Introduction
Spinal cord injury (SCI) often leads to paralysis,
has a high morbidity rate and occurs in both genders equally
(1,2). A series of pathophysiological
responses lead to progressive spinal cord tissue degeneration and
necrosis following SCI. It is thought this is due to
microcirculation disorder and neuronal biochemical imbalances
involving prostaglandins, calcium, neurotransmitters and free
radicals (3). Despite numerous
advances in medical science, no definitive treatment for SCI has
been developed. Ongoing research aiming to resolve this issue has
proposed various treatment options including medicinal, cell and
gene therapies (4–6). The lack of success observed in this
research may be attributed to the complicated pathophysiology of
SCIs and associated factors. For example, the inhibiting factor
chondroitin sulfate proteoglycan (CSPG) is associated with glial
scars and is thought to limit the effectiveness of SCI treatment
(7,8).
It has been proposed that the primary factors
influencing the outcome of patients with SCI are glial scarring and
chronic inflammation (9,10). CSPG is an extracellular matrix
molecule that is expressed following SCI and reaches its maximal
expression two weeks following injury (11). CSPG activates a cascade that leads
to the activation of glycogen synthase kinase-3β (GSK3β), a key
factor in the inhibition of tissue and axon recovery in the central
nervous system (12).
Chondroitinase ABC (ChABC) is an enzyme produced by the bacteria
Proteus vulgaris that cleaves disaccharides and
tetrasaccharides (13). It was
demonstrated that the digestion of glycosaminoglycan (GAG) by ChABC
may enhance axonal regeneration and improve neuromotor function
following SCI (14,15). A loss of GAG occurs in
intervertebral disc degeneration and thus, the injection of ChABC
may be used to construct a model of this condition (16). ChABC administration following SCI
has moderate efficacy and its effectiveness may be improved by the
simultaneous administration of other medications (17–20).
Previous studies have demonstrated that lipid
oxidation by free radicals has important implications in the
outcome of SCI (3,21). Certain reports suggest that
hyperbaric oxygen (HBO) treatment induces GAG synthesis, which
reduces oxygen free radical generation in the body and subsequently
reduces lipid oxidation and promotes SCI repair. However, other
studies have concluded the opposite (16,22–24).
Therefore, the effect of HBO on nerve injury is not fully
understood. A previous study demonstrated that HBO may protect
Sprague-Dawley rats induced by ChABC treatment from intervertebral
disc degeneration (16). However,
less is known about the combined effect of HBO and ChABC therapy in
a rat model of SCI. In the present study, the efficacy of
simultaneous HBO and ChABC treatment in rats following SCI was
examined. Serum malondialdehyde (MDA) and superoxide dismutase
(SOD) levels were examined to evaluate the extent of lipid
peroxidation in injured cells (21).
Materials and methods
Experimental animals
The Ethics Committee of the Yantai Yuhuangding
Hospital (Yantai, China) approved the protocol of animal care and
handling in the present study. A total of 48 healthy male Wistar
rats (150–170 g) were provided by The Experimental Animal Center of
Shandong University (Jinan, China). Rats had free access to food
and water and were housed together in a 12 h light/dark cycle at
25°C and 75% relative humidity, in specific pathogen-free
conditions. Rats were randomly divided into six groups (Table I).
 | Table I.Experimental groups. |
Table I.
Experimental groups.
| Experimental groups
(n=8) | Procedure |
|---|
| Sham | Animals were
surgically exposed and treated with regular air, however not
subjected to the SCI procedure. |
| SCI | Animals with SCI
treated with regular air (no treatment). |
| Vehicle | Animals with SCI
that received 10 µl buffer phosphate saline injection and treated
with regular air (intra-spinal) |
| HBO | Animals with SCI
treated by HBO (80% oxygen at 0.3 MPa) |
| Enzyme | Animals with SCI
that received 10 µl of 100 U/ml of chondroitinase ABC injection and
treated with regular air (intra spinal) |
| HBO + enzyme | Animals with spinal
cord injury treated with HBO and chondroitinase ABC |
Experimental SCI model
The experimental SCI model was induced in rats using
the clip compression method (18,25).
Rats were anesthetized by intraperitoneal injection of ketamine (80
mg/kg) and xylasine (10 mg/kg). The laminectomy was performed at
the T13-L1 level following skin and muscle incision. The spinal
cord was compressed by a microvascular clip (Fine Science Tools
GmbH, Heidelberg, Germany) that induced 20 g/cm2
pressure for 90 sec. Animals were monitored for 4 weeks following
surgery.
HBO treatment
HBO treatment began 2 h following SCI establishment
and was continued once daily for 14 days. A medical hyperbaric
oxygen chamber (Ningbo Hyperbaric Oxygen Corporation, Ningbo,
China) was prepared with a flush of pure oxygen for 10 min. SCI-HBO
rats were placed into the HBO chamber and exposed to 80% oxygen at
0.3 MPa (3 ATA) for 60 min, followed by depressurization for 30
min. Control animals were treated with regular air.
ChABC injection
The injury site was re-exposed in anesthetized rats
7 days following surgery and ChABC (0.1 U/µl; total, 10 µl;
Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) diluted in 0.01%
bovine serum albumin (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and PBS was slowly intraspinally injected over 2
min using a 30 µm glass micropipette connected to a Hamilton
syringe (depth, 1 mm), 2 mm rostral to injury site (18). The needle was kept in the injection
site for 1 min following the termination of the injection to
prevent backflow.
Neuromotor function assessment
The Basso-Beattie-Bresnahan (BBB) locomotor rating
scale (26) and the inclined plane
assessment were used to examine neuromotor function prior to
surgery and for 4 weeks thereafter (26). For the inclined plane assessment,
the inclination angle of the board is freely adjustable. The
maximum inclination angle of the board on which the rat stayed for
5 sec without falling off was recorded (27). The BBB rating scale has a range of
0–21 points, judged by parameters including the coordination of
limb movement, paw placement and tail balance. No visible movement
of the hind legs was scored as zero points. For the maximum 21
points, the rat had to walk continuously on its paws, with a cocked
tail, good fore and hind limb motor coordination and trunk
stability. Data was quantified as the average score of the two hind
limbs.
SOD and MDA assays
Tail blood (1.5 ml) was obtained at 0, 2 and 4 weeks
following SCI. SOD activity was measured using the xanthine oxidase
assay (cat no. A001-1-1; Nanjing Jiancheng Bioengineering
Institute, Nanjing, China) and MDA levels were detected using the
thiobarbituric acid assay (cat no. A003-4; Nanjing Jiancheng
Bioengineering Institute), according to the manufacturer's
protocols.
Western blot analysis
Following the removal of the dura, spinal cord
(100–300 µg) from the T13-L1 level was homogenized on ice in
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China). Protein concentration was measured
with a Nano dropper (Thermo Fisher Scientific, Inc.). The proteins
(25 µg/lane) were electrophoresed on 10% SDS-PAGE gel for 1 h at
120 V and subsequently transferred to polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA). The blots were
blocked with 5% non-fat dry milk for 1 h at 37°C prior to
incubation with the primary antibodies rabbit polyclonal
anti-aquaporin 4 (AQP4; 1:100; catalog no. ab46182; Abcam,
Cambridge, UK) and rabbit polyclonal anti-GSK3β (1:100; catalog no.
ab15314, Abcam) overnight at 4°C. Following incubation with
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (1:1,000; cat no. A0208; Beyotime Institute of
Biotechnology) for 1 h at 37°C, the protein bands were visualized
using the chemiluminescence substrate kit (Beyotime Institute of
Biotechnology). GAPDH (1:2,000; cat no. sc-69778; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) was used as an internal
control. Densitometry analysis performed using the Image J software
version 1.3 (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All data is presented as the mean ± standard error.
Statistical analyses were performed with SPSS software 15.0 (SPSS,
Inc., Chicago, IL, USA). Data were analyzed using the two-sided
repeated measures analysis of variance with the Bonferroni post-hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
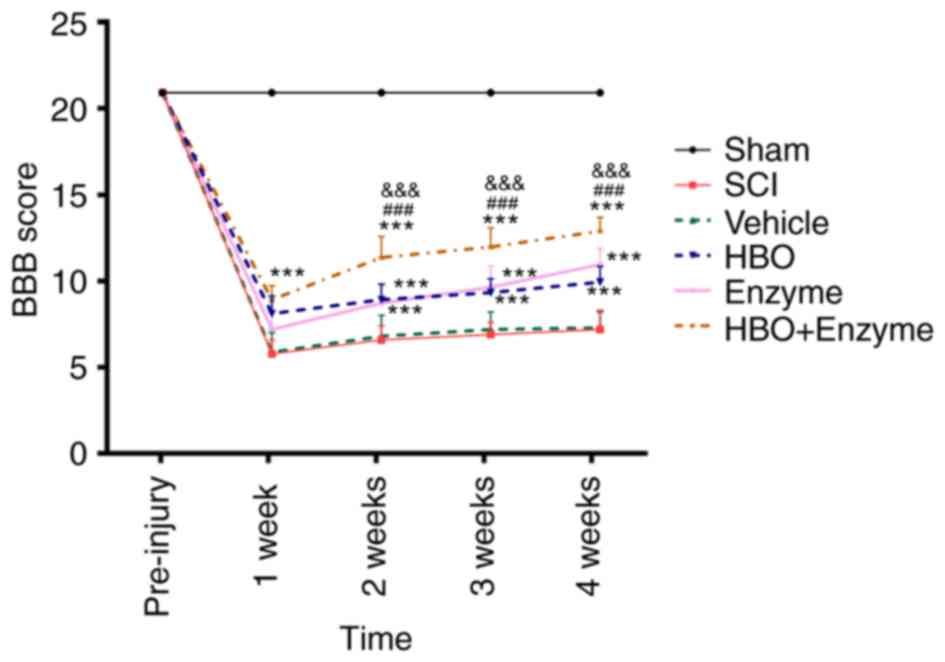
Hind limb neuromotor function
assessment
The BBB locomotor rating scale and the inclined
plane assessment were used to assess the hind limb neuromotor
function, which is an important index for SCI (26). Prior to establishment of the SCI,
rats in all groups had a baseline BBB score of 21 points. Following
SCI, the BBB score was significantly decreased in all groups
compared with the control rats (P<0.0001), indicating that motor
functions were reduced following SCI. During the 4 week follow up,
a certain degree of motor function recovery was observed, however
the baseline level was not recovered. Sham rats had a BBB score of
21 throughout the study (Fig.
1).
The BBB score in the combination therapy group (HBO
+ enzyme group) was significantly increased compared with the SCI
rats 1 week following SCI establishment (P<0.001). At week 2, 3
and 4, the BBB score was increased in the HBO, enzyme and HBO +
enzyme groups compared with the SCI group (P<0.001). The BBB
score increase in the HBO + enzyme group was significantly greater
compared with the HBO and enzyme groups at week 2 (P<0.001), 3
(P<0.001) and 4 (P<0.001).
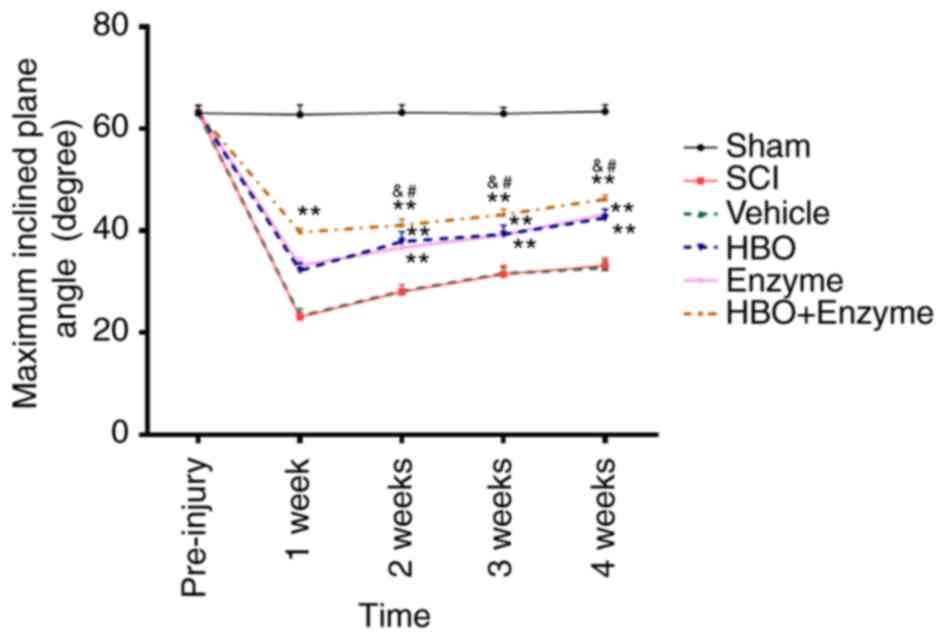
For the inclined plane assessment, rats in all
groups achieved a maximum angle of ~63° prior to SCI establishment.
The maximum inclination angles were significantly reduced and
subsequently increased at 2, 3 and 4 weeks in all groups following
SCI (P<0.01). The maximum angle in the HBO + enzyme, HBO and
enzyme groups were significantly increased compared with the SCI
group (P<0.01). The angle in the HBO + enzyme group was
significantly increased compared with the HBO and enzyme groups
(P<0.05; Fig. 2). Therefore,
the combination of HBO and ChABC significantly improved the motor
function recovery following SCI compared with HBO or ChABC
treatment alone.
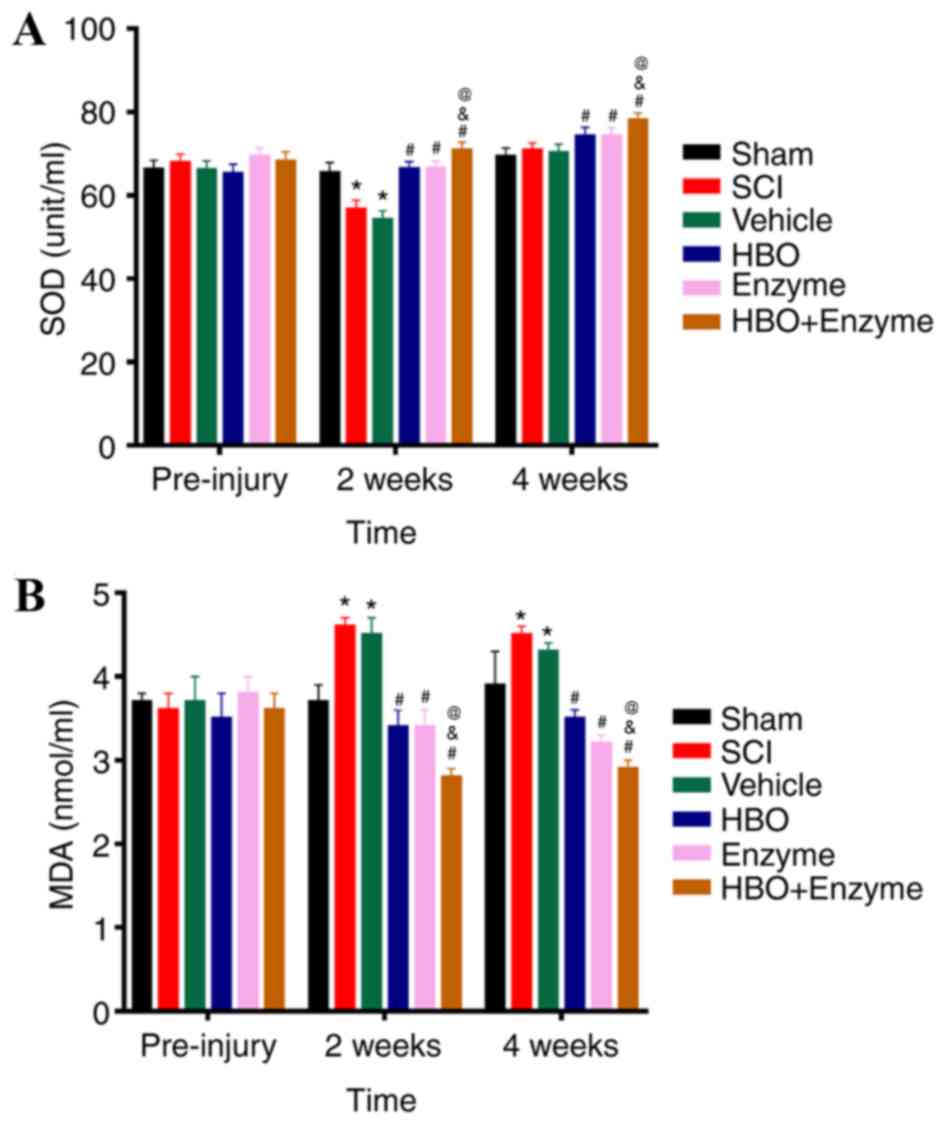
Serum SOD and MDA content
To detect alterations in the generation of oxygen
free radicals, serum SOD and MDA levels were detected. Following
SCI, serum SOD levels were significantly decreased and serum MDA
levels were significantly increased compared with the sham group.
Serum SOD activity in HBO + enzyme, HBO and enzyme groups were
significantly increased compared with sham and SCI rats 2 weeks
following SCI (Fig. 3A).
Conversely, MDA levels were significantly decreased in the HBO +
enzyme, HBO and enzyme groups compared with the sham and SCI groups
(Fig. 3B). SOD and MDA levels in
HBO + enzyme group were respectively increased or decreased
compared with HBO and enzyme groups 2 and 4 weeks following SCI
establishment. There was no significant alteration in SOD or MDA
levels in the HBO and enzyme groups at 2 or 4 weeks.
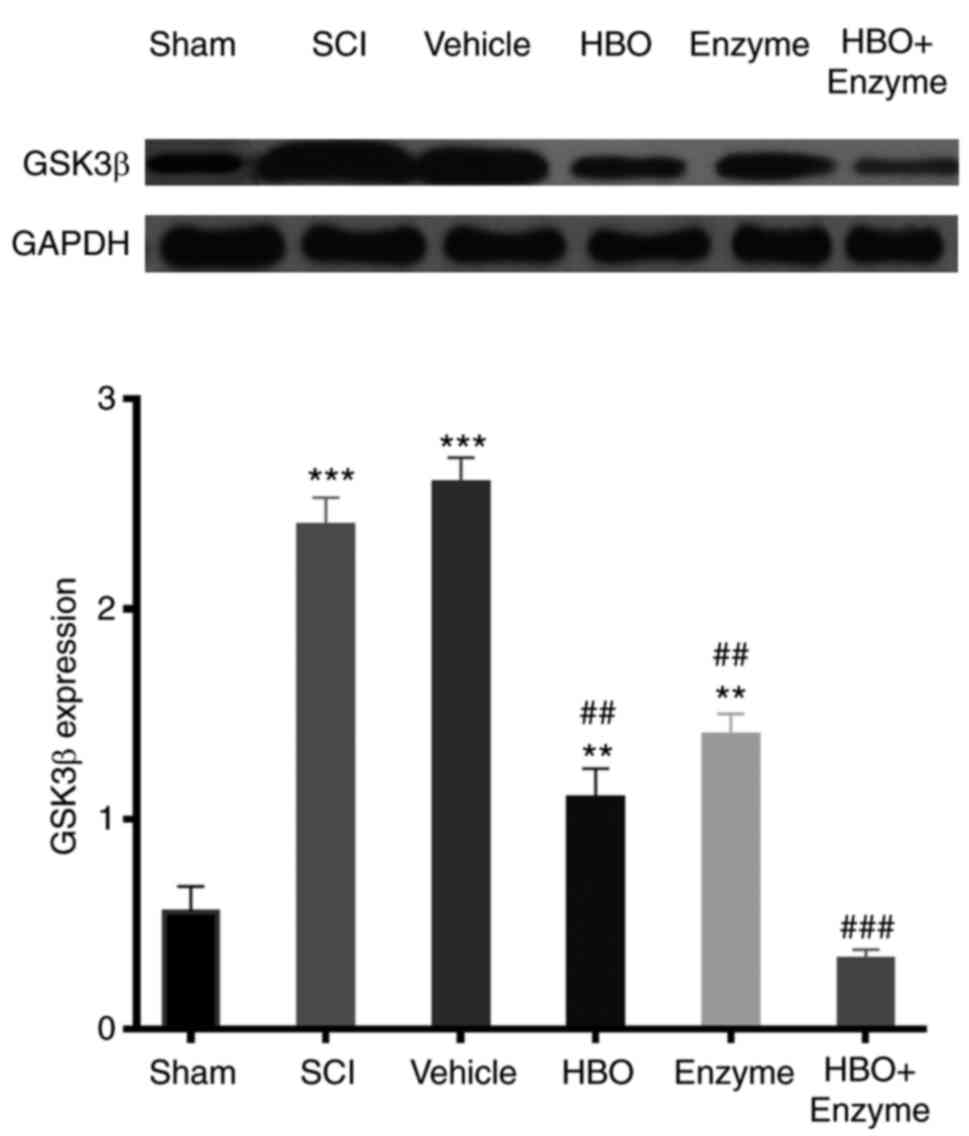
GSK3β expression in the spinal
cord
GSK3β is involved in the prevention of axon
regeneration following SCI (19,28).
Western blot analysis indicated that GSK3β expression was
significantly upregulated compared with baseline following SCI
establishment (P<0.001) 4 weeks post-surgery. HBO (P<0.01),
enzyme (P<0.01) and HBO + enzyme (P<0.001) treatments all
significantly inhibited GSK3β expression. The combination of HBO
and ChABC reduced GSK3β expression to sham rat expression levels,
indicating that this combination may be effective in improving the
outcome of SCI (Fig. 4).
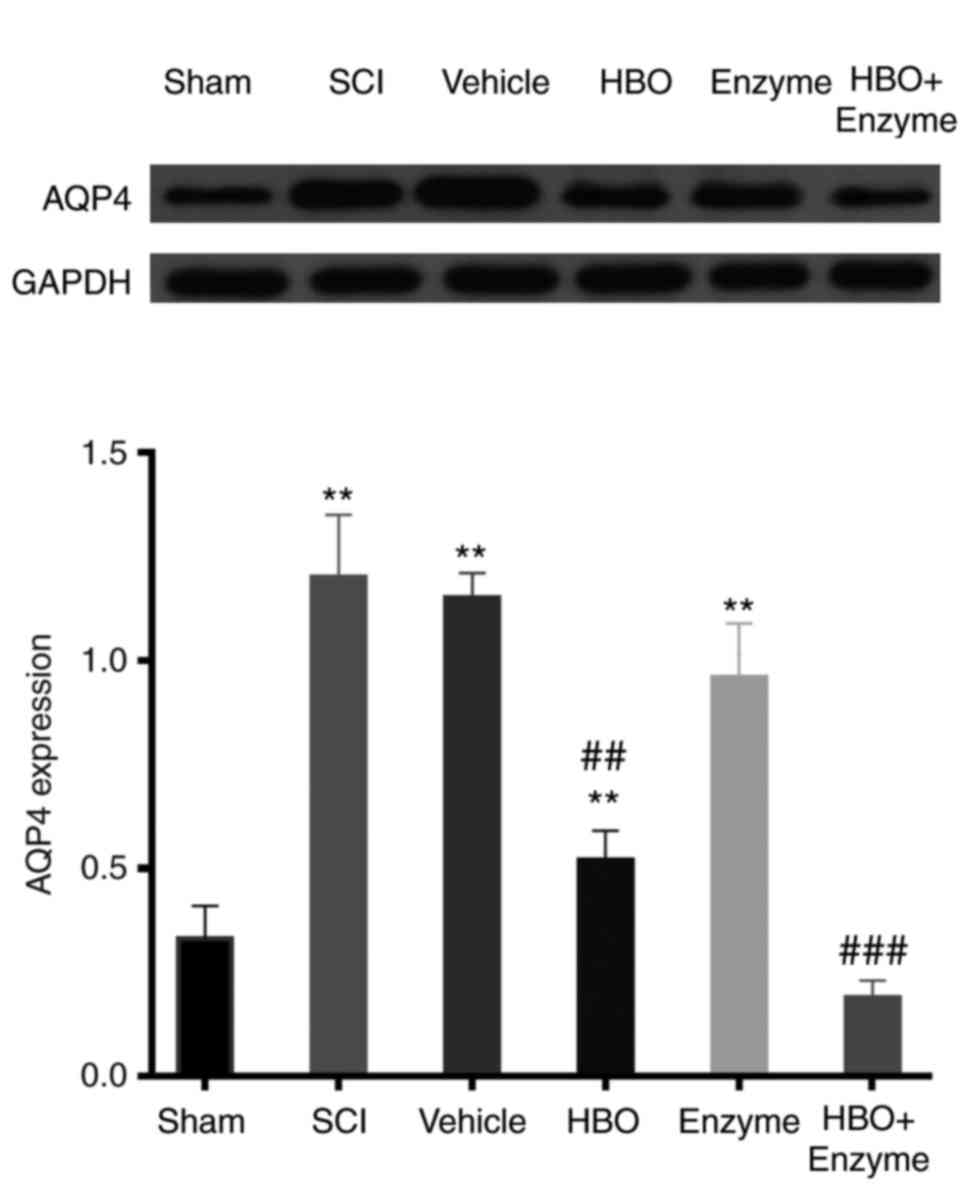
Assessment of AQP4 expression in the
spinal cord
Increased AQP4 expression impedes SCI recovery
(29,30). Western blot analysis determined
that AQP4 expression was significantly increased compared with the
sham group following SCI establishment (P<0.001). HBO
(P<0.01) and HBO + enzyme (P<0.001) treatment significantly
inhibited the expression of AQP4 compared with the SCI group. The
enzyme treatment alone did not significantly inhibit the expression
of AQP4 compared with the SCI group. The strongest inhibition of
AQP4 expression was observed in the HBO + enzyme treatment group
(Fig. 5).
Discussion
In the present study, the therapeutic effects of HBO
and ChABC administrated alone and in combination in SCI rats was
examined. The combination of HBO and ChABC was demonstrated to have
the most beneficial effects on BBB scores, motor function recovery
and the expression of SOD, MDA, GSK3β and AQP4, compared with the
administration of either treatment alone.
Lipid peroxidation and the extent of neuronal damage
may be assessed by the detection of inflammatory mediators,
including SOD and MDA (21). SOD
is a naturally occurring neuroprotective free radical scavenger
that converts harmful superoxide radicals into hydrogen peroxide.
MDA is a key product of membrane lipid peroxidation, which is
involved in the mitochondrial respiratory chain complex and
exacerbates damage to the membrane. In the present study, HBO
treatment significantly improved neuromotor function compared with
the sham and SCI rats, suggesting an increase in SOD activity and
reduced lipid peroxidation by oxygen free radicals. This indicates
that oxygen free radical inhibition may be one of the mechanisms by
which HBO treatment improves neuromotor function in SCI rats.
There are two primary obstacles that impede recovery
following SCI: The expression of inflammatory factors and the
production of CSPG (31). A direct
correlation exists between the production of inflammatory cytokines
and CSGP (31). In SCI, CSGP is
involved in processes of cell death, demyelination, cavitation,
microglial invasion and eventually the impairment of neuromotor
function (14,15). Inflammation is associated with an
increase in GSK3β expression in SCI (28,32).
GSK3β is involved in the induction of demyelination and Wallerian
degeneration (12,28). GSK3β also regulates the production
of CSGP and thus prevents axonal regeneration following SCI
(19,28). Increased CSGP expression may also
contribute to neuromotor function impairment through the disruption
of axonal connections and axonal regeneration (28). All these mechanisms may be involved
in the BBB decrease observed in SCI rats and the recovery of
neuromotor function following HBO + enzyme treatment in the present
study. ChABC enzyme administration may directly overcome the
endothelial barrier through the degradation of CSPG (33), thus creating a permeable
environment that allows axonal regeneration (34,35).
A previous study indicated that enzyme secretion begins within a
day of injury and reaches its maximum expression following 1 week
(11). Therefore, the enzyme was
injected 1 week following SCI establishment. ChABC may exhibit
anti-inflammatory properties (36). The results of the current study
revealed that GSK3β expression decreased in the combined treatment
and that neuromotor function following SCI was improved, which is
indicative of reduced demyelination and axonal regeneration
(32,37). The combined HBO and ChABC treatment
may have created a permeable environment by decreasing CSPG and
GSK3β to promote neuromotor function.
Previous studies have demonstrated that local edema
occurs immediately following SCI, due to endothelial barrier
permeability injury (38,39). Microglial invasion and inflammatory
cytokine production increases, leading to cell death,
demyelination, cavity formation, paralysis and even mortality
(40,41). AQP4 may reduce edema by
facilitating the excretion of excessive water (42). However, increased AQP4 expression
in the chronic stage of SCI may be indicative of continued water
retention and cytotoxic edema that impedes recovery (29,30).
A previous study suggested that the role of HBO in the
dysregulation of blood-brain barrier permeability should be taken
into consideration when patients are exposed to HBO (43). Additionally, Wang et al
(16) reported that HBO therapy
significantly suppresses the decrease in disc height caused by
ChABC injection, suggesting that HBO may protect the intervertebral
discs against ChABC-induced injury. Consistent with these results,
the present study demonstrated that combined HBO and ChABC therapy
exerted a protective effect in the SCI rat model. Although
alterations in the permeability of the spinal cord vasculature were
not directly detected, to the best of the author's knowledge, the
present study is the first to demonstrate the combined effect of
ChABC and HBO in SCI. It was revealed that the expression of AQP4
in SCI rats was not significantly decreased by ChABC alone,
indicating cytotoxic edema may be a limitation for this treatment.
Results also indicated that HBO may protect against permeability
barrier destruction by ChABC, however this conclusion should be
validated with further research. The association of HBO with ChABC
is still unclear and further investigation of the molecular
mechanisms and associated signaling pathways are required.
In conclusion, the results of the present study
demonstrated that although the application of HBO and ChABC alone
reduced the GSK3β level and improved neuromotor recovery in SCI
model rats, ChABC alone did not inhibit the induction of AQP4
expression following SCI, which may be a limitation to this
treatment. In the combined treatment group, the expression of GSK3β
and AQP4 was returned to baseline and the recovery of neuromotor
function was improved. Increased serum SOD activity and decreased
serum MDA content indicated a potential role of oxygen free
radicals in the pathophysiological consequences of SCI. These
alterations were reversed by the combined HBO and ChABC treatment.
Therefore, the combined treatment was concluded to be more
effective than application of either treatment alone. Improved
neuromotor function was likely due to a reduction in inflammation
by ChABC and HBO, as well as the degradation of CSPG by ChABC which
contributes to the preservation of tissue integrity. Future studies
should aim to further understand the underlying mechanisms.
References
|
1
|
Jackson A: Spinal-cord injury: Neural
interfaces take another step forward. Nature. 539:177–178. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ropper AE and Ropper AH: Acute spinal cord
compression. N Engl J Med. 376:1358–1369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hall ED: Lipid antioxidants in acute
central nervous system injury. Ann Emerg Med. 22:1022–1027. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ajiboye AB, Willett FR, Young DR, Memberg
WD, Murphy BA, Miller JP, Walter BL, Sweet JA, Hoyen HA, Keith MW,
et al: Restoration of reaching and grasping movements through
brain-controlled muscle stimulation in a person with tetraplegia: A
proof-of-concept demonstration. Lancet. 389:1821–1830. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Capogrosso M, Milekovic T, Borton D,
Wagner F, Moraud EM, Mignardot JB, Buse N, Gandar J, Barraud Q,
Xing D, et al: A brain-spine interface alleviating gait deficits
after spinal cord injury in primates. Nature. 539:284–288. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lebedev MA and Nicolelis MA: Brain-machine
interfaces: From basic science to neuroprostheses and
neurorehabilitation. Physiol Rev. 97:767–837. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bagherzadeh K, Maleki M, Golestani A,
Khajeh K and Amanlou M: Chondrotinase ABC I thermal stability is
enhanced by site-directed mutagenesis: A molecular dynamic
simulations approach. J Biomol Struct Dyn. 1–10. 2017.
|
|
8
|
Rauvala H, Paveliev M, Kuja-Panula J and
Kulesskaya N: Inhibition and enhancement of neural regeneration by
chondroitin sulfate proteoglycans. Neural Regen Res. 12:687–691.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan YM and He C: The glial scar in spinal
cord injury and repair. Neurosci Bull. 29:421–435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muramoto A, Imagama S, Natori T, Wakao N,
Ando K, Tauchi R, Hirano K, Shinjo R, Matsumoto T, Ishiguro N and
Kadomatsu K: Midkine overcomes neurite outgrowth inhibition of
chondroitin sulfate proteoglycan without glial activation and
promotes functional recovery after spinal cord injury. Neurosci
Lett. 550:150–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jones LL, Margolis RU and Tuszynski MH:
The chondroitin sulfate proteoglycans neurocan, brevican,
phosphacan, and versican are differentially regulated following
spinal cord injury. Exp Neurol. 182:399–411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dill J, Wang H, Zhou F and Li S:
Inactivation of glycogen synthase kinase 3 promotes axonal growth
and recovery in the CNS. J Neurosci. 28:8914–8928. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akbari M, Khaksari M, Rezaeezadeh-Roukerd
M, Mirzaee M and Nazari-Robati M: Effect of chondroitinase ABC on
inflammatory and oxidative response following spinal cord injury.
Iran J Basic Med Sci. 20:806–812. 2017.PubMed/NCBI
|
|
14
|
Cheng CH, Lin CT, Lee MJ, Tsai MJ, Huang
WH, Huang MC, Lin YL, Chen CJ, Huang WC and Cheng H: Local delivery
of high-dose chondroitinase ABC in the sub-acute stage promotes
axonal outgrowth and functional recovery after complete spinal cord
transection. PLoS One. 10:e01387052015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Graham JB and Muir D: Chondroitinase C
selectively degrades chondroitin sulfate glycosaminoglycans that
inhibit axonal growth within the endoneurium of peripheral nerve.
PLoS One. 11:e01676822016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang IC, Liu HT, Yu CM, Whu SW, Lin SS, Su
CI, Chen CH and Chen WJ: Effect of hyperbaric oxygenation on
intervertebral disc degeneration: An in vivo study with
sprague-dawley rats. Spine (Phila Pa 1976). 38:E137–E142. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alluin O, Delivet-Mongrain H, Gauthier MK,
Fehlings MG, Rossignol S and Karimi-Abdolrezaee S: Examination of
the combined effects of chondroitinase ABC, growth factors and
locomotor training following compressive spinal cord injury on
neuroanatomical plasticity and kinematics. PLoS One. 9:e1110722014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Janzadeh A, Sarveazad A, Yousefifard M,
Dameni S, Samani FS, Mokhtarian K and Nasirinezhad F: Combine
effect of Chondroitinase ABC and low level laser (660 nm) on spinal
cord injury model in adult male rats. Neuropeptides. 65:90–99.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sarveazad A, Babahajian A, Bakhtiari M,
Soleimani M, Behnam B, Yari A, Akbari A, Yousefifard M, Janzadeh A,
Amini N, et al: The combined application of human adipose derived
stem cells and Chondroitinase ABC in treatment of a spinal cord
injury model. Neuropeptides. 61:39–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xia T, Huang B, Ni S, Gao L, Wang J, Wang
J, Chen A, Zhu S, Wang B, Li G, et al: The combination of db-cAMP
and ChABC with poly(propylene carbonate) microfibers promote axonal
regenerative sprouting and functional recovery after spinal cord
hemisection injury. Biomed Pharmacother. 86:354–362. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hall ED: Inhibition of lipid peroxidation
in CNS trauma. J Neurotrauma. 8 Suppl 1:S31–S41. 1991.PubMed/NCBI
|
|
22
|
Dayan K, Keser A, Konyalioglu S, Erturk M,
Aydin F, Sengul G and Dagci T: The effect of hyperbaric oxygen on
neuroregeneration following acute thoracic spinal cord injury. Life
Sci. 90:360–364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Geng CK, Cao HH, Ying X, Zhang HT and Yu
HL: The effects of hyperbaric oxygen on macrophage polarization
after rat spinal cord injury. Brain Res. 1606:68–76. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thompson CD, Zurko JC, Hanna BF,
Hellenbrand DJ and Hanna A: The therapeutic role of interleukin-10
after spinal cord injury. J Neurotrauma. 30:1311–1324. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rong H, Liu Y, Zhao Z, Feng J, Sun R, Ma Z
and Gu X: Further standardization in the aneurysm clip: The effects
of occlusal depth on the outcome of spinal cord injury in rats.
Spine (Phila Pa 1976). 43:E126–E131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Turner RC, Seminerio MJ, Naser ZJ, Ford
JN, Martin SJ, Matsumoto RR, Rosen CL and Huber JD: Effects of
aging on behavioral assessment performance: Implications for
clinically relevant models of neurological disease. J Neurosurg.
117:629–637. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nagai J, Owada K, Kitamura Y, Goshima Y
and Ohshima T: Inhibition of CRMP2 phosphorylation repairs CNS by
regulating neurotrophic and inhibitory responses. Exp Neurol.
277:283–295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nesic O, Guest JD, Zivadinovic D, Narayana
PA, Herrera JJ, Grill RJ, Mokkapati VU, Gelman BB and Lee J:
Aquaporins in spinal cord injury: The janus face of aquaporin 4.
Neuroscience. 168:1019–1035. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nesic O, Lee J, Ye Z, Unabia GC, Rafati D,
Hulsebosch CE and Perez-Polo JR: Acute and chronic changes in
aquaporin 4 expression after spinal cord injury. Neuroscience.
143:779–792. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shechter R, Raposo C, London A, Sagi I and
Schwartz M: The glial scar-monocyte interplay: A pivotal resolution
phase in spinal cord repair. PLoS One. 6:e279692011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cuzzocrea S, Genovese T, Mazzon E,
Crisafulli C, Di Paola R, Muià C, Collin M, Esposito E, Bramanti P
and Thiemermann C: Glycogen synthase kinase-3 beta inhibition
reduces secondary damage in experimental spinal cord trauma. J
Pharmacol Exp Ther. 318:79–89. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zeng Y, Ebong EE, Fu BM and Tarbell JM:
The structural stability of the endothelial glycocalyx after
enzymatic removal of glycosaminoglycans. PLoS One. 7:e431682012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xia Y, Yan Y, Xia H, Zhao T, Chu W, Hu S,
Feng H and Lin J: Antisense vimentin cDNA combined with
chondroitinase ABC promotes axon regeneration and functional
recovery following spinal cord injury in rats. Neurosci Lett.
590:74–79. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ni S, Xia T, Li X, Zhu X, Qi H, Huang S
and Wang J: Sustained delivery of chondroitinase ABC by
poly(propylene carbonate)-chitosan micron fibers promotes axon
regeneration and functional recovery after spinal cord hemisection.
Brain Res. 1624:469–478. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Didangelos A, Iberl M, Vinsland E, Bartus
K and Bradbury EJ: Regulation of IL-10 by chondroitinase ABC
promotes a distinct immune response following spinal cord injury. J
Neurosci. 34:16424–16432. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kingwell K: Spinal cord injury: Clamping
down on calpains to treat injury-induced spasticity. Nat Rev Drug
Discov. 15:3102016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zeng Y: Endothelial glycocalyx: Novel
insight into atherosclerosis. J Biomed. 2:109–116. 2017. View Article : Google Scholar
|
|
39
|
Zeng Y: Endothelial glycocalyx as a
critical signalling platform integrating the extracellular
haemodynamic forces and chemical signalling. J Cell Mol Med.
21:1457–1462. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Iwahashi K, Hayashi T, Watanabe R,
Nishimura A, Ueta T, Maeda T and Shiba K: Effects of orthotic
therapeutic electrical stimulation in the treatment of patients
with paresis associated with acute cervical spinal cord injury: A
randomized control trial. Spinal Cord. Jun 27–2017.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shultz RB and Zhong Y: Minocycline targets
multiple secondary injury mechanisms in traumatic spinal cord
injury. Neural Regen Res. 12:702–713. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cabrera-Aldana EE, Ruelas F, Aranda C,
Rincon-Heredia R, Martínez-Cruz A, Reyes-Sánchez A, Guizar-Sahagún
G and Tovar-Y-Romo LB: Methylprednisolone administration following
spinal cord injury reduces aquaporin 4 expression and exacerbates
edema. Mediators Inflamm. 2017:47929322017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tatar S, Orhan N, Yilmaz CU, Arican N,
Ahishali B, Kucuk M, Elmas I, Kaya M and Toklu AS: Hyperbaric
oxygen therapy for five days increases blood-brain barrier
permeability. Undersea Hyperb Med. 44:345–355. 2017. View Article : Google Scholar : PubMed/NCBI
|