Introduction
Hepatic carcinoma is associated with high levels of
morbidity and mortality (1). In
addition, hepatocellular carcinoma is the second most common type
of cancer, the incidence of which is increasing worldwide, which
accounts for >90% of primary liver cancer cases (2,3). At
present, the common clinical therapeutic strategies for hepatic
carcinoma include surgery, chemotherapy and radiotherapy; however,
these treatments present only modest efficacy and often induce side
effects in patients (4,5). In addition, the efficacy of these
conventional therapeutic strategies remain limited, particularly
for patients with late stage, advanced hepatic carcinoma (6). Although the efficacy of aggressive
surgery is limited for patients with cancer, tumor resection is the
most common clinical strategy used to treat patients with hepatic
carcinoma. Therefore, various anesthetics have been developed and
applied in tumor resection to attenuate surgical pain for patients
with hepatic carcinoma during the perioperative period. Additional
functions of anesthetics have also been reported and further
analyzed in hepatic carcinoma cells and tissues.
Isoflurane is a volatile general anesthetic that can
be applied for the induction and maintenance of general anesthesia,
in order to abolish the behavioral responsiveness of patients
during tumor resection (7). It has
previously been reported that pretreatment with isoflurane
influences the cytokine response to cancer surgery during the
perioperative period (8). In
addition, research has indicated that the effects of isoflurane may
activate the caspase-induced apoptotic signaling pathway; this
cellular response is consistent with the neuropathogenesis of
senile dementia (9). In addition,
Liu revealed that isoflurane can increase serum levels of
interleukin (IL)-8 and IL-10 in patients with cancer (10). Furthermore, emulsified isoflurane
treatment can inhibit the cell cycle and respiration of human
bronchial epithelial 16HBE cells in a p53-independent manner
(11). The anesthetic efficacy of
isoflurane has also been investigated in patients undergoing
craniotomy for primary brain tumor excision (12). These data suggest that is of lurane
may regulate various signaling pathway in tumor cells during the
perioperative period. Therefore, it may be hypothesized that
isoflurane inhibits hepatic carcinoma growth and aggressiveness,
and promotes apoptosis via the phosphoinositide 3-kinase/protein
kinase B (PI3K/AKT)-mediated nuclear factor (NF)-κB signal
pathway.
Oncogenic Ras signaling, resulting in activation of
the PI3K/AKT pathway, has been analyzed during tumor maintenance
(13). The PI3K/AKT signaling
pathway serves an essential role in cell growth, proliferation and
survival under physiological conditions (14). A previous study suggested that
inhibition of PI3K/AKT signaling could induce apoptosis, and impair
mammary tumor outgrowth and metastasis (15). In addition, isoflurane-induced
neuroapoptosis via the PI3K/AKT pathway has been investigated in
vivo and the expression of PI3K and AKT has been reported to
affect neuroapoptosis (16).
Furthermore, the PI3K/AKT-induced NF-κB signaling pathway is
associated with tumor angiogenesis and provides a novel insight
into the mechanisms underlying cancer cell growth and
aggressiveness (17). In addition,
Miao and Zhao indicated that inhibition of the PI3K/AKT/NF-κB
signaling pathway could suppress tumor invasion in follicular
thyroid carcinoma (18). These
reports suggest that the PI3K/AKT-mediated NF-κB signal pathway may
serve an essential role in the initiation and progression of
carcinoma growth and aggressiveness. Therefore, the present study
investigated the expression and activity of the PI3K/AKT-mediated
NF-κB signaling pathway in hepatic carcinoma cells following
treatment with isoflurane.
In the present study, the anesthetic and cellular
effects of isoflurane on hepatic carcinoma cell biology were
investigated, in order to better understand the mechanisms
underlying isoflurane-mediated tumor suppression in patients with
hepatic carcinoma. The present study also evaluated the molecular
mechanism underlying isoflurane-induced apoptosis and tumor therapy
for patients with hepatic carcinoma.
Materials and methods
Ethics statement
The present study was directed according to the
Guide for the Care and Use of Clinical Investigation of
Anesthesiology of Linyi Cancer Hospital (Linyi, China). The present
study was approved by the ethics committee of Linyi Cancer
Hospital. All patients provided written informed consent.
Patients
A total of 10 patients with hepatic carcinoma were
recruited in Linyi Cancer Hospital between June 2013 and May 2014.
The mean age was 46.7 years old (range, 38.5–62.5 years old).
Patients with a history of cancer were excluded from this study.
None of the patients had received anti-cancer treatments before
tumorectomy. Patients were treated with total intravenous
anesthesia isoflurane (n=5, 10 mg/kg) or propofol (n=5, 2
mg/kg).
Pharmacodynamics analysis
Serum concentrations of isoflurane and Cmax
concentrations of isoflurane were analyzed in patients with hepatic
carcinoma after anesthesia. Serum concentrations of isoflurane were
recorded at 0–120 min (15 min interval). Cmax concentrations of
isoflurane were evaluated at 0–25 mg/kg (5 mg/kg interval).
Concentrations of isoflurane (Cmax) were determined by High
Performance Liquid Chromatography as described previously (19).
Cell culture
Hepatic carcinoma cells were isolated from patients
with hepatic carcinoma and were cultured in Dulbecco's modified
Eagle's medium (DMEM; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) supplemented with 5% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Tumor cells were
cultured at 37°C in a humidified atmosphere containing 5%
CO2. Cells were treated with isoflurane (2 mg/ml) and/or
PI3K inhibitor (2 mg/ml) for 12 h at 37°C for further analysis.
MTT assay
Hepatic carcinoma cells (1×103
cells/well) were incubated in 96-well plates for 72 h at 37°C in
triplicate. Cells were then treated with isoflurane (2 mg/ml) or
isoflurane (2 mg/ml) for 48 h at 37°C. Subsequently, 20 µl MTT
solution (5 mg/ml) was added to the cells and the plates were
incubated for 2 h at 37°C. The medium was then removed and 100 µl
dimethyl sulfoxide was added to the wells to solubilize the
crystals. Absorbance was measured using an ELISA reader at a
wavelength of 450 nm.
Cell viability assay
Hepatic carcinoma cells (1×103
cells/well) were seeded in 96-well plates and cultured for 12 h at
37°C. Cells were then treated with isoflurane (2 mg/ml) or
isoflurane (2 mg/ml) for 48 h at 37°C. The CCK-8 detection kit
(Sigma-Aldrich; Merck KGaA) was used to measure cell viability
according to the manufacturer's instructions.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from hepatic carcinoma cells
using RNAeasy Mini kit (Qiagen, Inc., Gaithersburg, MD, USA). mRNA
expression levels were measured by RT-qPCR using an RT-qPCR kit
(A15300; Thermo Fisher Scientific, Inc.). All the forward and
reverse primers were synthesized by Invitrogen (Thermo Fisher
Scientific, Inc., Table I).
Thermocycling conditions included 45 amplification cycles,
denaturation at 95°C for 45 sec, primer annealing at 62.5°C for 30
sec with touchdown to 54°C for 45 sec and applicant extension at
72°C for 60 sec. The relative mRNA expression levels of B-cell
lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax), caspase-3 and
caspase-8 were calculated according to the 2−ΔΔCq method
(20). The results were analyzed
in triplicate according to the 2−ΔΔCq method, and were
normalized to β-actin.
 | Table I.Sequences of primers used in the
present study. |
Table I.
Sequences of primers used in the
present study.
|
| Sequence |
|---|
|
|
|
|---|
| Gene name | Forward | Reverse |
|---|
| Bax |
5′-TGGCAGCTGACATGTTTTCTGAC-3′ |
5′-TCACCCAACCACCCTGGTCTT-3′ |
| Bcl-2 |
5′-CGTCATAACTAAAGACACCCC-3′ |
5′-TTCATCTCCAGTATCCGACT-3′ |
| Caspase-3 |
5′-ATGGAGAACAACAAAACCTCAGT-3′ |
5′-TTGCTCCCATGTATGGTCTTTAC-3′ |
| Caspase-8 |
5′-CACTAGAAAGGAGGAGATGGAAAG-3′ |
5′-CTATCCTGTTCTCTTGGAGAGTCC-3′ |
| β-actin |
5′-ACGGTCAGGTCATCACTATCG-3′ |
5′-GGCATAGAGGTCTTTACGGATG-3′ |
Western blot analysis
Hepatic carcinoma cells (1×105
cells/well) were seeded in 6-well plates and cultured for 12 h at
37°C. Cells were then treated with isoflurane (2 mg/ml) or
isoflurane (2 mg/ml) for 48 h at 37°C. Hepatic carcinoma cells
isolated from patients with hepatic cancer were homogenized in
lysis buffer containing protease inhibitor (P3480; Sigma-Aldrich;
Merck KGaA), and were centrifuged at 8,000 × g for 10 min at 4°C.
The supernatant was used to analyze the expression of target
proteins. Protein concentration was measured by a BCA protein assay
kit (Thermo Fisher Scientific, Inc.). Protein samples (20 µg) were
resolved by 15% SDS-PAGE and then transferred onto polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA). For western
blotting, primary mouse anti-human antibodies against p65 (ab16502;
1:2,000), PI3K and (ab40776; 1:2,000), AKT (ab8805; 1:1,000), tumor
necrosis factor (TNF)-α (ab6671; 1:2,000), IL-2 (cat. no. ab92381;
1:2,000), IκB kinase (IKK)-β (ab7547; 1:2,000), NF-κB inhibitor α
(IκBα; ab133478; 1:2,000), pAKT (ab38449; 1:12,000) and β-actin
(ab8827; 1:2,000; all from Abcam, Cambridge, UK) were added to the
membranes after blocking with 5% skimmed milk for 2 h at 37°C.
Following washing three times with PBS, membranes were incubated
with secondary rabbit anti-mouse antibodies (PV-6001; 1:2,000;
OriGene Technologies, Inc., Beijing, China) for 2 h at 37°C, in
order to detect target proteins. The results were visualized using
a chemiluminescence detection system (GE Healthcare Life Sciences,
Little Chalfont, UK) according to the manufacturer's protocol.
Cells migration and invasion
assays
Hepatic carcinoma cells were cultured in DMEM for 48
h at 37°C. Cells were suspended at a density of 1×105 in
500 µl serum-free DMEM. Hepatic carcinoma cells were then plated in
the upper chambers of a chamber inserts (BD Biosciences, San Jose,
CA, USA) with only DMEM and DMEM with 5% FBS in the lower chambers
according to the manufacturer's protocol. In addition, hepatic
carcinoma cells (1×106) were incubated with isoflurane
(2 mg/kg) or PBS (2 mg/kg) for 72 h at 37°C in a Matrigel-coated
membrane (BD Biosciences). The cells were fixed and stained for 30
min in a 0.1% crystal violet solution in PBS. The tumor cell
invasion and migration was counted in at least three random
fields/membrane, by light microscopy (Olympus Corporation, Tokyo,
Japan) at magnification, ×40.
Pain assessment
To determine the efficacy of isoflurane for
postoperative pain remission in patients (the same patients used
for cell collection) with hepatic carcinoma who had undergone tumor
resection, general appearance parameter (GAP) scores were used to
calculate the pain score 4 h post operation. GAP scoring was
conducted according to previously published parameters regarding
posture, activity and breathing pattern (21).
Apoptosis assay
TUNEL assays were used to analyze the apoptotic rate
of hepatic carcinoma cells from patients with hepatic carcinoma who
had undergone tumor resection following pretreatment with
isoflurane. The TUNEL assay was performed according to a previous
study (22).
NF-κB activity
Hepatic carcinoma cells were cultured and treated
with isoflurane (2 mg/kg) or PBS (2 mg/kg) for 12 h at 37°C.
Subsequently, NF-κB activity was analyzed according a method
described in a previous study (23).
Statistical analysis
All data are presented as the mean ± standard error
of the mean. Unpaired data were analyzed by Student's t-test. Data
were analyzed using GraphPad Prism version 5.0 software (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Analysis of the efficacy of isoflurane
on pain remission and biochemical indexes in patients with hepatic
carcinoma
In order to analyze the anesthetic effects of
isoflurane, 156 patients with hepatic carcinoma were recruited, who
had undergone tumor resection following pretreatment with
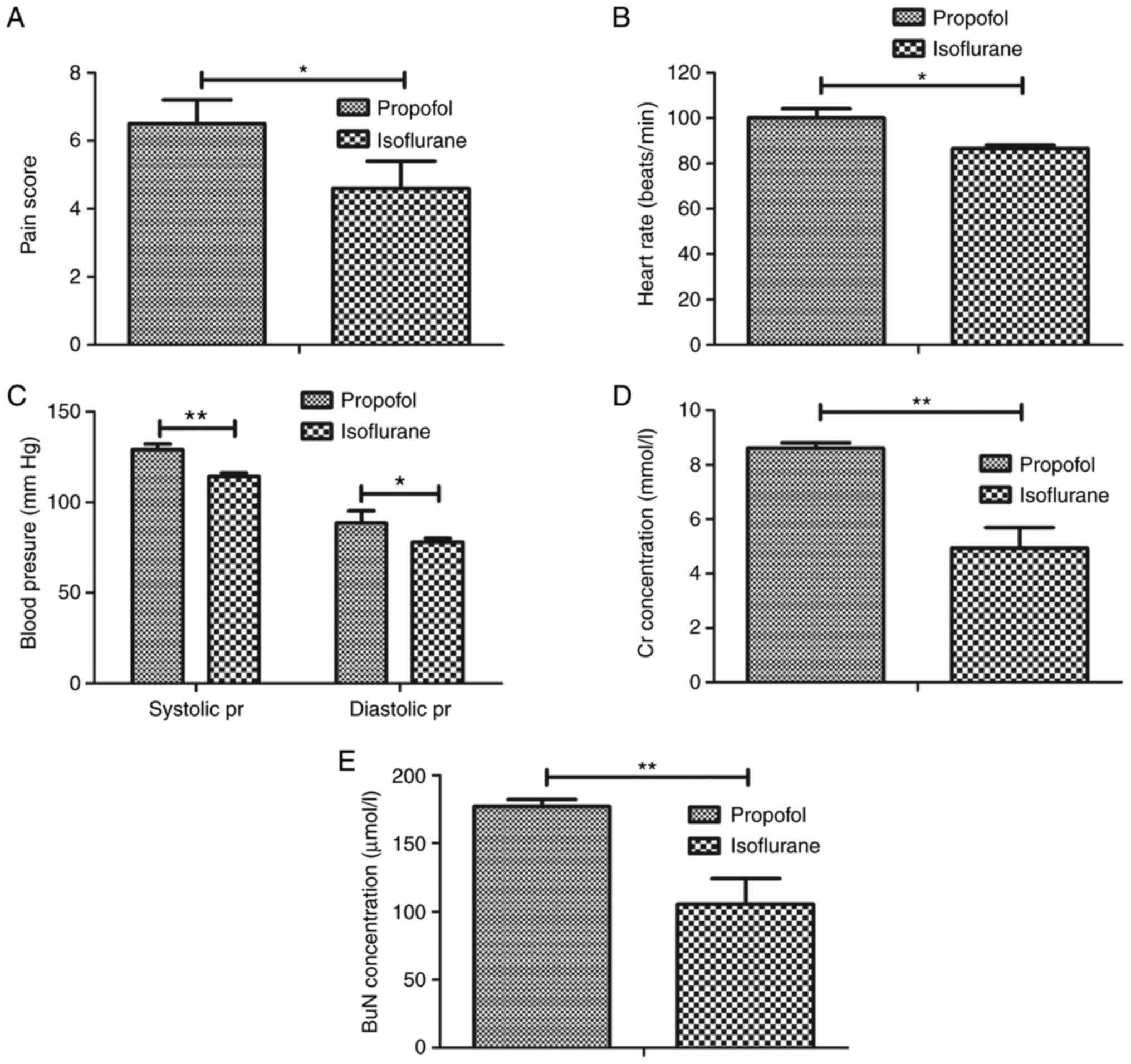
isoflurane. As presented in Fig.
1A, pretreatment with isoflurane significantly attenuated pain
in patients following tumor resection compared with in patients
pretreated with propofol. Heart rate and mean blood pressure of
patients were recorded from baseline to the 24-h anesthesia (24 h;
Fig. 1B and C). Pretreatment with
isoflurane reduced heart rate and mean arterial blood pressure in
patients that underwent tumor resection. Measurement of biochemical
indexes indicated that isoflurane pretreatment decreased creatinine
and blood urea nitrogen levels in patients prior to anesthesia and
at 24 h after anesthesia (Fig. 1D and
E). Taken together, these findings suggested that pretreatment
with isoflurane may efficiently attenuate pain remission for
patients undergoing tumor resection.
Effects of isoflurane on
proliferation, growth, migration and invasion of hepatic carcinoma
cells
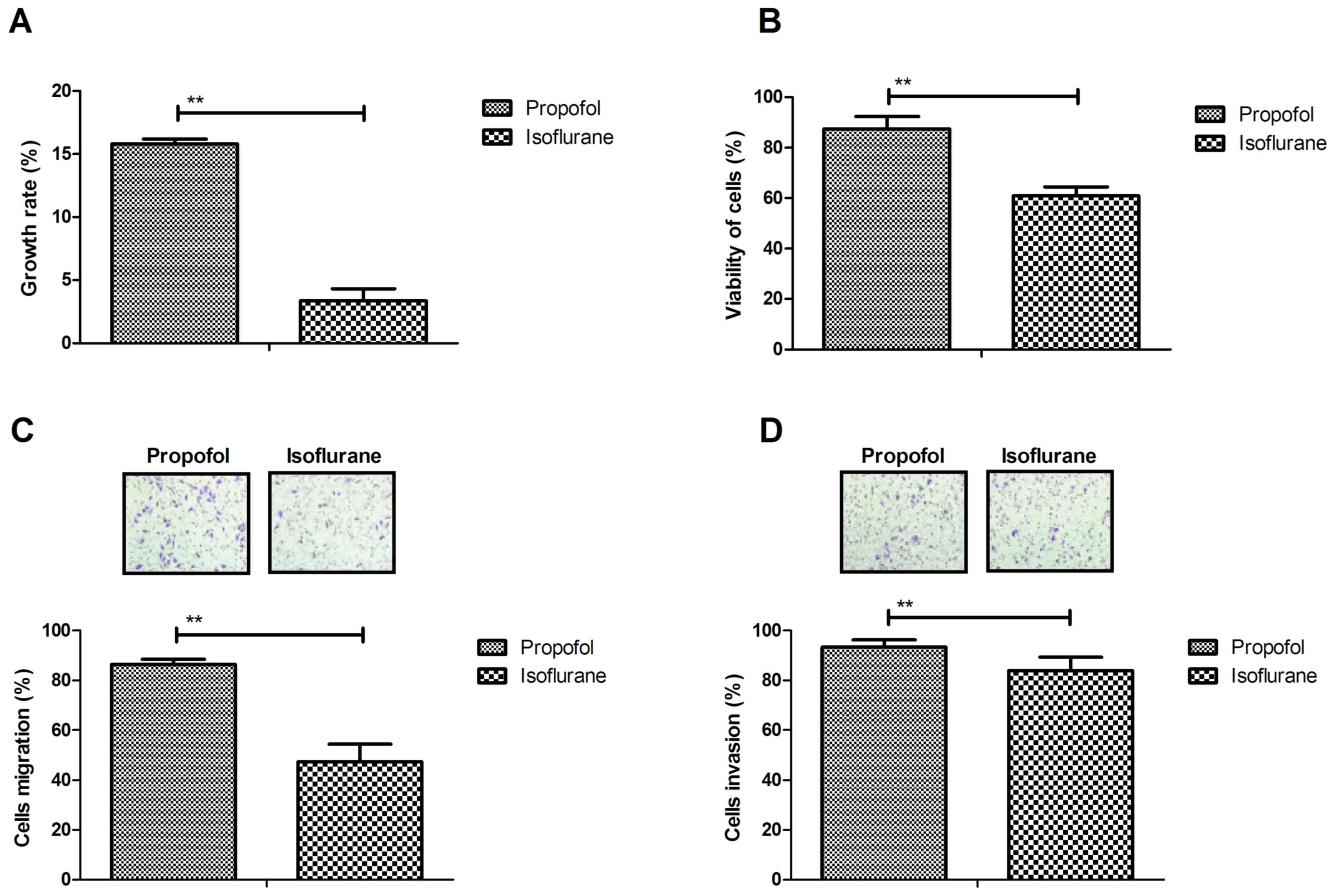
The present study investigated the efficacy of
pretreatment of isoflurane on hepatic tumor cells. The results
demonstrated that isoflurane significantly inhibited growth of
hepatic carcinoma cells isolated from patients with cancer that had
undergone tumor resection compared with propofol (Fig. 2A). Viability of hepatic carcinoma
cells was also decreased following treatment with isoflurane
compared with propofol (Fig. 2B).
The results of migration and invasion assays demonstrated that the
metastatic potential of hepatic carcinoma cells was reduced
following isoflurane pretreatment compared with propofol (Fig. 2C and D). These observations
suggested that isoflurane may inhibit growth, migration and
invasion of hepatic carcinoma cells.
Effects of isoflurane on apoptosis and
the expression levels of apoptotic genes in hepatic carcinoma
cells
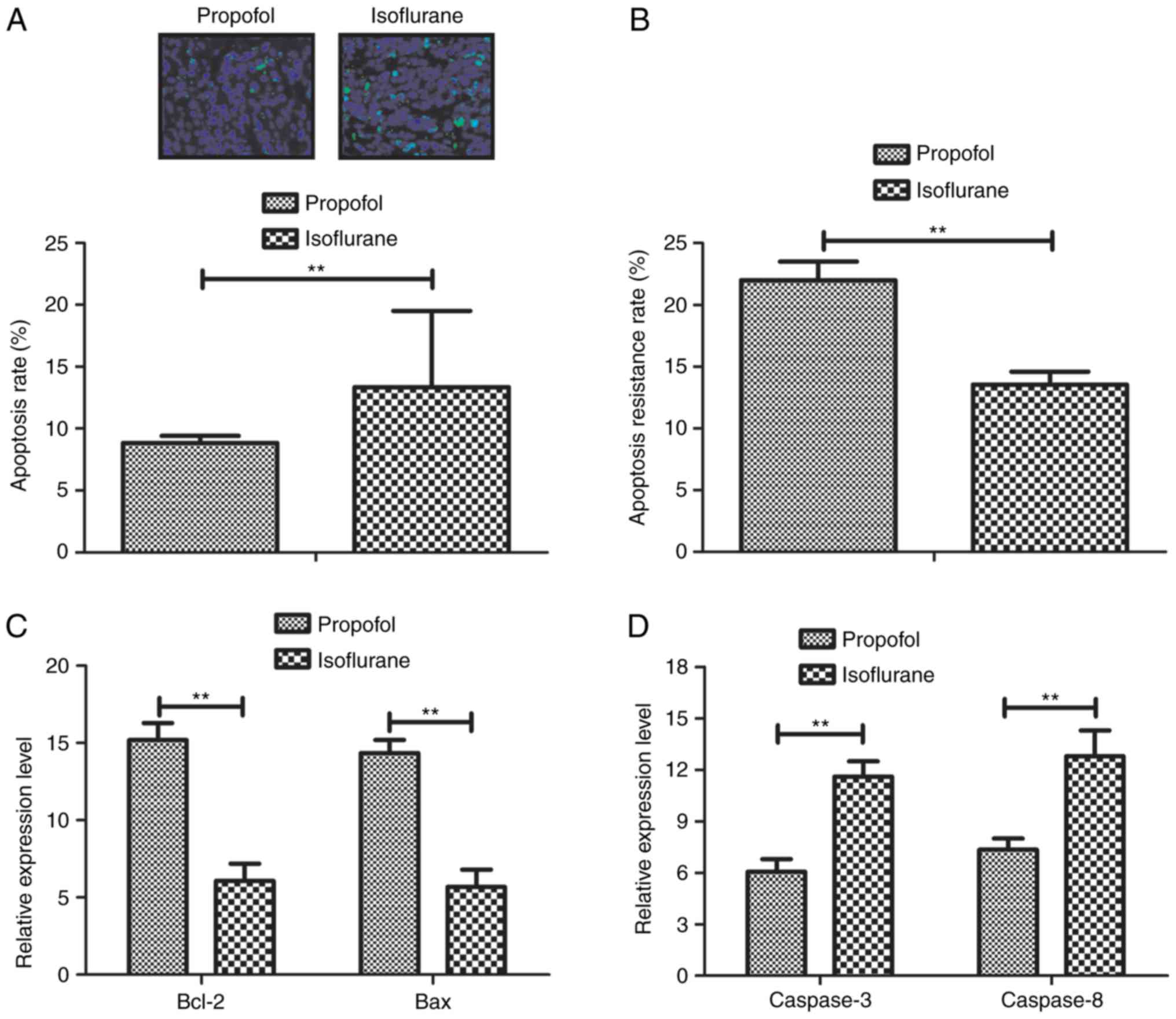
In order to investigate the anti-apoptotic effects
of isoflurane on hepatic carcinoma cells, the apoptosis and
survival rate of tumor cells isolated from patients were analyzed.
As presented in Fig. 3A,
isoflurane pretreatment increased the apoptosis of hepatic
carcinoma cells. In addition, the apoptotic rate of hepatic
carcinoma cells was increased in response to the anticancer
chemotherapeutic agent Taxol, as determined by TUNEL assay
(Fig. 3B). In addition, the
expression levels of Bcl-2, Bax, caspase-3 and caspase-8 were
detected in hepatic carcinoma cells. Data demonstrated that the
mRNA expression levels of Bcl-2 and Bax were downregulated in
hepatic carcinoma cells following isoflurane treatment compared
with control (Fig. 3C)
Furthermore, the mRNA expression levels of caspase-3 and caspase-8
were upregulated in hepatic carcinoma cells following treatment
with isoflurane compared with control (Fig. 3D). Collectively, these results
indicated that isoflurane may decrease the survival rate and
promote the apoptosis of hepatic carcinoma cells isolated from
patients with hepatic carcinoma.
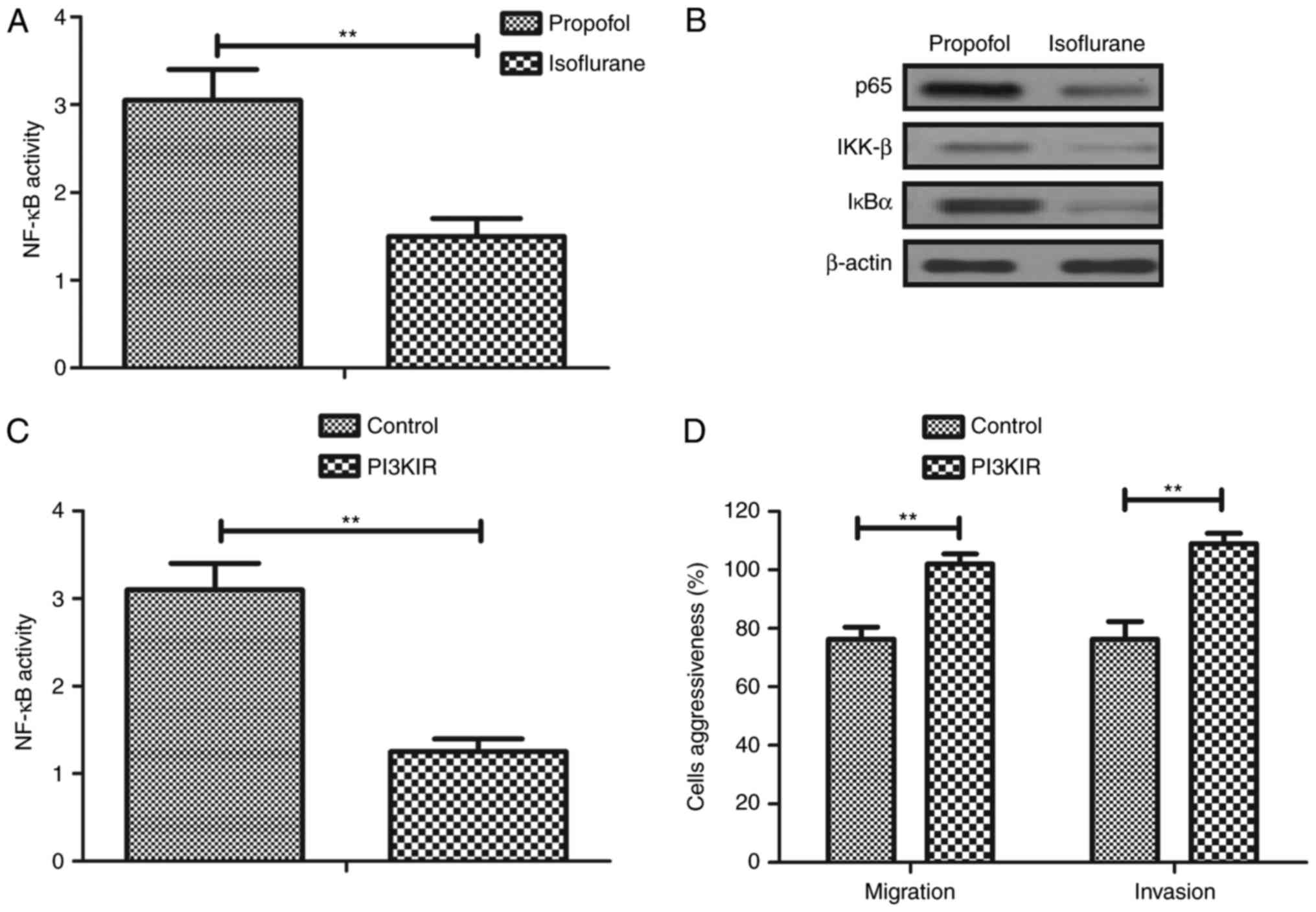
Isoflurane regulates aggressiveness of
hepatic carcinoma cells via the PI3K/AKT signaling pathway
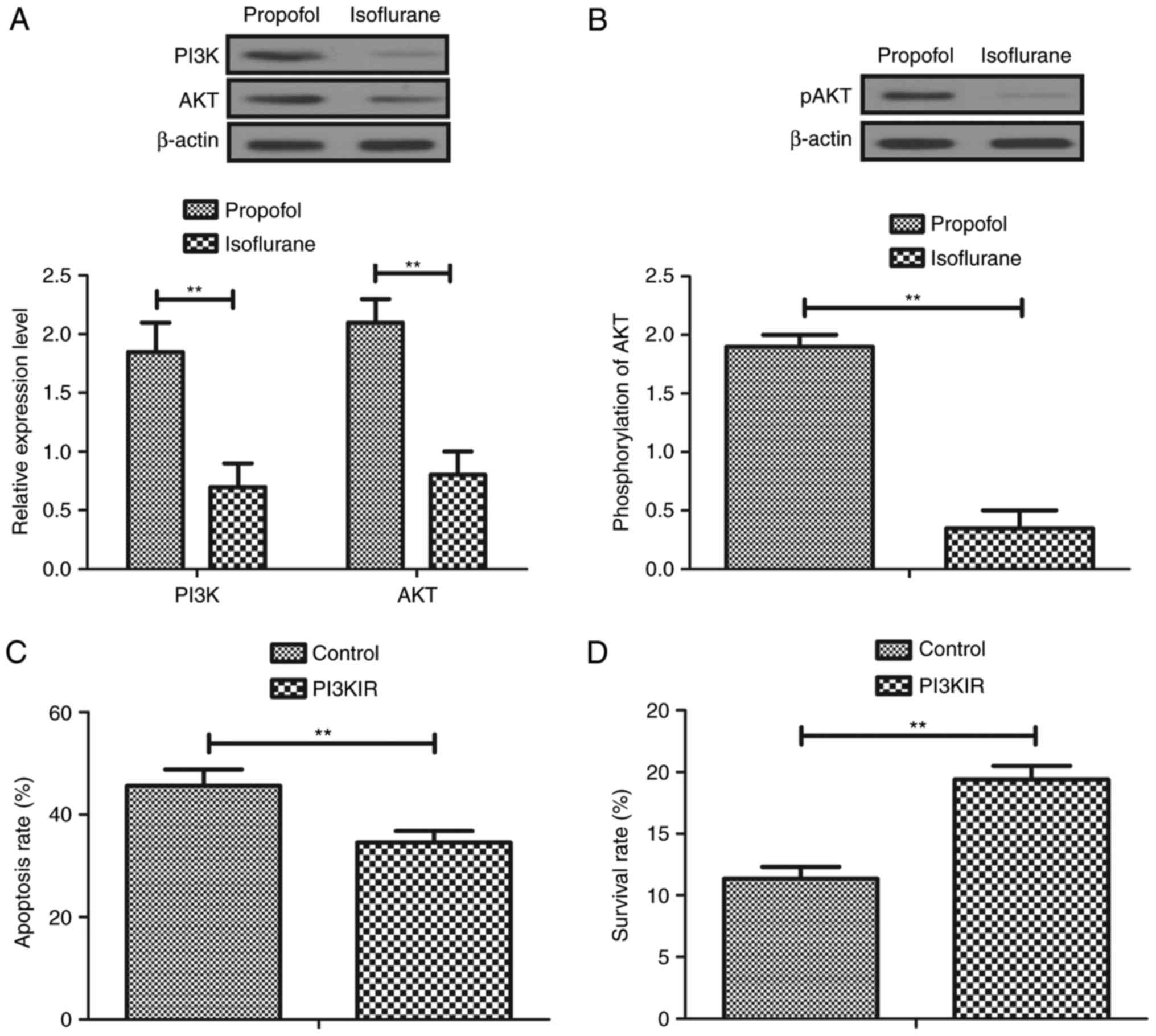
To investigate the molecular mechanism underlying
isoflurane-mediated inhibition of aggressiveness of hepatic
carcinoma cells, the PI3K/AKT signaling pathway was analyzed in
hepatic carcinoma cells. The results demonstrated that the
expression levels of PI3K and AKT were decreased in
isoflurane-treated hepatic carcinoma cells compared with in
propofol-treated cells (Fig. 4A).
In addition, phosphorylation levels of AKT were downregulated in
hepatic carcinoma cells following pretreatment with isoflurane
compared with propofol (Fig. 4B).
Furthermore, treatment with PI3KIR abolished isoflurane-induced
apoptosis of hepatic carcinoma cells compared with propofol
(Fig. 4C). Furthermore, PI3KIR
treatment abolished Taxol-inhibited survival of hepatic carcinoma
cells compared with propofol (Fig.
4D). Taken together, these results suggested that isoflurane
may significantly regulate growth and apoptosis of hepatic
carcinoma cells via the PI3K/AKT signaling pathway.
Isoflurane inhibits migration and
invasion via the PI3K/AKT-mediated NF-κB signaling pathway
The present study further analyzed the expression
levels of inflammatory factors and NF-κB in hepatic carcinoma
cells. Clinical data revealed that NF-κB activity was downregulated
in hepatic carcinoma cells isolated from patients that underwent
isoflurane pretreatment compared with propofol (Fig. 5A). NF-κB (p65, IKK-β and IκBα)
expression levels were also downregulated in hepatic carcinoma
cells isolated from clinical patients with isoflurane pretreatment
compared with propofol (Fig. 5B).
In addition, PI3KIR downregulated NF-κB activity in hepatic
carcinoma cells in vitro. PI3KIR also suppressed
isoflurane-inhibited migration and invasion of hepatic carcinoma
cells in vitro (Fig. 5D).
Taken together, these results suggested that isoflurane may
markedly inhibit migration and invasion via the PI3K/AKT-mediated
NF-κB signaling pathway.
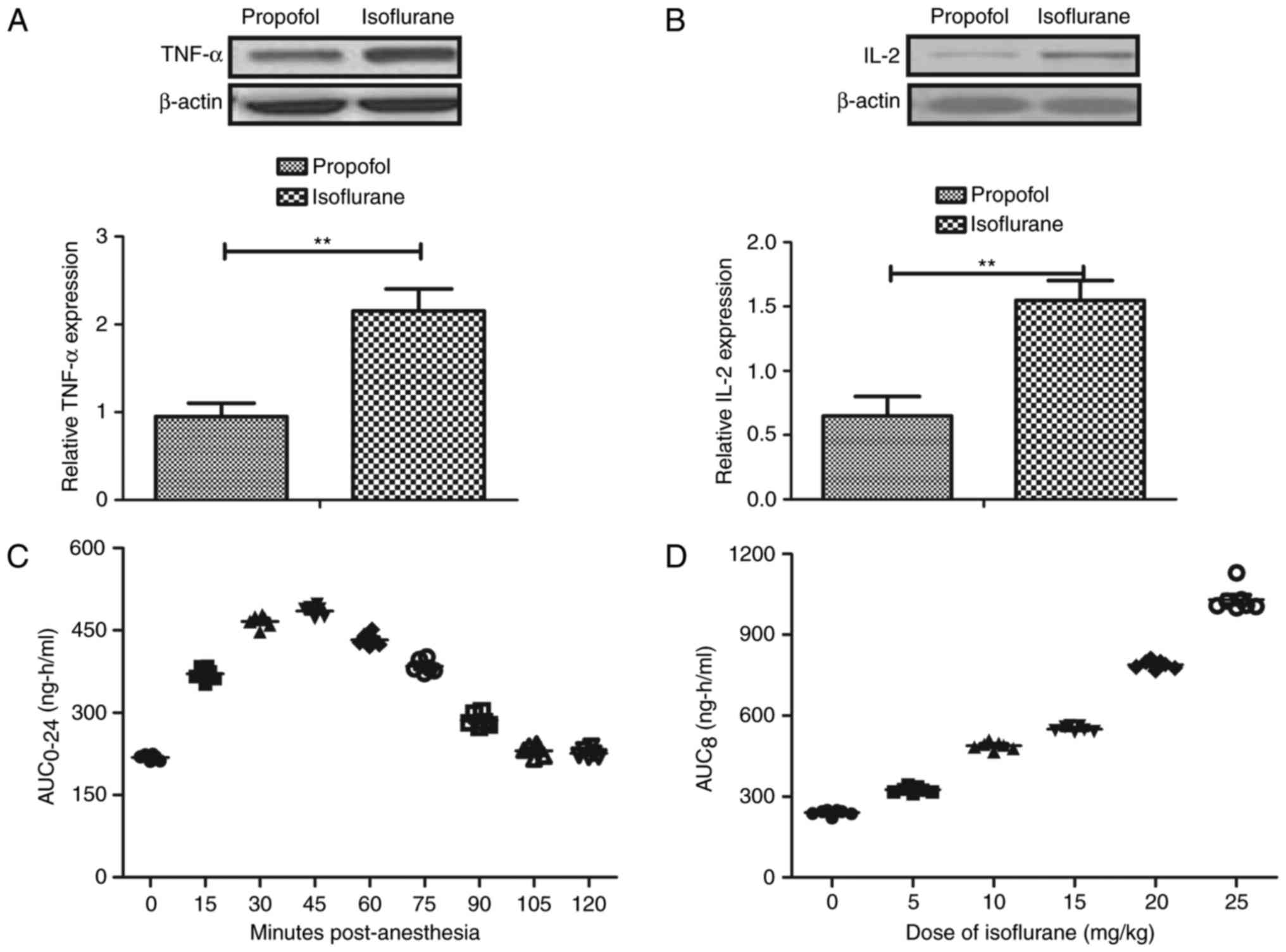
Pharmacodynamics of isoflurane in
patients with hepatic carcinoma during the perioperative
period
Inflammatory factor levels and pharmacodynamics of
isoflurane were investigated in patients with hepatic carcinoma
during the perioperative period. The expression levels of the
inflammatory factors, TNF-α and IL-2, were upregulated in the serum
of patients with hepatic carcinoma pretreated with isoflurane
during the perioperative period (Fig.
6A and B). Isoflurane was rapidly absorbed at a clinical dose
(10 mg/kg) within 30 min. In addition, serum concentrations of
isoflurane reached a maximum 45 min after injection (Fig. 6C). Furthermore, Cmax concentrations
of isoflurane (5–25 mg/kg) increased linearly with increasing dose
(Fig. 6D). There was no drug
accumulation after patients received 10 mg/kg body weight and it
was observed that Cmax values were at a steady state following
tumor resection. Collectively, these results suggested that
isoflurane pretreatment can be preserved at an efficient
concentration and may increase the expression levels of TNF-α and
IL-2 in patients with hepatic carcinoma during the perioperative
period.
Discussion
Previous studies have indicated that hepatic
carcinoma is associated with genetically complex, multifactorial
and heterogeneous tumors (24,25).
Although various novel cancer therapeutic strategies have been
proposed and have presented potential curative effects for the
treatment of patients with hepatic carcinoma, tumor resection is
still the most common treatment (26,27).
In order to attenuate the pain of patients during tumor resection,
anesthesia is used. In the present study, the anesthetic effects of
isoflurane were investigated on patients with hepatic carcinoma
during the perioperative period. In addition, the biological
effects of isoflurane on hepatic carcinoma were determined in
patients with hepatic carcinoma who had undergone tumor resection.
The molecular mechanism underlying isoflurane-induced apoptosis of
hepatic carcinoma cells isolated from patients with hepatic
carcinoma following surgery was also analyzed. The results
indicated that isoflurane not only significantly attenuated
postoperative pain, but also inhibited hepatic carcinoma growth and
aggressiveness, and promoted apoptosis via the PI3K/AKT-mediated
NF-κB signaling pathway.
Clinically, isoflurane is one of the most commonly
used volatile anesthetic agents, which is used extensively in
surgical procedures. However, the role of isoflurane in tumor
suppression is seldom reported. In the present study, the clinical
outcomes suggested that isoflurane may exert inhibitory effects on
hepatic carcinoma cells. A previous study indicated that isoflurane
can increase the expression levels of the proinflammatory cytokine
IL-6 in serum from patients with neuroglioma, resulting in
anticancer potential via the NF-κB signaling pathway. In addition,
isoflurane suppresses prostate cancer cell malignancy via
modulation of the hypoxia-inducible factor-1α signaling pathway to
regulate cancer recurrence (28).
However, Luo et al (29)
concluded that isoflurane can promote the malignant potential of
ovarian cancer cells through the upregulation of markers associated
with the cell cycle, growth, aggressiveness and angiogenesis.
Furthermore, isoflurane has the potential to induce cancer cell
apoptosis and inhibit apoptotic resistance (30). The results of the present study
confirmed that isoflurane serves an inhibitory role in the growth,
migration and invasion of hepatic carcinoma cells. In addition, it
was suggested that isoflurane regulates aggressiveness of hepatic
carcinoma cells via the PI3K/AKT-mediated NF-κB signaling
pathway.
Previous studies have reported the association
between the PI3K/AKT signaling pathway and progression of human
cancer (31–33). Kang et al (34) suggested that the expression of
proteins associated with the PI3K/AKT pathway may be considered
indictors of the hepatic-metastasis risk of colorectal cancer. In
addition, the effects of interferon-α on hepatic cancer via the
PI3K/AKT signaling pathway have been identified and clearly
elaborated in a previous mechanistic study (35). Furthermore, PI3K/AKT-mediated
cancer cell growth and aggression via the NF-κB signaling pathway
has been investigated in breast and gastric cancer cells (36,37).
Li et al (38) also
analyzed the association between invasion of cancer cells and the
PI3K/AKT/NF-κB signaling pathway. In the present study, the effects
of isoflurane were investigated on the PI3K/AKT and NF-κB signaling
pathways. The results indicated that isoflurane inhibited growth
and aggressiveness of hepatic carcinoma cells through
downregulation of PI3K/AKT-induced NF-κB signaling pathways. These
findings provide novel evidence and a potential molecular mechanism
underlying the anticancer effects of isoflurane.
Notably, the present findings demonstrated that
apoptosis of hepatic carcinoma cells was enhanced by isoflurane.
Clinically, apoptosis of tumors cells serves a crucial role in
tumor suppression and the treatment of human cancer (39,40).
The present study demonstrated that the mRNA expression levels of
caspase-3 and caspase-8 were upregulated in hepatic carcinoma cells
treated with isoflurane. Caspase-3 and caspase-8 upregulation
contributes to apoptosis of hepatic carcinoma cells, and decreases
apoptotic resistance (41,42). In addition, a previous study
suggested that apoptosis and cell proliferation are correlated with
Bcl-2 expression in human hepatocellular carcinoma (43). Bax-induced apoptosis, has been
investigated in numerous tumor cells (44). In the present study, isoflurane was
revealed to reduce apoptotic resistance via the upregulation of
caspase-3 and caspase-8 expression, and the downregulation of Bcl-2
and Bax expression. The downregulation of Bax induced by isoflurane
should be investigated in future studies.
In conclusion, the present study identified the
benefits of isoflurane pretreatment for patients with hepatic
carcinoma. This study indicated that the inhibitory effects of
isoflurane on hepatic cancer aggressiveness may be mediated by
regulation of the PI3K/AKT-induced NF-κB signaling pathway. In
addition, the results suggested that isoflurane may suppress
apoptotic resistance via activation of caspase-3 and caspase-8, and
suppression of Bcl-2 and Bax. These findings indicated that
isoflurane may be regarded as a preferable anesthetic and
additional antitumor agent for the clinical treatment of patients
with hepatic carcinoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
QGL designed the research. JH, JLH and HMJ performed
the research and analyzed data. JH and QGL wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was directed according to the
Guide for the Care and Use of Clinical Investigation of
Anesthesiology of Linyi Cancer Hospital (Linyi, China). The present
study was approved by the ethics committee of Linyi Cancer
Hospital.
Consent for publication
All patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kao HK, Guo LF, Cheng MH, Chen IH, Liao
CT, Fang KH, Yu JS and Chang KP: Predicting postoperative morbidity
and mortality by model for endstage liver disease score for
patients with head and neck cancer and liver cirrhosis. Head Neck.
33:529–534. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Menon KV, Hakeem AR and Heaton ND: Review
article: Liver transplantation for hepatocellular carcinoma-a
critical appraisal of the current worldwide listing criteria.
Aliment Pharmacol Ther. 40:893–902. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shariff MI, Cox IJ, Gomaa AI, Khan SA,
Gedroyc W and Taylor-Robinson SD: Hepatocellular carcinoma: Current
trends in worldwide epidemiology, risk factors, diagnosis and
therapeutics. Expert Rev Gastroenterol Hepatol. 3:353–367. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Philip-Ephraim EE, Eyong KI, Williams UE
and Ephraim RP: The role of radiotherapy and chemotherapy in the
treatment of primary adult high grade gliomas: Assessment of
patients for these treatment approaches and the common immediate
side effects. ISRN Oncol. 2012:9021782012.PubMed/NCBI
|
|
5
|
Bai P, Zhang R, Li XG, Ma SK, Wu LY and
Zhang WH: Efficiency and side effects of concurrent radiotherapy
and chemotherapy for advanced cervical cancers. Zhonghua Zhong Liu
Za Zhi. 29:467–469. 2007.(In Chinese). PubMed/NCBI
|
|
6
|
Huang YH, Wu JC, Chen SC, Chen CH, Chiang
JH, Huo TI, Lee PC, Chang FY and Lee SD: Survival benefit of
transcatheter arterial chemoembolization in patients with
hepatocellular carcinoma larger than 10 cm in diameter. Aliment
Pharmacol Ther. 23:129–135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Joseph JD, Peng Y, Mak DO, Cheung KH, Vais
H, Foskett JK and Wei H: General anesthetic isoflurane modulates
inositol 1,4,5-trisphosphate receptor calcium channel opening.
Anesthesiology. 121:528–537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu C, Luo YL, Xiao SS, Zhang Q and Chen
SL: Influence of propofol and isoflurane on cytokines response to
cancer surgery during perioperative period. Hua Xi Kou Qiang Yi Xue
Za Zhi. 25:554–556. 2007.(In Chinese). PubMed/NCBI
|
|
9
|
Jiang J and Jiang H: Effect of the inhaled
anesthetics isoflurane, sevoflurane and desflurane on the
neuropathogenesis of Alzheimer's disease (review). Mol Med Rep.
12:3–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu TC: Influence of propofol, isoflurane
and enflurance on levels of serum interleukin-8 and interleukin-10
in cancer patients. Asian Pac J Cancer Prev. 15:6703–6707. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang H, Deng J, Jiang Y, Chen J, Zeng X,
He Z, Jiang X, Li Z and Jiang C: Emulsified isoflurane treatment
inhibits the cell cycle and respiration of human bronchial
epithelial 16HBE cells in a p53-independent manner. Mol Med Rep.
14:349–354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miura Y, Kamiya K, Kanazawa K, Okada M,
Nakane M, Kumasaka A and Kawamae K: Superior recovery profiles of
propofol-based regimen as compared to isoflurane-based regimen in
patients undergoing craniotomy for primary brain tumor excision: A
retrospective study. J Anesth. 26:721–727. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lim KH and Counter CM: Reduction in the
requirement of oncogenic Ras signaling to activation of PI3K/AKT
pathway during tumor maintenance. Cancer cell. 8:381–392. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi LX, Wang JH and Shi XD: PI3K/AKT/mTOR
Pathway and Pediatric T acute lymphoblastic leukemia-review.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 24:1269–1274. 2016.(In
Chinese). PubMed/NCBI
|
|
15
|
Dey JH, Bianchi F, Voshol J, Bonenfant D,
Oakeley EJ and Hynes NE: Targeting fibroblast growth factor
receptors blocks PI3K/AKT signaling, induces apoptosis, and impairs
mammary tumor outgrowth and metastasis. Cancer Res. 70:4151–4162.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang CM, Cai XL and Wen QP: Astaxanthin
reduces isoflurane-induced neuroapoptosis via the PI3K/Akt pathway.
Mol Med Rep. 13:4073–4078. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao K, Song X, Huang Y, Yao J, Zhou M, Li
Z, You Q, Guo Q and Lu N: Wogonin inhibits LPS-induced tumor
angiogenesis via suppressing PI3K/Akt/NF-κB signaling. Eur J
Pharmacol. 737:57–69. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miao X and Zhao Y: ST6GalNAcII mediates
tumor invasion through PI3K/Akt/NF-κB signaling pathway in
follicular thyroid carcinoma. Oncol Rep. 35:2131–2140. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Al-Sobayil FA and Omer OH: Serum
biochemical values of adult ostriches (Struthio camelus)
anesthetized with xylazine, ketamine, and isoflurane. J Avian Med
Surg. 25:97–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nilsson U and Idvall E: Pain assessments
in day surgery patients. J Clin Nurs. 19:2942–2943. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng N, Wei X, Zhang D, Chai W, Che M,
Wang J and Du B: Hepatic resection or transarterial
chemoembolization for hepatocellular carcinoma with portal vein
tumor thrombus. Medicine (Baltimore). 95:e39592016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jia YS, Hu XQ, Gabriella H, Qin LJ and
Meggyeshazi N: Antitumor activity of tenacissoside H on esophageal
cancer through arresting cell cycle and regulating PI3K/Akt-NF-κB
transduction cascade. Evid Based Complement Alternat Med.
2015:4649372015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simonetti RG, Cammà C, Fiorello F, Politi
F, D'Amico G and Pagliaro L: Hepatocellular carcinoma. A worldwide
problem and the major risk factors. Dig Dis Sci. 36:962–972. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zidan A, Scheuerlein H, Schüle S,
Settmacher U and Rauchfuss F: Epidemiological pattern of hepatitis
B and hepatitis C as etiological agents for hepatocellular
carcinoma in iran and worldwide. Hepat Mon. 12:e68942012.PubMed/NCBI
|
|
26
|
Marabelle A and Gray J: Tumor-targeted and
immune-targeted monoclonal antibodies: Going from passive to active
immunotherapy. Pediatr Blood Cancer. 62:1317–1325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishimura Y, Tomita Y, Yuno A, Yoshitake Y
and Shinohara M: Cancer immunotherapy using novel tumor-associated
antigenic peptides identified by genome-wide cDNA microarray
analyses. Cancer Sci. 106:505–511. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang H, Benzonana LL, Zhao H, Watts HR,
Perry NJ, Bevan C, Brown R and Ma D: Prostate cancer cell
malignancy via modulation of HIF-1α pathway with isoflurane and
propofol alone and in combination. Br J Cancer. 111:1338–1349.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luo X, Zhao H, Hennah L, Ning J, Liu J, Tu
H and Ma D: Impact of isoflurane on malignant capability of ovarian
cancer in vitro. Br J Anaesth. 114:831–839. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Dong Y, Wu X, Lu Y, Xu Z, Knapp
A, Yue Y, Xu T and Xie Z: The mitochondrial pathway of anesthetic
isoflurane-induced apoptosis. J Biol Chem. 285:4025–4037. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mu GG, Zhang LL, Li HY, Liao Y and Yu HG:
Thymoquinone pretreatment overcomes the insensitivity and
potentiates the antitumor effect of gemcitabine through abrogation
of Notch1, PI3K/Akt/mTOR regulated signaling pathways in pancreatic
cancer. Dig Dis Sci. 60:1067–1080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Coco S, Truini A, Alama A, Dal Bello MG,
Venè R, Garuti A, Carminati E, Rijavec E, Genova C, Barletta G, et
al: Afatinib resistance in non-small cell lung cancer involves the
PI3K/AKT and MAPK/ERK signalling pathways and
epithelial-to-mesenchymal transition. Targeted Oncol. 10:393–404.
2015. View Article : Google Scholar
|
|
33
|
Yang Y, Zhang J, Zhu Y, Zhang Z, Sun H and
Feng Y: Follicle-stimulating hormone induced epithelial-mesenchymal
transition of epithelial ovarian cancer cells through
follicle-stimulating hormone receptor PI3K/Akt-Snail signaling
pathway. Int J Gynecol Cancer. 24:1564–1574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kang B, Hao C, Wang H, Zhang J, Xing R,
Shao J, Li W, Xu N, Lu Y and Liu S: Evaluation of
hepatic-metastasis risk of colorectal cancer upon the protein
signature of PI3K/AKT pathway. J Proteome Res. 7:3507–3515. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yan L and Shi G: Effect of IFN-α on
hepatic cancer SMCC-7721 cell via PI3K/Akt signaling pathway and
related mechanism research. Zhonghua Yi Xue Za Zhi. 95:2960–2963.
2015.(In Chinese). PubMed/NCBI
|
|
36
|
Sha M, Ye J, Zhang LX, Luan ZY, Chen YB
and Huang JX: Celastrol induces apoptosis of gastric cancer cells
by miR-21 inhibiting PI3K/Akt-NF-κB signaling pathway.
Pharmacology. 93:39–46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rabi T, Huwiler A and Zangemeister-Wittke
U: AMR-Me inhibits PI3K/Akt signaling in hormone-dependent MCF-7
breast cancer cells and inactivates NF-κB in hormone-independent
MDA-MB-231 cells. Mol Carcinog. 53:578–588. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li C, Li F, Zhao K, Yao J, Cheng Y, Zhao
L, Li Z, Lu N and Guo Q: LFG-500 inhibits the invasion of cancer
cells via down-regulation of PI3K/AKT/NF-κB signaling pathway. PloS
One. 9:e913322014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dastjerdi MN, Babazadeh Z, Rabbani M,
Gharagozloo M, Esmaeili A and Narimani M: Effects of disulfiram on
apoptosis in PANC-1 human pancreatic cancer cell line. Res Pharm
Sci. 9:287–294. 2014.PubMed/NCBI
|
|
40
|
Din Tengku TA, Seeni A, Khairi WN,
Shamsuddin S and Jaafar H: Effects of rapamycin on cell apoptosis
in MCF-7 human breast cancer cells. Asian Pac J Cancer Prev.
15:10659–10663. 2014.PubMed/NCBI
|
|
41
|
Russe OQ, Möser CV, Kynast KL, King TS,
Olbrich K, Grösch S, Geisslinger G and Niederberger E: LPS inhibits
caspase 3-dependent apoptosis in RAW264.7 macrophages induced by
the AMPK activator AICAR. Biochem Biophys Res Commun. 447:520–525.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mandal R, Raab M, Matthess Y, Becker S,
Knecht R and Strebhardt K: Perk 1/2 inhibit Caspase-8 induced
apoptosis in cancer cells by phosphorylating it in a cell cycle
specific manner. Mol Oncol. 8:232–249. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
EI-Emshaty HM, Saad EA, Toson EA, Malak
Abdel CA and Gadelhak NA: Apoptosis and cell proliferation:
Correlation with BCL-2 and P53 oncoprotein expression in human
hepatocellular carcinoma. Hepatogastroenterology. 61:1393–1401.
2014.PubMed/NCBI
|
|
44
|
Tsai CJ, Liu S, Hung CL, Jhong SR, Sung TC
and Chiang YW: BAX-induced apoptosis can be initiated through a
conformational selection mechanism. Structure. 23:139–148. 2015.
View Article : Google Scholar : PubMed/NCBI
|