Introduction
Esophageal cancer is one of the most common types of
cancer worldwide, and is associated with a particularly poor
prognosis. Esophageal squamous cell carcinoma (ESCC) is the most
common pathological type of esophageal cancer, accounting for 70%
of cases (1). The incidence and
mortality rates for ESCC are particularly high in Asian countries
compared with Western countries (2). A combination of treatments, including
surgery and chemotherapy, is the primary method for treating ESCC
(3,4). However, the prognosis of esophageal
cancer remains poor, and the overall five-year survival rate was
reported to be 15–25% (5). Tumor
metastasis is the main cause of mortality associated with
esophageal squamous cell carcinoma (6). Due to the lack of serous layer in the
esophageal wall, direct infiltration and metastasis can occur in
the early stage of esophageal cancer (7). It has been reported that 56% of
patients have suffered from blood vessel metastasis and 32% of
patients have suffered from lymph metastasis during tumor invasion
of the submucosal tissue (8). The
early symptoms and signs of esophageal cancer are not easily
distinguished; however, when identified, the disease tends to be
within the middle and late stage of pathogenesis. Subsequently,
distant metastasis occurs, leading to poor prognosis (4). Therefore, the mechanism of the
invasion and metastasis of esophageal cancer has been a focus of
research.
Aidi, an extract of ginseng, Astragalus
membranaceus, Acanthopanax and Mylabris, has been
researched and manufactured in abundance for clinical use in China
since 2002 (9). Aidi injection has
been widely used for the treatment of a range of cancer types,
including ESCC (10), gastric
carcinoma (11), primary liver
cancer (12) and colorectal cancer
(13). Modern pharmacological
research has demonstrated that treatment with Aidi produces various
pharmacological effects, including anti-tumor and immune regulatory
actions. The chemical constituents of Aidi have been identified
through spectral data from chromatography on Sephadex LH-20 gel
columns, and reverse phase semi-preparative high-performance liquid
chromatography; a total of 22 compounds were isolated and
identified (14). The results
showed that the 6 compounds were from Astragalus
membranaceus, 12 compounds were from ginseng, 4 compounds came
from Acanthopanax. The evaluation of pharmacological and
toxicological activity in compound preparation is still in
progress. The anti-tumor effects of treatment with Aidi include the
induction of apoptosis, the inhibition of cell proliferation and
angiogenesis, and the relief of chemotherapy-associated side
effects (15,16). However, the therapeutic effects of
Aidi on the inhibition of metastasis in ESCC are unclear.
The mechanisms of invasion and metastasis of tumor
are very complex involves hypoxia microenvironment, angiogenesis,
epithelial-mesenchymal transformation (EMT), and network regulation
of numerous signaling pathways and so on (17). In this study, we would elucidate
the anti-metastatic effects of treatment with Aidi in ESCC, and
identify the possible underlying mechanisms angiogenesis and EMT in
the anti-metastatic effects.
Materials and methods
Cell culture and drugs
The human ESCC cell lines EC9706 and KYSE70 (Cell
Bank of Chinese Academy of Sciences, Shanghai Institute of Cell
Biology, Shanghai, China) were cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS; both Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) in 5% CO2 at
37°C. Human umbilical vein endothelial cells (HUVECs; Zhong Qiao
Xin Zhou Biotech, Shanghai, China) were cultured in high-glucose
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) containing 20% FBS in 5% CO2 at 37°C.
Aidi was purchased from Guizhou Yibai Pharmaceutical Co., Ltd.
(Guizhou, China) with an initial concentration of 0.3 g/ml.
5-Fluorouracil (5-Fu; 0.025 g/ml) was purchased from Jin Yao Amino
Acid Co., Ltd. (Tianjin, China). The drugs were diluted to the
required concentration in a sterile environment with the cell
culture media mentioned above, and were used in in vitro
experiments; drugs diluted with sterile saline were used in in
vivo experiments.
Cell proliferation assay
The cells (2×103) were cultured in
96-well plates for 24 h at 37°C. Culture medium (100 µl) containing
1.5, 3, 6, 12, 24, 48 or 96 mg/ml Aidi was added following removal
of the original medium. The cells were cultured for 24 or 48 h at
37°C. A volume of 15 µl 5 mg/ml MTT (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was added and incubated for 4 h, and
subsequently 180 µl dimethyl sulfoxide (Amresco, LLC, Solon, OH,
USA) was added. Once the bluish violet crystalline formazan had
dissolved completely with gentle shaking for 10 min, the absorbance
(A) was detected at 490 nm using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The cell growth rate (%)
was determined as follows: (Acontrol
group-Aobserved group)/Acontrol group
×100.
Cell morphology observation
A total of 1×105 cells per well were
cultured in 6-well plates for 24 h at 37°C. Then cells were treated
with 3, 6 or 12 mg/ml Aidi, 32 mg/l 5-Fu, or without any drugs
respectively for 24 h at 37°C. Cells were observed and photographed
using bright field microscopy (magnification, ×100, BX41; Olympus
Corporation, Tokyo Japan).
Wound-healing assay
To detect the rate of migration, a wound-healing
assay was performed. Cells were cultured on 6-well plates for 24 h,
prior to being treated with 6, 12 or 24 mg/ml Aidi, 32 mg/l 5-Fu,
or without drugs for 24 h. A pipette tip was used to scratch the
monolayer in each well. Detached cells were removed by washing
twice with PBS. The remaining adherent cells were cultured with
complete culture medium for 24 h. The extent of space filling,
representing the cell migration, was evaluated using bright field
microscopy (magnification, ×100, BX41; Olympus Corporation, Tokyo
Japan). The migratory capacity of cells was calculated as (scratch
width of the treatment group-scratch width of the control
group)/scratch width of the control group ×100.
Matrigel invasion assay
To detect the invasive ability of the cells, Corning
Matrigel Invasion Chambers (Corning Incorporated, Corning, NY, USA)
were used. Matrigel was diluted with RPMI-1640 medium at a ratio of
1:8 and used to coat the upper chamber. RPMI-1640 or DMEM with 20%
FBS (500 µl) was added to the lower chamber. Cells were collected
following 24 h of treatment, as specified in the wound-healing
assay method. Cells (5×104) in serum-free RPMI-1640
medium were added to the upper chamber and cultured for 24 h at
37°C. The non-invading cells were removed by wiping gently with
cotton swabs. The invading cells on the lower membrane were fixed
with 100% methanol at room temperature for 15 min and stained with
0.1% crystal violet (Sigma-Aldrich; Merck KGaA) at room temperature
for 30 min. The invading cells were detected by light microscopy
(magnification, ×100, BX53M; Olympus Corporation). The invasive
capacity of cells was calculated as (number of invading cells in
the treatment group/number of invading cells in the control
group).
Tube formation assay
In the tube formation assay, HUVECs and ESCC KYSE70
cells were used to determine the rate of angiogenesis in a
vasculogenic mimicry (VM) formation assay. Matrigel diluted with
serum-free medium (1:1) was applied to a 96-well plate at 50
µl/well, and incubated at 37°C for 1 h. A total of 5×103
cells treated with drugs for 24 h suspended in 50 µl culture medium
were added to the matrix gel and incubated in 5% CO2 at
37°C for 16 h. The three-dimensional organization of the cells was
examined and imaged under an inverted microscope (magnification,
×100).
Western blotting
Total protein from EC9706 and KYSE70 cells treated
for 24 h was extracted by lysis in solubilizing buffer with 1
mmol/l phenylmethanesulfonyl fluoride and a protease inhibitor
cocktail (Beyotime Institute of Biotechnology, Haimen, China), and
subsequently centrifuged at 13,000 × g at 4°C for 15 min. Protein
concentration was detected by a Bicinchoninic Acid protein
measuring kit (Beyotime Institute of Biotechnology). A total of 30
µg protein per lane was separated by 10% SDS-PAGE and transferred
onto polyvinylidene fluoride membranes. The membranes were blocked
with 5% skimmed milk at room temperature for 2 h and incubated with
primary antibodies against cadherin-1 [Cell Signaling Technology
(CST), Inc., Danvers, MA, USA; cat. no. 3195; dilution, 1:1,000],
cadherin-2 (CST, Inc.; cat. no. 13116; dilution, 1:1,000), vimentin
(CST, Inc.; cat. no. 5741; dilution, 1:1,000) and vascular
endothelial growth factor (VEGF-A; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA; cat. no. sc-152; dilution, 1:500) overnight at
4°C. β-actin (CST, Inc.; cat. no. 4970; dilution, 1:1,000) was
utilized as a loading control. A horseradish peroxidase-conjugated
goat anti-rabbit antibody (CST, Inc.; cat. no. 7074; dilution,
1:2,000) was subsequently applied at room temperature for 2 h. An
enhanced chemiluminescent detection reagent (Thermo Fisher
Scientific, Inc.) was used to visualize the immunoreactive signals
with a gel imaging system (Bio-Rad Laboratories, Inc.).
Densitometry analysis of bands was calculated using Quantity One
analysis software, version 4.62 (Bio-Rad Laboratories, Inc.).
Experimental tumor metastasis
model
To evaluate the anti-metastatic effects of Aidi
injection, nude mouse peritoneal metastasis models were
established. A total of 24 female BALB/c NU mice (5 weeks old) were
purchased from the Comparative Medicine Laboratory Animal Center
(license no. scxk (SU) 2012–0004) of Yangzhou University (Jiangsu,
China). These nude mice weighed ~19 g. The nude mice were housed at
a constant temperature (25–27°C), constant humidity (45–50%),
specific pathogen free environment. Sterile purified water and food
were provided ad libitum. EC9706 cells (2×106)
were inoculated into the peritoneal cavity of 20 mice, and the rest
of 4 mice were not injected with the cells as the normal group.
After 14 days following tumor implantation, the mice were treated
with Aidi injection (1, 2 or 4 g/kg/day), 60 mg/kg 5-Fu, or the
same volume of saline (control group) via intraperitoneal injection
for 15 days. Each group contained four mice. At the end of
treatment, the mice were sacrificed by cervical dislocation and a
laparotomy was performed. The numbers of peritoneal cancer nodules
were examined. All experimental procedures were performed following
internationally accepted guidelines regarding the use of laboratory
animals (18) and the protocol of
the present study was reviewed and approved by the Institutional
Animal Care and Use Committee of Yangzhou University (Jiangsu,
China).
Immunohistochemistry
All tissues were fixed with 10% neutral buffered
formalin at room temperature overnight and embedded in paraffin.
Sections of 5-µm thickness of the tumor tissues temperature were
cut for immunohistochemical staining. The slides were dewaxed in
xylene and rehydrated a descending alcohol series. Hydrogen
peroxide (3%) was added to quench the endogenous peroxidase
activity for 10 min. Antigen retrieval was performed in citrate
buffer at 95°C for 5 min. Goat serum (10%; Beyotime Institute of
Biotechnology) was used to block for 20 min at room temperature.
Subsequently, the aforementioned primary antibodies (vimentin,
dilution 1:200; cadherin-1, dilution 1:200; VEGF-A, dilution 1:100)
were incubated with the sections at 4°C overnight. Following this
incubation, the slides were incubated with an appropriate secondary
antibody conjugated with HRP (CST, Inc.; undiluted; cat. no. 8114)
at 37°C for 20 min. The slides were stained with
3,3-diaminobenzidine at room temperature for 10 min following
washing with PBS. The slides were counterstained with hematoxylin
for 15 min at room temperature. The slides were subsequently
dehydrated and mounted with neutral gum for imaging with inverted
microscope (magnification, ×200).
Statistical analysis
Each experiment was repeated three times. All values
are presented as the mean ± standard deviation. Data from the in
vitro and in vivo experiments were compared using a
one-way analysis of variance with Bonferroni's post hoc test, or a
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference. All analysis was performed
using SPSS software version 16.0 (SPSS, Inc., Chicago, IL,
USA).
Results
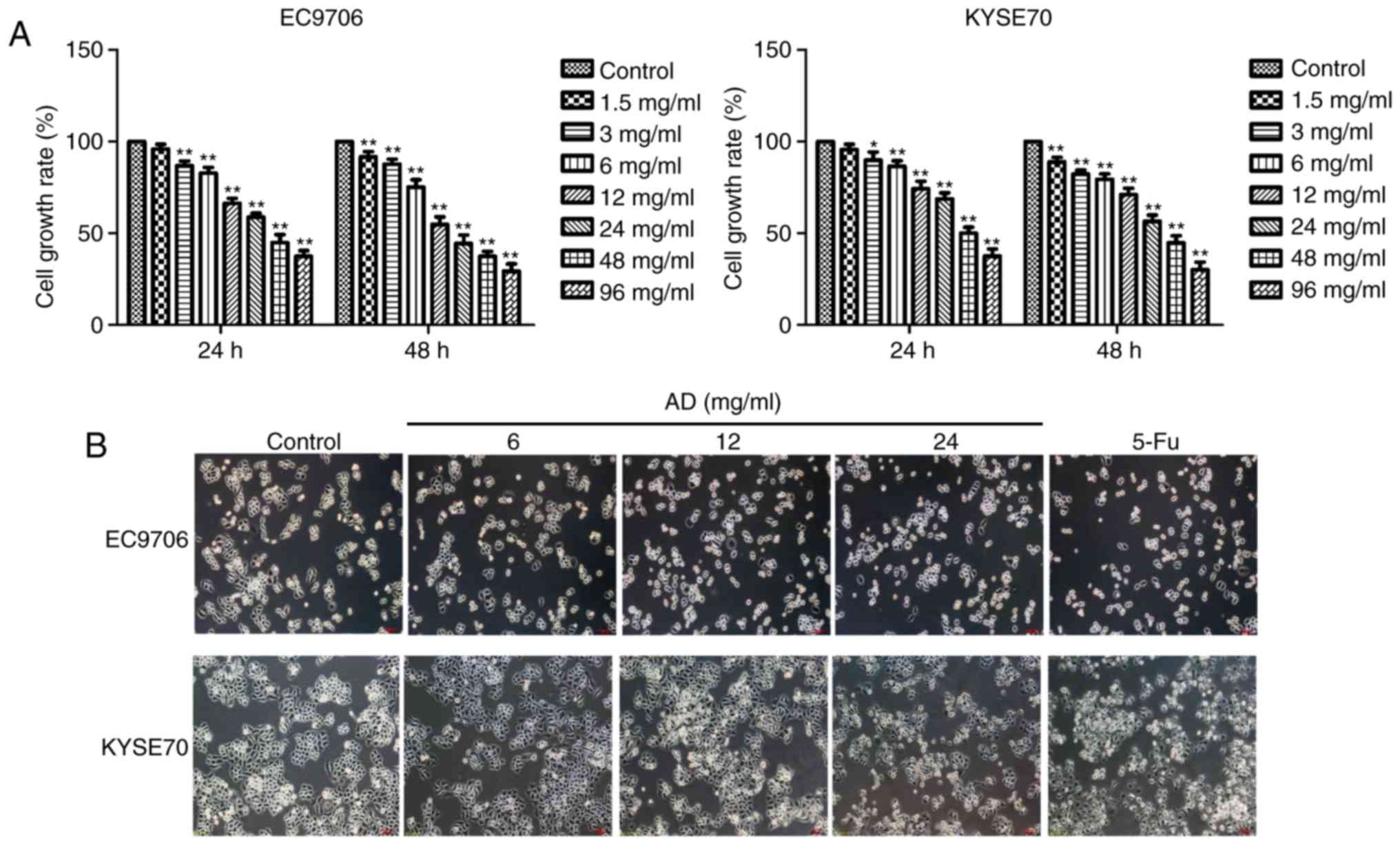
Aidi inhibits the viability of ESCC
cells
EC9706 and KYSE70 cells treated with varying
concentrations (3, 6, 12, 24, 48 or 96 mg/ml) of Aidi for 24 or 48
h revealed significantly inhibited growth (P<0.05, P<0.01;
Fig. 1A) in a concentration and
time-dependent manner. The cell growth rates of EC9706 for 24 h
were 1.5 mg/ml (95.95±2.67%), 3 mg/ml (86.79±2.64%), 6 mg/ml
(82.76±3.10%), 12 mg/ml (66.27±2.74%), 24 mg/ml (58.84±2.12%), 48
mg/ml (44.99±4.29%), 96 mg/ml (37.44±3.07%), respectively. The cell
growth rates of KYSE70 for 24 h were 1.5 mg/ml (95.68±2.91%), 3
mg/ml (89.95±4.34%), 6 mg/ml (86.61±2.94%), 12 mg/ml (74.36±4.07%),
24 mg/ml (68.65±3.41%), 48 mg/ml (50.11±3.24%), 96 mg/ml
(39.57±3.87%), respectively. The half maximal inhibitory
concentration at 24 h for EC9706 and KYSE70 cells was 25.89 mg/ml
and 32.58 mg/ml, respectively. Aidi was administered via
intravenous injection once a day, so 24 h treatment was selected
and Aidi concentrations of 6, 12 and 24 mg/ml which growth
inhibition rate was <50% were used to avoid cytotoxic effects of
excessive concentration in subsequent experiments; 32 mg/ml 5-Fu
was used as a control. The morphological characteristics of the
cells were observed following the selected treatments. The
intercellular space increased, cell numbers were reduced and the
cytoplasm shrank in response to Aidi and 5-Fu treatments compared
with in the control group of the two cell lines (Fig. 1B).
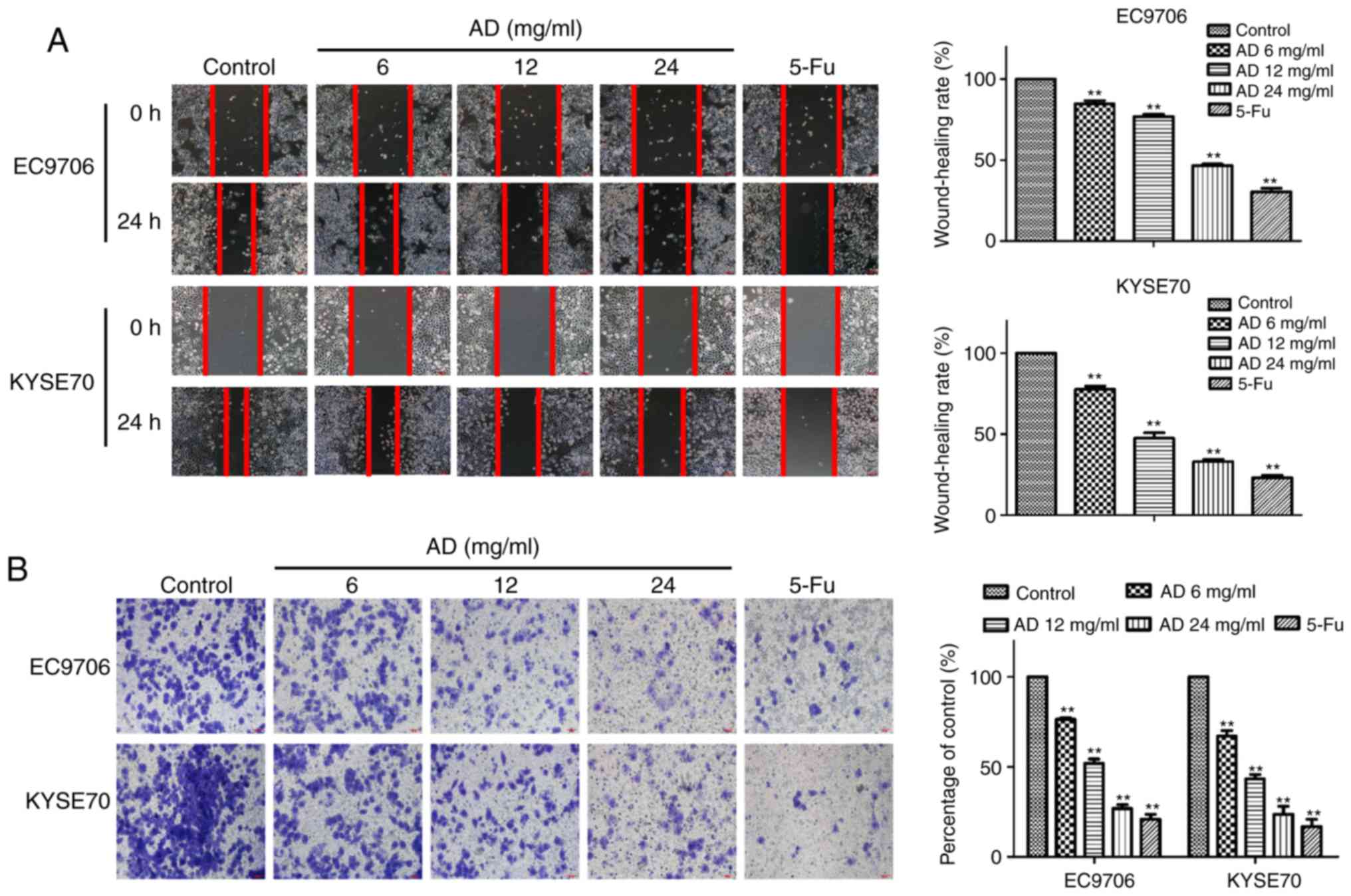
Aidi suppresses the migration and
invasion of ESCC cells
The migratory capacity of the cells was tested with
a wound-healing assay. Treatment with Aidi for 24 h significantly
inhibited the migration of EC9706 and KYSE70 cells in a
dose-dependent manner (P<0.01; Fig.
2A). The wound-healing rates of the EC9706 cells following
treatment with 6, 12 and 24 mg/ml Aidi and 32 mg/ml 5-Fu were
84.77±3.46%, 76.88±2.27%, 46.70±1.65% and 30.44±3.85%,
respectively, while the wound-healing rates of the KYSE70 cells
were 77.55±3.63%, 47.27±5.54%, 32.84±1.95% and 23.06±2.83%,
respectively. The trends of the rates in the two cell types, with
different degrees of differentiation, are consistent. The number of
invading cells was significantly reduced by treatment with Aidi, in
a dose-dependent manner (P<0.01; Fig. 2B). The invasion inhibition rates of
treatment with 6, 12 and 24 mg/ml Aidi and 32 mg/ml 5-Fu were
76.46±1.45%, 51.97±4.15%, 26.90±3.52% and 20.85±4.86% in EC9706
cells, and 67.03±5.36%, 43.26±4.29%, 23.67±7.77% and 16.86±6.86% in
KYSE70 cells, respectively. These results demonstrated that
treatment with Aidi induced an inhibition of ESCC migratory and
invasive capacity in a concentration-dependent manner, suggesting
an inhibitory effect on metastasis.
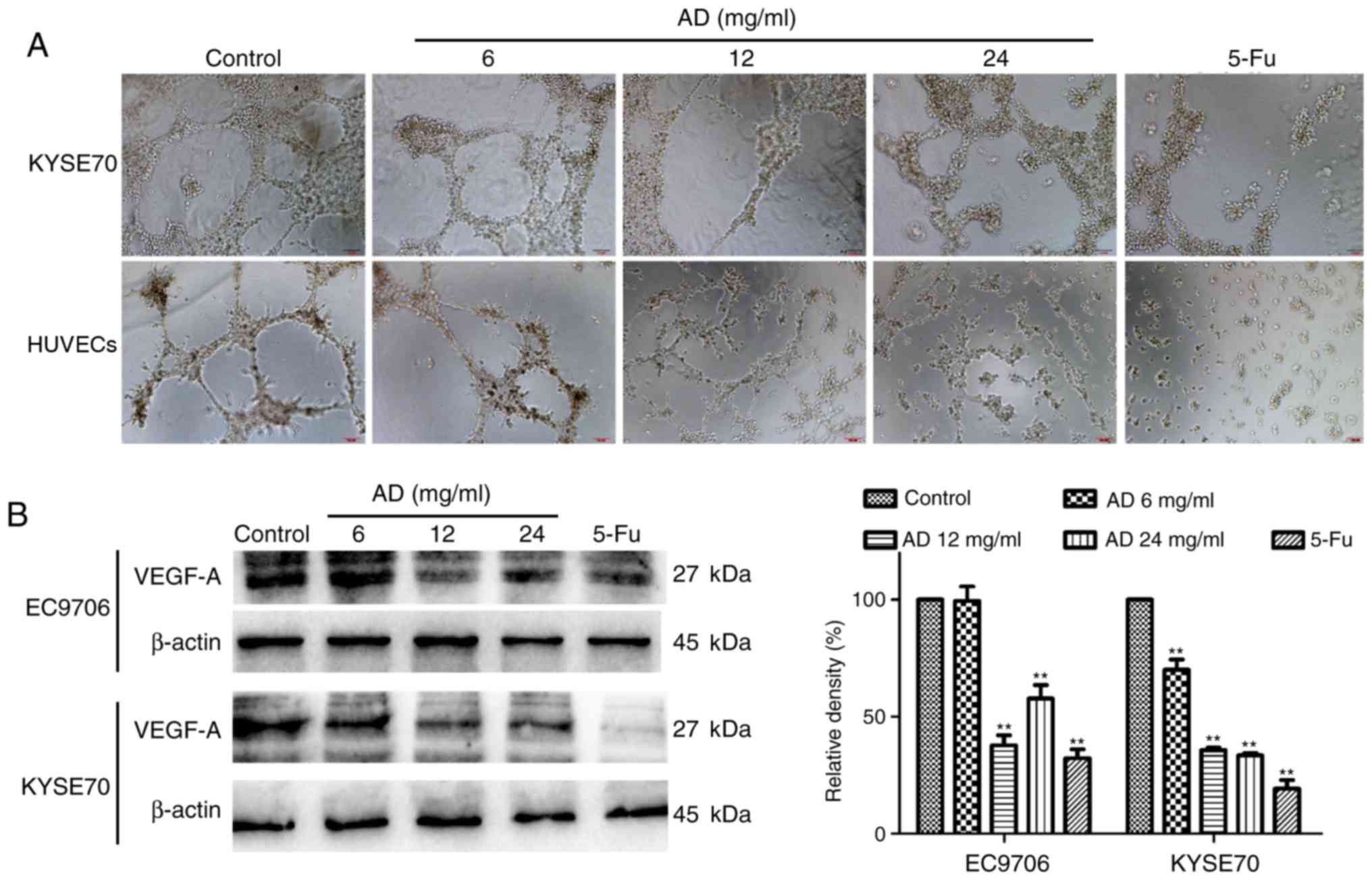
Aidi inhibits the angiogenesis of
ESCC
An angiogenesis assay was performed to investigate
the effect of treatment with Aidi on angiogenesis in vitro.
HUVECs resemble the principal constituent of tumor blood vessels;
it was identified that treatment with Aidi may reduce the formation
of a network of capillary-like tubes in HUVECs (Fig. 3A). To better understand the
mechanism of angiogenesis, VM formation in ESCC cells was examined.
As EC9706 cells are highly differentiated and less malignant, they
may not form capillary-like tubes well, while KYSE70 cells have
higher malignancy, and were therefore selected. The results showed
treatment with Aidi downregulated VM formation in KYSE70 cells
(Fig. 3A). Western blot analysis
demonstrated that treatment with Aidi decreased the expression of
VEGF-A in EC9706 and KYSE70 cells (Fig. 3B). These data indicated that
treatment with Aidi may inhibit angiogenesis.
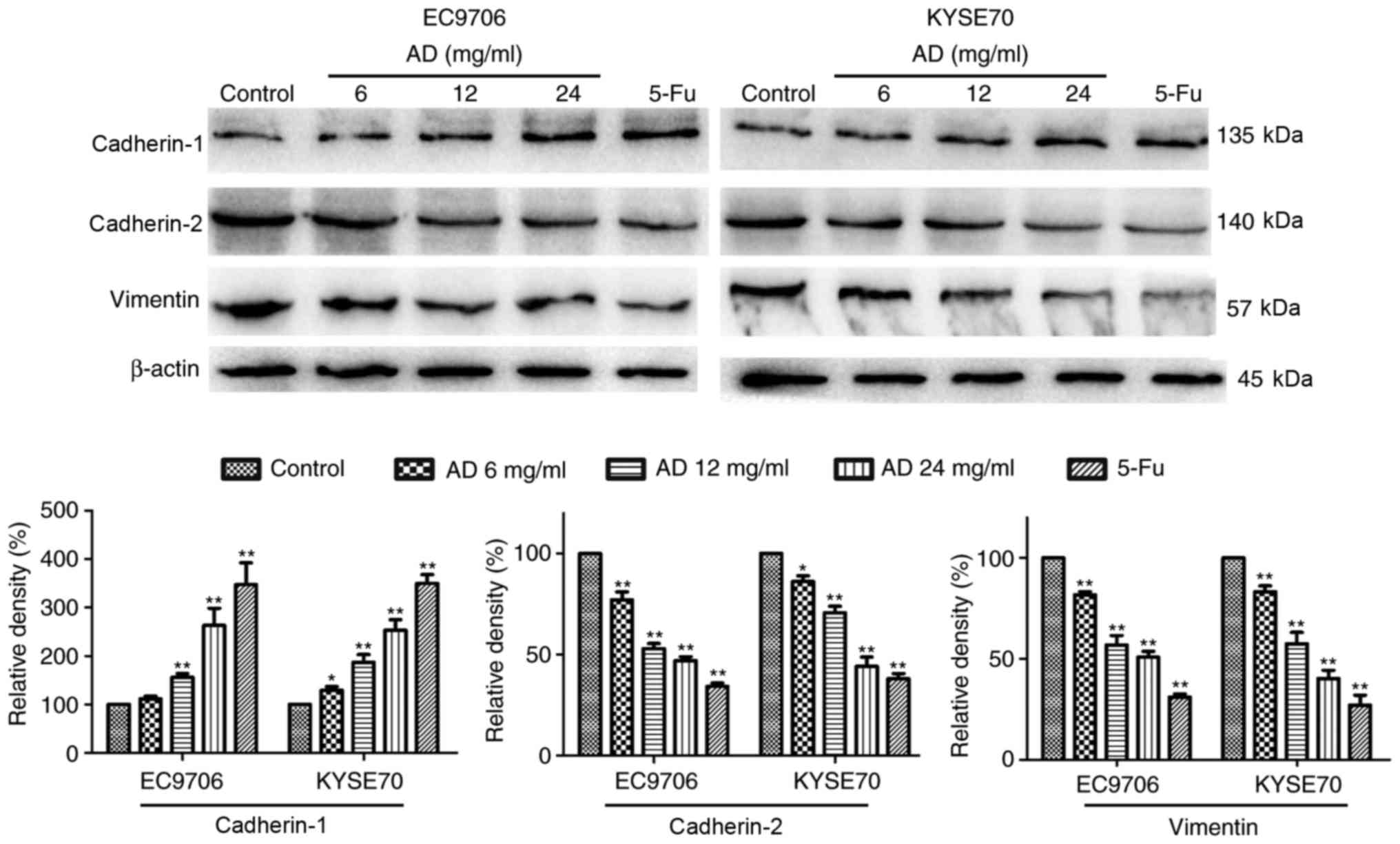
Aidi inhibits epithelial-mesenchymal
transition (EMT) signaling
EMT signaling contributes to tumor metastasis. To
investigate whether the inhibition of EMT was associated with the
effects of treatment with Aidi, EMT signaling in EC9706 and KYSE70
cells was examined. The expression levels of cadherin-1, associated
with epithelial morphology, were increased, while the expression of
cadherin-2 and vimentin, associated with mesenchymal morphology,
were decreased in a dose-dependent manner following treatment with
Aidi (Fig. 4).
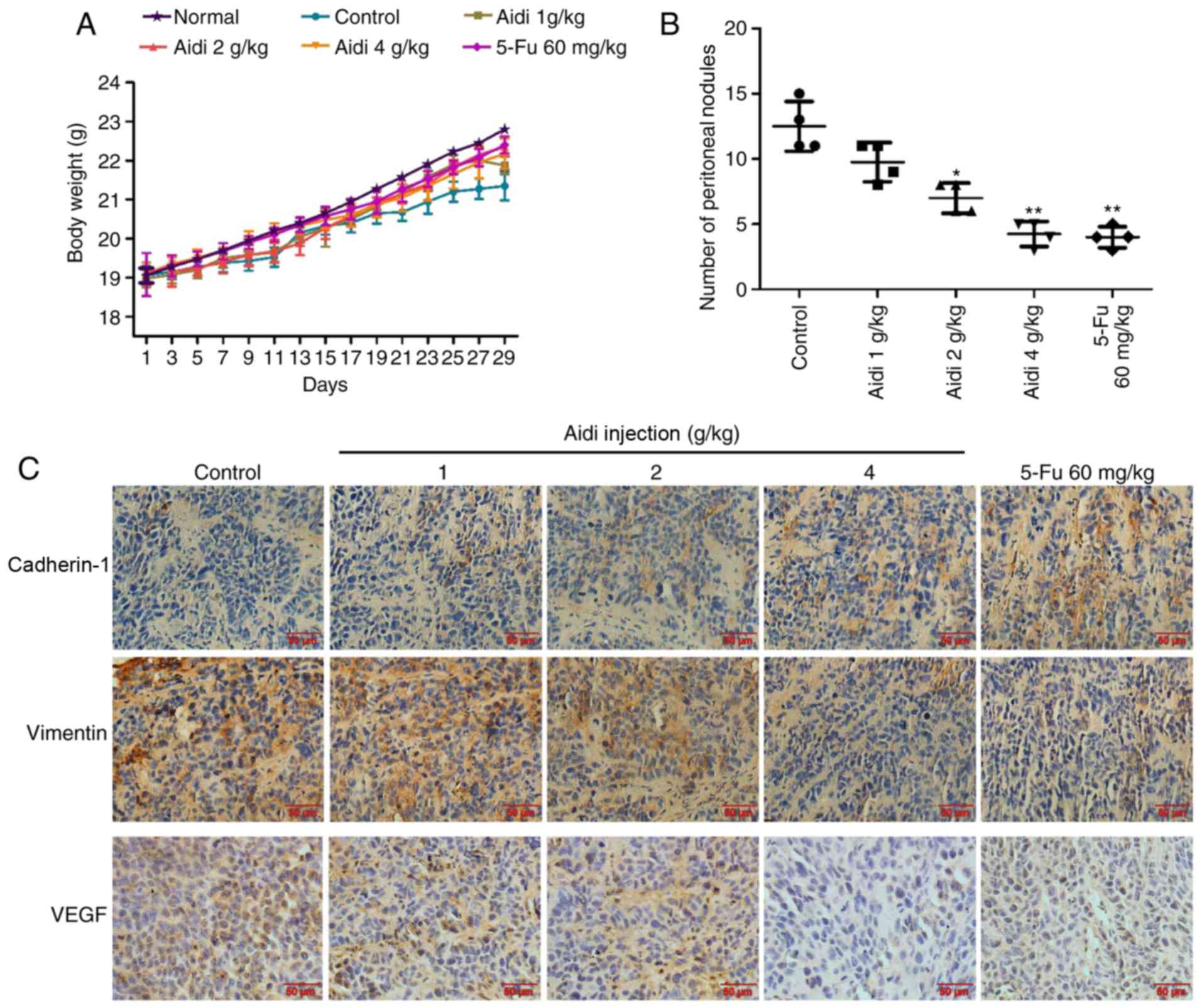
Aidi injection inhibits peritoneal
dissemination in an experimental metastasis model
To evaluate the effects of Aidi injection on the
metastasis of ESCC, a mouse abdominal tumor model was established.
The weight of nude mice in the treatment groups was compared with
the negative control group, which were injected with saline; there
was no significant alteration (Fig.
5A), indicating that no toxicity had occurred in any of the
treated groups. Aidi injection inhibited peritoneal dissemination,
as the number of macroscopic tumor nodules in the Aidi injection
groups was fewer compared with that in the control group (Fig. 5B). These data suggested that Aidi
injection may inhibit ESCC metastasis.
Aidi injection suppresses EMT and
VEGF-A expression in peritoneal cancer nodules
To better understand the mechanism of Aidi injection
on the suppression of metastasis in vivo, the expression
levels of cadherin-1, vimentin and VEGF-A were examined in the
tumor tissue of the nude mice by immunohistochemical analysis;
cadherin-2 expression was not analyzed in the present study as the
respective antibody could not be applied for immunohistochemical
analysis. The results revealed that Aidi injection increased the
expression of cadherin-1 and decreased the expression of vimentin
and VEGF-A in the tumor tissue (Fig.
5C). The data collectively indicated that Aidi may suppress
metastasis by inhibiting EMT and angiogenesis.
Discussion
Metastasis is the most fatal characteristic of
cancer, accounting for >90% of cancer-associated mortality
(19). The process of cancer
metastasis is complex, including alterations to the tumor
microenvironment, cell growth and transformation, angiogenesis,
invasion, dissemination, and the subsequent adhesion and
colonization of a secondary organ or tissue (17). The identification of drugs that may
prevent tumor metastasis is crucial. There is previous evidence to
suggest that traditional Chinese medicine may serve a role in
treating patients with cancer (20), including Aidi (21), shenmai (22), Brucea javanica (23) and Kanglaite® (24). Aidi is a Chinese medicine extracted
from Chinese drugs such as Mylabris, Radix ginseng,
Astragalus membranaceus Bge and Radix Acanthopanacis
Senticosi (25). At present, Aidi
injection is widely used in the treatment of esophageal cancer,
lung cancer and colorectal carcinoma (10–14).
However, whether Aidi may inhibit metastasis, and the underlying
mechanisms of Aidi injection in ESCC, remain to be investigated. To
identify whether Aidi injection is appropriate to prevent ESCC
metastasis in clinical use, the effects of Aidi injection on
viability, migration and invasion were observed. The present in
vitro experimental results demonstrated that treatment with
Aidi inhibited the viability, migration and invasion of EC9706 and
KYSE70 cells in a dose-dependent manner. Furthermore, Aidi
inhibited peritoneal dissemination in a mouse metastasis model.
These present results demonstrated that Aidi injection may
effectively reduce the extent of metastasis.
Angiogenesis is a principal driving force and an
essential pathological feature of tumor progression, which serves a
key role in tumor metastasis and metabolic deregulation (26). Tumor angiogenesis is a popular
research topic in the clinical treatment of cancer (27). There are two characteristic types
of angiogenesis in tumors; blood vessels formed by normal
endothelial cells and VM, vessel-like structures lined with tumor
cells instead of endothelial cells (28). The present data demonstrated that
treatment with Aidi may inhibit the tube formation of HUVECs and
the VM formation of KYSE70 cells. VEGF-A is a key growth factor for
endothelial cells in tumor angiogenesis (29); higher VEGF-A expression is
associated with a more advanced Tumor, Node, Metastasis stage and a
decreased overall survival in patients with esophageal cancer
(30). In the present study, Aidi
treatment decreased the expression of VEGF-A in HUVECs and KYSE70
cells. VEGF-A expression in the mouse tumor tissue, as determined
by immunohistochemistry, revealed the same trend.
EMT is an important process in tumor progression, in
which epithelial cells undergo a phenotypic conversion to
mesenchymal cells, which is closely associated with invasion,
metastasis and prognosis (31). A
reduction in cadherin-1 expression, and an increase in cadherin-2
and vimentin expression, are representative characteristics of EMT
(32). The present study
demonstrated that treatment with Aidi in EC9706 and KYSE70 cells
increased the levels of cadherin-1, and decreased the expression of
cadherin-2 and vimentin, in a dose-dependent manner, representing
the inhibition of EMT signaling. In vivo, Aidi injection
additionally increased the expression of cadherin-1 and decreased
the expression of vimentin in an implanted tumor model of human
ESCC in nude mice.
Additionally, EMT may promote angiogenesis and VM
formation. Recent studies have demonstrated that transcription
factors associated with EMT, including Twist-related protein 1
(TWIST1) and zinc-finger E-box-binding homeobox 1 (ZEB1), serve a
key role in angiogenesis in cancer (33,34).
Sun et al (35) identified
that the low expression of TWIST1 in liver cancer cells was
associated with a decreased capacity for VM formation. Liu et
al (34) observed that ZEB1
served an important role in the VM process. A future direction of
research may be to verify whether treatment with Aidi inhibits
tumor angiogenesis through an effect on EMT and to further clarify
the mechanism of the anti-metastatic effect of treatment with Aidi
on ESCC.
Aidi, a medicine extracted from Chinese drugs,
exhibits anti-tumor activity. The present data indicated that
treatment with Aidi may inhibit ESCC cell proliferation, migration,
invasion, angiogenesis and EMT. There was evidence that treatment
with Aidi effectively suppressed tumor metastasis in a nude mouse
model through the reduced expression of vimentin and VEGF-A, and
the increased expression of cadherin-1 in the tumor tissue. Aidi
may inhibit tumor metastasis by inhibiting EMT and angiogenesis in
human ESCC. The present results highlighted the anti-metastatic
activity of Aidi, potentially providing a theoretical basis for its
clinical use.
Acknowledgements
We thank Dr Yang Bao (The Affiliated Hospital of
Yangzhou University, Yangzhou, China) the support in statistical
analysis.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QS and ZD made substantial contributions to the
design of the present study. QS, YD and FJ conducted the
experiments. QS and YD made substantial contributions of data
analysis. ZD supervised all the work and critically revised the
manuscript for important intellectual content. All the authors have
approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were performed following
internationally accepted guidelines regarding the use of laboratory
animals, and the protocol of the present study was reviewed and
approved by the Institutional Animal Care and Use Committee of
Yangzhou University (Jiangsu, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
EMT
|
epithelial-mesenchymal transition
|
|
ESCC
|
esophageal squamous cell carcinoma
|
|
FBS
|
fetal bovine serum
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
TWIST1
|
Twist-related protein 1
|
|
VEGF-A
|
vascular endothelial growth
factor-A
|
|
VM
|
vasculogenic mimicry
|
|
ZEB1
|
zinc finger E-box-binding homeobox
1
|
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li S, Qin X, Chai S, Qu C, Wang X and
Zhang H: Modulation of E-cadherin expression promotes migration
ability of esophageal cancer cells. Sci Rep. 6:217132016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang L, Ma J, Han Y, Liu J, Zhou W, Hong
L and Fan D: Targeted therapy in esophageal cancer. Expert Rev
Gastroenterol Hepatol. 10:595–604. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arnal Domper MJ, Ferrández Arenas Á and
Arbeloa Lanas Á: Esophageal cancer: Risk factors, screening and
endoscopic treatment in Western and Eastern countries. World J
Gastroenterol. 21:7933–7943. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zeng H, Zheng R, Zhang S, Zuo T, Xia C,
Zou X and Chen W: Esophageal cancer statistics in China, 2011:
Estimates based on 177 cancer registries. Thorac Cancer. 7:232–237.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jain R, Gupta S, Pasricha N, Faujdar M,
Sharma M and Mishra P: ESCC with metastasis in the young age of
caustic ingestion of shortest duration. J Gastrointest Cancer.
41:93–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ludmir EB, Robey B, Shelby E, Patel-Nguyen
SV, Rittershaus A and Contarino MR: Skeletal muscle metastasis from
signet ring cell esophageal adenocarcinoma. Transl Gastroenterol
Hepatol. 1:372016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang H, Deng F, Liu Q and Ma Y: Prognostic
significance of lymph node metastasis in esophageal squamous cell
carcinoma. Pathol Res Pract. 213:842–847. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiao Z, Wang C, Zhou R, Hu S, Yi N, Feng
J, Zhou M, Liu S, Chen L, Ding J, et al: Can Aidi injection improve
overall survival in patients with non-small cell lung cancer? A
systematic review and meta-analysis of 25 randomized controlled
trials. Complement Ther Med. 37:50–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu J, Ju WZ and Tan HS: Study of
pharmacological effects and clinical application of Aidi injection.
Pharm Clin Res. 1:48–51. 2012.(In Chinese).
|
|
11
|
Zhang H, Jiang H, Hu X and Jia Z: Aidi
injection combined with radiation in the treatment of non-small
cell lung cancer: A meta-analysis evaluation the efficacy and side
effects. Cancer Res Ther. 11 Suppl 1:C118–C121. 2015. View Article : Google Scholar
|
|
12
|
Lou HZ, Pan HM and Jin W: Clinical study
on treatment of primary liver cancer by Aidi injection combined
with cool-tip radiofrequency ablation. Zhongguo Zhong Xi Yi Jie He
Za Zhi. 27:393–395. 2007.(In Chinese). PubMed/NCBI
|
|
13
|
Ji B and Yuan J: Meta-analysis of the
clinical efficacy and safety about Aidi injection in the treatment
of colorectal cancer. China Pharm. 40:3797–3799. 2011.(In
Chinese).
|
|
14
|
Zhang MM, Liu YL, Chen Z, Li XR, Xu QM and
Yang SL: Studies on chemical constituents from Aidi injection. Chin
Tradit Herbal Drugs. 8:1462–1470. 2012.(In Chinese).
|
|
15
|
Jiancheng W, Long G, Ye Z, Jinlong L, Pan
Z, Lei M and Kehu Y: Effect of aidi injection plus chemotherapy on
gastric carcinoma: A meta-analysis of randomized controlled trials.
J Tradit Chin Med. 35:361–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Q, He X, Tian J, Wang X, Ru P, Ruan Z
and Yang K: A meta-analysis of aidi injection plus taxotere and
cisplatin in the treatment of non-small cell lung cancer. Zhongguo
Fei Ai Za Zhi. 13:1027–1034. 2010.(In Chinese). PubMed/NCBI
|
|
17
|
Jin X, Zhu Z and Shi Y: Metastasis
mechanism and gene/protein expression in gastric cancer with
distant organs metastasis. Bull Cancer. 101:E1–E12. 2014.PubMed/NCBI
|
|
18
|
Jones-Bolin S: Guidelines for the care and
use of laboratory animals in biomedical research. Curr Protoc
Pharmacol Appendix 4: Appendix 4B. 2012.doi:
10.1002/0471141755.pha04bs59. View Article : Google Scholar
|
|
19
|
Robert J: Biology of cancer metastasis.
Bull Cancer. 100:333–342. 2013.(In French). PubMed/NCBI
|
|
20
|
Tao W, Luo X, Cui B, Liang D, Wang C, Duan
Y, Li X, Zhou S, Zhao M, Li Y, et al: Practice of traditional
Chinese medicine for psycho-behavioral intervention improves
quality of life in cancer patients: A systematic review and
meta-analysis. Oncotarget. 6:39725–39739. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiao Z, Liang R, Wang CQ, Xu S, Li N, He
Y, Tang F, Chen L and Ma H: Can aidi injection alleviate the
toxicity and improve the clinical efficacy of radiotherapy in lung
cancer? A meta-analysis of 16 randomized controlled trials
following the PRISMA guidelines. Medicine (Baltimore).
95:e45172016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang L, Huang XE and Cao J: Clinical study
on safety of cantharidin sodium and shenmai injection combined with
chemotherapy in treating patients with breast cancer
postoperatively. Asian Pac J Cancer Prev. 15:5597–600. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ji ZQ, Huang XE, Wu XY, Liu J, Wang L and
Tang JH: Safety of Brucea javanica and cantharidin combined with
chemotherapy for treatment of NSCLC patients. Asian Pac J Cancer
Prev. 15:8603–8605. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Zhang W, Wang XJ and Liu S:
Antitumor effect of Kanglaite® injection in human
pancreatic cancer xenografts. BMC Complement Altern Med.
14:2282014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiao Z, Wang C, Chen L, Tang X, Li L, Li
N, Li J, Gong Q, Tang F, Feng J and Li X: Has aidi injection the
attenuation and synergistic efficacy to gemcitabine and cisplatin
in non-small cell lung cancer? A meta-analysis of 36 randomized
controlled trials. Oncotarget. 8:1329–1342. 2017.PubMed/NCBI
|
|
26
|
Wang Z, Dabrosin C, Yin X, Fuster MM,
Arreola A, Rathmell WK, Generali D, Nagaraju GP, El-Rayes B,
Ribatti D, et al: Broad targeting of angiogenesis for cancer
prevention and therapy. Semin Cancer Biol. 35 Suppl:S224–S243.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu J, Zhang Y, Leung LH, Liu L, Yang F and
Yao X: Efficacy and safety of angiogenesis inhibitors in advanced
gastric cancer: a systematic review and meta-analysis. J Hematol
Oncol. 9:1112016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qiao L, Liang N, Zhang J, Xie J, Liu F, Xu
D, Yu X and Tian Y: Advanced research on vasculogenic mimicry in
cancer. J Cell Mol Med. 19:315–326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gianni-Barrera R, Trani M, Fontanellaz C,
Heberer M, Djonov V, Hlushchuk R and Banfi A: VEGF over-expression
in skeletal muscle induces angiogenesis by intussusception rather
than sprouting. Angiogenesis. 16:123–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu XL, Ling ZQ, Chen W, Xu YP and Mao WM:
The overexpression of VEGF in esophageal cancer is associated with
a more advanced TMN stage: A meta-analysis. Cancer Biomark.
13:105–113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Davis FM, Stewart TA, Thompson EW and
Monteith GR: Targeting EMT in cancer: Opportunities for
pharmacological intervention. Trends Pharmacol Sci. 35:479–488.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Banyard J and Bielenberg DR: The role of
EMT and MET in cancer dissemination. Connect Tissue Res.
56:403–413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Che N, Zhao XL, Sun T, Zhao XM, Gu Q, Dong
XY, Zhao N, Liu YR, Yao Z and Sun BC: The role of Twist1 in
hepatocellular carcinoma angiogenesis: A clinical study. Hum
Pathol. 42:840–847. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu Z, Sun B, Qi L, Li H, Gao J and Leng
X: Zinc finger E-box binding homeobox 1 promotes vasculogenic
mimicry in colorectal cancer through induction of
epithelial-to-mesenchymal transition. Cancer Sci. 103:813–820.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun T, Zhao N, Zhao XL, Gu Q, Zhang SW,
Che N, Wang XH, Du J, Liu YX and Sun BC: Expression and functional
significance of Twist1 in hepatocellular carcinoma: Its role in
vasculogenic mimicry. Hepatology. 51:545–556. 2010. View Article : Google Scholar : PubMed/NCBI
|