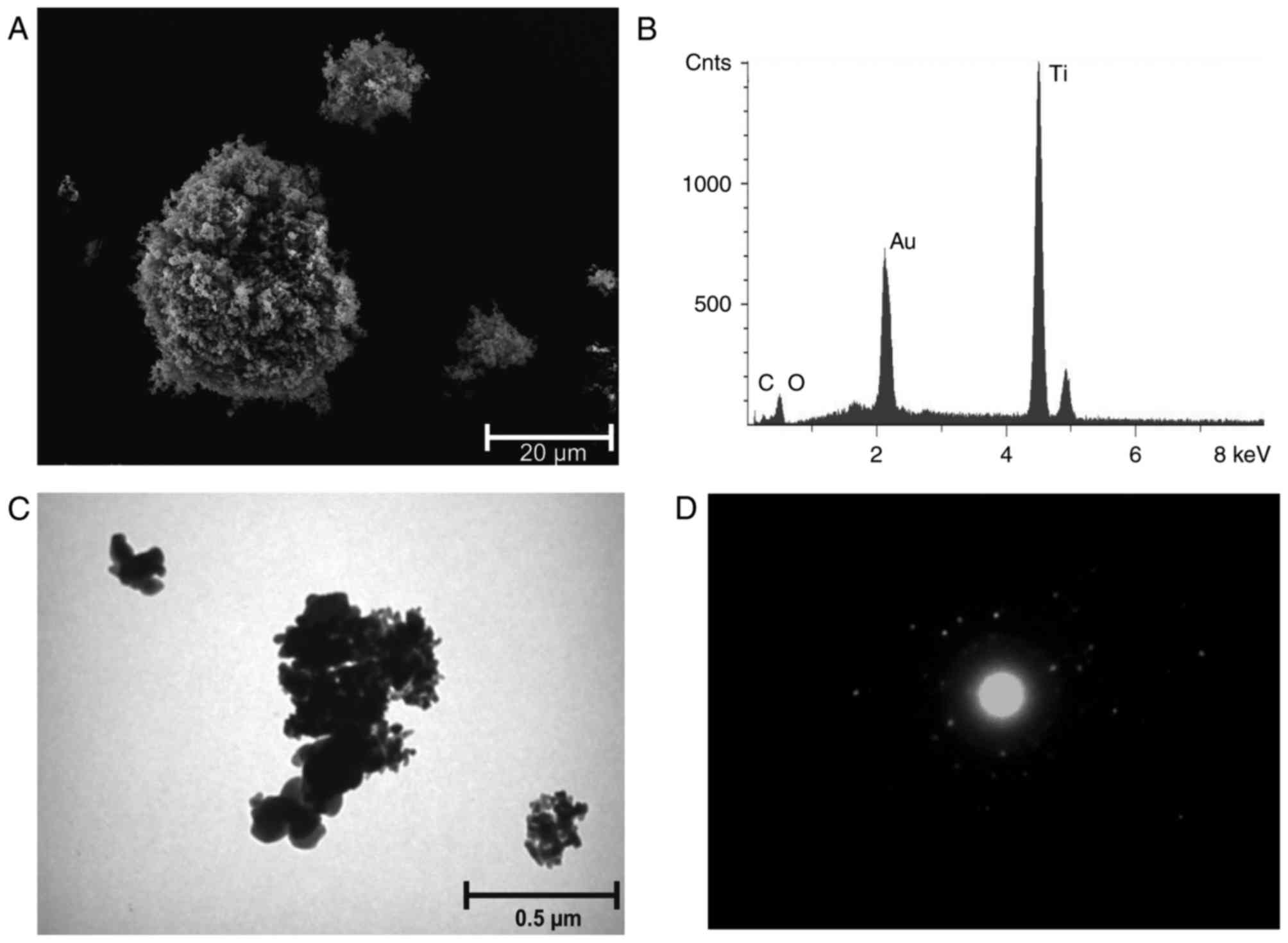
TiO2 ma is irregularly shaped and
crystalline. The microsized aggregates (diameter, 1–3 µm) were
composed of ~20 primary particles with a 100–200 nm diameter. The
specific surface area (BET) of TiO2 ma was 9.9
m2/g (35). These
details are summarised in Table
II. A detailed characterisation of TiO2 ma is also
provided in a previous manuscript (30).
 | Table II.Characterisation of granular
particles. |
Table II.
Characterisation of granular
particles.
| Particle | Size | Structure | Diameter aa,
µm | pp, nm | pp/aa | BET,
m2/g |
|---|
| TiO2
ma | Microsized | Anatase | 1–3 | 100–200 | ~20 | 9.9 |
| TiO2
na | Nanosized | Anatase | 0.1–2 | ~20 | 10–500 |
>120 |
| TiO2
nr | Nanosized | Rutile | 0.1–2 | ~20 | 10–500 | 50–150 |
| Hematite | Nanosized | NA | 0.2–2 | ~20 | 50–500 | 50–150 |
TiO2 na consisted of ~20 nm spherical
agglomerated primary particles. Agglomerates of 0.1–2 µm were
formed by 10–500 primary particles. The BET was specified as
>120 m2/g. A circular diffraction pattern was
observed for TiO2 na, indicating a lower state of
crystallisation (disorder) compared to TiO2 nr (Fig. 1 and Table II). TiO2 nr comprised
~20 nm spherical agglomerated crystalline primary particles
(Fig. 2). Agglomerates of 0.1–2 µm
were formed by 10–500 primary particles. The BET was specified with
50–150 m2/g (Table
II). Hematite is a spherical formed, nanosized material.
Agglomerates of 0.2–2 µm were formed by 50–500 primary particles
with a diameter of ~20 nm (Table
II). Additionally, smaller aggregates (<100 nm) were
detected by electron microscopy (data not shown). Hematite
crystallises in different forms, including hexagonal flats, rod
shapes and spherical forms. Due to precipitation reactions in
aqueous solutions, additional water molecules can be incorporated
into the crystal structure of hematite. The nanosized hematite used
was selected as the anhydrous primary particles were of comparable
sizes to both of the TiO2 nanoparticles used.
Furthermore, the hematite nanoparticles are able to produce ROS by
Fenton's reactions through to their iron (III) content. A detailed
characterisation of nanosized hematite is provided in a previous
manuscript (30). For the granular
particles, volatile impurities are not observed by TG (30,31).
An overview of the granular particles characteristics is presented
in Table II.
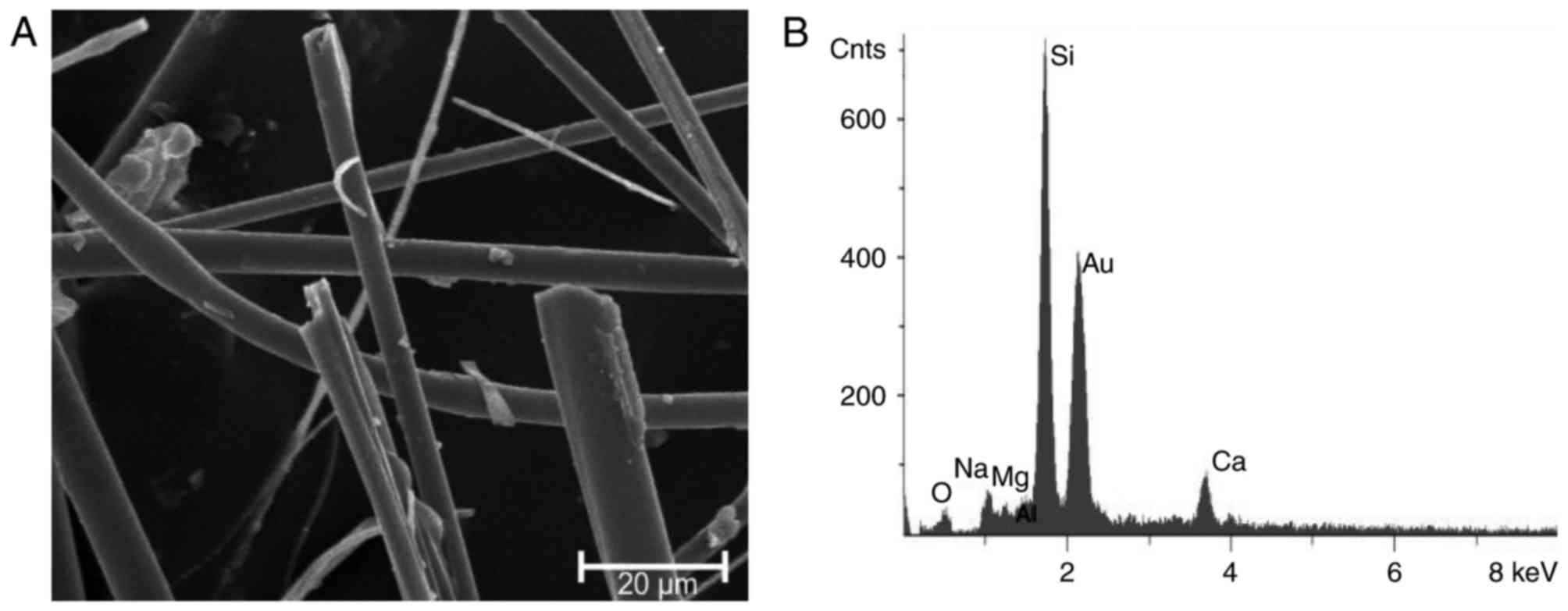
The chemical composition, 70.0% SiO2,
14.3% CaO, 9.7% Na2O, 2.5% MgO, 2.3% K2O,
1.2% Al2O3, of the glass fibres was detected
by EDX analysis (Fig. 3). The WHO
fraction [length of >5 µm and a diameter of <3 µm; length:
Diameter ration 3:1 (35)] of the
fibres resulted in an average of 260,000 F/mg. The length to
diameter ratio was >3:1 (Table
III). UICC chrysotile ‘A’ Rhodesian
[Mg6(Si4O10(OH)8)] was
demonstrated to have 800×106 F/mg at a length of >5
µm and a diameter of <3 µm. The length to diameter ratio was
>3:1 (WHO fraction; Table
III). Chrysotile has a curly, pliable structure with close to
equal Mg/Si distribution. UICC crocidolite South African
[Na2Fe3Fe2(Si8O22(OH)2)]
was demonstrated to have 130×106 F/mg at a length of
>5 µm and a diameter of <3 µm. The length to diameter ratio
was >3:1 (WHO fraction; Table
III). Crocidolite is a rigid and rod-like fibre with
characteristic iron content. A detailed characterisation of
chrysotile asbestos (UICC, Rhodesian) and crocidolite asbestos
(UICC, South African) is provided in a previous manuscript
(30). An overview of the fibrous
particles characteristics is presented in Table III.
 | Table III.Characterisation of fibrous
particles. |
Table III.
Characterisation of fibrous
particles.
| Fibre | Composition | WHO fraction,
F/mg |
|---|
| Glass fibres | 70.0%
SiO2, 14.3% CaO, 9.7% Na2O, 2.5% MgO, 2.3%
K2O, 1.2% Al2O3 | 260,000 |
| Chrysotile
asbestos |
Mg6[Si4O10(OH)8] |
800×106 |
| Crocidolite
asbestos |
Na2Fe3Fe2[Si8O22(OH)2] |
130×106 |
Dosimetry of particles (Table IV)
TiO2 ma particles (>90%) settled out
of the suspension onto the cell layer after 30 min. After 30 and
240 min, respectively, ~60 and >70% nanosized TiO2
particles (TiO2 na TiO2 nr) settled out of
the suspension onto the cell layer. For hematite particles, ~30 and
>90% settled out of the suspension onto the cell layer after 30
and 240 min, respectively. Glass fibres (~90%) settled out of the
suspension onto the cell layer after 5 min. Following 240 min
>70% of the UICC chrysotile asbestos fibres settled out of the
suspension onto the cell layer whereas following the same time
period only ~30% of the UICC crocidolite asbestos fibres settled
out of the suspension onto the cell layer.
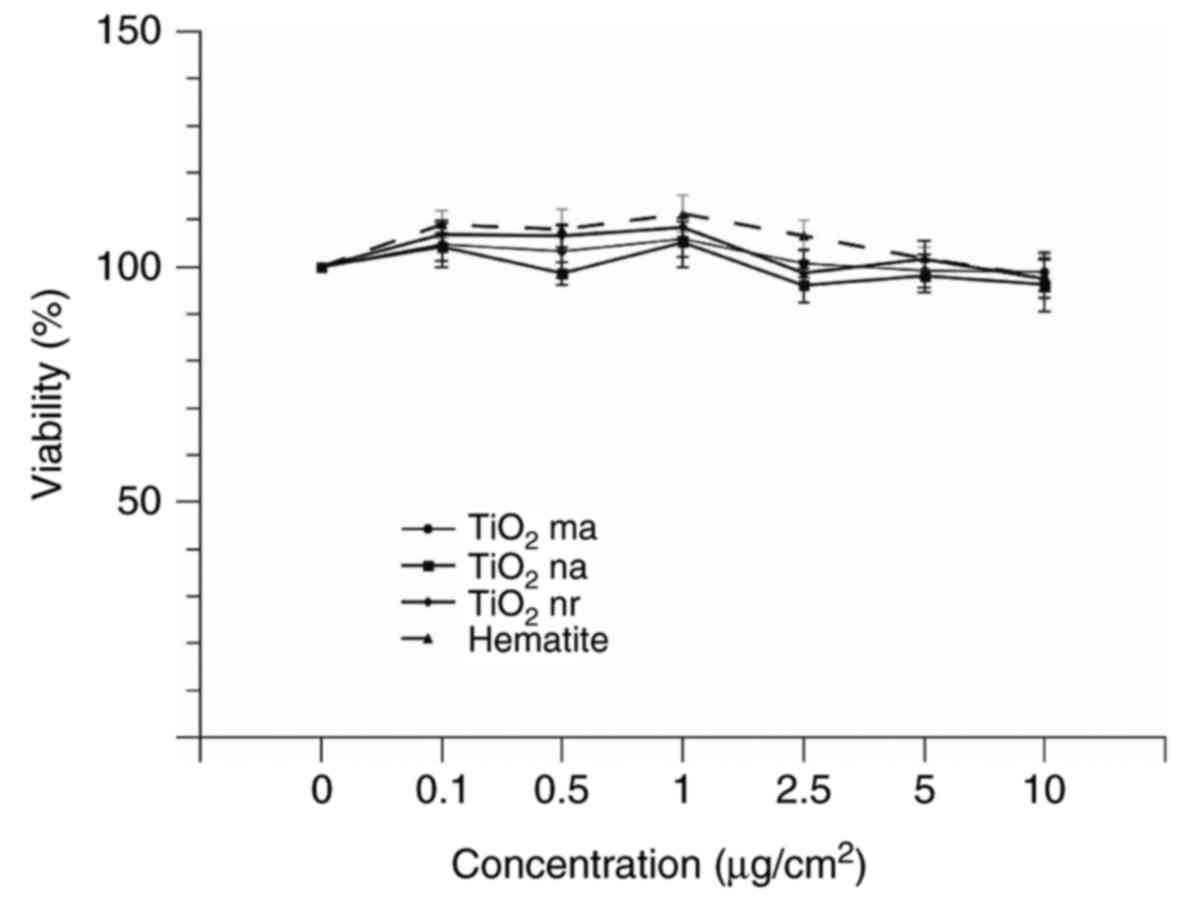
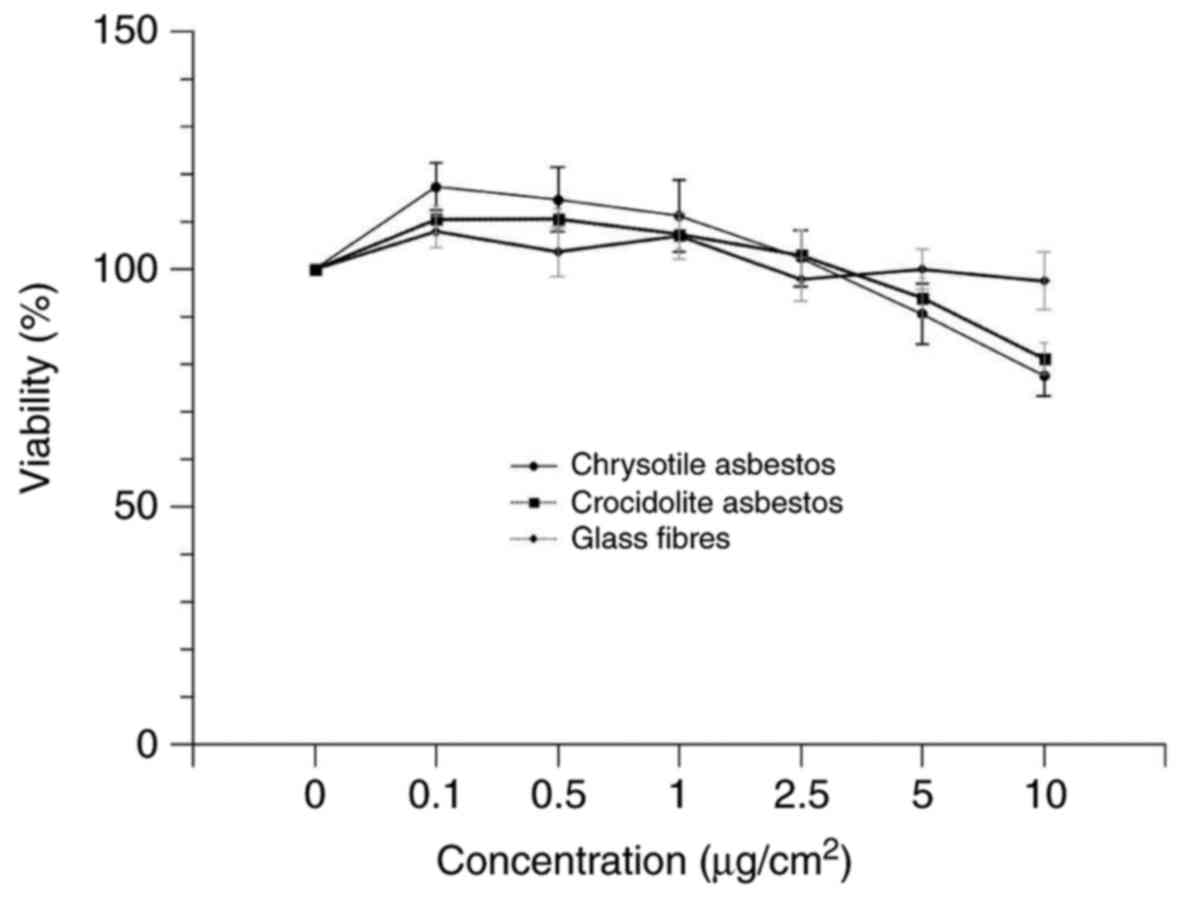
Cell viability
Cell viability was determined following the
incubation at 37°C of A549 cells with granular or fibrous particles
of varying concentrations (0.1–10 µg/cm2) over 24 h.
Loss of viability was not observed when A549 cells were exposed to
the granular particles: TiO2 ma, TiO2 nr,
TiO2 na and hematite (Fig.
4). Similar to the results for granular particles, no loss of
cell viability was observed following incubation of cells with
glass fibres. However, chrysotile and crocidolite markedly reduced
cell viability at concentrations ≥5 µg/cm2 (Fig. 5).
ROS production following exposure to
granular particles
All investigated granular particles significantly
induced ROS production in A549 lung carcinoma cells within 24 h.
Although the TiO2 species investigated in the present
study have the same chemical composition, they differ in their size
and crystal structure modification. Of the three TiO2
particles investigated, TiO2 na exhibited the greatest
ability to generate ROS at concentrations ≥1 µg/cm2 and
induced higher levels of ROS compared with hematite at
concentrations ≥5 µg/cm2. TiO2 nr particles
generated significantly higher ROS in comparison with controls but
exhibited the lowest level of ROS production of all nanosized
particles investigated (TiO2 na, TiO2 nr and
hematite). TiO2 ma particles induced high ROS production
at a concentration of 10 µg/cm2, while lower
concentrations generated ROS to a lower extent, which was
comparable with the ROS generation of fibrous particles. Exposure
to low concentrations (<1 µg/cm2) of granular
particles, with the exception of hematite, induced relatively small
increases in ROS. Above concentrations of 1 µg/cm2,
TiO2 na, with its disordered surface (demonstrated by
TEM diffraction in Fig. 1),
exhibited the greatest ability to generate ROS. The nanosized
TiO2 particles investigated (TiO2 na and
TiO2 nr) exhibit the same characteristics but differ in
their structural modification. Comparing the ROS generation in A549
cells between both nanosized TiO2 particles revealed a
statistically significant difference of P=0.02. Therefore, the
surface of the particle appears to be a modifying factor. Hematite
induced ROS in a dose-dependent manner. Potentially due to the iron
content of hematite, which induces ROS by Fenton's reaction,
significant differences were revealed in comparison to
TiO2 ma (P=0.031) and TiO2 nr (P=0.005). The
results described in this paragraph are all presented in Fig. 6.
ROS production following exposure to
fibrous particles
In the present study, all investigated fibrous
particles induced ROS production in A549 lung carcinoma cells at a
lower level compared with granular particles (Figs. 6 and 7). A potential reason for this finding
may be that fibrous particles are comparatively larger and
therefore have fewer particles and a smaller surface area. None of
the fibrous particles induced ROS levels to a level induced by
hematite even at its lowest concentrations. Chrysotile exhibited
dose-dependent ROS induction, while the ROS-generating ability of
crocidolite did not increase any further above concentrations of
0.5 µg/cm2 (Fig. 7). No
statistically significant differences in ROS generation were
observed between chrysotile and crocidolite asbestos. However,
ROS-production by crocidolite at concentrations of 0.5, 1 and 5
µg/cm2 exceeded those by chrysotile asbestos despite
lower fibre concentrations (factor 6,2) and slower sedimentation
(Fig. 7). Higher ROS production by
crocidolite may be explained by the iron content inducing ROS by
Fenton's reaction. Glass fibres did not significantly induce ROS
generation in A549 cells at any concentration compared with the
control group (Fig. 7).
Gene expression in A549 cells
following exposure to granular and fibrous particles
The mRNA expression levels of genes encoding
relevant enzymes of the GSH and the thioredoxin systems, as well as
SOD, were determined in the present study. Compared with an
unexposed control, the examined particles did not alter the mRNA
expression levels of GSH peroxidase 2 (GPX2), GSH reductase (GR),
GSH S transferase pi (GSTpi), thioredoxin 1 (TRX1), thioredoxin
reductase 1 (TRXR1), thioredoxin domain-containing 5 (TXNDC5) or
N-Myc downstream gene (NDRG1) within 24 h of exposure (data not
shown). GSH and thioredoxin systems act together with SOD1 and
SOD2. RT-qPCR results also demonstrated that SOD1 was marginally,
but not significantly, downregulated (P=0.150; data not shown),
while SOD2 was significantly upregulated (P=0.008; Fig. 8). The SOD2 gene expression,
compared with an unexposed control, was significantly upregulated
for chrysotile (P=0.001), crocidolite (P=0.030), glass fibres
(P=0.005), hematite (P=0.037) and TiO2 nr (P<0.001),
as demonstrated in Fig. 8.
Additionally, the mRNA levels of a gene involved in
apoptosis were measured. X-linked inhibitor of apoptosis (XIAP), a
gene encoding a protein that belongs to a family of apoptotic
suppressor proteins (4), was
marginally, but not significantly, downregulated (P=0.065; data not
shown).
Discussion
In the present study the ROS-generating ability of
various particles within A549 cells was investigated using the
fluorescent probe DCF. It is reported that H2-DCF may be
located within the cytosol of the cells and is oxidised by
cellular-derived ROS into the highly fluorescent form, DCF
(2). The ability of a particle to
stimulate cellular oxidant production may be determined by this
procedure, but this method cannot distinguish between the
oxidant-generating properties of the particles themselves
(acellular) and the stimulation of oxidant generation
(intracellular). H2-DCF is not prevented from migrating
out of cells and therefore may be oxidised by ‘acellular’ ROS
(2). Additionally, it has been
reported that TiO2 nanoparticles are rapidly
internalised within A549 cells (36). Jiang et al (37) demonstrated that the acellular
oxidant-generating capacity of TiO2 nanoparticles was
dependent on the crystal phase; a higher ROS activity for
TiO2 na was observed compared with TiO2 nr.
Of all investigated particles in the present study, at higher
concentrations, TiO2 na appeared to exhibit the greatest
ability to generate ROS within A459 cells. As TiO2 nr
and TiO2 na have the same size and sinking rates in
suspension, other properties may contribute to this effect.
Characterisation of the particles revealed that TiO2 na
agglomerated to the smallest units of all investigated nanosized
particles; therefore, TiO2 na is the sample with the
highest particle amount. Additionally, the crystal phase appears to
be an important factor. A circular diffraction pattern, as observed
for TiO2 na, indicated a lower state of crystallization
(disorder). Therefore, the crystalline structure of the
TiO2 na particles surface may be more disordered
compared with an ideal crystallised particle, such as
TiO2 nr. Collectively, TiO2 na has an
electronically-defective surface structure with reactive bonding
properties, that induces high ROS generation. Usually, a disordered
surface results in a higher chemical reactivity (38), which is affirmed by the results of
ROS experiments in the present study. Wang and Fan (25) reported that anatase appears to be
the most active form of TiO2, while rutile is considered
to be inert in vitro within cell culture systems. Sayes
et al (39) proposed that
the crystal phase of nanosized TiO2 particles, rather
than the surface area, is the most important parameter for
toxicity; TiO2 na particles induced an LC50
of 3,6 µg/ml, while TiO2 nr particles induced an
LC50 of 550 µg/ml. A greater toxicity of TiO2
na compared to TiO2 nr was observed within A549 cells,
as measured by lactate dehydrogenase (LDH) release and a
colorimetric assay for assessing cell metabolic activity via an MTT
assay.
Using the MTT assay, Simon-Deckers et al
(36) reported the cell death rate
after 48 h of exposure to 100 µg/ml TiO2 na
(TiO2 12 nm, 26% and TiO2 25 nm, 24%) to be
slightly more toxic compared with exposure to TiO2 nr
(TiO2 68 nm, 10%). However, an XTT assay revealed only a
low level of cytotoxicity (15% cell death after 48 h with 100
µg/ml) for all investigated nanoparticles. After 6 h of exposure,
TiO2 particles, independent of their size (12–140 nm),
were observed in the cytoplasm of the majority of cells. Theses
variations in cytotoxicity may have been associated with particle
size and potentially the internalisation pathway; smaller
TiO2 particles (12 and 25 nm) were markedly more toxic
than larger TiO2 particles (140 nm) (36).
In the present study, the results demonstrated that
exposure of A549 cells to hematite for 24 h led to the generation
of ROS in a dose-dependent manner. Of all the particles
investigated, hematite exhibited the greatest ability to induce ROS
generation at concentrations ≤1 µg/cm2 due to its iron
content, which allows the induction of ROS via Fenton's reaction.
Concerning the toxicity of hematite, there are contrasting results
in previous studies. Khan et al (40) demonstrated that iron oxide
nanoparticles with an average size between 30 and 65 nm induced ROS
generation in a time-(1–24 h) and concentration-(10–100 µg/ml)
dependent manner within A549 cells. Wottrich et al (41) demonstrated the uptake of hematite
particles with an average size of 70 nm (50–90 nm) into A549 cells,
where they formed agglomerates. The cytotoxicity of hematite
exposure (24 h), measured by LDH release and the induction of
interleukin-6 and interleukin-8 release, were affected by the
particles in a dose-dependent manner (6.1 and 121
µg/cm2). The authors concluded that particle size and
particle composition, respectively, may be responsible for the
biological effects observed (41).
However, Freyria et al (42) reported that hematite particles ≤100
µg/cm2 did not increase LDH release within 24 h in A549
cells and are therefore a poorly reactive with low toxicity. They
also observed that reducing the size from 1–2 µm to 80–100 nm did
not cause increases in the surface reactivity associated with
oxidative stress, including the production of free radicals and
cysteine depletion. All hematite particles were considered to be
non-toxic and did not induce apoptosis or DNA damage in an A549
cell model within 24 h. Furthermore, Karlson et al (43) reported that there was no or low
toxicity, as observed by trypan blue staining for iron oxide
particles (Fe2O3 and
Fe3O4, size 30–60 nm), when A549 cells were
exposed at concentrations from 20–40 µg/cm2 over 18 h.
Additionally, an increase in intracellular ROS production was not
observed after 4 h exposure to 20 and 40 µg/cm2
Fe2O3 or Fe3O4
nanoparticles. Notably, nanoscaled hematite differs by its
manufacturing process and the H2O content of its crystal
lattice (31), which may explain
the different outcomes reported.
In general, fibrous particles appear to have a
lower ability to generate ROS than granular particles within A549
cells. Herzog et al (44)
observed only a low oxidative stress response within A549 cells
following crocidolite exposure, but the incubation time of the
cells was limited to 1 h. Furthermore, Baldys et al
(45) hypothesised that the
apoptosis of A549 cells mediated by crocidolite may also require
the inactivation of important cell growth and differentiation
pathways, rather than solely being a result of oxidant production.
These findings support the results from our previous study, in
which we demonstrated that granular particles induced the
signalling pathways responsible for oxidative/metabolic stress and
inflammation, while fibrous particles also altered the signalling
pathways responsible for carcinogenesis and proliferation (30). It is clear that asbestos fibres do
induce ROS significantly in A549 cells. In the present study,
asbestos fibres appeared to induce ROS to a lower extent than
granular particles, which may be explained by fewer particles and
subsequent lower surface area in fibrous particles compared with
granular particles. The generation of ROS induced by asbestos is
considered to be a major mediator of asbestos-associated toxicity
through at least two primary mechanisms, with one being that the
iron content of the fibres catalyses ROS production at the fibre
surface and induces certain inflammatory cells, including pulmonary
alveolar macrophages and neutrophils, which release further ROS in
an attempt to remove these fibres (19,23).
As aforementioned, in the current study, intracellular levels of
ROS within A549 cells were analysed after 24 h of incubation, which
may explain the reduced levels of ROS production following exposure
to fibrous particles compared with granular particles. Fibrous
particles are comparatively larger and therefore have a lower
particle number compared with granular particles. The lower surface
area and geometrical form (needle-like) of fibres also lead to
weaker contact with the cell surface compared with granular
particles, which may also contribute to the differences
observed.
In the present study, glass fibres did not reduce
cell viability or induce ROS generation in A549 cells
significantly. Conversely, Rapisarda et al (12) reported that exposure to glass
fibres (2.1, 21 and 42 µg/cm2) over 72 h significantly
reduced cell viability, as observed in an MTT assay; increased
oxidative stress within A549 cells was also observed via DCF
fluorescence analysis. Notably, the glass fibres employed in the
present study were free of boron oxide
(B2O3), while the glass fibres used by
Rapisarda et al (12)
contained a large amount (13%) of B2O3.
Additionally, it is possible that ROS may have been induced as a
result of the longer exposure duration of 72 h, compared with the
exposure duration of 24 h applied in the present study.
It has been reported that at low levels of ROS
production, cells may initiate a protective response to ensure
their survival, while excessive levels of ROS causes damage to
cellular compounds (1,46). For the gene expression analyses in
the present study, a relatively low exposure concentration of 1
µg/cm2 for fibrous and 0.1 µg/cm2 for
granular particles was selected. The idea was to determine whether
protective alterations in RNA expression occur at low effect levels
(ROS generation). Therefore, the mRNA levels of the genes coding
for antioxidant and antiapoptotic enzymes were analysed following
exposure of A549 cells to each particle for 24 h. Investigating
antioxidant enzymes of the GSH and the thioredoxin system, as well
as the SOD enzymes, the results demonstrated that only SOD2
expression was significantly upregulated by certain particles,
while SOD1 expression was rather stable or marginally
downregulated. Therefore, SOD2 rather than SOD1 can be used as a
marker for ROS-inducing processes at low concentrations. This
finding is consistent with the consideration that SOD2 is more
sensitive to intracellular or environmental stimuli, while SOD1 is
considered to be expressed constitutively (47). Also consistent with this, Hu et
al (4) demonstrated
upregulated transcript levels of SOD2 and stable transcript levels
of SOD1 in A549 cells under resveratrol treatment for 24 h. XIAP is
a potent inhibitor of apoptosis and is able to directly inhibit the
initiation and execution of the caspase cascade (48,49).
Under high dosage of resveratrol (for 24 h), an increased mRNA
level of XIAP has been reported in A549 cells (4). This protective effect might be true
for high dosage but was not observed in the low exposure
concentration of granular and fibrous particles employed in the
presents study.
In conclusion, the ability of biopersistent
granular dust to generate ROS is due to the number of particles as
well as substance-specific properties. The crystalline surface
structure of the particles may be a factor that influences their
ability to generate ROS. Hematite induced high ROS production even
at low concentrations in the present study, which may be attributed
to the Fenton's reactions that occur due to the iron content of
hematite. As fibrous particle are comparatively larger and have
fewer particles and a lower surface area compared with granular
particles, they induce ROS at a much lower level. In the case of
crocidolite and chrysotile asbestos, the lower sinking rate under
experimental conditions should also be considered. The present
study demonstrated that particles of the same composition and size
may induce different effects in biological systems. Therefore, it
is of high importance to nvestigate and characterize these
particles in more detail further.
Acknowledgements
Certain results in this manuscript were included in
the thesis of Mrs. Julia Putzier. The authors thank Mrs. Monika
Philipp and Mrs. Daniela Schreiber (Institute and Outpatient Clinic
for Occupational and Social Medicine, Justus-Liebig University and
Physiologisches Institut, Justus-Liebig-Universität Giessen) for
their technical assistance in cell culture experiments and Dipl.
Ing. Natalia Haibel, Dipl. Ing. Rolf Arhelger and Dipl. Ing. Bernd
Brückel (Institute and Outpatient Clinic for Occupational and
Social Medicine, Justus-Liebig University, Giessen) for their work
in particle characterisation.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
SH provided the conception and administrative
support, provision of study materials, acquisition, data analysis
and interpretation of data and write the manuscript. SW provided
the conception and design, support in ROS analysis and drafted the
manuscript. JP provided the acquisition, collection and assembly of
data and interpretation of data. DW provided the conception,
acquisition, and characterisation of dust material and data
interpretation. HM provided the conception and design, and drafted
the manuscript. JS provided the conception and design, data
analysis and interpretation, critically revised and partially wrote
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Roesslein M, Hirsch C, Kaiser JP, Krug HF
and Wick P: Comparability of in vitro tests for bioactive
nanoparticles: A common assay to detect reactive oxygen species as
an example. Int J Mol Sci. 14:24320–24337. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Held P: An Introduction to Reactive Oxygen
Species. Measurement of ROS in CellsWhite Paper. BioTek
Instruments, Inc.; Winooski, VT: 2015
|
|
3
|
Nel A, Xia T, Mädler L and Li N: Toxic
potential of materials at the nanolevel. Science. 311:622–627.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu Y, Rahlfs S, Mersch-Sundermann V and
Becker K: Resveratrol modulates mRNA transcripts of genes related
to redox metabolism and cell proliferation in non-small-cell lung
carcinoma cells. Biol Chem. 388:207–219. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu J and Holmgren A: The thioredoxin
antioxidant system. Free Radic Biol Med. 66:75–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Becker K, Gromer S, Schirmer RH and Müller
S: Thioredoxin reductase as a pathophysiological factor and drug
target. Eur J Biochem. 267:6118–6125. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiao GG, Wang M, Li N, Loo JA and Nel AE:
Use of proteomics to demonstrate a hierarchical oxidative stress
response to diesel exhaust particle chemicals in a macrophage cell
line. J Biol Chem. 278:50781–50790. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oberdörster G, Oberdörster E and
Oberdörster J: Nanotoxicology: An emerging discipline evolving from
studies of ultrafine particles. Environ Health Perspect.
113:823–839. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Phaniendra A, Jestadi DB and Periyasamy L:
Free radicals: Properties, sources, targets, and their implication
in various diseases. Indian J Clin Biochem. 30:11–26. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Murphy MP, Holmgren A, Larsson NG,
Halliwell B, Chang CJ, Kalyanaraman B, Rhee SG, Thornalley PJ,
Partridge L, Gems D, et al: Unraveling the biological roles of
reactive oxygen species. Cell Metab. 13:361–366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Federico A, Morgillo F, Tuccillo C,
Ciardiello F and Loguercio C: Chronic inflammation and oxidative
stress in human carcinogenesis. Int J Cancer. 121:2381–2386. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rapisarda V, Loreto C, Ledda C, Musumeci
G, Bracci M, Santarelli L, Renis M, Ferrante M and Cardile V:
Cytotoxicity, oxidative stress and genotoxicity induced by glass
fibers on human alveolar epithelial cell line A549. Toxicol In
Vitro. 29:551–557. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Knaapen AM, Borm PJ, Albrecht C and Schins
RP: Inhaled particles and lung cancer. Part A: Mechanisms. Int J
Cancer. 109:799–809. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
DFG: List of MAK and BAT valuesList of MAK
and BAT Values 2016: Permanent Senate Commission for the
Investigation of Health Hazards of Chemical Compounds in the Work
Area. Report No. 52. Wiley-VCH; Weinheim: 2016
|
|
15
|
Walter D: Primary
particles-Agglomerates-Aggregates. In: NanomaterialsDeutsche
Forschungsgemeinschaft (DFG) (ed). Wiley-VCH Verlag GmbH & Co.
KGaA.; Weinheim: pp. 9–24. 2013
|
|
16
|
Stone V, Johnston H and Clift MJ: Air
pollution, ultrafine and nanoparticle toxicology: Cellular and
molecular interactions. IEEE Trans Nanobioscience. 6:331–340. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oberdörster G: Pulmonary effects of
inhaled ultrafine particles. Int Arch Occup Environ Health. 74:1–8.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mossman BT and Churg A: Mechanisms in the
pathogenesis of asbestosis and silicosis. Am J Respir Crit Care
Med. 157:1666–1680. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kamp DW: Asbestos-induced lung diseases:
An update. Transl Res. 153:143–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shukla A, MacPherson MB, Hillegass J,
Ramos-Nino ME, Alexeeva V, Vacek PM, Bond JP, Pass HI, Steele C and
Mossman BT: Alterations in gene expression in human mesothelial
cells correlate with mineral pathogenicity. Am J Respir Cell Mol
Biol. 41:114–123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Robledo R and Mossman B: Cellular and
molecular mechanisms of asbestos-induced fibrosis. J Cell Physiol.
180:158–166. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lemaire I and Ouellet S: Distinctive
profile of alveolar macrophage-derived cytokine release induced by
fibrogenic and nonfibrogenic mineral dusts. J Toxicol Environ
Health. 47:465–478. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kamp DW and Weitzman SA: The molecular
basis of asbestos induced lung injury. Thorax. 54:638–652. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Riedel E: Allgemeine und Anorganische
Chemie. Walter de Gruyter; Berlin, New York: 1999
|
|
25
|
Wang J and Fan Y: Lung injury induced by
TiO2 nanoparticles depends on their structural features: Size,
shape, crystal phases, and surface coating. Int J Mol Sci.
15:22258–22278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liochev SL: The role of iron-sulfur
clusters in in vivo hydroxyl radical production. Free Radic Res.
25:369–384. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liochev SI and Fridovich I: The relative
importance of HO* and ONOO-in mediating the toxicity of O*-. Free
Radic Biol Med. 26:777–778. 1999.PubMed/NCBI
|
|
28
|
Liochev SI and Fridovich I: The
Haber-Weiss cycle-70 years later: An alternative view. Redox Rep.
7(55–57): 59–60. 2002.
|
|
29
|
Schneider J, Walter D, Brückel B and
Rödelsperger K: Primary particles and their agglomerate formation
as modifying risk factors of nonfibrous nanosized dust. J Toxicol
Environ Health A. 76:131–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Helmig S, Dopp E, Wenzel S, Walter D and
Schneider J: Induction of altered mRNA expression profiles caused
by fibrous and granular dust. Mol Med Rep. 9:217–228. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Walter D: Characterization of synthetic
hydrous hematite pigments. Thermochimica Acta. 445:195–199. 2006.
View Article : Google Scholar
|
|
32
|
Rhodes JM: Introduction to Patricle
Technology. 2nd edition. John Wiley & Sons Ltd.; Chichester:
2008, View Article : Google Scholar
|
|
33
|
Helmig S, Hadzaad B, Döhrel J and
Schneider J: Relative quantification of Cytochrome P450 1B1 gene
expression in peripheral leukocytes using lightcycler. Cancer
Genomics Proteomics. 6:13–17. 2009.PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rödelsperger K, Brückel B, Podhorsky S and
Schneider J: Charakterisierung von ultrafeinen Partikeln für den
Arbeitsschutz-Teil 2. Bundesanstalt für Arbeitsschutz und
Arbeitsmedizin, Dortmund/Berlin/Dresden. 2009.https://www.baua.de/DE/Angebote/Publikationen/Berichte/F2075.pdf?__blob=publicationFile
|
|
36
|
Simon-Deckers A, Gouget B, Mayne-L'hermite
M, Herlin-Boime N, Reynaud C and Carrière M: In vitro investigation
of oxide nanoparticle and carbon nanotube toxicity and
intracellular accumulation in A549 human pneumocytes. Toxicology.
253:137–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang J, Oberdörster G, Elder A, Gelein R,
Mercer P and Biswas P: Does nanoparticle activity depend upon size
and crystal phase? Nanotoxicology. 2:33–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ertl G and Knözinger H: Handbook of
Heterogeneous Catalysis. Wiley-VCH; Weinheim: 1997, View Article : Google Scholar
|
|
39
|
Sayes CM, Wahi R, Kurian PA, et al:
Correlating nanoscale titania structure with toxicity: a
cytotoxicity and inflammatory response study with human dermal
fibroblasts and human lung epithelial cells. Toxicol Sci.
92:174–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Khan MI, Mohammad A, Patil G, Naqvi SA,
Chauhan LK and Ahmad I: Induction of ROS, mitochondrial damage and
autophagy in lung epithelial cancer cells by iron oxide
nanoparticles. Biomaterials. 33:1477–1488. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wottrich R, Diabaté S and Krug HF:
Biological effects of ultrafine model particles in human
macrophages and epithelial cells in mono- and co-culture. Int J Hyg
Environ Health. 207:353–361. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Freyria FS, Bonelli B, Tomatis M, et al:
Hematite nanoparticles larger than 90 nm show no sign of toxicity
in terms of lactate dehydrogenase release, nitric oxide generation,
apoptosis, and comet assay in murine alveolar macrophages and human
lung epithelial cells. Chem Res Toxicol. 25:850–861. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Karlsson HL, Cronholm P, Gustafsson J and
Moller L: Copper oxide nanoparticles are highly toxic: a comparison
between metal oxide nanoparticles and carbon nanotubes. Chem Res
Toxicol. 21:1726–1732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Herzog E, Byrne HJ, Davoren M, Casey A,
Duschl A and Oostingh GJ: Dispersion medium modulates oxidative
stress response of human lung epithelial cells upon exposure to
carbon nanomaterial samples. Toxicol Appl Pharmacol. 236:276–281.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Baldys A, Pande P, Mosleh T, Park SH and
Aust AE: Apoptosis induced by crocidolite asbestos in human lung
epithelial cells involves inactivation of Akt and MAPK pathways.
Apoptosis. 12:433–447. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Otsuki T, Maeda M, Murakami S, Hayashi H,
Miura Y, Kusaka M, Nakano T, Fukuoka K, Kishimoto T, Hyodoh F, et
al: Immunological effects of silica and asbestos. Cell Mol Immunol.
4:261–268. 2007.PubMed/NCBI
|
|
47
|
Zelko IN, Mariani TJ and Folz RJ:
Superoxide dismutase multigene family: A comparison of the CuZn-SOD
(SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures,
evolution, and expression. Free Radic Biol Med. 33:337–349. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Eckelman BP, Salvesen GS and Scott FL:
Human inhibitor of apoptosis proteins: Why XIAP is the black sheep
of the family. EMBO Rep. 7:988–994. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Caballero-López MJ, Nieto-Diaz M, Yunta M,
Reigada D, Muñoz-Galdeano T, Del Águila Á, Navarro-Ruíz R,
Pita-Thomas W, Lindholm D and Maza RM: XIAP interacts with and
regulates the activity of FAF1. Biochim Biophys Acta.
1864:1335–1348. 2017. View Article : Google Scholar
|