Introduction
Gliomas are one of the most common and most
aggressive types of central nervous system tumor. Gliomas account
for approximately 35–60% of all primary intracranial tumors
(1). Gliomas are derived from
various types of glial cell tumor, which are divided into four
categories, including astrocytoma, medulloblastoma,
oligodendroglioma and ependymoma. Among these, glioblastomas are
the most common, accounting for ~55% of all intracranial neoplasms.
According to the pathological grading defined by the World Health
Organization in 2007, gliomas are divided into grade I, II, III and
IV (2). Glioblastoma multiforme
(GBM) are grade IV. The clinical features of glioma include rapid
growth, high infiltration, and with no obvious boundary between
tumor tissue and normal brain tissue. It is difficult to achieve
complete removal of tumor tissues via surgery; thus, the recurrence
rate is high. In addition, due to the existence of the blood-brain
barrier, antineoplastic drugs do not readily enter the brain.
Although chemotherapy combined with radiotherapy is often used to
treat glioma, the outcome remains unsatisfactory. Previous evidence
indicates that the median survival time in patients with newly
diagnosed GBM is only 14.6 months despite the use of radiotherapy,
chemotherapy and surgical intervention (3). A large number of studies have shown
that gliomas remain one of the worst types of tumor with poor
prognosis (4,5). Therefore, it is considered to be of
great importance to elucidate the underlying mechanism of the
occurrence and development of gliomas, and develop more effective
targets to improve therapeutic strategies against gliomas.
Angiogenesis is an important basis for the growth of
solid tumors, including gliomas (6,7). The
rapid growth, invasion, metastasis and tumor recurrence of gliomas
are closely associated with angiogenesis. Studies have demonstrated
that glioma vascular endothelial hyperplasia increased
significantly with glioma malignancy, which provides strong
evidence that angiogenesis is an important feature of the
development of glioma (8,9). Vascular endothelial growth factor
(VEGF) is important in glioma angiogenesis (10). The use of anti-VEGF antibody in rat
models of glioma in vivo demonstrated that angiogenesis was
significantly inhibited, while there no inhibitory effect was
observed in glioma cells in vitro (11,12).
Thus, VEGF promotes the proliferation of ECs, and angiogenesis in
glioma. Currently, microvascular proliferation is considered to be
one of the most important criteria for malignant grading of
glioma.
Gliomas exhibit a strong invasive ability (13). Tumor angiogenesis is vital in tumor
invasion. In recent years, with the discovery of microRNAs (miRNA)
and their function in in-depth investigations, it was identified
that miRNA contributes to the regulation of angiogenesis in glioma
formation, thus affecting the invasion ability of gliomas (14,15).
Previous studies demonstrated that miR-24 was upregulated in
glioblastoma tissue (16–18) and promotes cell proliferation in
glioma cells via cooperative regulation of MAX interactor 1,
dimerization protein (19).
Furthermore, miR-24 regulates the proliferation and invasion of
gliomas through suppression of tumorigenicity 7 like via
β-catenin/transcription factor 4 signaling (20). The migration of human umbilical
vein endothelial cells (HUVECs) is an important process (21), and HUVECs are a major contributor
of angiogenesis (22,23). The aim of the present study was to
investigate whether the dysregulation of miR-24 in glioma cells
promotes microvascular proliferation of ECs, and to investigate the
possible underlying mechanism.
Materials and methods
Cell culture and transfection
The human U251 glioma cell line and HUVECs were
purchased from Shanghai Chinese Academy of Sciences (Shanghai,
China). Cells were cultured in Dulbecco's modified Eagle's medium
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) containing
Gibco 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), 100 U/ml penicillin and 100 µg/ml streptomycin
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2.
The miR-24 mimics and inhibitor were chemically
synthesized and purified by high-performance liquid choromatography
(Shanghai GenePharma Co., Ltd., Shanghai, China). The primer
sequences were as follows: Forward, 5′-UUCUCCGAACGUGUCACGUTT-3′ and
reverse, 5′-ACGUGACACGUUCGGAGAATT-3′ for the negative control (NC);
CUGUUCCUGCUGAACUGAGCCA for the miR-24 inhibitor; forward,
5′-UGGCUCAGUUCAGCAGGAACAG-3′ and reverse,
5′-GUUCCUGCUGAACUGAGCCAUU-3′ for the miR-24-3p mimic. Cell
transfection was performed using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. For 48 h post-transfection, the changes of target
genes were confirmed by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR). The expression levels of
miR-24 in the culture medium were detected by RT-qPCR. The levels
of VEGFA was detected using an ELISA kit (cat. no. RS10115B;
SHRQSW, Shanghai, China; http://www.shrqsw.com/) according to the
manufacturer's instructions.
RNA extraction and RT-qPCR
Total RNA was extracted from cells that were
transfected with mimic NC, mimic, inhibitor NC or inhibitor using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and
were reverse-transcribed using a miR-Cute miRNA First Strand cDNA
kit (KR201-02; TianGen Biotech Co., Ltd., Beijing, China). The
sequences of the primers for mir-24 were as follows: Forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′ forhsa-miR-24-3p; and forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′AACGCTTCACGAATTTGCGT-3′ for
U6. The RNA was quantified and checked for purity by
spectrophotometry. The expression level of miR-24-3p was
quantitated using a miRcute miRNA qPCR detection kit (FP401;
TianGen Biotech Co., Ltd.) and U6 RNA served as an internal
standard. The thermocycling conditions were as follows: 94°C for 2
min, followed by 40 cycles of 94°C for 20 sec and 60°C for 34 sec.
The comparative Cq (ΔΔCq) method (24) was used to determine the expression
fold change.
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay
lysis buffer (BioTeke Corporation, Beijing, China) and
concentration was determined using a bicinchoninic acid protein
assay kit (Beyotime Institute of Biotechnology, Shanghai, China).
Proteins (30 µg per lane) were loaded for 10% SDS-PAGE and
transferred to an Immobilon-P membrane (EMD Millipore, Billerica,
MA, USA). The membrane was incubated with the following primary
antibodies: Anti-VEGF (cat. no. ab46154; 1:1,000; Abcam, Cambridge,
USA), anti-tumor growth factor (TGF)-β1 (cat. no. ab92486; 1:1,000;
Abcam), anti-β-catenin (cat. no. ab32572; 1:1,000; Abcam), anti-AKT
(cat. no. 4691s; 1:2,000; Cell Signaling Technology, Inc., Danvers,
MA, USA), anti-pAKT (cat. no. 4060s; 1:2,000; Cell Signaling
Technology, Inc.) and anti-GAPDH (cat. no. ab181602; 1:5,000;
Abcam) overnight at 4°C, and incubated for 1 h with a horseradish
Peroxidase-conjugated goat anti-rabbit (cat. no. 65-6120; 1:3,000;
Invitrogen; Thermo Fisher Scientific, Inc.) at room temperature.
The immunoblot was detected by Enhanced chemiluminescence
Supersignal® western blotting detection reagents
(Pierce; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol using ChemiDox XRS + (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), and densitometry was analyzed by Gelpro
analyze 4.2 (Media Cybernetics, Inc., Rockville, MD, USA).
Cell viability
Cells (2×103 cells/well) were cultured in
96-well plates at 24, 48 and 72 h post-transfection. MTT reagent
(20 µl; 5 mg/ml) was added directly to the medium and incubated at
37°C, in a 5% CO2 incubator for 4 h. After removing the
supernatants, 150 µl dimethyl sulfoxide was added to dissolve the
formazan crystals and mixed thoroughly for 10 min. Optical
densities (ODs) at a wavelength of 570 nm were measured using the
culture medium as a blank control.
Cell migration
A Transwell assay was performed to quantify in
vitro migration of HUVECs. Cells pretreated with conditional
medium or normal medium for 48 h were digested with trypsin
containing 0.25% ethylenediaminetetraacetic acid (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). Following centrifugation (1,200 ×
g, 6 min, 4°C), cells were diluted to 5×105/ml, and then
seeded in the upper chamber in serum-free medium. Medium containing
20% FBS was added to the lower chamber. Following a 24-h incubation
at 37°C, in a 5% CO2 incubator for 24 h. Then, cells
were fixed with 4% paraformaldehyde for 20 min at 4°C, and the
cells that had not migrated were removed using a cotton swab. The
migrated cells were stained with 0.05% crystal violet for 20 min at
room temperature. Ffollowing two washes for 3 min, the number of
migrated cells were observed under an inverted microscope,
photographed and the migration rate was calculated.
In vitro angiogenesis assay
Matrigel (cat. no. 356237; Corning Incorporated,
Corning, NY, USA) was added to a pre-cooled 96-well plate and
placed in the incubator for 30 min to allow Matrigel
solidification. Then, 100 µl cells (2×105 cell/ml) were
added to each well. To confirm the role of AKT and β-catenin in
angiogenesis, LY294002 (1 µM; cat. no. S1105; Selleck Chemicals,
Houston, TX, USA) and KYA1797K (0.75 µM; cat. no. S8327; Selleck
Chemicals) were added to the medium. Cells were imaged after 6 h
and the total length, number of branches, and mean mesh size was
calculated to examine the angiogenesis ability of HUVECs using
imageJ software (version 1.4; National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
of at least three independent experiments. The data were analyzed
using the GraphPad Prism 5.0 software (GraphPad Software, Inc., La
Jolla, CA, USA). Statistical analyses were performed with Student's
t-test or one-way analysis of variance with the least significant
difference post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression level of miR-24 in glioma
cells
Following transfection with miR-24 mimics,
inhibitors or NC for 48 h, the miR-24 expression levels in U251
cells were detected by RT-qPCR (Fig.
1). After 48 h, miR-24 was significantly upregulated by miR-24
mimics (Fig. 1A) and significantly
downregulated by miR-24 inhibitors (Fig. 1B). miR-24 was also detected in the
culture medium (Fig. 1C). miR-24
in the conditional medium was not significantly changed. Thus, the
direct effect of miR-14 on ECs may be negligible. Subsequently, the
culture medium was collected and prepared for HUVEC culture.
Expression level of VEGFA in culture
medium from miR-24 mimic- or inhibitor-transfected U251 cells
VEGFA was detected in the culture medium (Fig. 1D). Results demonstrated that VEGFA
was significantly increased in the culture medium from miR-24
mimic-transfected U251 cells, whereas it was significantly
decreased in the culture medium from miR-24 inhibitor-transfected
U251 cells. As miR-24 in the culture medium was not significantly
changed, miR-24 may not influence the response to VEGFA in ECs. The
VEGFA expressed by U251 may promote EC viability and
angiogenesis.
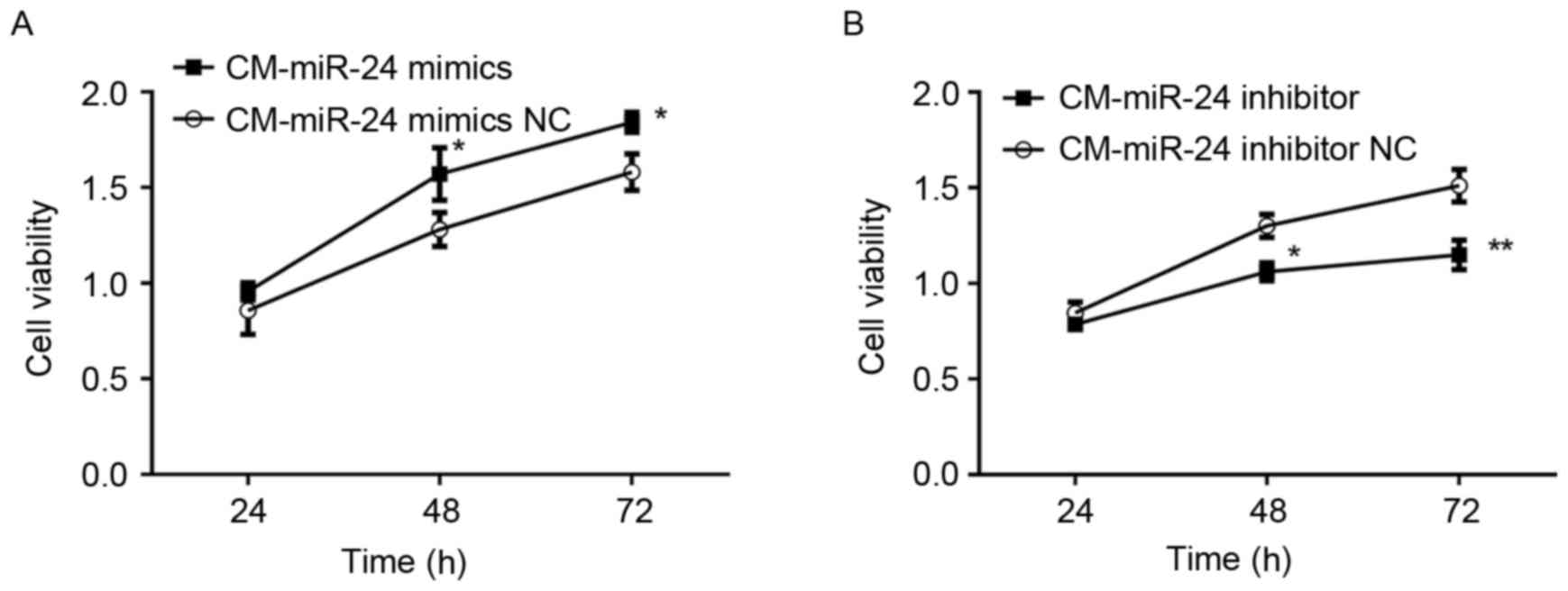
Effect of conditional medium from
miR-24 mimic- or inhibitor-transfected U251 cells on cell viability
of HUVECs
To detected whether the overexpression of miR-24 in
U251 cells affects the cell viability of HUVECs, the conditional
medium samples collected from miR-24 mimic- or
inhibitor-transfected U251 cells were used to culture the HUVECs
for 24 h and cell viability was examined by MTT (Fig. 2). The conditional medium from
miR-24 mimic-transfected U251 cells demonstrated significantly
increased cell viability of HUVECs (Fig. 2A). By contrast, the conditional
medium from miR-24 inhibitor-transfected U251 cells exhibited
significantly decreased cell viability of HUVECs (Fig. 2B).
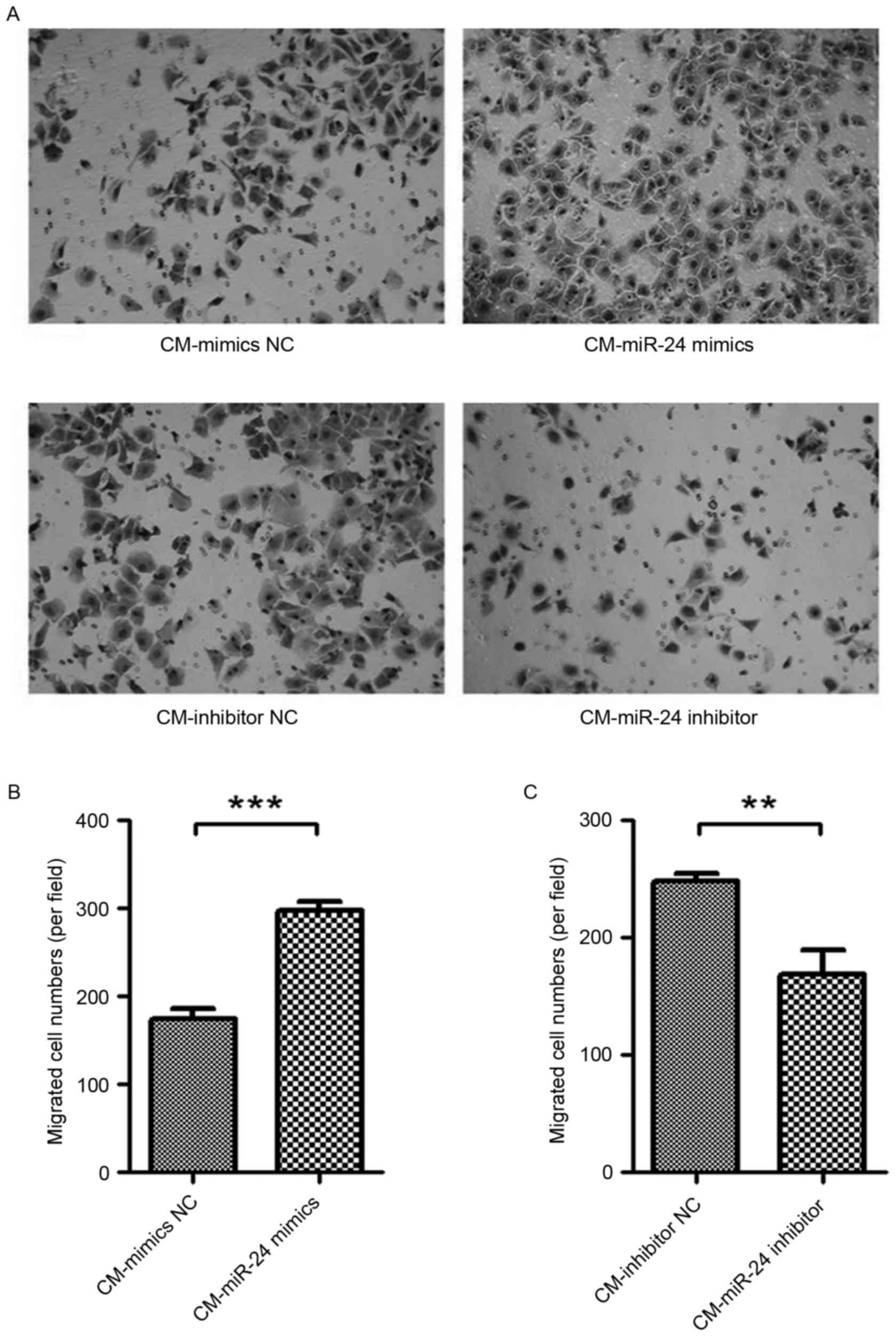
Effect of conditional medium from
miR-24 mimic- or inhibitor-transfected U251 cells on cell migration
of HUVECs
To detected whether the overexpression of miR-24 in
U251 cells affects the cell migration of HUVECs, the conditional
medium samples collected from miR-24 mimic- or
inhibitor-transfected U251 cells were used to culture the HUVECs
for 24 h, and cell migration was examined by Transwell assay
(Fig. 3). The conditional medium
from miR-24 mimic-transfected U251 cells exhibited significantly
increased cell migration of HUVECs (Fig. 3A and B). By contrast, the
conditional medium from miR-24 inhibitor-transfected U251 cells
demonstrated significantly decreased cell migration of HUVECs
(Fig. 3A and C).
Effect of conditional medium from
miR-24 mimic- or inhibitor-transfected U251 cells on angiogenesis
of HUVECs
To further detect whether the overexpression of
miR-24 in U251 cells affects the angiogenesis of HUVECs, the
conditional medium collected from miR-24 mimic- or
inhibitor-transfected U251 cells were used to culture the HUVECs
for 24 h, and the tube formation ability was examined (Fig. 4). The conditional medium from
miR-24 mimic-transfected U251 cells demonstrated significantly
increased tube formation of HUVECs (Fig. 4A-D). By contrast, the conditional
medium from miR-24 inhibitor-transfected U251 cells exhibited
significantly decreased tube formation of HUVECs (Fig. 4A and E-G).
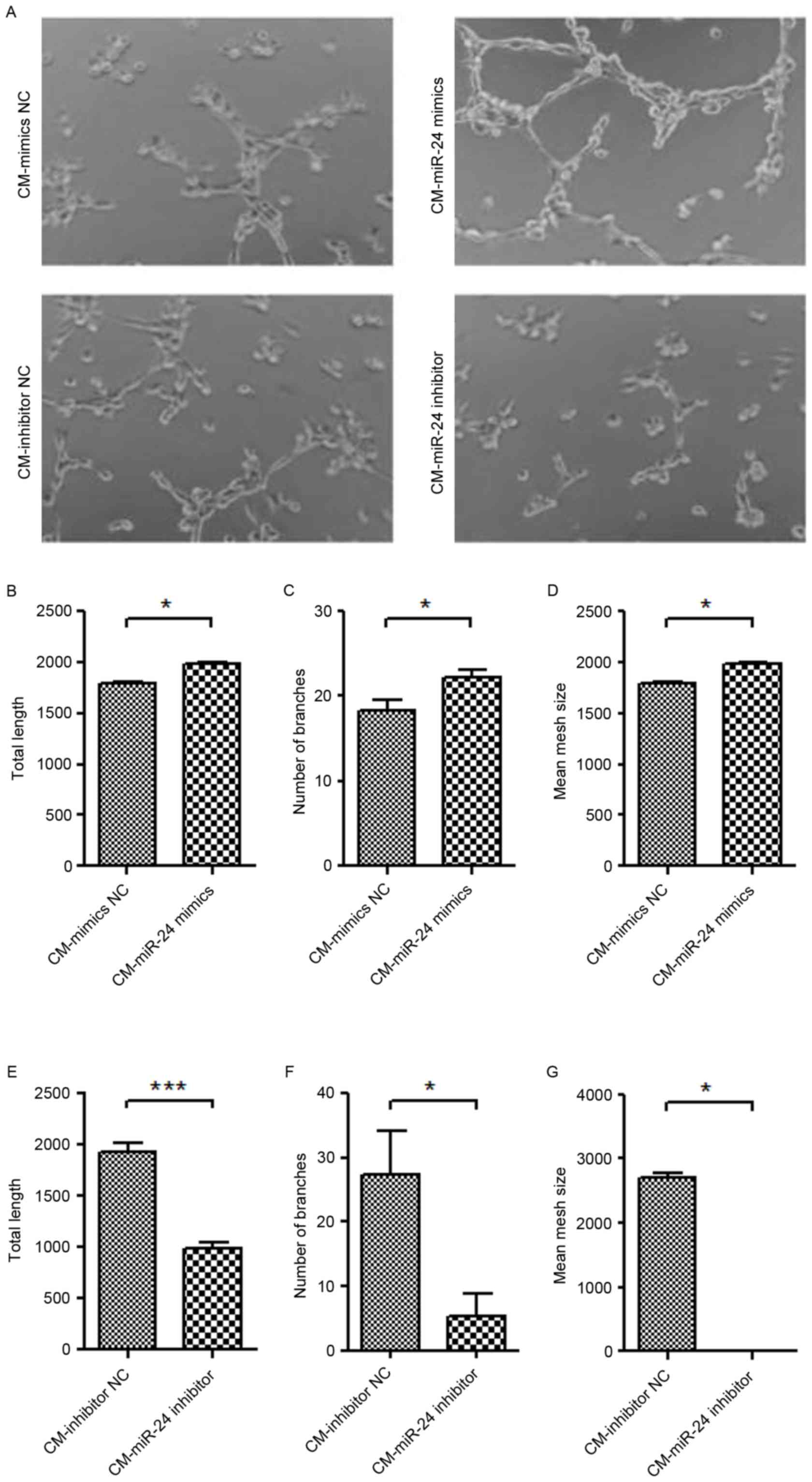 | Figure 4.Effect of conditional medium from
miR-24 mimic- or inhibitor-transfected U251 cells on angiogenesis
of HUVECs. The CM collected from miR-24 mimic- or
inhibitor-transfected U251 cells was used to culture the HUVECs for
24 h, and the tube formation was examined. (A) Tube formation
(magnification, ×100). Quantification data of the mimics groups,
including (B) total length, (C) number of branches and (D) mean
mesh size. Quantification data of inhibitor groups, including (E)
total length, (F) number of branches and (G) mean mesh size.
*P<0.05, ***P<0.001 vs. NC. miR, microRNA; HUVEC, human
umbilical vein endothelial cell; CM, conditional medium; NC,
negative control. |
Regulatory mechanism of angiogenesis
in conditional medium from miR-24 mimic- or inhibitor-transfected
U251 cells
The regulatory mechanism of angiogenesis in HUVECs
from conditional medium from miR-24 mimic- or inhibitor-transfected
U251 cells was investigated (Figs.
5–7). The mRNA expression
levels of VEGF, basic fibroblast growth factor (bFGF), epidermal
growth factor (EGF), TGF-β, matrix metalloproteinase (MMP)-2 and
MMP-9 in U251 cells were significantly increased by miR-24 mimics
(Fig. 5A-F), and were
significantly decreased by miR-24 inhibitors (Fig. 5G-L). Western blot detection
confirmed the increase of VEGF and TGF-β protein expression levels
in U251 by miR-24 mimics (Fig.
5M), and the decrease of VEGF and TGF-β protein expression
levels in U251 by miR-24 inhibitors (Fig. 5N). It was proposed that the change
of VEGF and TGF-β expression levels in U251 by miR-24 may
associated with the angiogenesis of HUVECs.
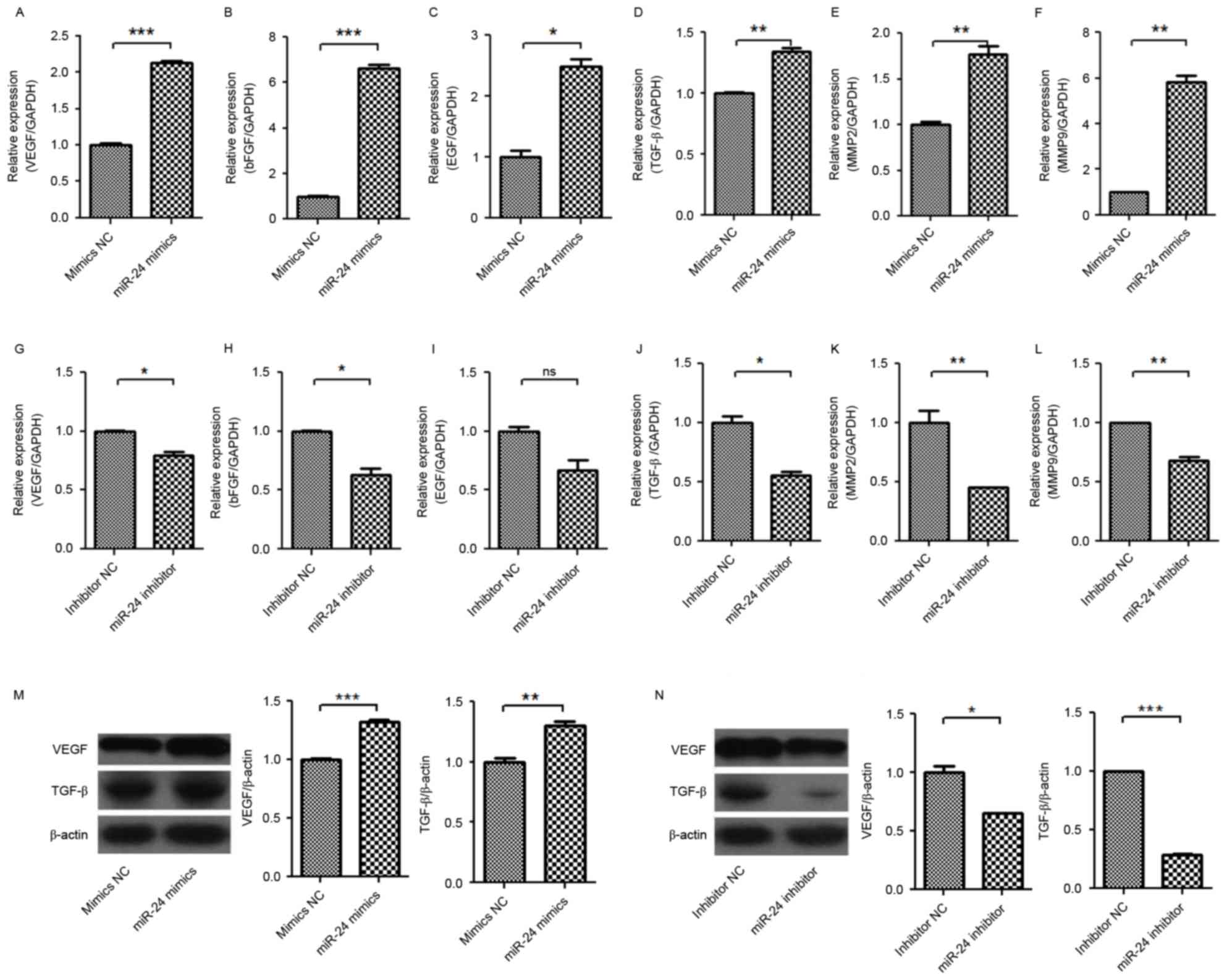 | Figure 5.Effect of miR-24 on mRNA expression
levels of VEGF, bFGF, EGF, TGF-β, MMP-2 and MMP-9, and protein
expression levels of VEGF and TGF-β in U251 cells. The U251 cells
were transfected with miR-24 mimics or inhibitors. Following 48 h,
the mRNAs and protein was detected by reverse
transcription-quantitative polymerase chain reaction and western
blot analysis. Expression levels of (A) VEGF mRNA, (B) bFGF mRNA,
(C) EGF mRNA, (D) TGF-β mRNA, (E) MMP-2 mRNA and (F) MMP-9 mRNA
induced by miR-24 mimics. Expression levels of (G) VEGF mRNA, (H)
bFGF mRNA, (I) EGF mRNA, (J) TGF-β mRNA, (K) MMP-2 mRNA, (L) MMP-9
mRNA inhibited by miR-24 inhibitors. Expression levels of VEGF and
TGF-β (M) induced by mir-24 mimics and (N) inhibited by miR-24
inhibitors. *P<0.05, **P<0.01; ***P<0.001 vs. NC. ns, not
significant; miR, microRNA; VEGF, vascular endothelial growth
factor; bFGF, basic fibroblast growth factor; EGF, epidermal growth
factor; TGF-β, transforming growth factor β; MMP, matrix
metalloproteinase; NC, negative control. |
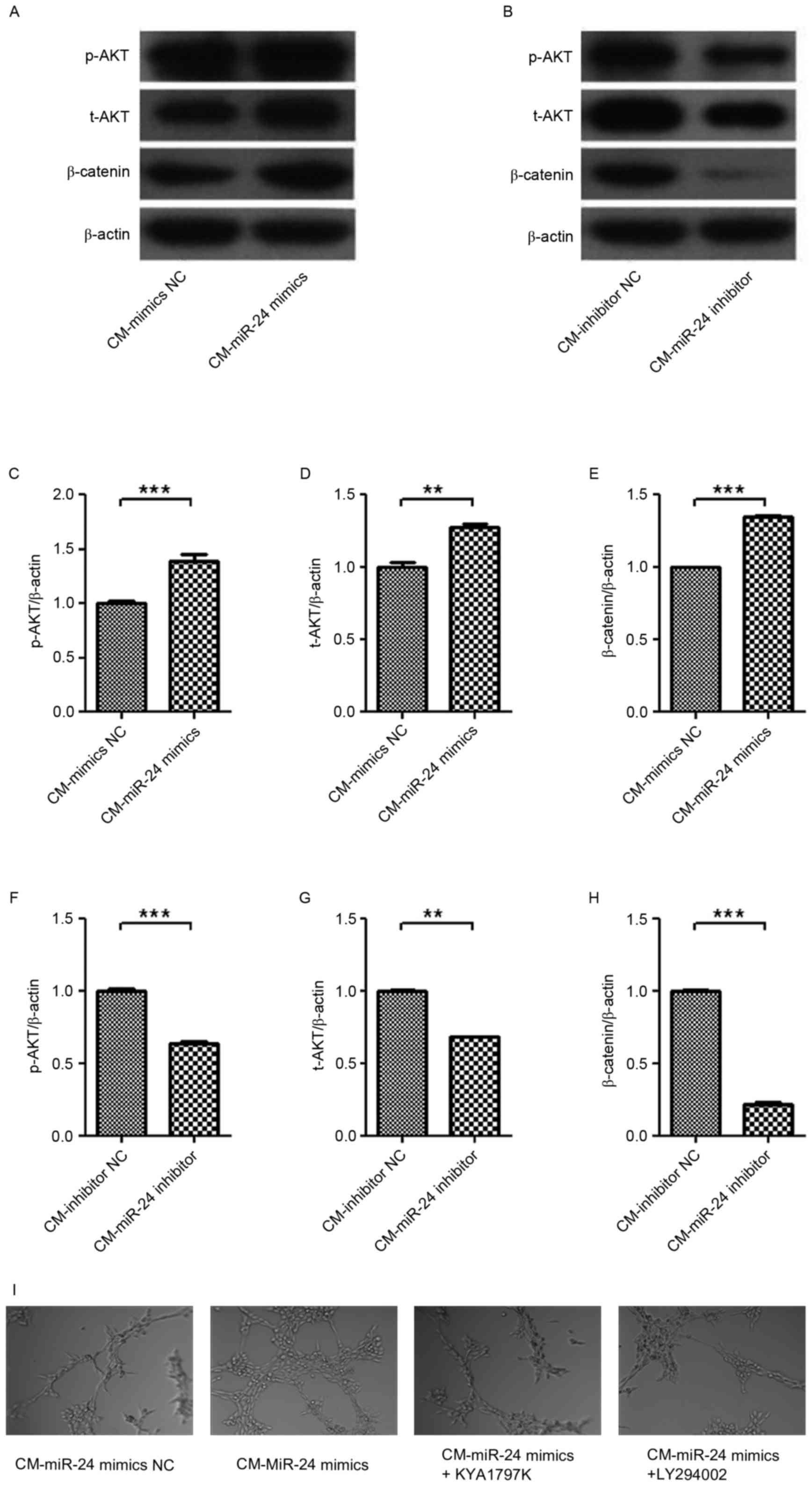 | Figure 7.Effect of CM from miR-24 mimic- or
inhibitor-transfected U251 cells on the expression levels of p-AKT,
t-AKT and β-catenin signaling in HUVECs. The U251 cells were
transfected with miR-24 mimics or inhibitors, and then the CM was
collected. Following treatment with the CM for 24 h, the expression
levels of p-AKT, t-AKT and β-catenin in HUVECs were detected by
western blotting: (A) CM-mimics and (B) CM-inhibitors, and
quantification data for (C-E) CM-mimics and (F-H) CM-inhibitors.
The roles of AKT and β-catenin in angiogenesis were confirmed using
LY294002 and KYA1797K, respectively, in HUVECs. (I) Tube formation
(magnification, ×100). **P<0.01; ***P<0.001 vs. NC. CM,
conditional medium; HUVEC, human umbilical vein endothelial cell;
NC, negative control. |
Subsequently, the expression levels of
angiogenesis-associated genes in HUVECs were further detected
(Fig. 6). The mRNA expression
levels of VEGF, TGF-β, MMP-2 and MMP-9 in HUVECs were significantly
increased by treatment of conditional medium from miR-24
mimic-transfected U251 cells (Fig.
6A-E), but were significantly decreased by treatment of
conditional medium from miR-24 inhibitor-transfected U251 cells
(Fig. 6F-J). However, the
expression level of bFGF mRNA was not significantly changed by the
conditional mediums from miR-24 mimic- and inhibitor-transfected
U251 cells.
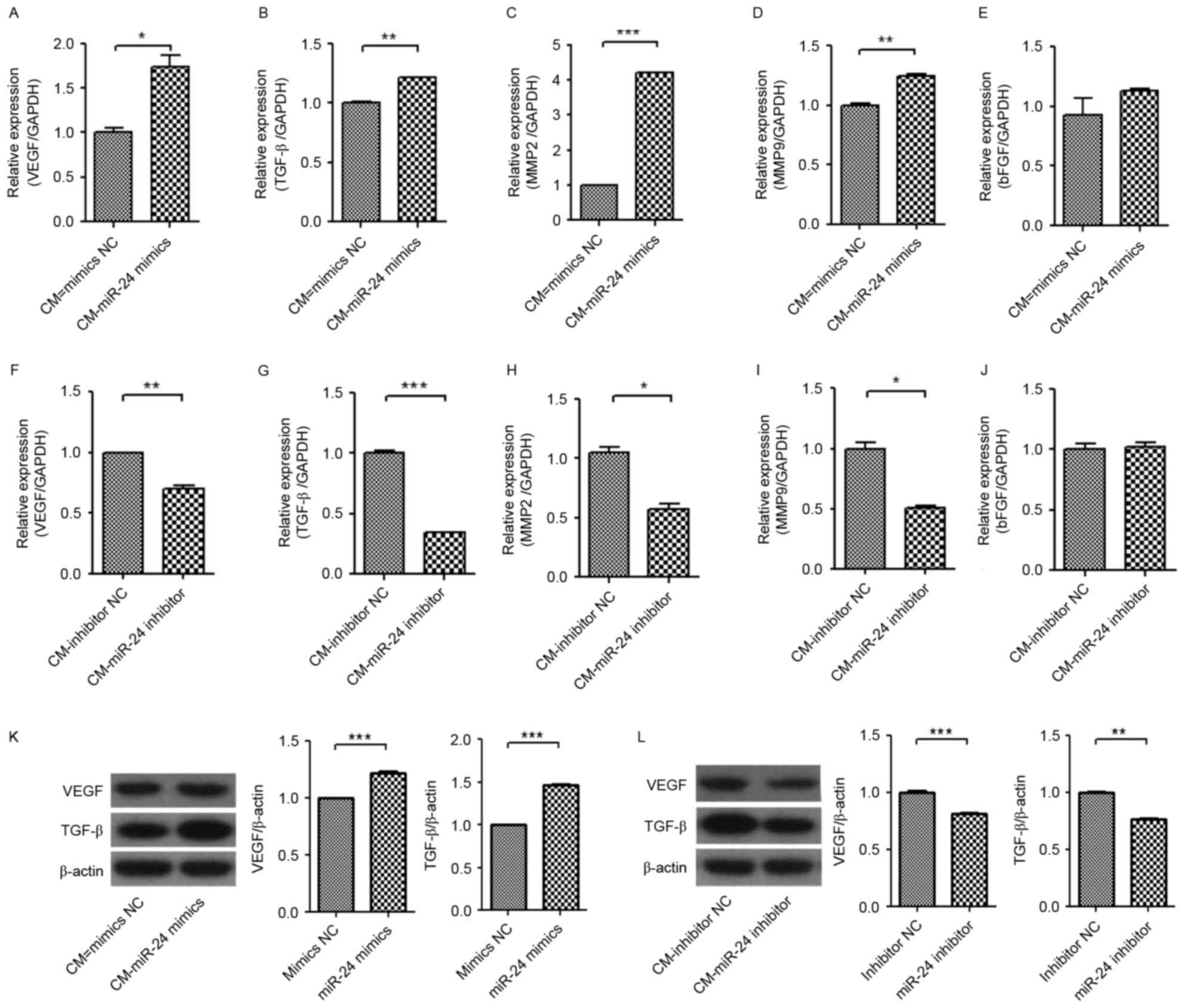 | Figure 6.Effect of CM from miR-24 mimic- or
inhibitor-transfected U251 cells on mRNA expression levels of VEGF,
TGF-β, MMP-2, MMP-9 and bFGF and protein expression levels of VEGF
and TGF-β in HUVECs. The U251 cells were transfected with miR-24
mimics or inhibitors. Following treatment with the CMs for 24 h,
the mRNAs and proteins were detected by reverse
transcription-quantitative polymerase chain reaction and western
blot analysis. mRNA expression levels of (A) VEGF, (B) TGF-β, (C)
MMP-2, (D) MMP-9 and (E) bFGF induced by miR-24 mimics. mRNA
expression levels of (F) VEGF, (G) TGF-β, (H) MMP-2, (I) MMP-9 and
(J) bFGF inhibited by miR-24 inhibitors. Protein expression levels
of VEGF and TGF-β (K) induced by mir-24 mimics and (L) inhibited by
miR-24 inhibitors. *P<0.05, **P<0.01; ***P<0.001 vs. NC.
CM, conditional medium; miR, microRNA; VEGF, vascular endothelial
growth factor; bFGF, basic fibroblast growth factor; EGF, epidermal
growth factor; TGF-β, transforming growth factor β; MMP, matrix
metalloproteinase; NC, negative control. |
Western blot detection confirmed the increase of
VEGF and TGF-β protein expression levels in U251 in the conditional
medium from miR-24 mimic-transfected U251 cells (Fig. 6K), and the decreased protein
expression level of VEGF and TGF-β in U251 cells by conditional
medium from miR-24 inhibitor-transfected U251 cells (Fig. 6L). It was further indicated that
the changed expression levels of VEGF and TGF-β in U251 cells by
miR-24 may be associated with the angiogenesis of HUVECs.
To investigate intracellular signaling, the
expression of p-AKT, t-AKT and β-catenin were detected in HUVECs by
treatment of conditional medium from miR-24 inhibitor-transfected
U251 cells (Fig. 7A-H). p-AKT,
t-AKT and β-catenin expression levels in HUVECs were upregulated by
conditional medium from miR-24 mimic-transfected U251 cells, and
were decreased by conditional medium from miR-24
inhibitor-transfected U251 cells. The roles of AKT and β-catenin in
angiogenesis were confirmed by using LY294002 and KYA1797K in
HUVECs, respectively (Fig. 7I).
Results indicated that the miR-24 mimic-induced angiogenesis was
inhibited by LY294002 and KYA1797K. Thus, AKT and β-catenin are
involved in angiogenesis in gliomas.
Discussion
The current study demonstrated that enforced
expression of miR-24 in U251 cells promotes cell viability and
angiogenesis of HUVECs, and increased the expression levels of
angiogenesis-associated factors, including VEGF, TGF-β, MMP-2 and
MMP-9. By contrast, reduced expression of miR-24 in U251 cells may
inhibit cell viability and angiogenesis of HUVECs, and downregulate
expression levels of angiogenesis-associated factors, including
VEGF, TGF-β, MMP-2 and MMP-9. The miR-24 in U251 cells may be
important in the angiogenesis of HUVECs via VEGF and TGF-β, and the
intracellular signaling AKT and β-catenin involved in this
process.
It was demonstrated that the expression level of
VEGF mRNA in patients with grade III and IV gliomas was
significantly higher than that in patients with grade I and II
gliomas, and the expression level of VEGF mRNA in patients with
grade I and II gliomas was significantly higher than that in normal
brain tissues (25–27). In addition, the cell viability,
migration and invasion, and angiogenesis of gliomas may be
inhibited by inhibition of the pituitary tumor transforming gene
that was positively associated with the glioma grade and tumor
microvessel density (28), and was
considered as an important target of glioma antiangiogenic therapy.
It was also demonstrated that certain angiogenic chemokines
secreted by tumor cells may directly effect vascular ECs, which
induces angiogenesis (29).
Furthermore, angiogenesis is regulated by the receptor of those
chemokines, including vascular endothelial growth factor receptor,
epidermal growth factor receptor and platelet-derived growth factor
receptor. Currently, studies have demonstrated that in the newly
diagnosed grade III malignant glioma patients, ~61% of patients
achieved a good outcome following treatment with Avastin (VEGF mAb)
combined with irinotecan in the second phase of clinical trials,
and adverse reactions were decreased compared with the use of
irinotecan alone (30). shRNA
plasmids encapsulated with urokinase-type plasminogen activator
(uPA) and the corresponding receptor (uPAR), and
metalloproteinases, such as MMP-9, significantly inhibit
angiogenesis and tumor growth in mice (31). Similarly, in animal models of
transplanted tumors, injection of a VEGF siRNA plasmid
significantly inhibits tumor angiogenesis (32).
miR-24 promotes tumors and angiogenesis in various
types of cancer, such as pancreatic carcinoma (33). miR-24 expression was downregulated
in glioma samples and glioma cells (19,20,34).
Overexpression of miR-24-3p may promote cell proliferation, as
observed by MTT assay (19). The
suppression of miR-24 expression inhibits cell proliferation and
invasion, indicating that that miR-24 acts as an oncogene in
gliomas (20). Certain genes
closely associated with angiogenesis (such as VEGF, bFGF, EGF,
TGF-β, MMP-2 and MMP-9) were detected. It was found that enforced
expression of miR-24 in U251 cells significantly increased the mRNA
expression levels of VEGF, bFGF, EGF, TGF-β, MMP-2 and MMP-9 in
U251 cells, as well as the VEGF and TGF-β protein expression levels
in U251 cells. By contrast, reduced expression of miR-24 in U251
cells significantly decreased the mRNA expression levels of VEGF,
bFGF, EGF, TGF-β, MMP-2 and MMP-9 in U251 cells, as well as the
VEGF and TGF-β protein expression levels in U251 cells. To detected
whether the dysregulation of miR-24 in glioma cells promotes
microvascular proliferation of ECs and investigate its potential
underlying mechanism, conditioned media was used to investigate the
secretion of pro-angiogenic molecules (such as VEGF, bFGF, EGF,
TGF-β, MMP-2, MMP-9) following miR-24 transfection. All these
pro-angiogenic molecules have demonstrated critical roles in tube
formation of HUVECs. VEGFA was significantly increased in the
culture medium from miR-24 mimic-transfected U251 cells, whereas it
was significantly decreased in the culture medium from miR-24
inhibitor-transfected U251 cells. As the miR-24 in the culture
medium was not significantly changed, miR-24 may not influence the
response to VEGFA in ECs. Therefore, miR-24 may contribute to
angiogenesis in glioma via upregulation of VEGF and TGF-β
expression levels in U251 cells. This was further confirmed using
the HUVECs by treatment of the of conditional medium from miR-24
mimic- or inhibitor-transfected U251 cells. Although the bFGF mRNA
was not significantly changed by the conditional mediums from
miR-24 mimic- and inhibitor-transfected U251 cells, conditional
medium from miR-24 mimic-transfected U251 cells significantly
increased the mRNA expression levels of VEGF, TGF-β, MMP-2 and
MMP-9, while the conditional medium from miR-24
inhibitor-transfected U251 cells significantly decreased. Thus,
miR-24 contributes to angiogenesis in gliomas via upregulation of
VEGF and TGF-β expression in U251 cells.
AKT and β-catenin signaling are two important
signaling pathways involved in the angiogenesis of HUVECs. p-AKT,
t-AKT and β-catenin expression levels were upregulated in
conditional medium from miR-24 mimic-transfected U251 cells, and
were decreased in the conditional medium from miR-24
inhibitor-transfected U251 cells. The miR-24 mimic-induced
angiogenesis was inhibited by LY294002 and KYA1797K, indicating
that AKT and β-catenin are involved in angiogenesis in gliomas.
In conclusion, miR-24 may contribute to the
angiogenesis in gliomas via upregulation of VEGF and TGF-β
expression levels in U251 cells and the intracellular AKT and
β-catenin signaling pathway. However, the present study was only
performed in vitro using one cell line, the human U251
glioma cell line. A further study with more cell lines and an in
vivo investigation is required in future. However, the current
findings may contribute to the therapy of gliomas.
Acknowledgements
The present study was supported by a grant from the
National Science Foundation of China (grant no. 81502163).
References
|
1
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 world health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bourne TD and Schiff D: Update on
molecular findings, management and outcome in low-grade gliomas.
Nat Rev Neurol. 6:695–701. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohgaki H and Kleihues P: Epidemiology and
etiology of gliomas. Acta Neuropathol. 109:93–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Poleszczuk J, Hahnfeldt P and Enderling H:
Therapeutic implications from sensitivity analysis of tumor
angiogenesis models. PLoS One. 10:e01200072015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao Z, Cheng P, Xue Y and Liu Y: Vascular
endothelial growth factor participates in modulating the C6
glioma-induced migration of rat bone marrow-derived mesenchymal
stem cells and upregulates their vascular cell adhesion molecule-1
expression. Exp Ther Med. 4:993–998. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Damert A, Machein M, Breier G, Fujita MQ,
Hanahan D, Risau W and Plate KH: Up-regulation of vascular
endothelial growth factor expression in a rat glioma is conferred
by two distinct hypoxia-driven mechanisms. Cancer Res.
57:3860–3864. 1997.PubMed/NCBI
|
|
10
|
Jensen RL: Growth factor-mediated
angiogenesis in the malignant progression of glial tumors: A
review. Surg Neurol. 49:189–196. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim TY, Kim J, Choo HY and Kwon HJ:
Inhibition of 5-lipoxygenase suppresses vascular endothelial growth
factor-induced angiogenesis in endothelial cells. Biochem Biophys
Res Commun. 478:1117–1122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim KJ, Li B, Winer J, Armanini M, Gillett
N, Phillips HS and Ferrara N: Inhibition of vascular endothelial
growth factor-induced angiogenesis suppresses tumour growth in
vivo. Nature. 362:841–844. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li R and Li X, Ning S, Ye J, Han L, Kang C
and Li X: Identification of a core miRNA-pathway regulatory network
in glioma by therapeutically targeting miR-181d, miR-21, miR-23b,
β-Catenin, CBP, and STAT3. PLoS One. 9:e1019032014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu S, Yin F, Zhang J, Wicha MS, Chang AE,
Fan W, Chen L, Fan M and Li Q: Regulatory roles of miRNA in the
human neural stem cell transformation to glioma stem cells. J Cell
Biochem. 115:1368–1380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao W, Guo G, Zhang Q, Fan L, Wu N and Bo
Y: The application of multiple miRNA response elements enables
oncolytic adenoviruses to possess specificity to glioma cells.
Virology. 458–459:69–82. 2014. View Article : Google Scholar
|
|
16
|
Ciafré SA, Galardi S, Mangiola A, Ferracin
M, Liu CG, Sabatino G, Negrini M, Maira G, Croce CM and Farace MG:
Extensive modulation of a set of microRNAs in primary glioblastoma.
Biochem Biophys Res Commun. 334:1351–1358. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Landgraf P, Rusu M, Sheridan R, Sewer A,
Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M,
et al: A mammalian microRNA expression atlas based on small RNA
library sequencing. Cell. 129:1401–1414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu D, Han L, Wu X, Yang X, Zhang Q and
Jiang F: Genome-wide microRNA changes in human intracranial
aneurysms. BMC Neurol. 14:1882014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu W, Liu M, Peng X, Zhou P, Zhou J, Xu K,
Xu H and Jiang S: miR-24-3p and miR-27a-3p promote cell
proliferation in glioma cells via cooperative regulation of MXI1.
Int J Oncol. 42:757–766. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen L, Zhang A, Li Y, Zhang K, Han L, Du
W, Yan W, Li R, Wang Y, Wang K, et al: MiR-24 regulates the
proliferation and invasion of glioma by ST7L via β-catenin/Tcf-4
signaling. Cancer Lett. 329:174–180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan Z, Liu J, Xie L, Liu X and Zeng Y:
Role of heparan sulfate in mediating CXCL8-induced endothelial cell
migration. Peer J. 4:e16692016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zeng Y, Liu XH, Tarbell J and Fu B:
Sphingosine 1-phosphate induced synthesis of glycocalyx on
endothelial cells. Exp Cell Res. 339:90–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeng Y and Liu J: Role of glypican-1 in
endothelial NOS activation under various steady shear stress
magnitudes. Exp Cell Res. 348:184–189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang N, Jain RK and Batchelor TT: New
directions in anti-angiogenic therapy for glioblastoma.
Neurotherapeutics. 14:321–332. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seystahl K, Wick W and Weller M:
Therapeutic options in recurrent glioblastoma-an update. Crit Rev
Oncol Hematol. 99:389–408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Codrici E, Enciu AM, Popescu ID, Mihai S
and Tanase C: Glioma stem cells and their microenvironments:
Providers of challenging therapeutic targets. Stem Cells Int.
2016:57284382016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cui L, Xu S, Song Z, Zhao G, Liu X and
Song Y: Pituitary tumor transforming gene: A novel therapeutic
target for glioma treatment. Acta Biochim Biophys Sin (Shanghai).
47:414–421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang H, Yang L, Teng X and Liu Z, Liu C,
Zhang L and Liu Z: The chemokine receptor CXCR7 is a critical
regulator for the tumorigenesis and development of papillary
thyroid carcinoma by inducing angiogenesis in vitro and in vivo.
Tumour Biol. 37:2415–2423. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Desjardins A, Reardon DA, Herndon JE II,
Marcello J, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S,
Sampson J, Bailey L, et al: Bevacizumab plus irinotecan in
recurrent WHO grade 3 malignant gliomas. Clin Cancer Res.
14:7068–7073. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gondi CS, Lakka SS, Dinh DH, Olivero WC,
Gujrati M and Rao JS: Downregulation of uPA, uPAR and MMP-9 using
small, interfering, hairpin RNA (siRNA) inhibits glioma cell
invasion, angiogenesis and tumor growth. Neuron Glia Biol.
1:165–176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Niola F, Evangelisti C, Campagnolo L,
Massalini S, Buè MC, Mangiola A, Masotti A, Maira G, Farace MG and
Ciafrè SA: A plasmid-encoded VEGF siRNA reduces glioblastoma
angiogenesis and its combination with interleukin-4 blocks tumor
growth in a xenograft mouse model. Cancer Biol Ther. 5:174–179.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu R, Zhang H, Wang X, Zhou L, Li H, Deng
T, Qu Y, Duan J, Bai M, Ge S, et al: The miR-24-Bim pathway
promotes tumor growth and angiogenesis in pancreatic carcinoma.
Oncotarget. 6:43831–43842. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiuju C, Zhen W and Yanchao S: SOX7
inhibits tumor progression of glioblastoma and is regulated by
miRNA-24. Open Med (Wars). 11:133–137. 2016.PubMed/NCBI
|