At present, 17 types of vitamin K-dependent proteins
(VKDPs) are reported to exist (1),
including coagulation factor prothrombin (II), proconvertin (VII),
antihemophilic factor (IX), Stuart factor (X, Stuart-Power factor),
matrix Gla protein (MGP), growth arrest-specific protein 6 (Gas6),
anticoagulant proteins C, S and Z, osteocalcin (OC), Gla-rich
protein (GRP), periostin (isoforms 1–4), periostin-like-factor
(PLF), proline-rich Gla protein (PRGP) 1, PRGP2, transmembrane Gla
protein (TMG) 3 and TMG4 (2–5). In
2002, only 14 types of vitamin K-dependent proteins had been
identified in the human body (2).
Vitamin K is the coenzyme for the glutamate γ-carboxylase (GGCX)
enzyme and promotes the transformation of vitamin K-dependent
protein glutamic acid (Glu) residues to γ-glutamic acid (Gla)
residues (6,7). Coagulation factors II, VII, IX and X,
and anticoagulation proteins C, S and Z, all depend on vitamin
K1 (3) in liver
synthesis. OC, MGP, Gas6 and GRP in the tissue outside the liver
rely on vitamin K for post-transcriptional modification (3). However, the function of PRGP1, PRGP2,
TMG3 and TMG4 remains unclear (2).
A portion of vitamin K remains in the liver in the form of
2,3-epoxide; the enzyme, cyclooxygenase, exhibits catalytic action
on the hydroquinone form of vitamin K, transforming it into
2,3-epoxide, and both cyclooxygenase and carboxylase are present
within the microsome. Epoxide is converted into the uniquinone form
of vitamin K by the action of epoxide reductase. Finally,
uniquinone may be reduced into the hydroquinone form of vitamin K.
In this way, the cycle of vitamin K is completed. The cycle
produces the epoxide form of vitamin K and thus ‘regenerates’
vitamin K (8).
Globally, osteoporosis is a major disease that is
associated with high trauma and/or fragility fracture (9). The population of the elderly and
postmenopausal women is continuously increasing and members of this
population are vulnerable to bone fracture. The incidence of hip
fracture was reported as 1.66 million cases globally in 1990, which
is estimated to increase to 6.26 million by 2050 (10). Supplemental calcium may be
beneficial for bone mineral density, the promotion of bone strength
and the prevention of osteoporosis. However, certain reports have
indicated that increased intake of calcium supplements may increase
the risk of heart disease and may be associated with enhanced
deposition of calcium within blood vessel walls and soft tissues
(11–16). In addition, the γ-carboxylated OC
aids the removal of calcium from the blood and its binding to the
bone matrix. It has been reported that the ability of OC to bind to
the mineral component of bone, termed hydroxyapatite, may partially
explain its ability to affect bone mineralisation (17).
Cardiovascular diseases (CVDs) are a leading cause
of death in the world. There were 12.59 million deaths (95% UI:
12.38 to 12.80 million deaths) due to CVDs in 1990, increasing to
17.92 million deaths (95% UI: 17.59 to 18.28 million deaths) in
2015. CVDs accounted for one-third of all deaths in 2015, and there
were an estimated 422 million prevalent cases (18,19).
The World Health Organisation estimated that~17.3 million deaths in
2013 were associated with CVDs, which has been forecasted to
increase to ~23.3 million in 2030 (20). MGP has been demonstrated to be a
potent inhibitor of vascular calcification (21). Calcification of the arteries is
commonly observed in elderly individuals; of those >70 years of
age, it has been reported that >96% exhibit aortic and coronary
artery calcification (22). This
observation is important as aortic calcification is associated with
an enhanced risk of atherosclerosis, myocardial infarction and
renal disease (23). Vitamin K
functions as a co-factor Gla carboxylation, which leads to the
formation of a modified amino acid that is termed γ-carboxyglutamic
acid (24), and vitamin K2 has
been reported to be associated with the inhibition of arterial
stiffening and arterial calcification (25,26).
In addition, a high intake of vitamin K was demonstrated to
decrease coronary artery calcium levels and the subsequent risk of
CVDs, coronary heart disease-associated mortality and the
calcification of arterial and aortic valves (23,27,28).
The present review discusses a number of different vitamin
K-dependent proteins.
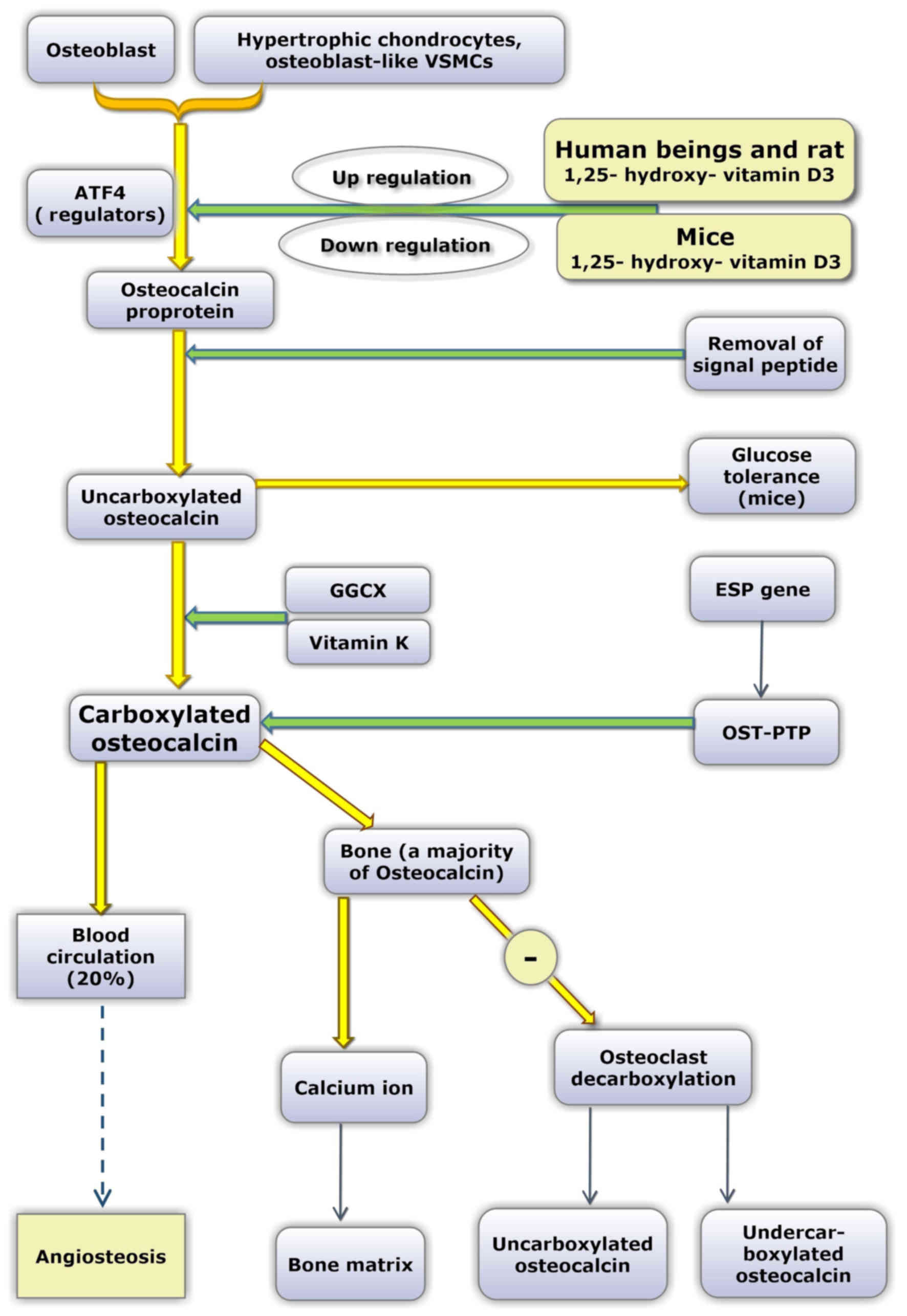
OC contains 49 amino acid residues and is
synthesized and secreted by osteoblasts, odontoblasts and
hypertrophic cartilage cells. The OC gene of humans is located on
chromosome 1, and the earliest form of the OC protein is the OC
proprotein. Subsequently, the signal peptide is removed (29). Uncarboxylated OC (uOC) is converted
into carboxylated OC due to the action of GGCX and vitamin K, which
acts as a coenzyme. The Glu amino acid residues are located at
positions 21, 24 and 27.
In the event of calcified plaques, the secretion of
inflammatory factors such as interleukin-8 and monocyte chemotactic
factor-1 was reduced in the blood vessels, compared with
non-calcified plaques, while bone morphogenetic protein (BMP)-6, OC
and other protein factors that promote bone formation were
increased in calcified plaques (38). It has been suggested that
calcification may exhibit a negative correlation with inflammation
in patients with carotid atherosclerosis (39,40).
However, the specific association and underlying mechanism of OC
and atherosclerosis require further investigation.
Laboratory mice are commonly employed as animal
models. However, in relation to OC, certain differences exist
between humans and mice. First, only a single OC gene has been
reported in humans, while three different OC genes have been
identified in mice. In mice, ~60% of protein sequences are
conserved compared with humans (45,46).
Secondly, in response to 1,25-dihydroxy vitamin D3, human OC genes
are upregulated in a dose-dependent manner by 1,25-dihydroxy
vitamin D3, whereas the mouse OC gene is downregulated by
1,25-dihydroxy vitamin D3 (47,48).
Additionally, in the majority of species, all three
vitamin-K-dependent γ-carboxyglutamic acid sites in the OC molecule
are fully carboxylated. However, in humans, OC in bone and serum is
incompletely carboxylated (undercarboxylated OC) (45). It has been suggested that as a
direct consequence of undercarboxylation of OC, human OC
concentration in bone and in the circulation are only 20% of that
exhibited by other species (49).
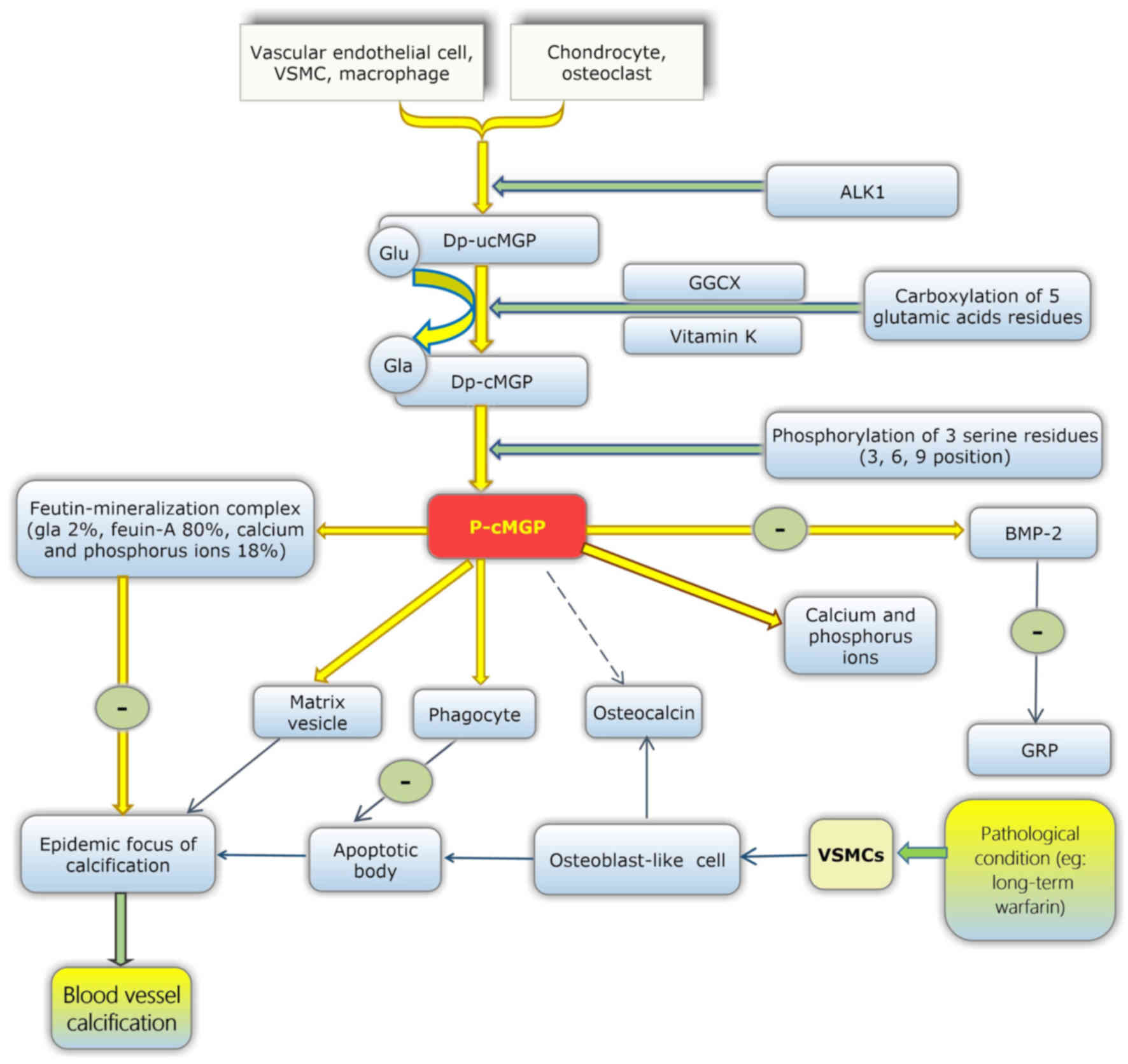
MGP is secreted from chondrocytes, arterial medial
vascular smooth muscle cells (VSMCs) (34), fibroblasts and endothelial cells.
MGP is also present in a large number of tissues, including the
arterial wall (50), the heart,
lung, kidney and skin. It has been reported that vitamin D in bone
cells may upregulate the expression of MGP (51).
The maturation of MGP involves two modification
steps. Initially, dephosphorylated-uncarboxylated MGP (dp-ucMGP) is
converted into undercarboxylated MGP (dp-cMGP). This process
enables five glutamate residues to be converted into γ-carboxylated
glutamate residues, which are termed Gla residues and provide
binding sites that target apoptotic bodies, calcium ions and matrix
vesicles. In the second process of modification, dp-cMGP is
converted into phosphorylated-carboxylated MGP (p-cMGP). This
process involves three serine residues (52,53).
However, the function of these serines remains unclear (Fig. 2).
According to epidemiological research, ectopic
calcification has an adverse effect on the occurrence and
development of CVDs (54). It has
been demonstrated via in vitro experiments that VSMC
calcification is induced by elevated inorganic phosphate (Pi)
uptake via a sodium-dependent phosphate cotransporter, and that
such calcification is also caused by phenotypic transition from
VSMCs to osteoblast-like cells and apoptotic cell death (55–61).
VSMCs' osteoblastic differentiation is regulated by the
upregulation of numerous osteogenic genes, including osteopontin,
runt related transcription factor 2 and OC (53,57).
VSMCs still affect the production of matrix vesicles. The matrix
vesicles may provide a suitable microenvironment for the deposition
of calcium in the vessel wall (62). Fibroblasts and mesenchymal stem
cells (MSCs) in the outer membrane may also be involved in arterial
calcification (63).
MGP acts as an inhibitor in the deposition and
crystallisation of calcium in the blood vessel wall. Carboxylated
MGP inhibits ectopic mineralisation by combining with calcium
crystals, thus inhibiting their growth, and also functions through
the binding and inhibition of BMP-2 (64–66).
Additionally, fetuin-mineralisation complexes have been observed in
animal experiments, which consist of MGP (2%), fetuin-A (80%), and
calcium and phosphorus ions (18%) (Fig. 2). Fetuin-mineralisation complexes
may effectively inhibit the growth, aggregation and deposition of
minerals. Therefore, MGP may also affect ectopic calcification via
fetuin-MGP-mineralisation complexes (67). Thus, reduced vitamin K may lead to
reduced carboxylated MGP and subsequently reduced inhibition of
blood vessel calcification. Therefore, the deficiency of vitamin K
may increase the risk of vascular calcification.
Osteophyma, also recognised as bone hyperplasia or
bone spur, refers to the formation of new bone tissue in the edge
of a bone, and the calcification of cartilage and meniscus is a
symptom of OA. This condition is associated with a poor quality of
life in the elderly population. Currently, no suitable treatment
measures are available to slow down the progression of OA (68). In epidemiological studies, low
levels of circulating vitamin K have been associated with hand and
knee OA cross-sections (69), with
substantial knee OA progression and cartilage loss longitudinally
(70).
Research has demonstrated that vitamin K deficiency
in subclinical conditions may contribute to an increased risk of
developing radiographic knee OA and magnetic resonance
imaging-based cartilage lesions (70). In fact, cartilage cells derived
from normal and OA conditions are able to produce MGP. Cartilage
cells derived from OA primarily produce uncarboxylated MGP, and
this form of MGP has no function. Meanwhile, cartilage cells from
normal cartilage produce carboxylated MGP, the functional form of
MGP, which indicates that OA may be associated with non-functional
MGP (71). Therefore, we
hypothesized that a deficiency in MGP carboxylation may be an
important cause of OA.
Consistently low dietary consumption of vitamin K,
leading to vitamin K deficiency, may lead to the inhibition of
vitamin K-dependent MGP and Gas6 functions, subsequently regulating
OA pathogenesis through effects on osteophytosis and cartilage
destruction (72). According to a
review published in 2003 (73),
three VKDPs are observed in the bone: OC, MGP and protein S. In
addition, certain studies have demonstrated that GRP and Gas6 are
also present within the bone and cartilage (70,74).
Thus, to a certain extent, the present review hypothesizes that
vitamin K, particularly vitamin K2, may serve a beneficial role in
the prevention of osteophytes. The specific underlying mechanism
and effect require further investigation.
The circulating total-uncarboxylated MGP (t-ucMGP)
concentration is 1,000 times higher compared with dp-ucMGP levels,
and t-ucMGP has been suggested to consist predominantly of
phosphorylated ucMGP (p-ucMGP) species. dp-ucMGP has no calcium
binding group and therefore cannot be retained in blood vessels
(50). In addition, a multivariate
logistic analysis suggested that dp-ucMGP represents a predictor of
peripheral arterial calcification independent from age, gender,
previous CVD and t-ucMGP levels; and is positively associated with
peripheral artery calcification (75). High levels of dp-ucMGP have also
been associated with aortic calcification in patients at different
stages of chronic kidney disease (CKD) (75,76).
It was previously reported that high dp-ucMGP levels
were associated with an elevated risk of CVD, particularly PAD and
heart failure, in patients with type 2 diabetes, while no
associations between alternative MGP species and CVD risk were
observed (50,77). Dalmeijer et al (77) demonstrated that low vitamin K
levels may be associated with an enhanced risk of CVD. In fact,
serum dp-ucMGP concentration was employed as a marker of vitamin K
concentration in vascular smooth muscle and vascular calcification
(78). In a previous study
(79), it was revealed that that
patients undergoing hemodialysis expressing the highest tertile of
dp-ucMGP levels had a significantly higher calcification score than
the patients in the lowest tertile. Furthermore, reduced levels of
non-phosphorylated carboxylated MGP have been revealed to be
associated with increased cardiovascular mortality and vascular
calcifications in patients undergoing dialysis (53). Schurgers et al (75) demonstrated a positive association
between dp-ucMGP levels and the calcification score in 107 patients
with CKD, including 40 patients undergoing hemodialysis (75). In addition, dp-ucMGP has been
associated with the severity of aortic calcification in patients
with CKD (78). Furthermore, high
dp-ucMGP levels have been suggested to be independently associated
with below-knee arterial calcification score in patients with type
2 diabetes exhibiting normal or slightly altered kidney function,
thus suggesting that estimated glomerular filtration rate remains a
strong predictor of MGP levels (50).
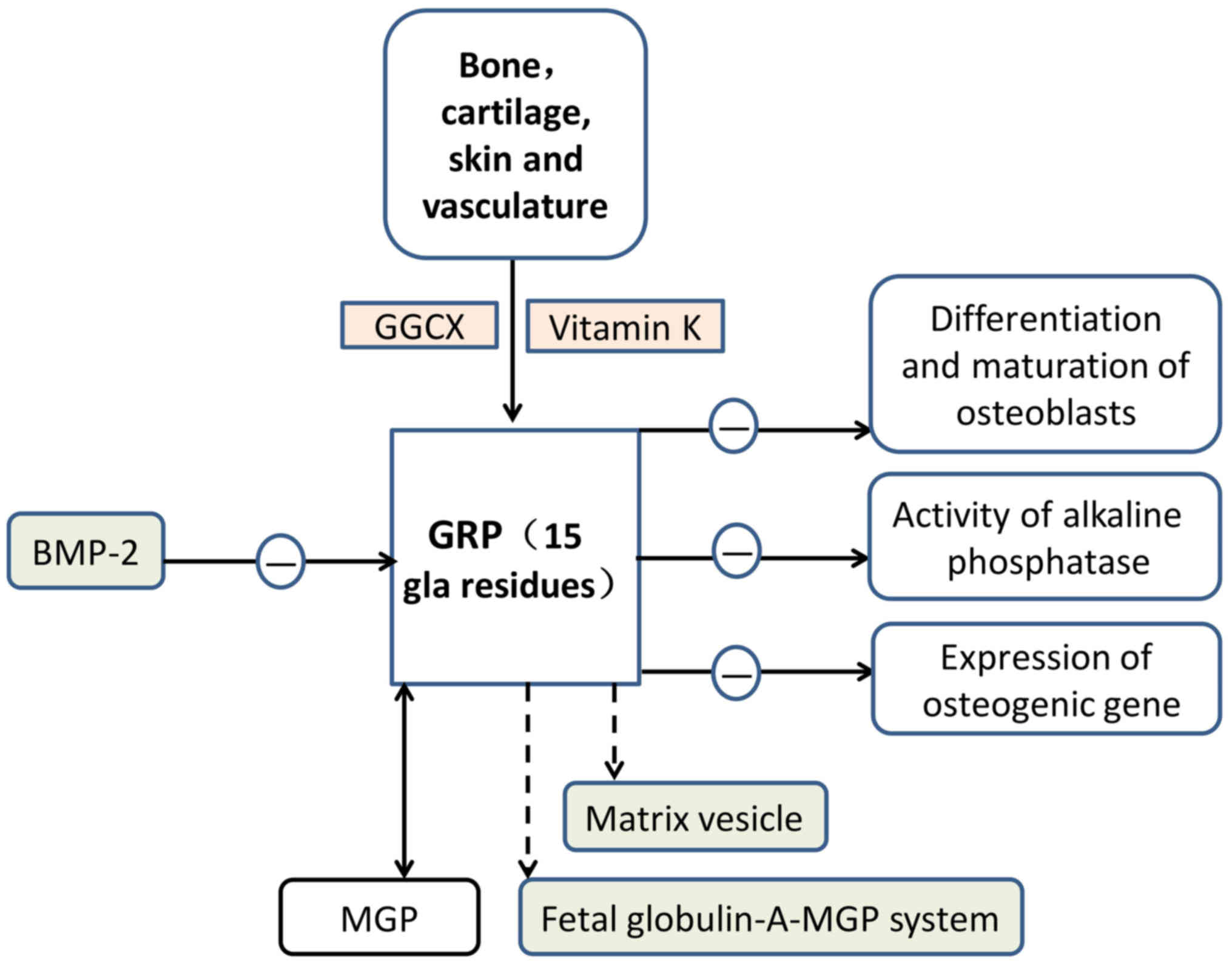
GRP, the most recently identified member of the VKDP
family, derives its name from the large number of Gla residues it
contains (68). GRP was first
identified in sturgeon-calcified cartilage (GRP of calcified
cartilage of Adriatic sturgeon has 16 Gla residues) and is
characterised by the presence of 15 putative Gla residues in man
(69,80–83).
Its distribution is primarily in the bone, cartilage, skin and
vasculature, where it serves an important role in inhibiting
vascular calcification (80,81,84,85).
GRP is considered to function as a negative
regulator of osteogenic differentiation (82). GRP-F5 mice have knocked-out exons
2, while GRP-F6 mice possess knocked-out exons 3. Therefore, GRP-F5
mice exhibit total loss of γ-carboxylation action, whereas GRP-F6
mice exhibit partial loss of secretory function (86). GRP-deficient mice demonstrated no
significant phenotypic alterations in the growth or calcification
of skeletal structures (86). GRP
may slow down the differentiation and maturation of osteoblasts and
decrease the activity of alkaline phosphatase and the expression of
osteogenic genes (87). In
addition, GRP has been implicated in the crosstalk between
inflammation and the calcification of articular tissues in OA
(87). BMP-2, a protein associated
with bone, was reported to be able to antagonise the expression of
GRP in chondrocytes (88). Certain
data has indicated that GRP may be upregulated by runt-related
transcription factor 2 and osterix, subsequently inducing the
differentiation of osteoblasts and the formation of nodules
(89). In addition, a previously
published paper demonstrated that GRP exhibited similar effects on
calcification and inflammation in control and OA-derived cells
(87). Therefore, GRP may have
potential as a therapeutic target in OA as it has effects on
calcification and inflammation processes. Comparative data
indicates the co-localisation of undercarboxylated GRP at sites of
ectopic calcification in cartilage and the synovial membrane in OA
(86). Therefore, vitamin K
insufficiency is associated with Knee OA (69,70,72).
In addition, Rafael et al (86) demonstrated that the association
between OA and the carboxylation deficiency of both GRP and MGP is
consistent.
GRP has been reported to function as an inhibitor of
calcification in the cardiovascular system (85). According to the association between
GRP and BMP-2, GRP may serve a role in the regulation of VSMC
differentiation into osteoblast-like cells and vascular
calcification. GRP has been associated with mineralisation
processes in numerous diseases that involve ectopic calcification
(81,82,85,86,90).
Similar to MGP, GRP acts as am inhibitor of calcification in
vascular and articular tissues. The modification of GRP by
γ-carboxylation is considered to be essential for its role as a
mineralisation inhibitor. GRP was also reported to be involved in
the mineralisation competence of VSMC-derived extracellular
vesicles (44,89). Furthermore, GRP may potentially be
involved in the fetuin-A-MGP calcification inhibitory system
(Fig. 3).
Periostin, with a molecular weight of ~90 kDa, is a
recently discovered VKDP. It contains 150 amino acid-long repeat
domains and is evolutionarily conserved (91). Periostin, also termed
osteoblast-specific factor 2, is an extracellular matrix (ECM)
protein and a member of the fascilin-1 protein family (92). Periostin was first identified in
the bones and located in the cortical periosteum (periosteum cells)
and periodontal ligament. Periostin and PLF are both present in
high-order vertebrates (93–96).
Periostin has been reported to affect heart development (97) and cardiac remodelling (98) during the embryonic period. However,
during ventricular hypertrophy and fibrosis, an increase in the
expression of periostin was observed (99). Periostin is a secretory protein
secreted by ECM proteins and is lipid soluble. Periostin has four
repetitive fascilin domains and may interact with other ECM
proteins to exhibit a variety of functions (100,101).
Periostin is primarily produced and secreted by
osteoblasts and their precursor cells. However, it is also secreted
by fibroblasts and is expressed in the bone and heart valves in
adult mammals. Periostin has a low degree of expression in the lung
and kidney (101). Periostin is
an adhesion molecule that, by binding to cell surface receptors,
promotes the differentiation, aggregation, adhesion and
proliferation of osteoblasts. Its involvement in collagen folding
is reported to be crucial for matrix assembly, which is responsible
for its association with bone strength (102). Periostin has also been
demonstrated to have an important effect on the development of the
heart (103), and the expression
of periostin in the ventriculus cordis may serve an important role
in atherosclerosis (104),
endocardial cushion formation and heart valve formation (97,98).
Increased expression levels of periostin may be
employed as a marker of sustained high pressure of bone tissue. The
level of PLF may also be used as an early indicator of adaptive
bone remodelling, serving an important role in the early diagnosis
and treatment of occupational musculoskeletal disorders. In adult
bone, PLF is reported to be upregulated under conditions of
fracture healing (105). Both
periostin and PLF were expressed under conditions of mechanical
overload or injury and repair of the musculoskeletal system
(106). In addition, periostin
markedly increased the ability of MC3T3-E1 cells to adhere to
type-1 collagen or fibronectin-coated surfaces, which are
established stimulators of MC3T3-E1 cell attachment (107,108).
Periostin in the connective tissue is associated
with mechanical force. For example, periostin was demonstrated to
be expressed in the heart valve and in the glomerulus of a patient
with a renal lesion (113), and
was also highly upregulated following cardiac tissue injury
(102). Periostin is also one of
the transcription products of vascular injury. Lindner et al
(114) demonstrated that
periostin mRNA was detected in the smooth muscle cell inner
membrane and tunica media in rats following carotid arteries
sacculus injury. Both periostin and PLF are vitamin K γ-carboxylate
proteins and are expressed in CVDs (106).
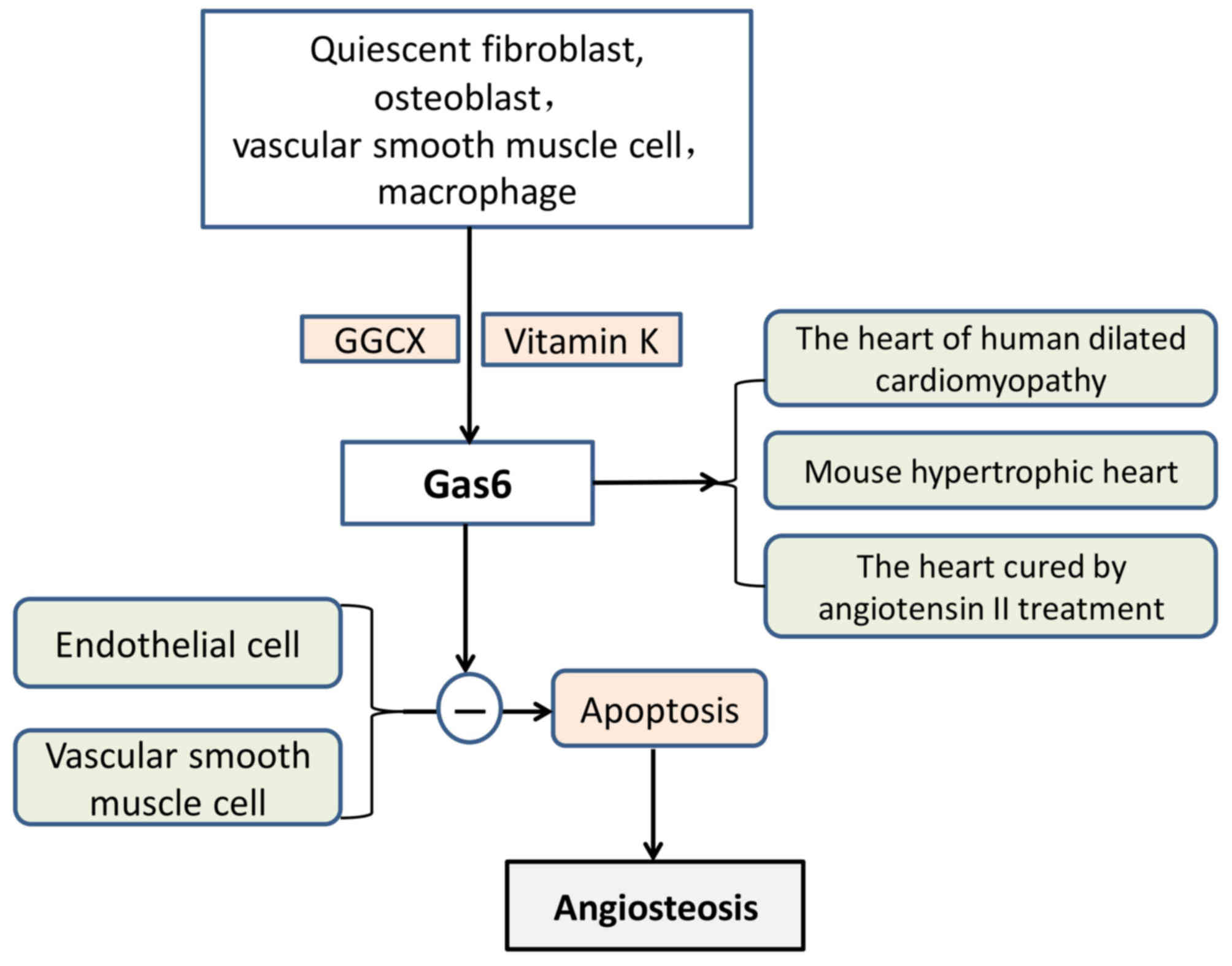
Gas6 and plasma anticoagulant protein S have 44%
homology, and the end of its amino acid sequence contains 11–12 Gla
residues (116). Gas6 includes
three parts, which are an amino terminal, a region that consists of
repeated proteins and a carboxyl terminal. Gas6 was originally
isolated from quiescent fibroblasts (117) and was also reported to be
secreted from osteoblasts (118).
When vitamin K is present, GGCX modifies Glu residues located in
the glutamic acid residue accumulation zone and converts them to
γ-Glu residues. Gas6 inhibited vascular calcification by inhibiting
VSMC apoptosis (111) (Fig. 5). In addition, Gas6 may be produced
by platelets and blood vessel walls, which affect thrombosis and
cell formation (92).
Gas6 is highly expressed in the heart, lung,
intestine, kidney, brain, spleen, ovary, testis, bone marrow, VSMCs
and macrophages; however, the level of expression in the liver is
low (107,108). Under pathological conditions,
VSMCs differentiate into osteoblast-like cells with a secretory
function, and osteoblast-like cells may synthesise and secrete
osteogenesis-associated proteins. Gas6 has been demonstrated to be
involved in vascular remodelling, homeostasis and atherosclerosis.
There is an established association between apoptotic bodies and
vascular calcifications. Gas6 has also been reported to protect
endothelial cells and VSMCs against apoptosis (119,120). It has also been demonstrated that
Gas6 is upregulated in human-dilated cardiomyopathy, cardiac
hypertrophy of mice and in hearts treated with angiotensin II
(121). Gas6 impairs the
adaptation of the ventricle to chronic pressure overload by the
activation of the mitogen-activated protein kinase kinase
1/2-extracellular signal-regulated kinase 1/2 signalling pathway
(121). Notably, a lack of Gas6
has been reported to weaken deoxycorticosterone-induced cardiac
hypertrophy and fibrosis (122).
VKDPs complete γ-carboxylation under the action of
vitamin K, where vitamin K acts as a cofactor (24). Carboxylated VKDPs exhibit
protective roles in the bone and cardiovascular system, and promote
the correct deposition of calcium. MGP inhibits calcium deposition
in the inner wall of the vascular internal wall and may even
reverse abnormal deposition to a certain extent to enhance calcium
entry into bone. In addition, carboxylated OC may attract and bind
calcium ions for the translocation of calcium ions to the bone
matrix, and is therefore beneficial for blood vessels and bone
(17,21,25–28).
These mechanisms are now clear and other VKDPs may also be
involved. VKDPs, vitamin K2 in particular, have been reported to
exert a protective effect on the bone and cardiovascular system. To
date, no serious side effects have been reported regarding vitamin
K (123,124).
Finally, the authors of the current review
hypothesize an association between the skeleton and the
cardiovascular system. In human embryonic development, the
cardiovascular system is differentiated from mesoderm mesenchyme.
The skeletal system originates from the ventral mesoderm and it may
derive from the mesenchyme in situ, excluding the sclerotome
(134–136). Therefore, their sources have
similarities. In addition, MSCs have been employed in clinical and
preclinical applications (137,138), including musculoskeletal tissue
bioengineering (139,140) and the treatment of heart disease
(141,142). A previous study has also revealed
that the endothelium acts as a template for the formation of new
bone tissues by bone-forming cells, indicating that vascular
patterning may guide bone formation (143). Based on this knowledge, the
cardiovascular system and bone may both originate from the mesoderm
during the period of embryonic development. The mesenchyme may
serve an important role in this process and the bone and
cardiovascular system may therefore be closely associated.
Thus, the authors of the current review formulated
the following hypotheses. The cardiovascular system and bone are
different structures of biological organisms and they bear
different physiological functions. However, they derive from
similar regions during embryonic development. When the body
matures, they differentiate into different tissues or organs but
maintain certain similar characteristics when they experience
identically short or sustained stimulation. For example, a lack of
vitamin K leads to the deficiency of carboxylated MGP, which
results in future ectopic calcification. Furthermore, calcification
of blood vessels is not a passive deposition process of calcium and
phosphorus. In blood vessels, VSMCs differentiate into
osteoblast-like cells, and certain similarities are observed with
the bone formation mechanism (61). In bone, osteoporosis and OA may
exist, key features of which include loss of articular cartilage,
osteophyte formation and other degenerative diseases (37,70).
When the stimulus is pressure, persistent ventricular hypertension
may induce heart failure and fibrosis. High pressure on joint or
heel induces osteophytes. In addition, the secretion of proteins,
such as periostin, occurs in the periosteum and also in the
cardiovascular system (93–96,99,109,111).
The authors of this study also have the following
conjectures. During the embryonic development period, if the
development position of different tissues and organs is closely
associated and have a common source, these tissues and organs may
retain a similar development tendency or secretion ability, such as
pressure stimulation and periostin, in the sustained stimulation of
the same nature. However, in general, no such similar performance
is observed when no common specific stimulus is found. In
conclusion, firstly, there may be certain intrinsic synergism and
antagonism between different VKDPs, thus maintaining internal
homeostasis. Secondly, the location of bone and the cardiovascular
system is closely associated during embryonic development. This may
be an important reason why vascular VSMCs may differentiate into
osteoblast-like cells, secrete BMP-2 and other osteogenic proteins,
and the heart produces periostin under load.
These statements are the authors' speculations and
assumptions. Further evidence and research with in-depth
understanding of these issues is required. Hopefully, the answer to
these questions may be obtained.
Not applicable.
This study was funded by the National Nature
Science Foundation of China (grant no. 30971065), the Science and
Technology Plan of Dalian (grant no. 2012E12SF074) and the
Education Fund Item of Liaoning province (grant no. 2009 A
194).
Not applicable.
SL supervised the writing of the present review as
well as directing its structure, and provided the final approval of
the version to be published. LW designed the concept of the review
and its structure, wrote and revised the manuscript, and agreed to
be accountable for all aspects of the work in ensuring that
questions related to the accuracy or integrity of any part of the
work are appropriately investigated and resolved. JC and LD were
involved in the writing of the review.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Vermeer C: Vitamin K: The effect on health
beyond coagulation-an overview. Food Nutrition Res. 56:53292012.
View Article : Google Scholar
|
|
2
|
Presnell SR and Stafford DW: The vitamin
K-dependent carboxylase. Thromb Haemost. 87:937–946. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Flore R, Ponziani FR, Di Rienzo TA, Zocco
MA, Flex A, Gerardino L, Lupascu A, Santoro L, Santoliquido A, Di
Stasio E, et al: Something more to say about calcium homeostasis:
The role of vitamin K2 in vascular calcification and osteoporosis.
Eur Rev Med Pharmacol Sci. 17:2433–2440. 2013.PubMed/NCBI
|
|
4
|
Taniyama Y, Katsuragi N, Sanada F, Azuma
J, Iekushi K, Koibuchi N, Okayama K, Ikeda-Iwabu Y, Muratsu J, Otsu
R, et al: Selective blockade of periostin exon 17 preserves cardiac
performance in acute myocardial infarction. Hypertension.
67:356–361. 2016.PubMed/NCBI
|
|
5
|
El Asmar MS, Naoum JJ and Arbid EJ:
Vitamin K dependent proteins and the role of vitamin K2 in the
modulation of vascular calcification: A review. Oman Med J.
29:172–177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tie JK, Jin DY, Straight DL and Stafford
DW: Functional study of the vitamin K cycle in mammalian cells.
Blood. 117:2967–2974. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lanham S, Cagampang FR and Oreffo ROC:
Maternal high fat diet affects offspring's vitamin K-dependent
proteins expression levels. PLoS One. 10:e01387302015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fusaro M, Crepaldi G, Maggi S, Galli F,
D'Angelo A, Calò L, Giannini S, Miozzo D and Gallieni M: Vitamin K,
bone fractures and vascular calcifications in chronic kidney
disease: An important but poorly studied relationship. J Endocrinol
Invest. 34:317–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mithal A, Dhingra V, Lau E, Stenmark J and
Nauroy L: The Asian Audit: Epidemiology, costs and burden of
osteoporosis in Asia China. International Osteoporosis Foundation
Publication; 2009
|
|
10
|
Dhanwal DK, Cooper C and Dennison EM:
Geographic variation in osteoporotic hip fracture incidence: The
growing importance of Asian influences in coming decades. J
Osteoporos Aug. 2:7571022010.
|
|
11
|
Bolland MJ, Avenell A, Baron JA, Grey A,
MacLennan GS, Gamble GD and Reid IR: Effect of calcium supplements
on risk of myocardial infarction and cardiovascular events:
Meta-analysis. BMJ. 341:c36912010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bolland MJ, Grey A, Avenell A, Gamble GD
and Reid IR: Calcium supplements with or without vitamin D and risk
of cardiovascular events: Reanalysis of the Women's Health
Initiative limited access dataset and meta-analysis. BMJ.
342:d20402011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li K, Kaaks R, Linseisen J and Rohrmann S:
Associations of dietary calcium intake and calcium supplementation
with myocardial infarction and stroke risk and overall
cardiovascular mortality in the Heidelberg cohort of the European
Prospective Investigation into Cancer and Nutrition study
(EPIC-Heidelberg). Heart. 98:920–925. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Michaëlsson K, Melhus H, Lemming Warensjö
E, Wolk A and Byberg L: Long term calcium intake and rates of all
cause and cardiovascular mortality: Community based prospective
longitudinal cohort study. BMJ. 346:f2282013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pentti K, Tuppurainen MT, Honkanen R,
Sandini L, Kröger H, Alhava E and Saarikoski S: Use of calcium
supplements and the risk of coronary heart disease in
52–62-year-old women: The Kuopio osteoporosis risk factor and
prevention study. Maturitas. 63:73–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao Q, Murphy RA, Houston DK, Harris TB,
Chow WH and Park Y: Dietary and supplemental calcium intake and
cardiovascular disease mortality: The national institutes of
health-AARP diet and health study. JAMA Intern Med. 173:639–646.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hoang QQ, Sicheri F, Howard AJ and Yang
DS: Bone recognition mechanism of porcine osteocalcin from crystal
structure. Nature. 425:977–980. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Clark H: NCDs: a challenge to sustainable
human development. Lancet. 381:510–511. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roth GA, Johnson C, Abajobir A, Abd-Allah
F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, et al:
Global, Regional, and National Burden of Cardiovascular Diseases
for 10 Causes, 1990 to 2015. J Am Coll Cardiol. 70:1–25. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
World Health Organisation (WHO), .
Cardiovascular disease. WHO; Geneva: 2013, http://www.who.int/cardiovasculardiseases/en/March
27–2015
|
|
21
|
Schurgers LJ, Dissel PE, Spronk HM, Soute
BA, Dhore CR, Cleutjens JP and Vermeer C: Role of vitamin K and
vitamin K-dependent proteins in vascular calcification. Z Kardiol.
90 Suppl 3:S57–S63. 2001. View Article : Google Scholar
|
|
22
|
Lahtinen AM, Havulinna AS, Jula A, Salomaa
V and Kontula K: Prevalence and clinical correlates of familial
hypercholesterolemia founder mutations in the general population.
Atherosclerosis. 238:64–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Doherty TM, Asotra K, Fitzpatrick LA, Qiao
JH, Wilkin DJ, Detrano RC, Dunstan CR, Shah PK and Rajavashisth TB:
Calcification in atherosclerosis: Bone biology and chronic
inflammation at the arterial crossroads. Proc Natl Acad Sci USA.
100:11201–11206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abdulameer AH, Sulaiman SABS and Kader
MBSA: An assessment of osteoporotic conditions among users and
Non-users of warfarin: A case-control study. J Clin Diagn Res.
11:OC21–OC24. 2017.PubMed/NCBI
|
|
25
|
Beulens JW, Bots ML, Atsma F, Bartelink
ML, Prokop M, Geleijnse JM, Witteman JC, Grobbee DE and van der
Schouw YT: High dietary menaquinone intake is associated with
reduced coronary calcification. Atherosclerosis. 203:489–493. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Geleijnse JM, Vermeer C, Grobbee DE,
Schurgers LJ, Knapen MH, van der Meer IM, Hofman A and Witteman JC:
Dietary intake of menaquinone is associated with a reduced risk of
coronary heart disease: The Rotterdam study. J Nutr. 134:3100–3105.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shea MK and Booth SL: Role of vitamin K in
the regulation of calcification. Int Congr Ser. 1297:165–178. 2007.
View Article : Google Scholar
|
|
28
|
Shea MK, O'Donnell CJ, Hoffmann U, Dallal
GE, Dawson-Hughes B, Ordovas JM, Price PA, Williamson MK and Booth
SL: Vitamin K supplementation and progression of coronary artery
calcium in older men and women. Am J Clin Nutr. 89:1799–1807. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hauschka PV and Reid ML: Vitamin D
dependence of a calcium-binding protein containing
gamma-carboxyghtamic acid in chicken bone. J Biol Chem.
253:9063–9068. 1978.PubMed/NCBI
|
|
30
|
Miyake N, Hoshi K, Sano Y, Kikuchi K,
Tadano K and Koshihara Y: 1,25-Dihydroxyvitamin D3 promotes vitamin
K2 metabolism in human osteoblasts. Osteoporos Int. 12:680–687.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shiraki M: Health benefits and demerits of
calcium nutrition or supplementation in older people. Nihon Rinsho.
73:1770–1776. 2015.(In Japanese). PubMed/NCBI
|
|
32
|
Zoch ML, Clemens TL and Riddle RC: New
insights into the biology of osteocalcin. Bone. 82:42–49. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iwamoto J: Vitamin K2 therapy
for postmenopausal. Nutrients. 6:1971–1980. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Neve A, Corrado A and Cantatore FP:
Osteocalcin: Skeletal and extra-skeletal effects. J CellPhysiol.
228:1149–1153. 2013.
|
|
35
|
Koshihara Y and Hoshi K: Vitamin K2
enhances osteocalcin accumulation in the extracellular matrix of
human osteoblasts in vitro. J Bone Miner Res. 12:431–438. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yunker LA, Undersander A, Lian JB, Stein
GS, Carlson CS and Mauro LJ: The tyrosine phesphatase, OST-PTP, is
expressed in mesenchymal progenitor cellsearly during
skeletagenosis in the mouse. J Cell Biochem. 93:761–773. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Naito K, Watari T, Obayashi O, Katsube S,
Nagaoka I and Kaneko K: Relationship between serum
undercarboxylated osteocalcin and hyaluronan levels in patients
with bilateral knee osteoarthritis. Int J Mol Med. 29:756–760.
2012.PubMed/NCBI
|
|
38
|
Zheng W, Kang H, Shu C, Tang ML, Fang PZ,
Xie J, He J and Wang M: Expression and significance of inflammatory
factors and bone formation mediators in carotid atherosclerotic
plaque. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 33:746–750. 2008.(In
Chinese). PubMed/NCBI
|
|
39
|
Orimo H, Nakamura T, Hosoi T, Iki M,
Uenishi K, Endo N, Ohta H, Shiraki M, Sugimoto T, Suzuki T, et al:
Japanese 2011 guidelines for prevention and treatment of
osteoporosis-executive summary. Arch Osteoporos. 7:3–20. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hunt JL, Fairman R, Mithell ME, Carpenter
JP, Golden M, Khalapyan T, Wolfe M, Neschis D, Milner R, Scoll B,
et al: Bone formation in carotid plaques: A clinicopathological
study. Stroke. 33:1214–1219. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Inaba N, Sato T and Yamashita T: Low-dose
daily intake of vitamin K2 (Menaquinone-7) improves osteocalcin
γ-carboxylation: A double-blind. randomized controlled trials. J
Nutr Sci Vitaminol (Tokyo). 61:471–480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brugè F, Bacchetti T, Principi F, Littarru
GP and Tiano L: Olive oil supplemented with menaquinone-7
significantly affects osteocalcin carboxylation. Br J Nutr.
106:1058–1062. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sato T, Schurgers LJ and Uenishi K:
Comparison of menaquinone-4 and menaquinone-7 bioavailability in
healthy women. Nutr J. 11:932012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Price PA: Role of vitamin K-dependent
proteins in bone metabolism. Annu Rev Nutr. 8:565–583. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Booth SL, Centi A, Smith SR and Gundberg
C: The role of osteocalcin in human glucose metabolism: Marker or
mediator? Nat Rev Endocrinol. 9:43–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Veldhuis-Vlug AG, Fliers E and Bisschop
PH: Bone as a regulator of glucose metabolism. Neth J Med.
71:396–400. 2013.PubMed/NCBI
|
|
47
|
Kerner SA, Scott RA and Pike JW: Sequence
elements in the human osteocalcin gene confer basal activation and
inducible response to hormonal vitamin D3. Proc Natl Acad Sci USA.
86:4455–4459. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lian J, Stewart C, Puchacz E, Mackowiak S,
Shalhoub V, Collart D, Zambetti G and Stein G: Structure of the rat
osteocalcin gene and regulation of vitamin D-dependent expression.
Proc Natl Acad Sci USA. 86:1143–1147. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cairns JR and Price PA: Direct
demonstration that the vitamin K-dependent bone Gla protein is
incompletely gamma-carboxylated in humans. J Bone Miner Res.
9:1989–1997. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liabeuf S, Bourron O, Vemeer C, Theuwissen
E, Magdeleyns E, Aubert CE, Brazier M, Mentaverri R, Hartemann A
and Massy ZA: Vascular calcification in patients with type 2
diabetes: The involvement of matrix Gla Protein. Cardiovasc
Diabetol. 3:852014. View Article : Google Scholar
|
|
51
|
Wallin R, Cain D and Sane DC: Matrix Gla
protein synthesis and gamma-carboxylation in the aortic vessel wall
and proliferating vascular smooth muscle cells-A cell system which
resembles the system in bone cells. Thromb Haemost. 82:1764–1767.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Harbuzova Viu and Ataman OV: Matrix
Gla-protein and its role in vascular wall calcification. Fiziol Zh.
57:96–112. 2011.(In Ukrainian). PubMed/NCBI
|
|
53
|
Schlieper G, Westenfeld R, Krüger T,
Cranenburg EC, Magdeleyns EJ, Brandenburg VM, Djuric Z, Damjanovic
T, Ketteler M, Vermeer C, et al: Circulating nonphosphorylated
carboxylated matrix gla protein predicts survival in ESRD. J Am Soc
Nephrol. 22:387–395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Leopold JA: Vascular calcification:
Mechanism of vascular smooth muscle cell calcification. Trends
Cardiovasc Med. 25:267–274. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
de Cavanagh EM, Inserra F, Ferder M and
Ferder L: From mitochondria to disease: Role of the
renin-angiotensin system. Am J Nephrol. 27:545–553. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li X, Yang HY and Giachelli CM: Role of
the sodium-dependent phosphate cotransporter, Pit-1, in vascular
smooth muscle cell calcification. Circ Res. 98:905–912. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Reynolds JL, Joannides AJ, Skepper JN,
McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL
and Shanahan CM: Human vascular smooth muscle cells undergo
vesicle-mediated calcification in response to changes in
extracellular calcium and phosphate concentrations: A potential
mechanism for accelerated vascular calcification in ESRD. J Am Soc
Nephrol. 15:2857–2867. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Son BK, Akishita M, Iijima K, Eto M and
Ouchi Y: Mechanism of pi-induced vascular calcification. J
Atheroscler Thromb. 15:63–68. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Son BK, Kozaki K, Iijima K, Eto M, Nakano
T, Akishita M and Ouchi Y: Gas6/Axl-PI3K/Akt pathway plays a
central role in the effect of statins on inorganic
phosphate-induced calcification of vascular smooth muscle cells.
Eur J Pharmacol. 556:1–8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Steitz SA, Speer MY, Curinga G, Yang HY,
Haynes P, Aebersold R, Schinke T, Karsenty G and Giachelli CM:
Smooth muscle cell phenotypic transition associated with
calcification: Upregulation of Cbfa1 and downregulation of smooth
muscle lineage markers. Circ Res. 89:1147–1154. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kim H, Kim HJ, Lee K, Kim JM, Kim HS, Kim
JR, Ha CM, Choi YK, Lee SJ, Kim JY, et al: α-Lipoic acid attenuates
vascular calcification via reversal of mitochondrial function and
restoration of Gas6/Axl/Akt survival pathway. J Cell Mol Med.
16:273–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Otsuka F, Sakakura K, Yahagi K, Joner M
and Virmani R: Has our understanding of calcification in human
coronary atherosclerosis progressed? Arterioscler Thromb Vasc Biol.
34:724–736. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cheng SL, Shao JS, Charlton-Kachigian N,
Loewy AP and Towler DA: MSX2 promotes osteogenesis and suppresses
adipogenic differentitation of multipotent mesenchymal progenitors.
J Biol Chem. 278:45969–45977. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wallin R, Cain D, Hutson SM, Sane DC and
Loeser R: Modulation of the binding of matrix Gla protein (MGP) to
bone morphogenetic protein-2 (BMP-2). Thromb Haemost. 84:1039–1044.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Roy ME and Nishimoto SK: Matrix Gla
protein binding to hydroxyapatite is dependent on the ionic
environment: Calcium enhances binding affinity but phosphate and
magnesium decrease affinity. Bone. 31:296–302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Nakase T, Miyaji T, Tomita T, Kaneko M,
Kuriyama K, Myoui A, Sugamoto K, Ochi T and Yoshikawa H:
Localization of bone morphogenetic protein-2 in human
osteoarthritic cartilage and osteophyte. Osteoarthritis Cartilage.
11:278–284. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Price PA, Williamson MK, Nguyen TM and
Than TN: Serum levels of the fetuin-mineral complex correlate with
artery calcification in the rat. J Biol Chem. 279:1594–1600. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Shea MK, Kritchevsky SB, Hsu FC, Nevitt M,
Booth SL, Kwoh CK, McAlindon TE, Vermeer C, Drummen N, Harris TB,
et al: The association between vitamin K status and knee
osteoarthritis features in older adults: The Health, Aging and Body
Composition Study. Osteoarthritis Cartilage. 23:370–378. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Neogi T, Booth SL, Zhang YQ, Jacques PF,
Terkeltaub R, Aliabadi P and Felson DT: Low vitamin K status is
associated with osteoarthritis in the hand and knee. Arthritis
Rheum. 54:1255–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Misra D, Booth SL, Tolstykh I, Felson DT,
Nevitt MC, Lewis CE, Torner J and Neogi T: Vitamin K deficiency is
associated with incident knee osteoarthritis. Am J Med.
126:243–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wallin R, Schurgers LJ and Loeser RF:
Biosynthesis of the vitamin K-dependent matrix Gla protein (MGP) in
chondrocytes: A fetuin-MGP protein complex is assembled in vesicles
shed from normal but not from osteoarthritic chondrocytes.
Osteoarthritis Cartilage. 18:1096–1103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Oka H, Akune T, Muraki S, En-yo Y, Yoshida
M, Saika A, Sasaki S, Nakamura K, Kawaguchi H and Yoshimura N:
Association of low dietary vitamin K intake with radiographic knee
osteoarthritis in the Japanese elderly population: Dietary survey
in a population-based cohort of the ROAD study. J Orthop Sci.
14:687–692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bügel S: Vitamin K and bone health. Proc
Nutr Soc. 62:839–843. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Shearer MJ, Fu X and Booth SL: Vitamin K
nutrition, metabolism and requirements: Current concepts and future
research. Adv Nutr. 3:182–195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Schurgers LJ, Barreto DV, Barreto FC,
Liabeuf S, Renard C, Magdeleyns EJ, Vermeer C, Choukroun G and
Massy ZA: The circulating inactive form of matrix gla protein is a
surrogate marker for vascular calcification in chronic kidney
disease: A preliminary report. Clin J Am Soc Nephrol. 5:568–575.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Boxma PY, van den Berg E, Geleijnse JM,
Laverman GD, Schurgers LJ, Vermeer C, Kema IP, Muskiet FA, Navis G,
Bakker SJ and de Borst MH: Vitamin k intake and plasma
desphospho-uncarboxylated matrix Gla-protein levels in kidney
transplant recipients. PLoS One. 7:e479912012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Dalmeijer GW, van der Schouw YT,
Magdeleyns EJ, Vermeer C, Verschuren WM, Boer JM and Beulens JW:
Matrix Gla protein species and risk of cardiovascular events in
type 2 diabetic patients. J Diabetes Care. 36:3766–3771. 2013.
View Article : Google Scholar
|
|
78
|
Tsugawa N: Cardiovascular diseases and fat
soluable vitamins: Vitamin D and Vitamin K. J Nutr Sci Vitaminol
(Tokyo). 61:S170–S172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Delanayc P, Krzesinski JM, Warling X,
Moonen M, Smelten N, Médart L, Pottel H and Cavalier E:
Dephosphorglated-uncarboxylated Matrix Gla protein concentration is
predictive of vitamin K status and is correlated with vascular
calcification in a cohort of hemodialysis patients. BMC Nephrol.
15:1452014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Viegas CS, Simes DC, Laizé V, Williamson
MK, Price PA and Cancela ML: Gla-rich protein (GRP), a new vitamin
K-dependent protein identified from sturgeon cartilage and highly
conserved in vertebrates. J Biol Chem. 283:36655–36664. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Viegas CS, Cavaco S, Neves PL, Ferreira A,
João A, Williamson MK, Price PA, Cancela ML and Simes DC: Gla-rich
protein is a novel vitamin K-dependent protein present in serum
that accumulates at sites of pathological calcifications. Am J
Pathol. 175:2288–2298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Surmann-Schmitt C, Dietz U, Kireva T, Adam
N, Park J, Tagariello A, Onnerfjord P, Heinegård D,
Schlötzer-Schrehardt U, Deutzmann R, et al: Ucma, a novel secreted
cartilage-specific protein with implications in osteogenesis. J
Biol Chem. 11:7082–7893. 2008. View Article : Google Scholar
|
|
83
|
Le Jeune M, Tomavo N, Tian TV, Flourens A,
Marchand N, Camuzeaux B, Mallien-Gerin F and Duterque-Coquillaud M:
Identification of four alternatively spliced transcripts of the
Ucma/GRP gene, encoding a new Gla-containing protein. J Exp Cell
Res. 316:203–215. 2010. View Article : Google Scholar
|
|
84
|
Tagariello A, Luther J, Streiter M,
Didt-Koziel L, Wuelling M, Surmann-Schmitt C, Stock M, Adam N,
Vortkamp A and Winterpacht A: Ucma, a novel-secreted factor
represents a highly specific marker for distal chondrocytes. Matrix
Biol. 27:3–11. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Viegas CS, Rafael MS, Enriquez JL,
Teixeira A, Vitorino R, Luis IM, Costa RM, Santos S, Cavaco S,
Neves J, et al: Gla-rich protein (GRP) acts as a calcification
inhibitor in the human cardiovascular system. Arterioscler Thromb
Vasc Biol. 35:399–408. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Rafael MS, Cavaco S, Viegas CS, Santos S,
Ramos A, Willems BA, Herfs M, Theuwissen E, Vermeer C and Simes DC:
Insights into the association of Gla-rich protein and
osteoarthritis, novel splice variants and γ-carboxylation status.
Mol Nutr Food Res. 58:1636–1646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Cavaco S, Viegas CS, Rafael MS, Ramos A,
Magalhães J, Blanco FJ, Vermeer C and Simes DC: Gla-rich protein is
involved in the cross-talk between calcification and inflammation
in osteoarthritis. Cell Mol Life Sci. 73:1051–1065. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Cancela ML, Conceição N and Laizé V:
Gla-rich protein, a new player in tissue calcification? Adv Nutr.
3:174–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lee YJ, Park SY, Lee SJ, Boo YC, Choi JY
and Kim JE: Ucma, a direct transcriptional target of Runx2 and
Osterix, promotes osteoblast differentiation and nodule formation.
Osteoarthritis Cartilage. 23:1421–1431. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Viegas CS, Herfs M, Rafael MS, Enriquez
JL, Teixeira A, Luís IM, van't Hoofd CM, João A, Maria VL, Cavaco
S, et al: Gla-rich protein is a potential new vitamin K target in
cancer: Evidences for a direct GRP-mineral interaction. Biomed Res
Int. 2014:3402162014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zinn K, McAllister L and Goodman CS:
Sequence analysis and neuronal expression of fasciclin I in
grasshopper and Drosophila. J Cell. 53:577–587. 1988. View Article : Google Scholar
|
|
92
|
Takeshita S, Kikuno R, Tezuka K and Amann
E: Osteoblast-specific factor 2: Cloning of a putative bone
adhesion protein with homology with the insect protein fasciclin I.
Biochem J. 294:271–278. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Politz O, Gratchev A, McCourt PA,
Schledzewski K, Guillot P, Johansson S, Svineng G, Franke P,
Kannicht C, Kzhyshkowska J, et al: Stabilin-1 and-2 constitute a
novel family of fasciclin-like hyaluronan receptor homologues.
Biochem J. 362:155–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Skonier J, Neubauer M, Madisen L, Bennett
K, Plowman GD and Purchio AF: CDNA cloning and sequence analysis of
beta ig-h3, a novel gene induced in a human adenocarcinoma cell
line after treatment with transforming growth factor-beta. DNA Cell
Biol. 11:511–522. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Horiuchi K, Amizuka N, Takeshita S,
Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF and Kudo A:
Identification and characterization of a novel protein, Periostin,
with restricted expression to periosteum and periodontal ligament
and increased expression by transforming growth factor beta. J Bone
Miner Res. 14:1239–1249. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Litvin J, Selim AH, Montgomery MO, Lehmann
K, Rico MC, Devlin H, Bednarik DP and Safadi FF: Expression and
function of periostin-isoforms in bone. J Biol Chem. 92:1044–1061.
2004.
|
|
97
|
Kruzynska-Frejtag A, Machnicki M, Rogers
R, Markwald RR and Conway SJ: Periostin (an osteoblast-specific
factor) is expressed within the embryonic mouse heart during valve
formation. Mech Dev. 103:183–188. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Stansfield WE, Andersen NM, Tang RH and
Selzman CH: Periostin is a novel factor in cardiac remodeling after
experimental and clinical unloading of the failing heart. Ann
Thorac Surg. 88:1916–1921. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Pohjolainen V, Rysä J, Näpänkangas J,
Kööbi P, Eräranta A, Ilves M, Serpi R, Pörsti I and Ruskoaho H:
Left ventricular periostin gene expression is associated with
fibrogenesis in experimental renal insufficiency. Nephrol Dial
Transplant. 27:115–122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Morita H and Komuro I: Periostin isoforms
and cardiac remodeling after myocardial infarction is the dispute
settled? Hypertension. 67:504–505. 2016.PubMed/NCBI
|
|
101
|
Iekushi K, Taniyama Y, Azuma J, Katsuragi
N, Dosaka N, Sanada F, Koibuchi N, Nagao K, Ogihara T and Morishita
R: Novel mechanisms of valsartan on the treatment of acute
myocardial infarction through inhibition of the antiadhesion
molecule periostin. Hypertension. 49:1409–1414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Merle B and Garnero P: The multiple facets
of periostin in bone metabolism. Osteoporos Int. 23:1199–1212.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Snider P, Standley KN, Wang J, Azhar M,
Doetschman T and Conway SJ: Origin of cardiac fibroblasts and the
role of periostin. Circ Res. 105:934–947. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hakuno D, Kimura N, Yoshioka M, Mukai M,
Kimura T, Okada Y, Yozu R, Shukunami C, Hiraki Y, Kudo A, et al:
Periostin advances atherosclerotic and rheumatic cardiac valve
degeneration by inducing angiogenesis and MMP production in humans
and rodents. J Clin Invest. 120:2292–2306. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhu S, Barbe MF, Liu C, Hadjiargyrou M,
Popoff SN, Rani S, Safadi FF and Litvin J: Periostin-like factor in
osteogenesis. J Cell Physiol. 218:584–592. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Rani S, Barbe MF, Barr AE and Litvin J:
Periostin-like-factor and periostin in an animal model of
work-related musculoskeletal disorder. Bone. 44:502–512. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Perrier A, Dumas V, Linossier MT, Fournier
C, Jurdic P, Rattner A, Vico L and Guignandon A: Apatite content of
collagen materials dose-dependently increases pre-osteoblastic cell
deposition of a cement line-like matrix. Bone. 47:23–33. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Freitas F, Jeschke M, Majstorovic I,
Mueller DR, Schindler P, Voshol H, Van Oostrum J and Susa M:
Fluoroaluminate stimulates phosphorylation of p130 Cas and Fak and
increases attachment and spreading preosteoblastic MC3T3-E1 cells.
Bone. 30:99–108. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wang DJ, Oparil S, Feng JA, Li P, Perry G,
Chen LB, Dai M, John SW and Chen YF: Effects of pressure overload
on extracellular matrix expression in the heart of the atrial
natriuretic peptide-null mouse. Hypertension J. 42:88–95. 2003.
View Article : Google Scholar
|
|
110
|
Litvin J, Blagg A, Mu A, Matiwala S,
Montgomery M, Berretta R, Houser S and Margulies K: Periostin and
periostin-like factor in the human heart: Possible therapeu tic
targets. Cardiovasc Pathol. 15:24–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Katsuragi N, Morishita R, Nakamura N,
Ochiai T, Taniyama Y, Hasegawa Y, Kawashima K, Kaneda Y, Ogihara T
and Sugimura K: Periostin as a novel factor responsible for
ventricular dilation. Circulation. 110:1806–1813. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Oka T, Xu J, Kaiser RA, Melendez J,
Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW II, Conway
SJ, et al: Genetic manipulation of periostin expression reveals a
role in cardiac hypertrophy and ventricular remodeling. Circ Res.
101:313–321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Sen K, Lindenmeyer MT, Gaspert A,
Eichinger F, Neusser MA, Kretzler M, Segerer S and Cohen CD:
Periostin is induced in glomerular injury and expressed de novo in
interstitial renal fibrosis. Am J Pathol. 179:1756–1767. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Lindner V, Wang Q, Conley BA, Friesel RE
and Vary CP: Vascular injury induces expression of periostin:
Implications for vascular cell differentiation and migration.
Arterioscler Thromb Vasc Biol. 25:77–83. 2005.PubMed/NCBI
|
|
115
|
Stanton LW, Garrard LJ, Damm D, Garrick
BL, Lam A, Kapoun AM, Zheng Q, Protter AA, Schreiner GF and White
RT: Altered patterns of gene expression in response to myocardial
infarction. Circ Res. 86:939–945. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Deng T, Zhang Y, Chen Q, Yan K and Han D:
Toll-like receptor-mediated inhibition of Gas6 and ProS expression
facilitates inflammatory cytokine production in mouse macrophages.
Immunology J. 135:40–50. 2012. View Article : Google Scholar
|
|
117
|
Bellosta P, Zhang Q, Goff SP and Basilico
C: Signaling through the ARK tyrosine kinase receptor protects from
apoptosis in the absence of growth stimulation. Oncogene.
15:2387–2389. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Shiozawa Y, Pedersen EA, Patel LR, Ziegler
AM, Havens AM, Jung Y, Wang J, Zalucha S, Loberg RD, Pienta KJ and
Taichman RS: GAS6/AXL axis regulates prostate cancer invasion,
proliferation, and survival in the bone marrow niche. Neoplasia.
12:116–127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Hasanbasic I, Rajotte I and Blostein M:
The role of gamma-carboxylation in the anti-apoptotic function of
gas6. J Thromb Haemost. 3:2790–2797. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Son BK, Kozaki K, Iijima K, Eto M, Kojima
T, Ota H, Senda Y, Maemura K, Nakano T, Akishita M and Ouchi Y:
Statins protect human aortic smooth muscle cells from inorganic
phosphate-induced calcification by restoring Gas6-Axl survival
pathway. Circ Res. 98:1024–1031. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Zhao YF, Xu DC, Zhu GF, Zhu MY, Tang K, Li
WM and Xu YW: Growth arrest-specific 6 exacerbates pressure
overload-induced cardiac hypertrophy. Hypertension. 67:118–129.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Park JK, Theuer S, Kirsch T, Lindschau C,
Klinge U, Heuser A, Plehm R, Todiras M, Carmeliet P, Haller H, et
al: Growth arrest specific protein 6 participates in DOCA-induced
target-organ damage. Hypertension. 54:359–364. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Cockayne S, Adamson J, Lanham-New S,
Shearer MJ, Gilbody S and Torgerson DJ: Vitamin K and the
prevention of fractures: Systematic review and meta-analysis of
randomized controlled trials. J Arch Intern Med. 166:1256–1261.
2006. View Article : Google Scholar
|
|
124
|
Pucaj K, Rasmussen H, Møller M and Preston
T: Safety and toxicological evaluation of a synthetic vitamin K2,
menaquinone-7. Toxicol Mech Methods. 21:520–532. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Danziger J, Young RL, Shea MK, Tracy RP,
Ix JH, Jenny NS and Mukamal KJ: Vitamin K-dependent protein
activity and incident ischemic cardiovascular disease: The
multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc
Biol. 36:1037–1042. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Litvina J, Blagga A, Mua A, Matiwalaa S,
Montgomerya M, Berrettaa R, Housera S and Marguliesa K: Periostin
and periostin-like factor in the human heart: possible therapeutic
targets. Cardiovasc Pathol. 15:24–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Severson AR, Ingram RT and Fitzpatrick LA:
Matrix proteins associated with bone calcification are present in
human vascular smooth muscle cells grown in vitro. In Vitro Cell
Dev Biol Anim. 31:853–857. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Dhore CR, Cleutjens JP, Lutgens E,
Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM,
Vermeer C and Daemen MJ: Differential expression of bone matrix
regulatory proteins in human atherosclerotic plaques. Arterioscler
Thromb Vasc Biol. 21:1998–2003. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Trion A and van der Laarse A: Vascular
smooth muscle cells and calcification in atherosclerosis. Am Heart
J. 147:808–814. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Dowd TL, Rosen JF, Li L and Gundberg CM:
The three-dimensional structure of bovine calcium ion-bound
osteocalcin using 1H NMR spectroscopy. Biochemistry. 42:7769–7779.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Hauschka PV and Carr SA: Calcium-dependent
alpha-helical structure in osteocalcin. Biochemistry. 21:2538–2547.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Gerbaix M, Vico L, Ferrari SL and Bonnet
N: Periostin expression contributes to cortical bone loss during
unloading. Bone. 71:94–100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Bonnet N, Gineyts E, Ammann P, Conway SJ,
Garnero P and Ferrari S: Periostin deficiency increases bone damage
and impairs injury response to fatigue loading in adult mice. PLoS
One. 8:e783472013. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Brent AE and Tabin CJ: Developmental
regulation of somite derivatives: Muscle, cartilage and tendon.
Curr Opin Genet Dev. 12:548–557. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Minguell JJ, Erices A and Conget P:
Mesenchymal stem cells. Exp Biol Med (Maywood). 226:507–520. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Le Blanc K and Pittenger M: Mesenchymal
stem cells: Progress toward promise. Cytotherapy. 7:36–45. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Reiser J, Zhang XY, Hemenway CS, Mondal D,
Pradhan L and La Russa VF: Potential of mesenchymal stem cells in
gene therapy approaches for inherited and acquired diseases. Expert
Opin Biol Ther. 5:1571–1584. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Hui JH, Ouyang HW, Hutmacher DW, Goh JC
and Lee EH: Mesenchymal stem cells in musculoskeletal tissue
engineering: A review of recent advances in National University of
Singapore. Ann Acad Med Singapore. 34:206–212. 2005.PubMed/NCBI
|
|
140
|
Caplan AI: Review: Mesenchymal stem cells:
Cell-based reconstructive therapy in orthopedics. Tissue Eng.
11:1198–1211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Menasché P: The potential of embryonic
stem cells to treat heart disease. Curr Opin Mol Ther. 7:293–299.
2005.PubMed/NCBI
|
|
142
|
Laflamme MA and Murry CE: Regenerating the
heart. Nat Biotechnol. 23:845–856. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Ben Shoham A, Rot C, Stern T, Krief S,
Akiva A, Dadosh T, Sabany H, Lu Y, Kadler KE and Zelzer E:
Deposition of collagen type I onto skeletal endothelium reveals a
new role for blood vessels in regulating bone morphology.
Development. 143:3933–3943. 2016. View Article : Google Scholar : PubMed/NCBI
|