Introduction
Postoperative cognitive dysfunction (POCD) is the
deterioration of cognitive performance after anesthesia (and/or
surgery), which presents as impaired memory or concentration
(1). Its clinical features include
deterioration in cognition, disturbance in attention and reduced
awareness of the environment, which result in higher morbidity,
mortality and greater utilization of social financial assistance.
Aging societies can expect an increase in the incidence of POCD
(2). POCD is a decrease in
cognition measured by neuropsychological tests after anesthesia and
surgery (3). In some studies, the
incidence of POCD has reached as high as 26% (4). Furthermore, at seven days after
surgery with propofol anesthesia, the incidence of POCD was 29.7%
(5).
It has been considered that cAMP response-element
binding protein (CREB) functions in hippocampal synaptic plasticity
and hippocampus-dependent long-term memory (6). CREB modulates the transcription of
genes, which contain a cAMP responsive element (CRE sites) in their
promoters, and appears to represent a key molecule in transforming
incoming information into long-term memory (7). Agents that disrupt the activity of
CREB specifically block the formation of long-term memory (8). A number of neurotransmitter receptors
and signaling pathways, such as protein kinase C (PKC),
phospholipase C (PLC), N-Methyl-D-Aspartate (NMDA) receptors and
MAPK/ERK kinase (MEK), contribute to transcriptional activation,
and these receptors are coupled to CREB activation through
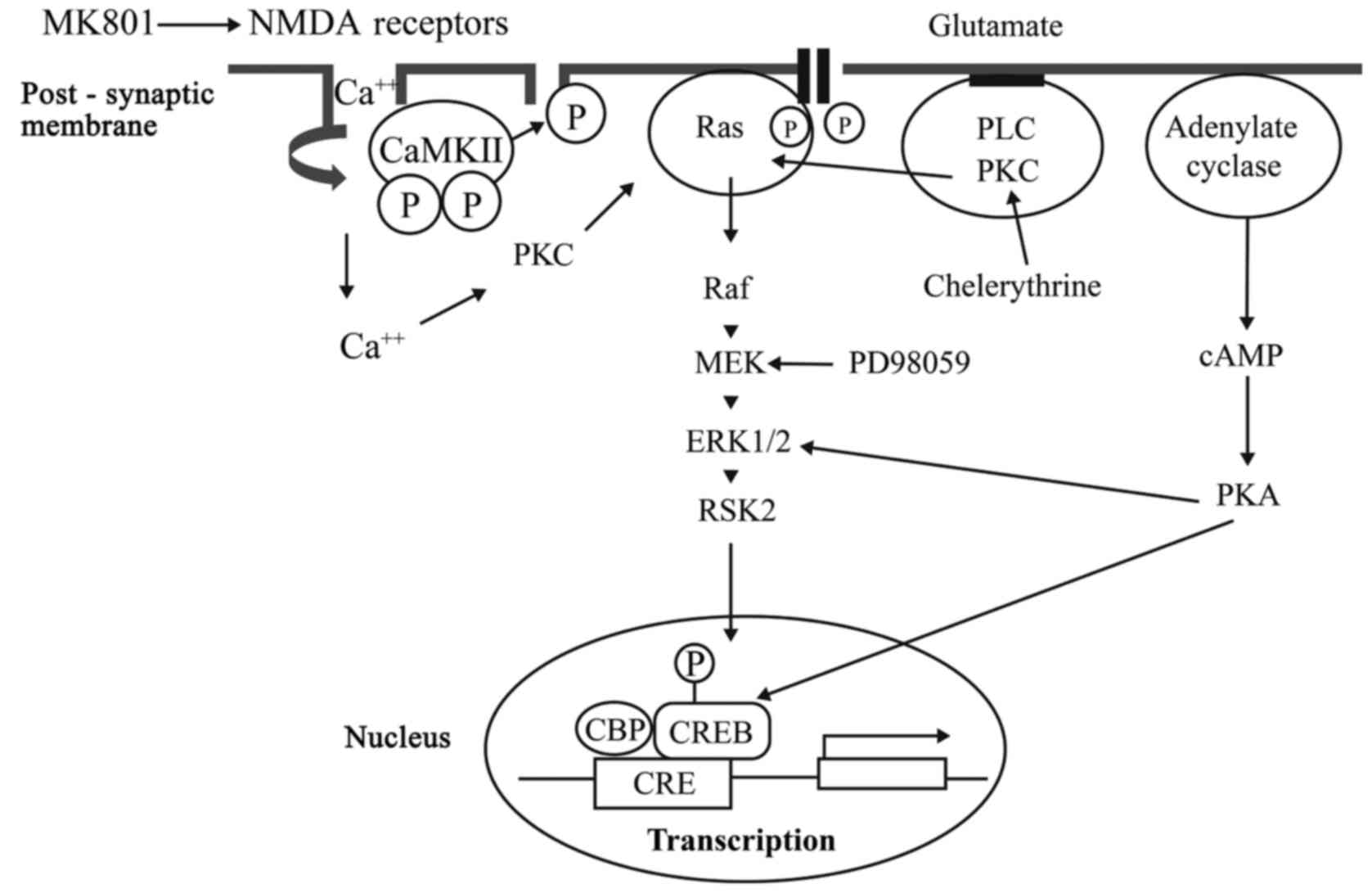
mitogen-activated protein kinase (MAPK) (Fig. 1) (9). The dysregulation of CREB activation
and cross-talking among relevant receptor pathways may be
responsible for the effects of intravenous anesthetics on
cognition.
It has been previously reported that ketamine
suppresses not only the morphine-induced phosphorylation of CREB,
but also the residue preference (10). A sub-anaesthetic dose of propofol
causes impairment of spatial memory retention, but not acquisition
inability, which was possibly mediated by the inhibition of CREB
signaling (11).
CREB may be the common target of anesthetics. The
effects of intravenous anesthetics on CREB phosphorylation in
hippocampal slices have not yet been studied. CREB phosphorylation
studies offer the opportunity to examine potential mechanisms that
contribute to POCD. We hypothesize that anesthesia might cause
memory impairment by inhibiting the phosphorylation of hippocampal
CREB. Previous studies have shown that CREB plays a key role in
hippocampal synaptic plasticity and memory formation. However, its
role in POCD remains to be determined.
Materials and methods
Handling procedures were made according to the Guide
for the Care and Use of Laboratory Animals. A total of 72 BALB/C
mice (both sex, weight: 18–22 g; Capital University of Medical
Science, Beijing, China) were used to perform the experiments. Mice
were housed on a 12:12 light/dark cycle, with food and water ad
libitum. The experimental protocol was approved by the
Institutional Animal Care and Use Committee of the Capital
University of Medical Science.
Preparation of hippocampal slices and
homogenates
Animals were sacrificed by decapitation. The brains
were quickly removed, and the hemispheres were separated. Each
hippocampi was carefully dissected and incubated in
Ca2+-free artificial cerebrospinal fluid (aCSF) [4°C,
116 mM of NaCl, 26.2 mM of NaHCO3, 5.4 mM of KCl, 0.9 mM
of NaH2PO4 and 5.6 mM of glucose adjusted to
pH 7.4, with 95%/5% (vol/vol) oxygen-carbon dioxide mixture].
The hippocampal slices (at a thickness of 450 µm)
prepared using a McIlwain tissue chopper (Campden Instruments Ltd.,
Loughborough, UK), transferred onto a six-well plate, and added
with 10 ml/well of aCSF (eight slices per well). The hippocampal
slices were allowed to stabilize for one hour under room
temperature. The diffusion of etomidate into the brain slices
requires approximately an hour to reach 80% equilibration at a
depth of 100 micromillimeters (12).
Slices were slowly warmed to 37°C and allowed to
equilibrate for 60 min without stimulation until pharmacologic
treatment. The aCSF in the chamber was maintained at 37°C for the
experiment.
At the end of the experiments, cerebrospinal fluid
was aspirated, and the slices were frozen in liquid nitrogen. The
tissues were homogenized by sonication in 100 µl of ice-cold
homogenization buffer, which consisted of 50 mM of Tris-HCL (pH
7.5), 1 mM of sodium orthovanadate, 2 mM of
ethylenediaminetetraacetic acid, 2 mM of ethylene glycol
tetraacetic acid, 1 mM of dithiothreitol, 5 mM of sodium
pyrophosphate, 5 mM of potassium fluoride, 100 nM of okadaic acid,
0.5% IGEPAL CA-630 (Np-40), and protease inhibitors (5 µg/ml of
leupeptin, 5 µg/ml of aprotinin, 5 µg/ml of pepstatin and 5 µg/ml
of chymostatin), and boiled for five min. The homogenates were
stored at −70°C until processing.
Chemicals and anesthetics
The effects of the following pharmacological and
anesthetic agents on CREB phosphorylation were studied alone or in
combination with the following agents: Propofol (1 nM-100 µM;
AstraZeneca, London, UK), etomidate (1 nM-100 µM; Enhua
Pharmaceuticals, Xuzhou, China), ketamine (1 nM-100 µM; SBPC,
Shanghai, China), midazolam (1 nM-100 µM; Roche, Basel,
Switzerland), NMDA (1 mM; Sigma, St. Louis, MO), MK801 (10 µM;
Sigma), chelerythrine (an inhibitor of PKC, 100 µM, Merck,
Kenilworth, NJ), phorbol 12-myristate 13-acetate (PMA, an activator
of PKC, 0.1 µM; Sigma), carbachol [100 µM, an activator of PLC;
Sigma], U73122 (50 µM, an inhibitor of PLC; Sigma), and PD 98059
(20 µM, an inhibitor of MEK; Sigma). Anesthetics were applied for
60 min, while chelerythrine, PMA, carbachol, U73122, MK801, NMDA
and PD 98059 were pre-incubated for one hour before adding any
other anesthetics.
In the time-response experiment, hippocampi in one
group was continuously exposed to 5 µM of propofol for 1, 2, 5, 7,
9, 12, 15, 30 and 60 min, respectively, while hippocampi in the
other group was incubated for five min only, and washed thoroughly
with plain aCSF for 2, 4, 7, 10 and 25 min, respectively, in order
to determine the recovery of CREB phosphorylation.
Immunoblot analysis
Protein concentration in the homogenates was
determined by bicinchoninic acid-based assay using bovine serum
albumin as a standard. Equal amounts of protein (50 µg) were
separated on 10% polyacrylamide gel in the presence of sodium
dodecyl sulphate, and transferred onto nitrocellulose membranes
(Bio-Rad Laboratories, Inc.,, Hercules, CA, USA). The blots were
blocked with 10% non-fat dried milk in phosphate buffered saline
with 0.1% Tween-20 for one hour at room temperature with agitation.
Then, the membranes were incubated with rabbit anti-phosphor-CREB
antibody (1:800; Cell Signaling Technology, Beverly, MA, USA) at
4°C overnight. Next, horseradish peroxidase-conjugated anti-rabbit
IgG (Chemicon, Temecula, CA, USA) was used at a dilation of 1:5,000
and the immune complex was detected by enhanced chemiluminescence
western blotting detection reagents (Chemicon). In order to
determine the percentage of phosphorylated CREB, the membranes were
stripped and incubated with rabbit anti-CREB antibody (1:800; Cell
Signaling Technology), and processed, as aforementioned.
Immunoreactive bands were quantified using a computer-assisted
densitometer, and expressed as a ratio between active CREB signal
and total CREB.
Statistical analysis
Statistical analysis was performed on raw data using
one-way analyses of variance (ANOVA), and statistical differences
between the control and experimental groups were determined using
the least significant difference procedure for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference. Data (mean ± standard deviation) were
normalized against the P-CREB/total-CREB ratio of the controls.
Results
Propofol, etomidate, ketamine and
midazolam inhibited the phosphorylation of CREB
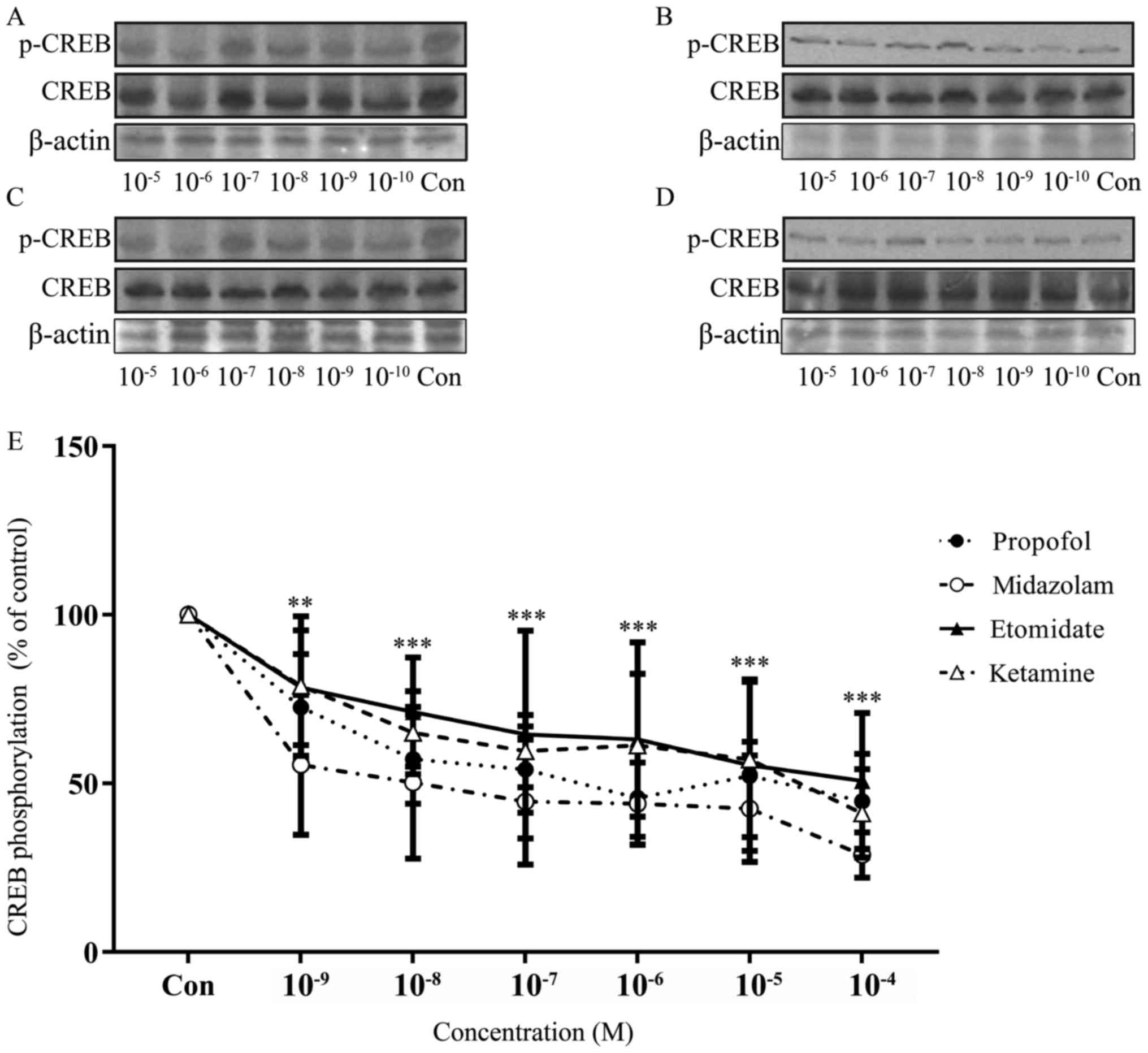
In order to evaluate the effects of propofol,
etomidate, ketamine and midazolam on the phosphorylation of CREB,
western blot analysis was performed on the hippocampus slices
exposed to a range of doses of individual drugs. The anesthetics
used at these concentrations markedly reduced the phosphorylation
of CREB at Ser133 in a dose-dependent manner (Fig. 2).
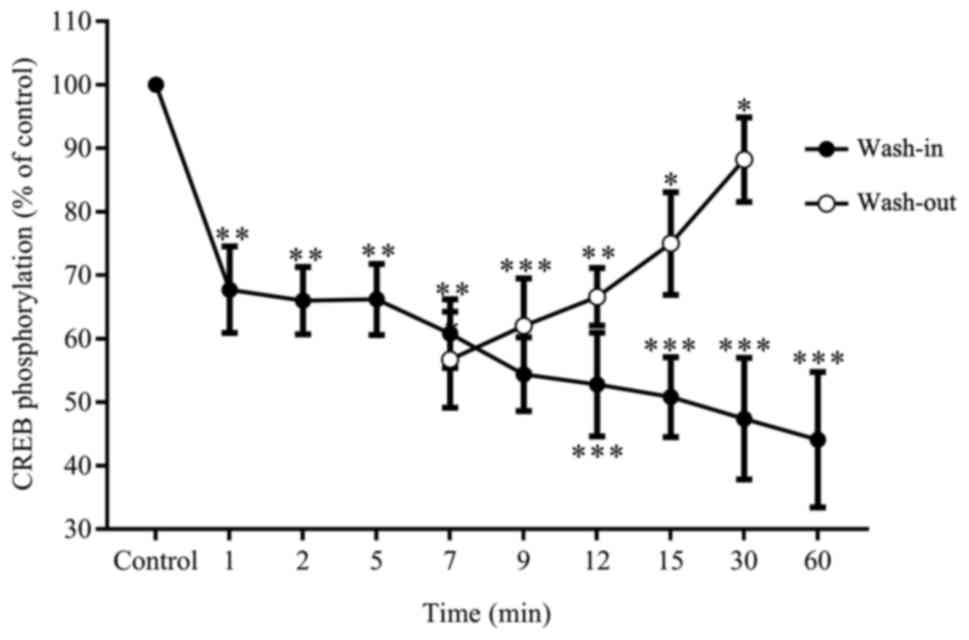
Next, it was determined whether the inhibition of
CREB phosphorylation by anesthetics was reversible using propofol,
which is a most commonly used intravenous anesthetic. One group of
hippocampi were continuously exposed to 5 µM of propofol for 1, 2,
5, 7, 9, 12, 15, 30 and 60 min, while the other group was
challenged for five min only. These were thoroughly washed and
analyzed in parallel for CREB phosphorylation. In these results,
exposure to 5 µM of propofol induced a reduction in CREB
phosphorylation, which proportionally decreased with exposure time.
Therefore, a 60-minute exposure to stimulating agents was selected
for further experiments. In contrast, the removal of propofol after
five min of exposure resulted in the recovery of CREB
phosphorylation starting from the seven-minute time point (Fig. 3).
Previous studies have demonstrated the involvement
of kinase receptor pathways in the interference with cognition by
intravenous anesthetics (13).
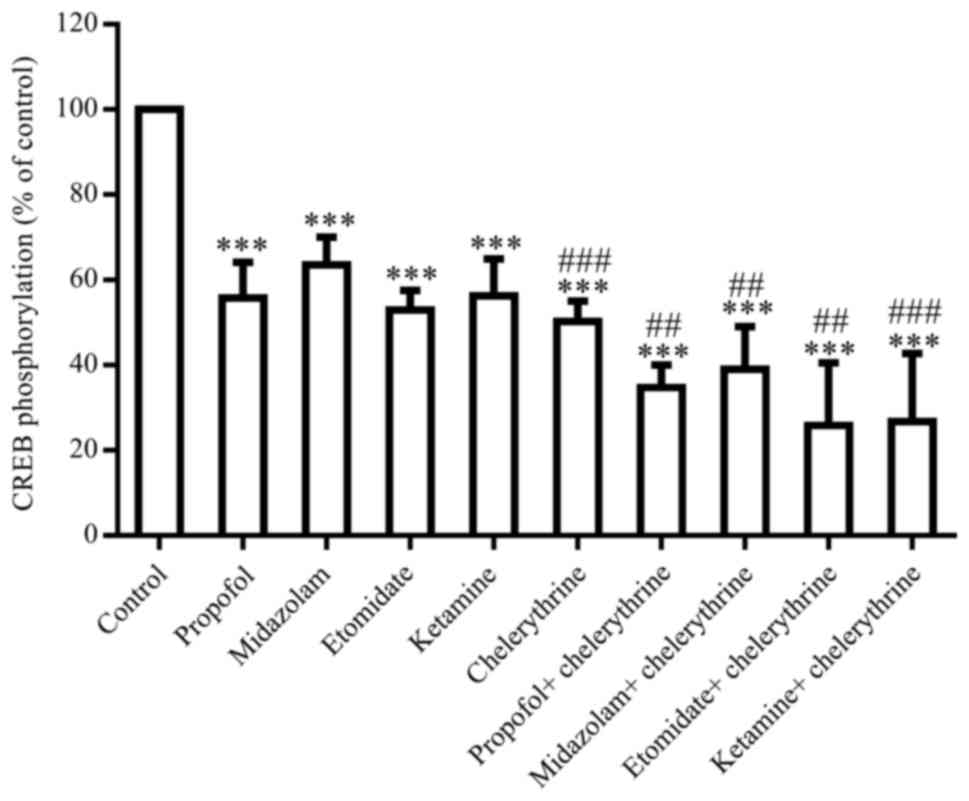
Therefore, the agonists and antagonists of PKC, PLC, MEK and NMDA
receptors were used in this study to characterize the functional
importance of individual pathways. The inhibition of PKC by
chelerythrine (100 µM) resulted in a 50.3% decrease in CREB
phosphorylation (P<0.05), and revealed the additive effects of
the anesthetic-induced decrease in CREB phosphorylation (Fig. 4). The activation of PKC by PMA (0.1
µM) induced a 65.9% decrease in CREB phosphorylation (P<0.05),
showing no effects on the anesthetic-induced decrease in CREB
phosphorylation (data not shown).
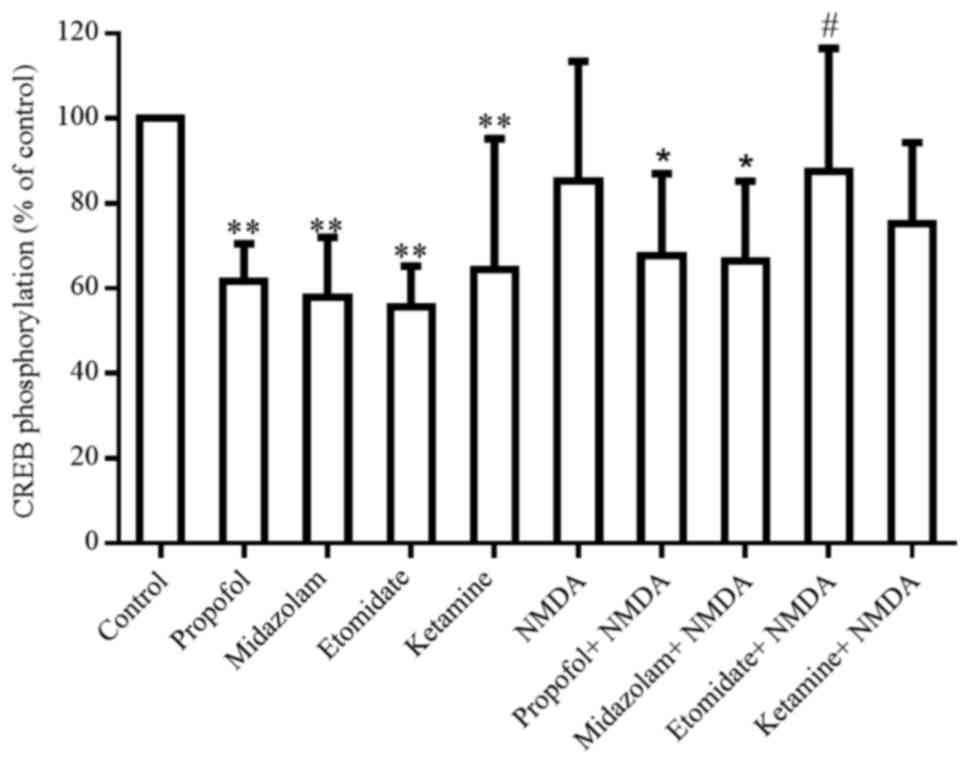
NMDA (1 mM) induced a weak but not significant
reduction in CREB phosphorylation (Fig. 5). This completely blocked the
etomidate-induced decrease in CREB phosphorylation, but only
partially suppressed the effects of other anesthetic agents
(Fig. 5).
In contrast, an NMDA receptor antagonist, MK801 (10
µM), was ineffective in blocking the anesthetic-induced decrease in
CREB phosphorylation. Moreover, the inhibition of PLC by U 73122
(50 µM) and the activation of PLC by carbachol (100 µM) also had no
effects on the anesthetic-induced suppression of CREB
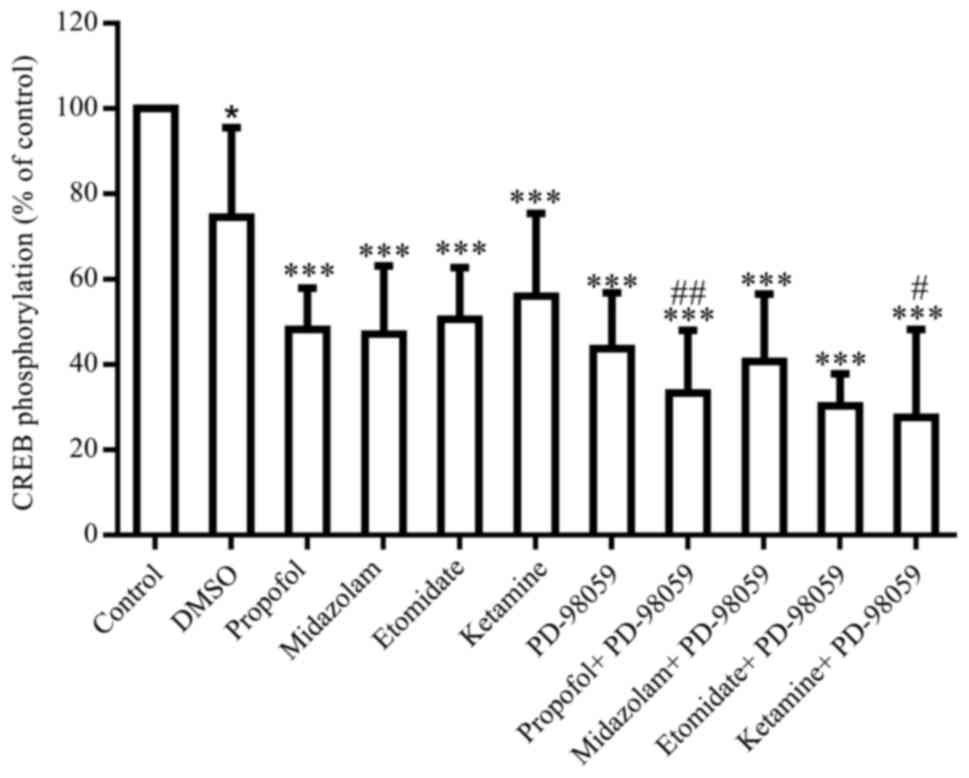
phosphorylation. PD-98059 (an inhibitor of MEK, 20 µM) induced a
significant decrease in CREB phosphorylation by 43.7% (P<0.05),
and revealed an additive or synergistic influence on the propofol-
and a ketamine-induced decrease in CREB phosphorylation (Fig. 6).
Discussion
In the present study, it was shown that clinically
relevant concentrations of intravenous anesthetic agents decreased
the phosphorylation of CREB in mice hippocampal slices. These
effects are likely to be mediated indirectly via the NMDA receptor,
PKC and MAPK/ERK signaling pathways. These present findings support
the notion that the phosphorylation of CREB represents a target for
anesthetic action in the central nervous system.
Cognitive side-effects such as emergence agitation
(EA), postoperative delirium (POD) and POCD do not infrequently
complicate postoperative care, especially in elderly and fragile
patients (14). POCD is a
recognized clinical entity characterized with cognitive deficits
after anesthesia and surgery, especially in elderly patients
(15).
Recent evidence has indicated that propofol and
midazolam impair memory for aversive and non-aversive experiences
at equianxiolytic doses that do not produce locomotor impairment in
rats (16). In humans, ketamine
selectively affects working, episodic and procedural memory, but
not perceptual priming, attention, or executive functioning.
9These findings suggest that intravenous anesthetics
disrupt memory formation, and may potentially suppress the activity
of transcription regulators that are considered to contribute to
memory processing. Transcriptional factor CREB is activated in
spatial learning (17). The radial
maze training in rats increases CREB phosphorylation in the
hippocampus in the course of spatial learning, which is followed by
spatial memory formation (17).
Furthermore, CREB is an evolutionarily conserved transcription
regulator essential for long-term memory formation (18), and plays a critical role in
long-term memory in invertebrates and vertebrates (19). In the present experiments, it was
observed that all intravenous anesthetic agents inhibited CREB
phosphorylation in proportion to dosages. This inhibitory effect on
CREB phosphorylation in the hippocampus coincided with the amnestic
effects of anesthetics in clinical practice. We also observed the
reversibility of these anesthetic effects on CREB phosphorylation,
at least for propofol, although the direct extrapolation to all
anesthetics could not be made. The lack of desensitization of its
effects over time and the reversibility of the propofol-induced
inhibition of CREB phosphorylation further support the relevance of
these present findings to anesthesia.
CREB is a vital part of many intracellular signaling
events that regulate multiple neural functions. Human recombinant
CREB can be phosphorylated by cAMP-dependent protein kinase and PKC
in vitro (20), but
preferentially at distinct serine residues. Two serines located in
the basic region of CREB, Ser-340 and Ser-367, are the major PKC
phosphorylation sites (20).
It has been previously shown that CREB
phosphorylation is principally regulated by PKC in immature
oligodendrocytes (21), and the
inhibition of PKC blocks the retinoic acid-mediated activation of
CREB (22). The PKC family
consists of several members, which can be divided into three major
groups: Classical PKC (α, βI, βII and γ), novel PKC (δ, ε, η and
θ), and atypical PKC (ζ and ι/λ) (23). GF 109203X inhibited the
conventional isoforms and novel isoforms of PKC (24,25).
A significant decrease in CREB phosphorylation was also observed
with the pre-treatment of 1 µM of GF 109203X, indicating that the
conventional and novel isoforms of PKC are responsible for CREB
phosphorylation after OX2R activation in CHO cells (26). The phosphorylation of CREB at
serine-133 induced by signaling through the B-cell antigen receptor
requires PKCδ (27). PKCε is
implicated in cytokine-induced serine-133 phosphorylation and the
activation of CREB-mediated transcription in human erythroleukemia
cell line TF-1. However, PKCε forms a component of the signal
cascade rather than act as the genuine CREB kinase (28). Chelerythrine is also a large
spectrum PKC inhibitor.
Furthermore, the apparent additive effect of PKC
inhibitor chelerythrine was observed on the anesthetic-induced
inhibition of CREB phosphorylation, while PKC activation by PMA
revealed no obvious changes. These results suggest that PKC is
responsible for the anesthetic-induced decrease in CREB
phosphorylation at Ser133, while its activation had no effect. The
phosphorylation of platelet protein P47, which is a marker of PKC
activation, is markedly inhibited by ketamine (350 µM) or midazolam
(15 and 30 µM) (29,30), and there is little direct evidence
on the effect of intravenous anesthetics on PKC.
Behavioral, anatomical and electrophysiological
studies have shown that hippocampal NMDA receptors are involved in
human memory (31). NMDA
stimulation induces the rapid phosphorylation of CREB on Ser133
during the development of hippocampal neurons in culture (32). The activation of NMDA receptors by
NMDA rapidly and concentration-dependently increases the number of
neurons expressing phosphorylated CREB, while antagonizing NMDA
signaling by MK801 reduces CREB phosphorylation (33). In addition, the transcription of
NMDAR1 is regulated by the c-AMP signaling pathway, which is most
likely through the binding of CREB and its activation through
signal-dependent phosphorylation (34). It is now clear that neither
AMPA/kainate receptors, nor NMDA receptors are sufficient to
independently stimulate a second messenger pathway that leads to
CREB phosphorylation (35). In the
present study, it was observed that NMDA treatment induced a weak
but not significant decrease in CREB phosphorylation. The NMDA
treatment itself did not increase CREB phosphorylation in the
present experiment. It was reported that an increase in p-CREB
protein level was observed from 6–12 h after NMDA injection in the
retina (36). Moreover, CREB
phosphorylation after exposure to glutamate was shown to be
dependent on CaMK II/IV in hippocampal neurons (37). However, this completely blocked the
etomidate-induced decrease in CREB phosphorylation, and partially
blocked the inhibition of other anesthetic agents. Lipid emulsions
have been used as carriers for hypnotics such as propofol and
etomidate. Lipid emulsions activate NMDA receptor channels in
cortical neurons (38). It was
observed that NMDA had an additive effect in the presence of
etomidate. It is noteworthy to mention that inhibiting NMDA
signaling by MK801 is not enough to block the anesthetic-induced
decrease in ERK1/2 phosphorylation. This present observation
supports the hypothesis that intravenous anesthetic agents may
induce a decrease in CREB phosphorylation via NMDA receptors. It
has been reported that there is a consecutive pathway from
AMPA/kainate receptors to NMDA receptors and from NMDA receptors to
L-type Ca2+ channels. AMPA/kainate receptors are
involved in relieving the Mg2+ block of NMDA receptors,
and NMDA receptors trigger the opening of L-type Ca2+
channels. The second messenger pathway that activates CREB
phosphorylation is likely activated by Ca2+entry through
L-type Ca2+ channels (39).
The MAPK pathway is another signaling cascade that
regulates CREB (39). MAPK
activation is required for the phosphorylation of CREB in response
to the activation of PKC, and is needed for PKA coupling to CREB
phosphorylation in the CA1 area (34). Furthermore, activated MAPK
increases the phosphorylation of CREB by stimulating its gene
expression (40). Hence, the
inhibition of ERK1/2 by PD98059 reduces CREB phosphorylation
(41). It was observed that
PD-98059 induced a significant decrease in CREB phosphorylation,
and revealed its additive influence on the propofol- and
ketamine-induced decrease in CREB phosphorylation. These results
indicate that intravenous anesthetic agents may induce a decrease
in CREB phosphorylation via the ERK1/2 signal transduction pathway.
Propofol, midazolam and etomidate act at GABAA receptors, and
ketamine act at NMDA receptors. The enhancement of GABAA
receptor-mediated inhibition is a property of most general
anesthetics, which is a candidate for the molecular mechanism of
anesthesia (42,43). Ketamine is an NMDA receptor
antagonist anesthetic agent (44).
Since GABAA is not related to MAPK-CREB, a GABA (A) agonist and
antagonist experiment was not performed.
Taken together, clinically relevant concentrations
of intravenous anesthetic agents decrease the phosphorylation of
CREB in mice hippocampal slices. These effects are likely to be
mediated indirectly via the NMDA receptor, PKC and ERK1/2 signaling
pathways. These findings support the idea that these inhibitory
effects on CREB phosphorylation may be potential mechanisms that
contribute to anesthetic-induced amnesia.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Davis N, Lee M, Lin AY, Lynch L,
Monteleone M, Falzon L, Ispahany N and Lei S: Postoperative
cognitive function following general versus regional anesthesia: A
systematic review. J Neurosurg Anesthesiol. 26:369–376. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Androsova G, Krause R, Winterer G and
Schneider R: Biomarkers of postoperative delirium and cognitive
dysfunction. Front Aging Neurosci. 7:1122015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Silbert BS, Evered LA and Scott DA:
Incidence of postoperative cognitive dysfunction after general or
spinal anaesthesia for extracorporeal shock wave lithotripsy. Br J
Anaesth. 113:784–791. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moller JT, Cluitmans P, Rasmussen LS, Houx
P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD,
et al: Long-term postoperative cognitive dysfunction in the elderly
ISPOCD1 study. ISPOCD investigators. International Study of
Post-Operative Cognitive Dysfunction. Lancet. 351:857–861. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang N, Ou C, Liu Y, Zuo Y and Bai Y:
Effect of inhalational anaesthetic on postoperative cognitive
dysfunction following radical rectal resection in elderly patients
with mild cognitive impairment. J Int Med Res. 42:1252–1261. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Middei S, Houeland G, Cavallucci V,
Ammassari-Teule M, D'Amelio M and Marie H: CREB is necessary for
synaptic maintenance and learning-induced changes of the AMPA
receptor GluA1 subunit. Hippocampus. 23:488–499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alberini CM: Genes to remember. J Exp
Biol. 202:2887–2891. 1999.PubMed/NCBI
|
|
8
|
Yin JC and Tully T: CREB and the formation
of long-term memory. Curr Opin Neurobiol. 6:264–268. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johnston MV, Alemi L and Harum KH:
Learning, memory, and transcription factors. Pediatr Res.
53:369–374. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao C, Che LW, Chen J, Xu XJ and Chi ZQ:
Ohmefentanyl stereoisomers induce changes of CREB phosphorylation
in hippocampus of mice in conditioned place preference paradigm.
Cell Res. 13:29–34. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang H, Zhang SB, Zhang QQ, Liu M, He XY,
Zou Z, Sun HJ, You ZD and Shi XY: Rescue of cAMP response
element-binding protein signaling reversed spatial memory retention
impairments induced by subanesthetic dose of propofol. CNS Neurosci
Ther. 19:484–493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Benkwitz C, Liao M, Laster MJ, Sonner JM,
Eger EI II and Pearce RA: Determination of the EC50 amnesic
concentration of etomidate and its diffusion profile in brain
tissue: Implications for in vitro studies. Anesthesiology.
106:114–123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Praticò C, Quattrone D, Lucanto T, Amato
A, Penna O, Roscitano C and Fodale V: Drugs of anesthesia acting on
central cholinergic system may cause post-operative cognitive
dysfunction and delirium. Med Hypotheses. 65:972–982. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jildenstål PK, Rawal N, Hallén JL,
Berggren L and Jakobsson JG: Perioperative management in order to
minimise postoperative delirium and postoperative cognitive
dysfunction: Results from a Swedish web-based survey. Ann Med Surg
(Lond). 3:100–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jia M, Liu WX, Sun HL, Chang YQ, Yang JJ,
Ji MH, Yang JJ and Feng CZ: Suberoylanilide hydroxamic acid, a
histone deacetylase inhibitor, attenuates postoperative cognitive
dysfunction in aging mice. Front Mol Neurosci. 8:522015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pain L, Angst MJ, LeGourrier L and
Oberling P: Effect of a nonsedative dose of propofol on memory for
aversively loaded information in rats. Anesthesiology. 97:447–453.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mizuno M, Yamada K, Maekawa N, Saito K,
Seishima M and Nabeshima T: CREB phosphorylation as a molecular
marker of memory processing in the hippocampus for spatial
learning. Behav Brain Res. 133:135–141. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taubenfeld SM, Wiig KA, Monti B, Dolan B,
Pollonini G and Alberini CM: Fornix-dependent induction of
hippocampal CCAAT enhancer-binding protein [beta] and [delta]
co-localizes with phosphorylated cAMP response element-binding
protein and accompanies long-term memory consolidation. J Neurosci.
21:84–91. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Josselyn SA and Nguyen PV: CREB, synapses
and memory disorders: Past progress and future challenges. Curr
Drug Targets CNS Neurol Disord. 4:481–497. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakurai A, Maekawa T, Sudo T, Ishii S and
Kishimoto A: Phosphorylation of cAMP response element-binding
protein, CRE-BP1, by cAMP-dependent protein kinase and protein
kinase C. Biochem Biophys Res Commun. 181:629–635. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shiga H, Yamane Y, Kubo M, Sakurai Y, Asou
H and Ito E: Differentiation of immature oligodendrocytes is
regulated by phosphorylation of cyclic AMP-response element binding
protein by a protein kinase C signaling cascade. J Neurosci Res.
80:767–776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aggarwal S, Kim SW, Cheon K, Tabassam FH,
Yoon JH and Koo JS: Nonclassical action of retinoic acid on the
activation of the cAMP response element-binding protein in normal
human bronchial epithelial cells. Mol Biol Cell. 17:566–575. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Steinberg SF: Structural basis of protein
kinase C isoform function. Physiol Rev. 88:1341–1378. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Toullec D, Pianetti P, Coste H,
Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P,
Boursier E, Loriolle F, et al: The bisindolylmaleimide GF 109203X
is a potent and selective inhibitor of protein kinase C. J Biol
Chem. 266:15771–15781. 1991.PubMed/NCBI
|
|
25
|
Le Panse R, Coulomb B, Mitev V, Bouchard
B, Lebreton C and Dubertret L: Differential modulation of human
fibroblast and keratinocyte growth by the protein kinase C
inhibitor GF 109203X. Mol Pharmacol. 46:445–451. 1994.PubMed/NCBI
|
|
26
|
Guo Y and Feng P: OX2R activation induces
PKC-mediated ERK and CREB phosphorylation. Exp Cell Res.
318:2004–2013. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blois JT, Mataraza JM, Mecklenbraüker I,
Tarakhovsky A and Chiles TC: B cell receptor-induced cAMP-response
element-binding protein activation in B lymphocytes requires novel
protein kinase Cdelta. J Biol Chem. 279:30123–30132. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gubina E, Luo X, Kwon E, Sakamoto K, Shi
YF and Mufson RA: betac cytokine receptor-induced stimulation of
cAMP response element binding protein phosphorylation requires
protein kinase C in myeloid cells: A novel cytokine signal
transduction cascade. J Immunol. 167:4303–4310. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang Y, Chen TL, Wu GJ, Hsiao G, Shen MY,
Lin KH, Chou DS, Lin CH and Sheu JR: Mechanisms involved in the
antiplatelet activity of ketamine in human platelets. J Biomed Sci.
11:764–772. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hsiao G, Shen MY, Chou DS, Chang Y, Lee
LW, Lin CH and Sheu JR: Mechanisms of antiplatelet and
antithrombotic activity of midazolam in in vitro and in vivo
studies. Eur J Pharmacol. 487:159–166. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grunwald T, Beck H, Lehnertz K, Blümcke I,
Pezer N, Kurthen M, Fernández G, Van Roost D, Heinze HJ, Kutas M
and Elger CE: Evidence relating human verbal memory to hippocampal
N-methyl-D-aspartate receptors. Proc Natl Acad Sci USA.
96:12085–12089. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sala C, Rudolph-Correia S and Sheng M:
Developmentally regulated NMDA receptor-dependent dephosphorylation
of cAMP response element-binding protein (CREB) in hippocampal
neurons. J Neurosci. 20:3529–3536. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mao L and Wang JQ: Interactions between
ionotropic and metabotropic glutamate receptors regulate cAMP
response element-binding protein phosphorylation in cultured
striatal neurons. Neuroscience. 115:395–402. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lau GC, Saha S, Faris R and Russek SJ:
Up-regulation of NMDAR1 subunit gene expression in cortical neurons
via a PKA-dependent pathway. J Neurochem. 88:564–575. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rajadhyaksha A, Barczak A, Macı'as W,
Leveque JC, Lewis SE and Konradi C: L-type Ca(2+) channels are
essential for glutamate-mediated CREB phosphorylation and c-fos
gene expression in striatal neurons. J Neurosci. 19:6348–6359.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takeda H, Kitaoka Y, Hayashi Y, Kumai T,
Munemasa Y, Fujino H, Kobayashi S and Ueno S:
Calcium/calmodulin-dependent protein kinase II regulates the
phosphorylation of CREB in NMDA-induced retinal neurotoxicity.
Brain Res. 1184:306–315. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mabuchi T, Kitagawa K, Kuwabara K,
Takasawa K, Ohtsuki T, Xia Z, Storm D, Yanagihara T, Hori M and
Matsumoto M: Phosphorylation of cAMP response element-binding
protein in hippocampal neurons as a protective response after
exposure to glutamate in vitro and ischemia in vivo. J Neurosci.
21:9204–9213. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Weigt HU, Georgieff M, Beyer C and Föhr
KJ: Activation of neuronal N-methyl-D-aspartate receptor channels
by lipid emulsions. Anesth Analg. 94:331–337. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Villarreal JS and Barea-Rodriguez EJ: ERK
phosphorylation is required for retention of trace fear memory.
Neurobiol Learn Mem. 85:44–57. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Roberson ED, English JD, Adams JP, Selcher
JC, Kondratick C and Sweatt JD: The mitogen-activated protein
kinase cascade couples PKA and PKC to cAMP response element binding
protein phosphorylation in area CA1 of hippocampus. J Neurosci.
19:4337–4348. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Alonso M, Vianna MR, Izquierdo I and
Medina JH: Signaling mechanisms mediating BDNF modulation of memory
formation in vivo in the hippocampus. Cell Mol Neurobiol.
22:663–674. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li GD, Chiara DC, Cohen JB and Olsen RW:
Numerous classes of general anesthetics inhibit etomidate binding
to gamma-aminobutyric acid type A (GABAA) receptors. J Biol Chem.
285:8615–8620. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Straub CJ, Lau HM, Parlato R, Schuetz G,
Fritschy JM and Rudolph U: Bidirectional regulation of intravenous
general anesthetic actions by α3-containing γ-aminobutyric acid A
receptors. Anesthesiology. 118:562–576. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dutta A, McKie S and Deakin JFW: Ketamine
and other potential glutamate antidepressants. Psychiatry Res.
225:1–13. 2015. View Article : Google Scholar : PubMed/NCBI
|