Introduction
Osteoarthritis (OA) is a disease of the joints; it
is the most common form of arthritis and is caused by the breakdown
of joint cartilage and underlying bone (1). In 2004, OA led to moderate-severe
disability in 43.4 million people worldwide (2). In 2010, 3.8% of the global population
were affected by OA (3,4).
OA affects work and normal daily activities, with
joint pain and stiffness being the most frequent symptoms. Abnormal
development of a joint or limb, inherited factors and previous
joint injury may result in OA, and the risk of developing OA is
greater in people who are overweight, have different leg lengths or
have jobs with high joint stress (5,6). OA
develops as cartilage is lost and the underlying bone is affected
(5). Balanced water content in
healthy cartilage is maintained by the compressive force, which
drives water out, and hydrostatic/osmotic pressures that draw water
in (7,8). Collagen fibres exhibit a compressive
force pushing water out which is balanced by the Gibbs-Donnan
effect and by cartilage proteoglycans that generate osmotic
pressure to draw water in (8).
However, during the progression of OA, the collagen matrix is
disorganized and the breakdown of collagen fibers lead to an
overall increase in water content (9).
SMAD family member 2 (SMAD2) is a protein that
serves a crucial role in transmitting the extracellular signals
from ligands of the transforming growth factor β (TGF-β)
superfamily into cell nucleus, which leads to the modulation of a
number of cellular processes, including cell proliferation,
apoptosis and differentiation (10,11).
In an OA mouse model, SMAD2 was demonstrated to be
hyperphosphorylated, which implied that its activation may be
connected to the development of OA (12). SMAD2 expression was also reported
to be decreased in OA tissues (13).
MicroRNAs (miRNAs) are small non-coding RNA
molecules that function in the post-transcriptional regulation of
gene expression (14,15). It is unknown whether there are
miRNAs that could target SMAD2 and serve a role in the treatment of
OA.
The present study aimed to investigate the
expression and role miR-486-5p in osteoarthritis, and demonstrated
that miR-486-5p was upregulated in OA. Furthermore, miR-486-5p may
inhibit chondrocyte proliferation and migration by targeting SMAD2,
indicating the crucial role of miR-486-5p in OA. Therefore,
miR-486-5p maybe a potential target in the treatment of OA.
Materials and methods
Patients
Articular cartilage tissues from 25 OA patients
undergoing total knee replacement surgery (male, 14; female, 11;
age 62.4±6.2 years) were considered to be the experimental group
and knee articular cartilage tissues from 25 non-OA patients (male,
16; female, 11; age 60.1±7.2 years) with femoral neck fracture who
had no known history of OA or rheumatoid arthritis served as the
control. All samples were collected at the Chinese People's
Liberation Army No. 149 Hospital from March 2015 to August 2016.
The present study was approved by the Ethics committee of
Lianyungang Oriental Hospital (Lianyungang, China). All of the
participants in the present study provided written informed consent
to the ethics committees of the Chinese People's Liberation Army
No. 149 Hospital (Lianyungang, China).
Cell culture
CHON-001 human chondrocyte cells were cultured in
Dulbecco's modified Eagle's medium/nutrient mixture F12 (DMEM/F12;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
supplemented with antibiotic and antimycotic agents (10,000
units/ml penicillin, 10,000 µg/ml streptomycin and 25 µg/ml
Fungizone; Gibco; Thermo Fisher Scientific, Inc.) in standard
culture conditions (37°C, 5% CO2 and 95% humidity).
Cell transfection
miR-486-5p mimics (50 nM; cat. no. miR10003130-1-5),
miR-486-5p inhibitor (100 nM; cat. no. miR20003130-1-5), the
negative control of miR-486-5p (NC, 50 nM; cat no. miR01101-1-5),
control siRNA (si-Control), and SMAD2 siRNA were purchased from
Guangzhou RiboBio Co., Ltd. (Guangzhou, China). To perform CHON-001
cell transfection, Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) was used according to the
manufacturer's protocol. Transfection efficiency was determined
using reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) as described below. The cells were then incubated for 24
h at 37°C and 5% CO2 until further analysis.
MTT assay
CHON-001 cells (5×103 cells/well) were
grown in 96-well plates. A total of 24 h following transfection
with either miR-486-5p mimics, miR-486-5p inhibitor or NC at 37°C.
Subsequently, MTT solution (20 µl) was added into each well at 24,
48 and 72 h, and the plates were incubated at 37°C for an
additional 4 h. The MTT solution was aspirated and dimethyl
sulfoxide (150 µl) was added prior to assessment of absorbance at
A570 nm; the value represents the cell proliferation of each well
examined.
Transwell migration assay
The migratory abilities of CHON-001 cells were
tested using 24-well Transwell chambers, with upper and lower
culture compartments separated by polycarbonate membranes. Cells
(2×104) were suspended in 100 µl serum-free DMEM and
seeded into the top chambers. The bottom chambers contained 0.5 ml
DMEM with 10% fetal bovine serum (FBS, Thermo Fisher Scientific,
Inc.). Following sub-culturing for ~24 h, cells on the upper
surface of membrane were removed by cotton tips. Cells that
migrated to the lower surface were fixed with 10% formalin for 30
min at room temperature. Migratory abilities were evaluated by
counting the number of cells stained with hematoxylin and eosin for
15 min at 37°C cells using an inverted microscope (magnification,
×200; Olympus Corporation, Japan).
Dual-luciferase assay
TargetScan bioinformatics software (TargetScanHuman
7.1; www.targetscan.org/vert_71) was used to predict the
target genes of miR-486-5p, which indicated that SMAD2 is potential
target of miR-486-5p. To verify that miR-486-5p directly targets
the 3′-untranslated region (UTR) of SMAD2, the vectors
SMAD2-3′UTR-wild type (WT,
GAGCTCTCCCAAAGGTTTATTAATAACAGTAGTAGTTATGTGTACAGGTAATGTATCATCTCGAG)
and SMAD2-3′UTR-mutant (MUT,
GAGCTCTCCCAAAGGTTTATTAATAACAGTAGTAGTTATGTCATGTCCTAATGTATCATCTCGAG)
of pMIR-REPORT (GeneCopoeia, Inc., Rockville, MD, USA), which
respectively contained the wild-type and mutated 3′UTR of SMAD2
mRNA, were established. For the luciferase reporter assay, CHON-001
cells (5×104 cells/well) were seeded into 24-well
plates. CHON-001 cells were co-transfected with either
SMAD2-3′UTR-WT or SMAD2-3′UTR-MUT and either miR-486-5p mimics or
miR-negative control using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 48 h.
Then, cells were collected and the luciferase activity was analyzed
using the Dual-Luciferase Reporter Assay system (Promega
Corporation, Madison, WI, USA) according to the manufacturer's
protocols. Renilla luciferase activity was normalized to
firefly luciferase activity.
Total RNA isolation and RT-qPCR
Micro-dismembrator was applied to homogenize
deep-frozen articular cartilage tissues at 4°C for 1 min at a speed
of 1,500 RPM. Total RNA was isolated using an RNeasy Mini kit
(Qiagen, Inc., Valencia, CA, USA) according to manufacturer's
protocol. cDNA was synthesized by reverse transcription using the
TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) and was used in the qPCR reactions
according to the manufacturer's protocol. Amplification conditions
for qPCR were as follows: 95°C for 5 min, followed by 40 cycles of
denaturation at 95°C for 15 sec and annealing/elongation at 60°C
for 30 sec. GAPDH was used as a reference gene. The
2−ΔΔCq method was used to calculate the relative
quantities of each gene (16).
Primer sequences are listed in Table
I.
 | Table I.Primer sequences for reverse
transcription-qPCR. |
Table I.
Primer sequences for reverse
transcription-qPCR.
| Gene | Primer sequence
(5′→3′) |
|---|
| SMAD2 | F:
ATGTCGTCCATCTTGCCATTC |
|
| R:
AACCGTCCTGTTTTCTTTAGCTT |
| Type II collagen | F:
CCCTGAGTGGAAGAGTGGAG |
|
| R:
GAGGCGTGAGGTCTTCTGTG |
| Aggrecan | F:
CTAGAGATCAGTGGACTGCCT |
|
| R:
TCTGGAGCTGTGCAGTCTAGTGG |
| miR-486-5p | F:
CTCGCTTCGGCAGCACA |
|
| F:
ACGCTTCACGAATTTGCGT |
| U6 | F: Universal_ RNU6B
_ Primer |
|
| R: Uni-miR qPCR
Primer |
| GAPDH | F:
CTTTGGTATCGTGGAAGGACTC |
|
| R:
GTAGAGGCAGGGATGATGTTCT |
Western blot analysis
Logarithmic growth phase cells (90% confluence) were
harvested and lysed by radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China). The
concentration of protein was detected by BCA assay (Thermo Fisher
Scientific, Inc.) per as the manufacturer's protocol. Protein
lysates (25 µg per lane) were separated by 12% SDS-PAGE,
transferred to polyvinylidene difluoride (PVDF) membranes.
Following blocking with 5% skimmed milk for 2 h at room
temperature, the membranes were incubated with primary antibodies
against SMAD2 (1:1,000; cat. no. 5339; Cell Signaling Technology,
Inc., Danvers, MA, USA), type II collagen (1:1,000; cat. no.
ab34712), aggrecan (1:1,000; cat. no. ab36861; both Abcam,
Cambridge, UK) and β-actin (1:2,000; cat. no. 4970; Cell Signaling
Technology, Inc.) at 4°C overnight. Subsequently, the PVDF
membranes were washed 3 times with TBS containing 0.1% Tween-20 and
incubated with Anti-rabbit IgG, HRP-linked Antibody (1:5,000; cat
no. 7074; Cell Signaling Technology, Inc.) at room temperature for
~1 h. Blots were developed with SuperSignal West Femto Maximum
Sensitivity Substrate (Pierce; Thermo Fisher Scientific, Inc.), and
the ChemiDoc XRS+ system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) was used for protein band observation according to the
manufacturer's protocols. Data were analyzed by densitometry using
Image Pro Plus v.6.0 software (Media Cybernetics, Inc., Rockville,
MD, USA). β-actin served as an internal control for all
experiments.
Statistical analysis
All the experiments were performed at least three
times. Data are presented as the mean ± standard deviation. One-way
analysis of variance with Tukey's post hoc test was used to
evaluate the effects of different treatments. Statistical analysis
was performed with SPSS software version 20.0 (IBM Corp., Armonk,
NY, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
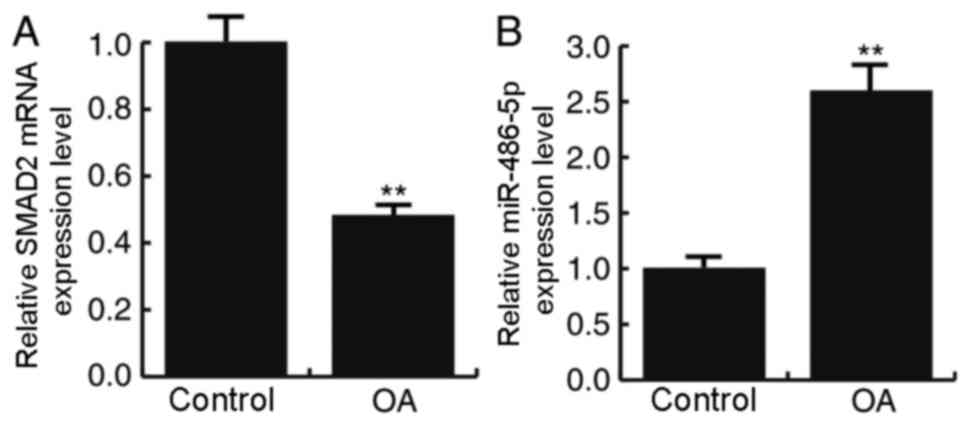
Patients with OA exhibit lower SMAD2
mRNA expression and higher miR-486-5p expression
mRNA expression levels of SMAD2 were examined by
RT-qPCR in the articular cartilage tissues of patients with and
without OA. The results demonstrated that SMAD2 expression was
significantly decreased in patients with OA compared with the
control patients without OA (P<0.01; Fig. 1A). The expression levels of
miR-486-5p in the two articular cartilage tissues were also
examined by RT-qPCR, and the results demonstrated a significantly
increased expression level of miR-486-5p in patients with OA
compared with the control patients (P<0.01; Fig. 1B).
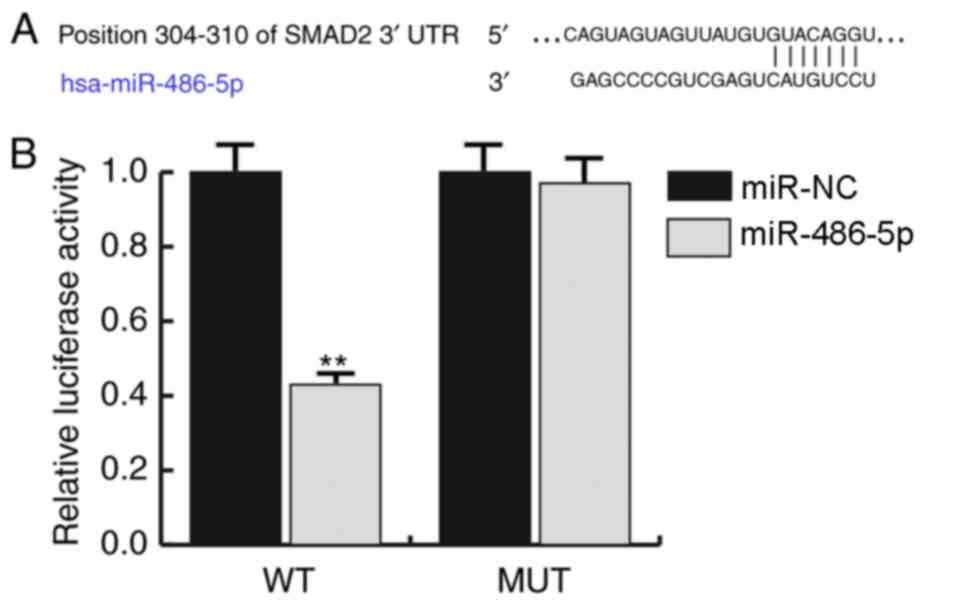
SMAD2 is a direct target of
miR-486-5p
TargetScan was used to predict miRNAs that may
target SMAD2 mRNA, and miR-486-5p exhibited a relatively high score
and was indicated to bind to the 3′-UTR of SMAD2 (Fig. 2A). SMAD2 was subsequently confirmed
as a direct target of miRNA-486-5p by dual-luciferase reporter
assay, which demonstrated that the relative luciferase activity was
significantly decreased in the SMAD2-3′UTR-WT group transfected
with miR-486-5p mimics compared with those cells co-transfected
with SMAD2-3′UTR-WT and miR-NC (P<0.01; Fig. 2B).
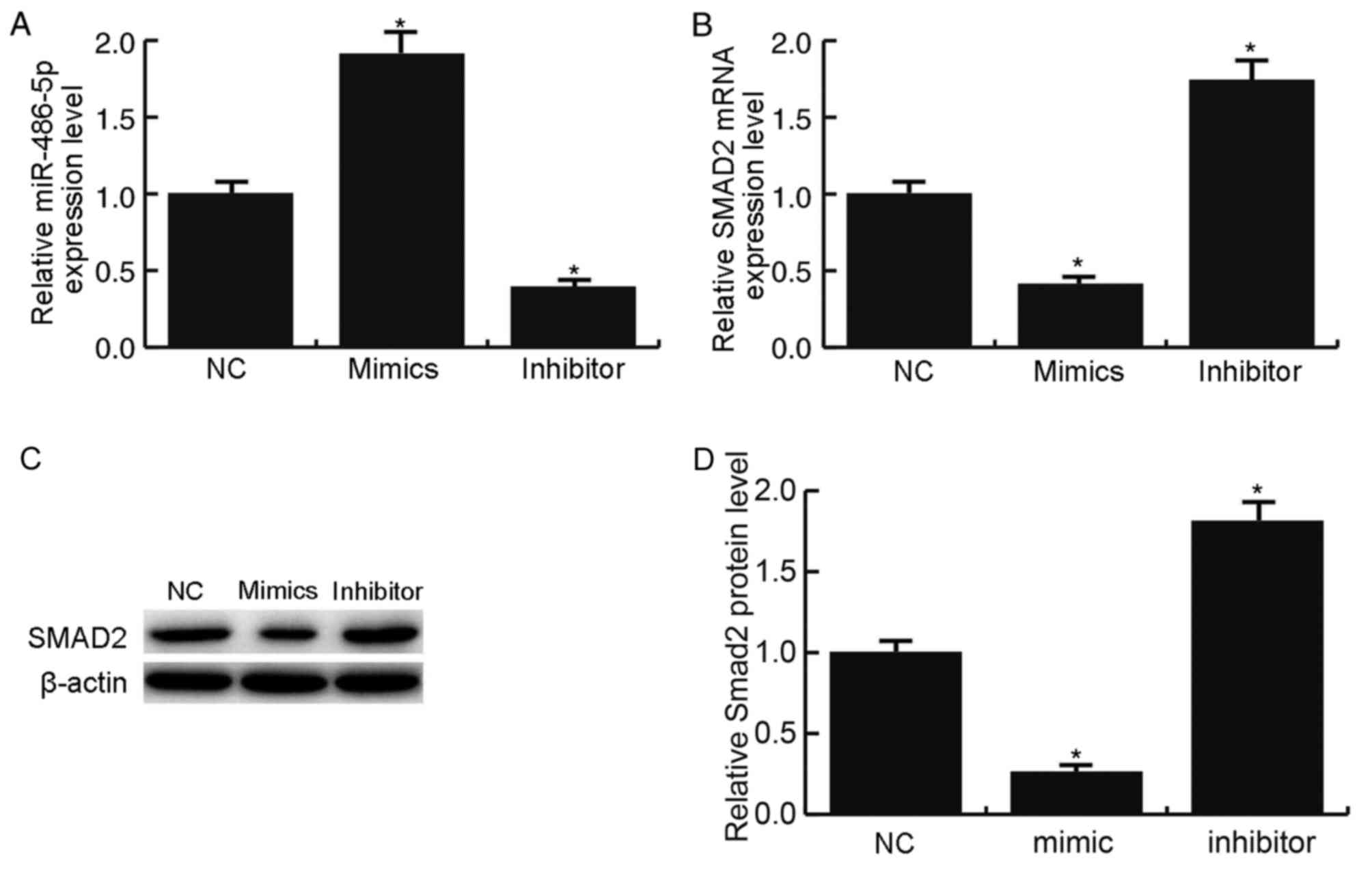
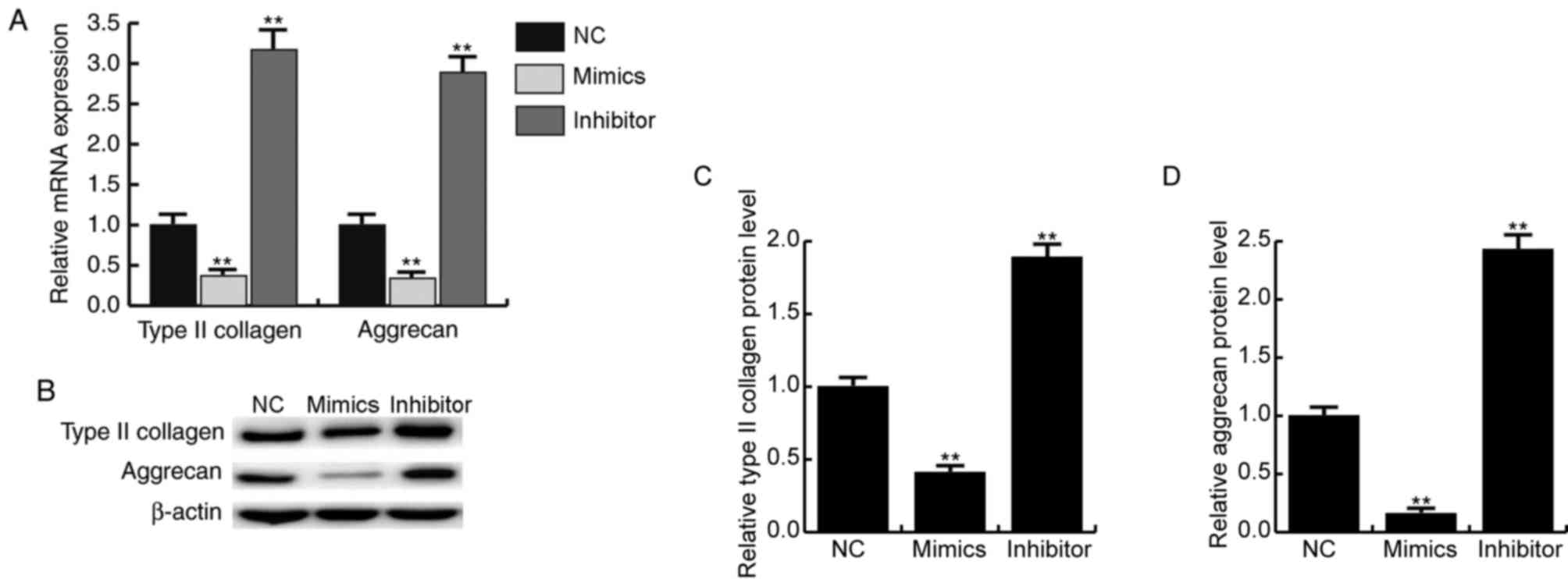
miR-486-5p mimics inhibit CHON-001
cell proliferation and migration and the expression of type II
collagen and aggrecan
To investigate the role of miR-486-5p in the
development of OA, CHON-001 cells were transfected with miR-486-5p
mimics or miR-486-5p inhibitor. miR-486-5p expression was
significantly enhanced by treatment with miR-486-5p mimics and
significantly inhibited by miR-486-5p inhibitor treatment
(P<0.05 vs. NC; Fig. 3A). As
expected, the miR-486-5p mimics significantly suppressed the
expression level of SMAD2 mRNA (P<0.05; Fig. 3B) and notably reduced SMAD2 protein
expression (Fig. 3C and D)
compared with NC group. Conversely, treatment with the miR-486-5p
inhibitor resulted in the opposite effects on SMAD2 mRNA and
protein expression levels (P<0.05; Fig. 3B-D).
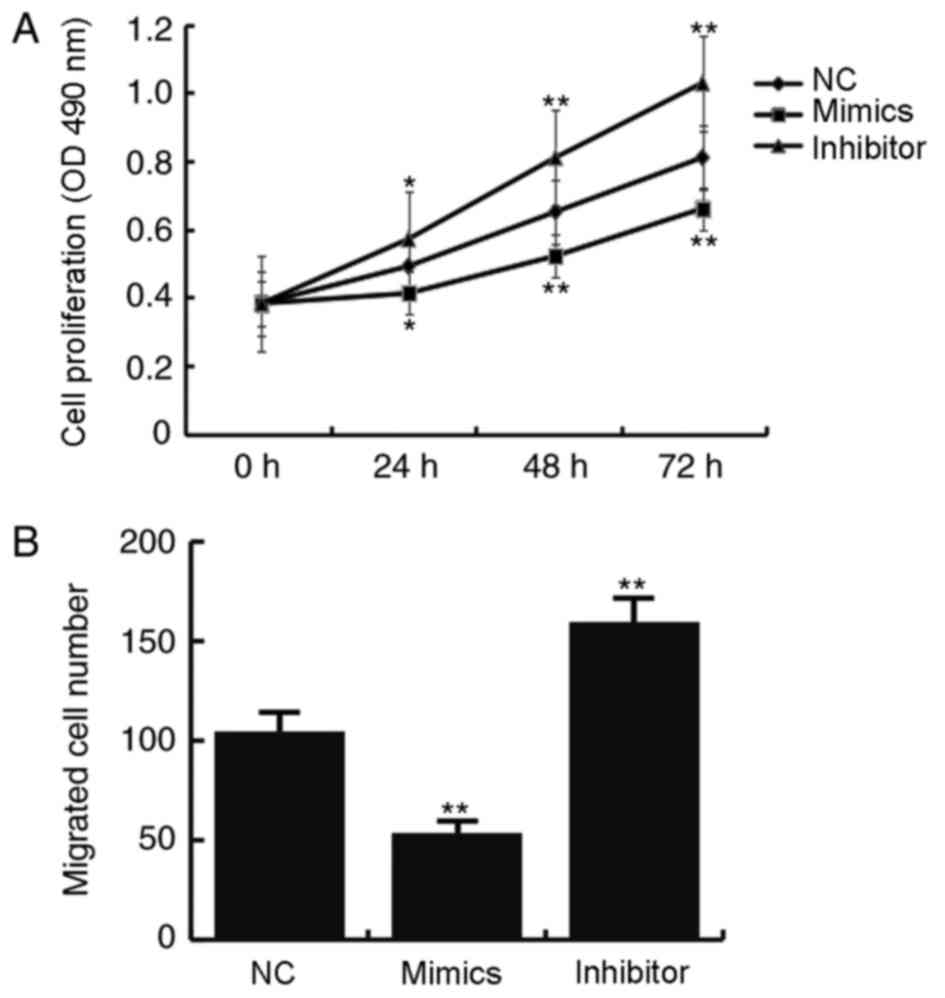
The effects of miR-486-5p on CHON-001 cell
proliferation and migration were determined by MTT and Transwell
migration assays, respectively. Compared with the miR-NC
transfected cells, the proliferative ability of cells transfected
with miR-486-5p mimics was significantly inhibited (P<0.05;
Fig. 4A). In addition, number of
migrated cells in the miR-486-5p mimics transfected group was
decreased compared with the miR-NC group, which indicated lower
migratory ability following transfection with miR-486-5p mimics
(Fig. 4B).
The expression of type II collagen and aggrecan are
important for the development of articular cartilage tissue.
RT-qPCR results demonstrated that the mRNA expression levels of
type II collagen and aggrecan were significantly decreased in
response to miR-486-5p mimics transfection compared with cells in
the miR-NC transfected group (P<0.01; Fig. 5A). Results from western blotting
also indicated that miR-486-5p mimics transfection notably reduced
the protein expression levels of type II collagen and aggrecan
(Fig. 5B-D).
miR-486-5p inhibitor promotes CHON-001
cell proliferation and migration and the expression of type II
collagen and aggrecan
To further confirm the role of miR-486-5p in OA
development, a miR-486-5p inhibitor was transfected into CHON-001
cells. Compared with the NC, cells transfected with miR-486-5p
inhibitor exhibited increased proliferative and migratory
capability (Fig. 4A and B,
respectively). In addition, the mRNA expression levels of type II
collagen and aggrecan were significantly increased in response to
miR-486-5p inhibitor treatment (P<0.01; Fig. 5A). Furthermore, the protein
expression levels of type II collagen and aggrecan significantly
increased following miR-486-5p inhibitor treatment (P<0.01;
Fig. 5B-D).
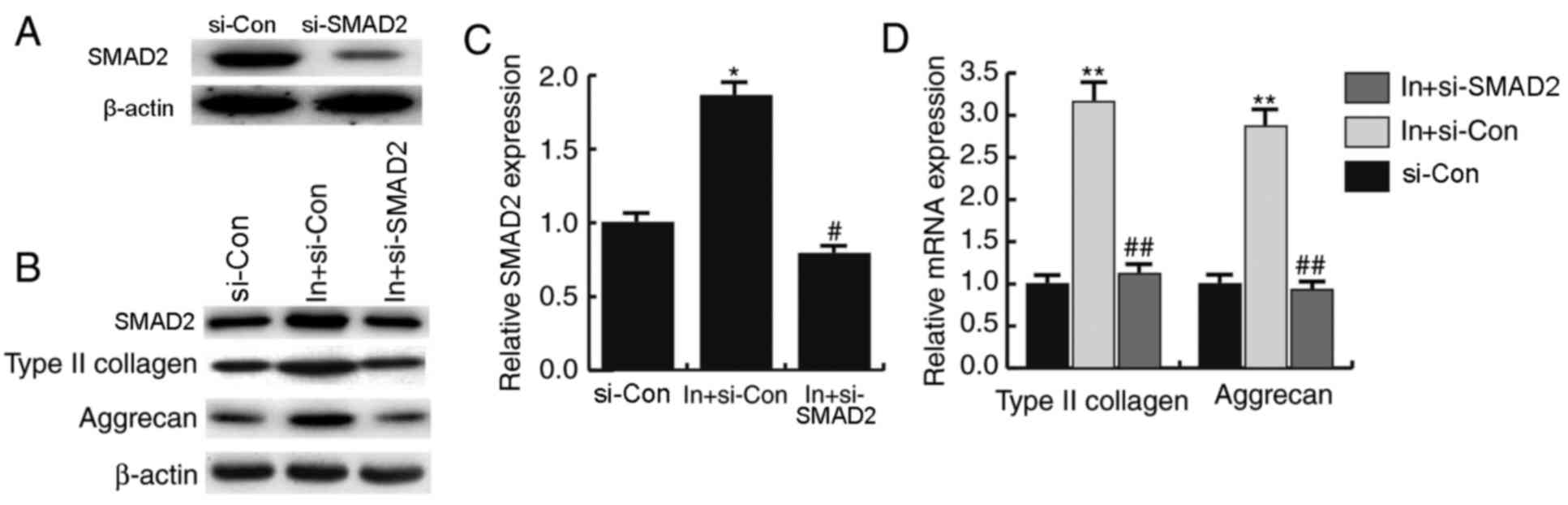
Silencing of SMAD2 reverses the
miR-486-5p inhibitor-induced upregulation of CHON-001 cell
proliferation and migration, and the expression levels of type II
collagen and aggrecan
SMAD2 serves a key role in OA development. To
determine if miRNA-486-5p affected chondrocytes through SMAD2,
Small interfering (si)RNAs were used to silence SMAD2 expression in
CHON-001 cells. Western blotting demonstrated that SMAD2 protein
expression level was reduced by SMAD2 siRNA transfection compared
with si-Control-treated cells (Fig.
6A). Western blotting and RT-qPCR results demonstrated that,
compared with cells transfected with miRNA-486-5p inhibitor +
si-Con, SMAD2 protein and mRNA expression levels were decreased in
cells transfected with miRNA-486-5p inhibitor + si-SMAD2
(P<0.05; Fig. 6B and C). In
addition, the miRNA-486-5p inhibitor-induced increase of type II
collagen and aggrecan expression levels were reversed by si-SMAD2
co-transfection (Fig. 6B and D).
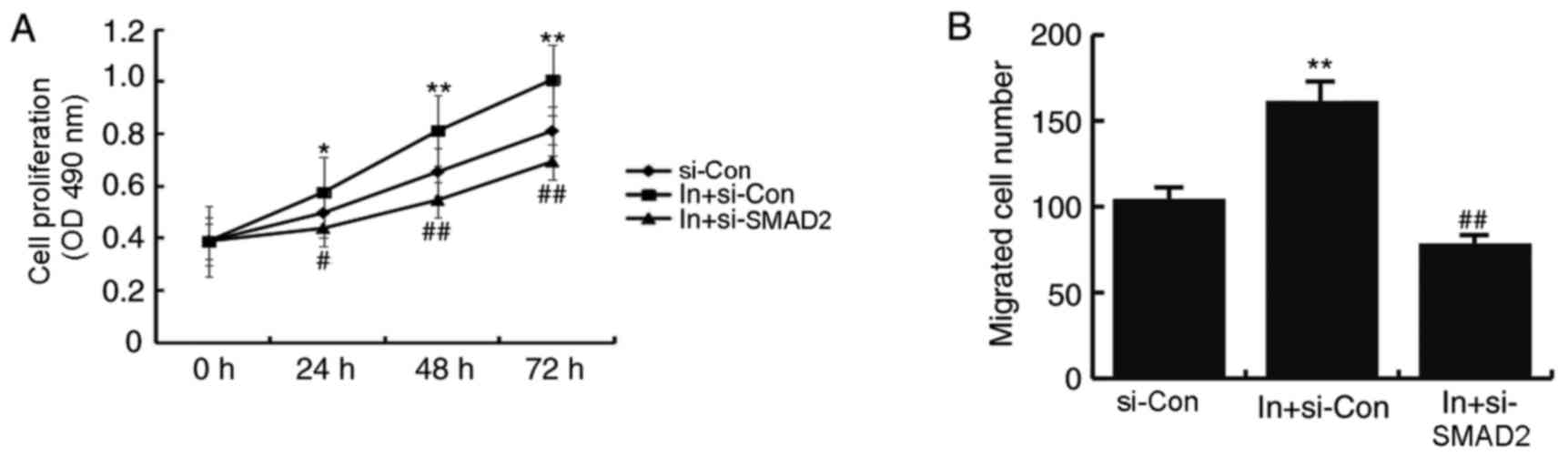
Notably, the miR-486-5p inhibitor-induced increase in proliferation
and migration was inhibited by SMAD2 silencing (Fig. 7A and B, respectively). These
results indicated that silencing of SMAD2 reversed the upregulation
of CHON-001 cell proliferation and migration and the level of type
II collagen and aggrecan expression that were induced by miR-486-5p
inhibitor treatment.
Discussion
OA is the most prevalent chronic joint disease that
affects the majority of people over 65 years old (17). The pathogenesis of OA is very
complex and involves several molecular mechanisms, such as
disregulation of the TGF-β signaling pathway and the Wnt/β-catenin
signaling pathway (18,19). miRNAs were previously indicated as
contributing to the disrupted signaling network in OA (20), and a number of specific miRNAs have
been identified to be crucial during OA pathogenesis (21). As a result, the discovery of key
miRNAs that may serve a role in the development of OA may provide
valuable insight for designing effective miRNA-targeted therapies.
In the present study, it was suggested that miR-486-5p may be
involved in development of OA and therefore may be a promising
target for the treatment of OA.
Loss of control of TGF-β signaling is frequently
observed in OA (22,23). A previous study indicated that
TGF-β signaling contributed to the development of OA through the
activin receptor-like kinase (ALK) 5/SMAD2/SMAD3 pathway or the
ALK1/SMAD2/SMAD5 pathway (24).
SMAD2 appeared to exhibit a stronger effect on chondrogenesis
compared with SMAD3 in vivo (25). In addition, a lower level of SMAD2
protein expression was detected in patients with OA compared with
in patients without OA (13).
TGF-β signaling may activate the mitogen-activated protein kinase
1/extracellular signal-regulated kinase signaling pathway and the
SMAD2/SMAD3 signaling pathway to induce the expression of type II
collagen and aggrecan in rat chondrocytes (26). In human chondrocyte cell line
CHON-001, silencing of SMAD2 expression was demonstrated to trigger
a decrease in type II collagen and aggrecan protein levels, which
suggested a crucial role for SMAD2 in OA development.
In addition to being regulated by TGF-β signaling,
SMAD2 is also controlled by several other molecules, including
miRNAs. For example, miR-155 was reported to directly target SMAD2
and repress SMAD2 expression (27). miR-486-5p regulates multiple
oncogenes and was demonstrated to be closely linked to the
development of cancer (28,29).
A previous study indicated that miR-486-5p may regulate SMAD2
expression in lens epithelial cells (30). However, the role of SMAD2 in OA
remains elusive. In the present study, it was demonstrated that
SMAD2 expression was decreased and that miR-486-5p expression was
elevated in patients with OA. The findings of the present study
were consistent with previous research (13,31).
Additionally, Liu et al (30) reported that miR-486-5p may regulate
SMAD2 expression in lens epithelial cells. The present study
investigated the association between miR-486-5p and SMAD2 in
CHON-001 cells and the results indicated that SMAD2 was
demonstrated to be a direct target of miR-486-5p. Furthermore, the
effect of miR-486-5p on chondrocyte were also investigated in the
present study. The findings suggested that overexpression of
miR-486-5p decreased chondrocyte proliferation and migration.
Conversely, inhibition of miR-486-5p resulted in increased
chondrocyte proliferation and migration. Notably, this increase in
proliferation and migration was reversed by SMAD2 silencing. The
expression levels of type II collagen and aggrecan were also
decreased in response to the miR-486-5p mimics transfection, which
may be enhanced by miR-486-5p inhibitor. SMAD2 silencing reversed
the miR-486-5p inhibitor-induced upregulated expression level of
type II collagen and aggrecan.
In conclusion, the present study demonstrated that
miR-486-5p targeted SMAD2 mRNA, a key gene in OA development. By
targeting SMAD2, miR-486-5p negatively regulated the expression
levels of type II collagen and aggrecan in chondrocytes. Therefore,
the loss of miR-486-5p may increase proliferation and migration of
chondrocytes. The results of the present study suggested a crucial
role for miR-486-5p in OA, which indicated that miR-486-5p may
represent a potential marker and target for the diagnosis and
treatment of OA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JS, SS and KG made substantial contributions to the
concept and design of the study, literature search and manuscript
preparation. JL and CL made substantial contributions to
statistical analysis and data interpretation.
Ethics approval and consent to
participate
The present study was approved by the Ethics
committee of Lianyungang Oriental Hospital (Lianyungang, China).
Written informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arden N, Blanco F, Cooper C, Guermazi A,
Hayashi D, Hunter D, Javaid MK, Rannou F, Roemer FW and Reginster
JY: Atlas of osteosteoarthritis. Springer Healthcare Ltd. 69–82.
2014.
|
|
2
|
World Health Organization, . The Global
Burden of Disease2004 Update. World Health Organization; Geneva:
2008
|
|
3
|
Cross M, Smith E, Hoy D, Nolte S, Ackerman
I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, et al:
The global burden of hip and knee osteoarthritis: Estimates from
the global burden of disease 2010 study. Ann Rheum Dis.
73:1323–1330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
March L, Smith EU, Hoy DG, Cross MJ,
Sanchez-Riera L, Blyth F, Buchbinder R, Vos T and Woolf AD: Burden
of disability due to musculoskeletal (MSK) disorders. Best Pract
Res Clin Rheumatol. 28:353–366. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chu CR, Millis MB and Olson SA:
Osteoarthritis: From Palliation to Prevention: AOA Critical Issues.
J Bone Joint Surg Am. 96:e1302014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Glyn-Jones S, Palmer AJ, Agricola R, Price
AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis. Lancet.
386:376–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sanchez-Adams J, Leddy HA, McNulty AL,
O'Conor CJ and Guilak F: The mechanobiology of articular cartilage:
Bearing the burden of osteoarthritis. Curr Rheumatol Rep.
16:4512014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maroudas AI: Balance between swelling
pressure and collagen tension in normal and degenerate cartilage.
Nature. 260:808–809. 1976. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chou MC, Tsai PH, Huang GS, Lee HS, Lee
CH, Lin MH, Lin CY and Chung HW: Correlation between the MR T2
value at 4.7 T and relative water content in articular cartilage in
experimental osteoarthritis induced by ACL transection.
Osteoarthritis Cartilage. 17:441–447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eppert K, Scherer SW, Ozcelik H, Pirone R,
Hoodless P, Kim H, Tsui LC, Bapat B, Gallinger S, Andrulis IL, et
al: MADR2 maps to 18q21 and encodes a TGFbeta-regulated MAD-related
protein that is functionally mutated in colorectal carcinoma. Cell.
86:543–552. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Riggins GJ, Thiagalingam S, Rozenblum E,
Weinstein CL, Kern SE, Hamilton SR, Willson JK, Markowitz SD,
Kinzler KW and Vogelstein B: Mad-related genes in the human. Nat
Genet. 13:347–349. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Long E, Motwani R, Reece D, Pettit N,
Hepworth J, Wong P, Reynolds P and Seegmiller R: The role of TGF-β1
in osteoarthritis of the temporomandibular joint in two genetic
mouse models. Arch Oral Biol. 67:68–73. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Madej W, Buma P and van der Kraan P:
Inflammatory conditions partly impair the mechanically mediated
activation of Smad2/3 signaling in articular cartilage. Arthritis
Res Ther. 18:1462016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dahaghin S, Bierma-Zeinstra SM, Ginai AZ,
Pols HA, Hazes JM and Koes BW: Prevalence and pattern of
radiographic hand osteoarthritis and association with pain and
disability. Ann Rheum Dis. 64:682–687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Davidson Blaney EN, Vitters EL, van der
Kraan PM and van den Berg WB: Expression of transforming growth
factor-beta (TGFbeta) and the TGFbeta signalling molecule SMAD-2P
in spontaneous and instability-induced osteoarthritis: Role in
cartilage degradation, chondrogenesis and osteophyte formation. Ann
Rheum Dis. 65:1414–1421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Min JL, Meulenbelt I, Riyazi N,
Kloppenburg M, Houwing-Duistermaat JJ, Seymour AB, Pols HA, van
Duijn CM and Slagboom PE: Association of the Frizzled-related
protein gene with symptomatic osteoarthritis at multiple sites.
Arthritis Rheum. 52:1077–1080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iliopoulos D, Malizos KN, Oikonomou P and
Tsezou A: Integrative microRNA and proteomic approaches identify
novel osteoarthritis genes and their collaborative metabolic and
inflammatory networks. PLoS One. 3:e37402008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sumiyoshi K, Kubota S, Ohgawara T, Kawata
K, Nishida T, Shimo T, Yamashiro T and Takigawa M: Identification
of miR-1 as a micro RNA that supports late-stage differentiation of
growth cartilage cells. Biochem Biophys Res Commun. 402:286–290.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Davidson Blaney EN, van der Kraan PM and
van den Berg WB: TGF-beta and osteoarthritis. Osteoarthritis
Cartilage. 15:597–604. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Itayem R, Mengarelli-Widholm S, Hulth A
and Reinholt FP: Ultrastructural studies on the effect of
transforming growth factor 1 on rat articular cartilage. Apmis.
105:221–218. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Finnson KW, Parker WL, Ten Dijke P,
Thorikay M and Philip A: ALK1 opposes ALK5/Smad3 signaling and
expression of extracellular matrix components in human
chondrocytes. J Bone Miner Res. 23:896–906. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang W, Song B, Anbarchian T, Shirazyan A,
Sadik JE and Lyons KM: Smad2 and Smad3 regulate chondrocyte
proliferation and differentiation in the growth plate. PLoS Genet.
12:e10063522016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu Y, Tao H, Jin C, Liu Y, Lu X, Hu X and
Wang X: Transforming growth factor-β1 induces type II collagen and
aggrecan expression via activation of extracellular
signal-regulated kinase 1/2 and Smad2/3 signaling pathways. Mol Med
Rep. 12:5573–9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Louafi F, Martinez-Nunez RT and
Sanchez-Elsner T: MicroRNA-155 targets SMAD2 and modulates the
response of macrophages to transforming growth Factor-{beta}. J
Biol Chem. 285:41328–41336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, Tian X, Han R, Zhang X, Wang X,
Shen H, Xue L, Liu Y, Yan X, Shen J, et al: Downregulation of
miR-486-5p contributes to tumor progression and metastasis by
targeting protumorigenic ARHGAP5 in lung cancer. Oncogene.
33:1181–1189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pang W, Tian X, Bai F, Han R, Wang J, Shen
H, Zhang X, Liu Y, Yan X, Jiang F and Xing L: Pim-1 kinase is a
target of miR-486-5p and eukaryotic translation initiation factor
4E, and plays a critical role in lung cancer. Mol Cancer.
13:2402014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu B, Sun J, Lei X, Zhu Z, Pei C and Qin
L: MicroRNA-486-5p suppresses TGF-β2-induced proliferation,
invasion and epithelial-mesenchymal transition of lens epithelial
cells by targetingSmad2. J Biosci. 42:575–584. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kong R, Gao J, Si Y and Zhao D:
Combination of circulating miR-19b-3p, miR-122-5p and miR-486-5p
expressions correlates with risk and disease severity of knee
osteoarthritis. Am J Transl Res. 9:2852–2864. 2017.PubMed/NCBI
|