Introduction
As one of the common age-associated diseases,
cataracts is a troublesome disease and is a leading cause of
non-traumatic blindness worldwide. The global progression of aging
has been associated with cataracts and resulting visual impairment,
which has placed a burden on society. According to an
epidemiological study published in 2011, ~96% of people aged >60
were reported to exhibit varying lens opacity (1). It is well known that various
morphological and functional alterations of lens cells, including
disordered cell cycle, DNA damage, lens epithelial cells (LECs)
excessive proliferation and abnormal epithelial-mesenchymal
transition (EMT) participate in the pathological formation of
cataracts (2–5). Therefore, investigation of a key
regulator associated with LECs' proliferation and EMT may
considerably contribute to the development of molecular therapy for
cataracts disease.
Long noncoding RNAs (lncRNAs) are macromolecules
grouped under noncoding RNAs, with length usually >200
nucleotides. They extensively exhibit varied roles in numerous
physiological and pathological processes, including cell
proliferation, cell differentiation, apoptosis, tumorigenesis and
EMT (6–8). LncRNA potassium voltage-gated channel
subfamily Q member 1 opposite strand/antisense transcript 1
(lncKCNQ1OT1) is located on human chromosome 11p15.5 and is
associated with the development of a wide array of diseases. Ren
et al (9) reported that
knockdown of KCNQ1OT1 suppresses the proliferation and invasion of
A549 cells, as well as advanced cellular apoptosis of A549 cells.
Jin et al (10) suggested
that KCNQ1OT1 may promote cataractogenesis, which may be dependent
upon microRNA-214 and activation of the caspase-1 pathway.
In the present study, the expression levels of
KCNQ1OT1 were investigated in cataract tissue specimens and in a
constructed cataract cell model induced by transforming growth
factor (TGF)-β2. In addition, the function of KCNQ1OT1 in LEC
proliferation and EMT was evaluated. The findings of the present
study may provide insight into a novel contributor to the process
of cataract formation.
Materials and methods
Patients and tissue samples
collection
A total of 30 cases (17 female and 13 male; mean age
63.5 years, range 58–73 years) of fresh posterior lens capsule
specimens with age-associated cataracts during phacoemulsification
and paired fresh posterior lens capsule specimens without cataracts
caused by ocular trauma during ophthalmectomy were collected at 4th
People's Hospital of Shenyang between July 2015 and July 2017.
Written informed consent was obtained from all patients for all
clinical investigations conducted and the present study was
approved by the Institute Research Medical Ethics Committee of 4th
People's Hospital of Shenyang (Shenyang, China).
Cell culture and TGF-β2
intervention
Human LEC cell line SRA01/04 was purchased from the
American Type Culture Collection (Manassas, VA, USA) and was
cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany),
100 U/ml penicillin (Baoman Biotechnology Co., Ltd., Shanghai,
China), and 100 U/ml streptomycin (Baoman Biotechnology Co., Ltd.).
The cell line was cultured at 37°C in a humidified atmosphere
containing 5% CO2 until 80–90% confluent. For the TGF-β2
intervention, 10 ng/ml recombinant human TGF-β2 (Biolegend, Inc.,
San Diego, CA, USA) was applied to induce a cataract cell model
according to a previous study (9).
Cell transfection
KCNQ1OT1 silencing plasmids short hairpin (sh)RNA
(KCNQ1OT1 shRNA) and negative control shRNA (NC shRNA), KCNQ1OT1
overexpression plasmids (pcDNA-KCNQ1) and corresponding empty
vector plasmids (pcDNA3.1), mothers against decapentaplegic homolog
4 (SMAD4) silencing plasmids (SMAD4 shRNA) and negative control
shRNA (NC shRNA) were constructed by Guangzhou RiboBio Co., Ltd.
(Guangzhou, China). TGF-β2-stimulated SRA01/04 cells were cultured
at 60–80% confluence and were then used for a further cell
transfection. Plasmids were transfected into TGF-β2-stimulated
SRA01/04 cells by using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. Then, 48 h after transfection, the cells were used for
further experimentation.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The procedure was carried out as previously
described (10). In brief, total
RNAs of tissue specimens and of the cells following different
interventions were extracted by TRIzol® (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols. cDNAs
were synthesis by PrimeScriptTM RT reagent kit (Takara
Biotechnology Co., Ltd., Dalian, China). qPCR was performed in a
total reaction volume of 50 µl with 35 cycles (denaturation at 95°C
for 5 sec, annealing at 60°C for 30 sec and extension 72°C for 5
sec) using a SYBR Premix Ex Taq II kit (Takara Biotechnology Co.,
Ltd.) and an Applied Biosystems 7500 Fluorescent Quantitative PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
expression of KCNQ1OT1 and SMAD4 were calculated using GAPDH as an
internal control by the 2−ΔΔCq method (11). The primers used were as follows:
KCNQ1OT1 forward, 5′-TGCAGAAGACAGGACACTGG-3′, reverse
5′-CTTTGGTGGGAAAGGACAGA-3′; SMAD4 forward,
5′-TGGGAAGAGATCACCCTGTC-3′ and reverse 5′-CCCAACGGTAAAAGACCTCA-3′;
GAPDH forward, 5′-GCACCGTCAAGGCTGAGAAC-3′, reverse
5′-TGGTGAAGACGCCAGTGGA-3′.
Western blot analysis
Total protein of tissue specimens and cells were
lysed by ice-cold radio immunoprecipitation assay (RIPA) buffer
(Sigma-Aldrich, Merck KGaA). Following protein concentration
quantification with a Bicinchoninic Acid protein assay kit (Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), 40 µg protein samples
were subjected to 10% SDS-PAGE and transferred onto a
polyvinylidene difluoride membrane (PVDF; Amresco, LLC, Solon, OH,
USA) and then blocked by 5% BSA (Cell Signaling Technology, Inc.,
Danvers, MA, USA) for 1 h at room temperature. Subsequently,
membranes were incubated with antibodies against SMAD4 (1:5,000;
cat no. ab40759; Abcam, Cambridge, MA, UK), E-cadherin (1:100; cat.
no. ab76055; Abcam), fibronectin (1 µg/ml; cat. no: ab23750; Abcam)
and GAPDH (1:10,000; cat. no. ab128915; Abcam) at 4°C overnight.
The following day, the membranes were incubated with secondary
antibodies [goat anti-rabbit IgG horseradish peroxidase (HRP),
1:2,000; cat. no. ab205718, Abcam] at room temperature for 1 h.
Following washing with TBST 3 times, an ECL Western Blotting
Substrate kit (cat. no. ab65623; Abcam) was used for
chemiluminescence imaging with Image J software version 2X
(National Institutes of Health, Bethesda, MD, USA).
Immunohistochemical (IHC)
staining
The procedure was carried out as previously
described (12). Briefly, the
collected cataract tissues were treated orderly: 4%
paraformaldehyde fixation, paraffin-embedding, section (4 µm
thickness), deparaffinization, a descending series of 100, 95, 80
and 70% alcohol were applied for rehydration followed by 3%
hydrogen peroxide incubation for 10 min, antigen retrieval,
blocking with 10% goat serum (BioWorld Technology, Inc., St. Louis
Park, MN, USA), primary antibody incubation (rabbit anti-SMAD4
antibody; 1:100; cat. no. ab40759; Abcam) at 4°C overnight,
secondary antibody incubation [goat anti-rabbit IgG horseradish
peroxidase (HRP), 1:2,000; cat. no. ab205718; Abcam] at 37°C for 20
min, streptavidin-horseradish peroxidase complex incubation,
3,3′-diaminobenzidine tetrahydrochloride (MedChemExpress, Monmouth
Junction, NJ, USA) staining, and hematoxylin (Amresco, LLC) was
used as a counterstain at room temperature for 1 min. All sections
were independently assessed by two experienced pathologists and
SMAD4 expression levels were evaluated by calculating the
proportion of positive staining and staining intensity of tumor
tissue. Sections with inconsistent results were re-examined by the
original two pathologists and a senior pathologist until a
consensus was attained.
5-Ethynyl-20-deoxyuridine (EdU)
incorporation assay and Cell Counting Kit-8 (CCK-8) assay
The procedure was carried out as previously
described (13). Briefly, SRA01/04
cells (5×103) transfected with KCNQ1OT1 or SMAD4
plasmids were seeded in a 96-well plate and cultured for 72 h. The
following assays were performed according to the manufacturer's
protocols of an EdU detection kit (cat. no. KGA331-500; Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China). The nuclei were observed
under a fluorescent microscope at an excitation wavelength of 350
nm (Leica Microsystems GmbH, Wetzlar, Germany). Images were
analyzed using Image-Pro Plus software version 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA). The quantitative data were
expressed as the percentage of EdU-positive nuclei relative to
total number of nuclei counted. In addition, a CCK-8 assay was
performed as previously described (14). SRA01/04 cells (2×103)
were cultured with DMEM supplemented with 10% FBS in a 96-well
plate for 24 h and followed by transfection of ddH2O
(mock group), KCNQ1OT shRNA (KCNQ1OT shRNA) and negative control
shRNA (NC shRNA group) for 24 h. At days 1, 2, 3, 4 and 5 following
transfection, 10 µl CCK-8 solution was added into each well and
incubated at 37 °C for 4 h. The absorbance was measured at an
optical density of 450 nm using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All experiments were repeated in triplicate and all
data from three independent experiments were expressed as mean ±
standard deviation. GraphPad Prism v5.0 software (GraphPad
Software, Inc., La Jolla, CA, USA) was used for statistical
analysis. For paired groups, the expression level of KCNQ1OT1 and
SMAD4, as well as alterations in proliferative ability were
analyzed by a two-tailed Student's t-test. For the comparison of
more than two groups, one-way analysis of variance was conducted
followed by a Student-Newman-Keuls post hoc test. Spearman
correlation analysis was performed to determine the correlation
between KCNQ1OT1 and SMAD4. P<0.05 was considered to indicate a
statistically significant difference.
Results
Increased KCNQ1OT1 expression levels
are observed in human cataract lens posterior capsular samples and
in TGF-β2 treated SRA01/04 cells
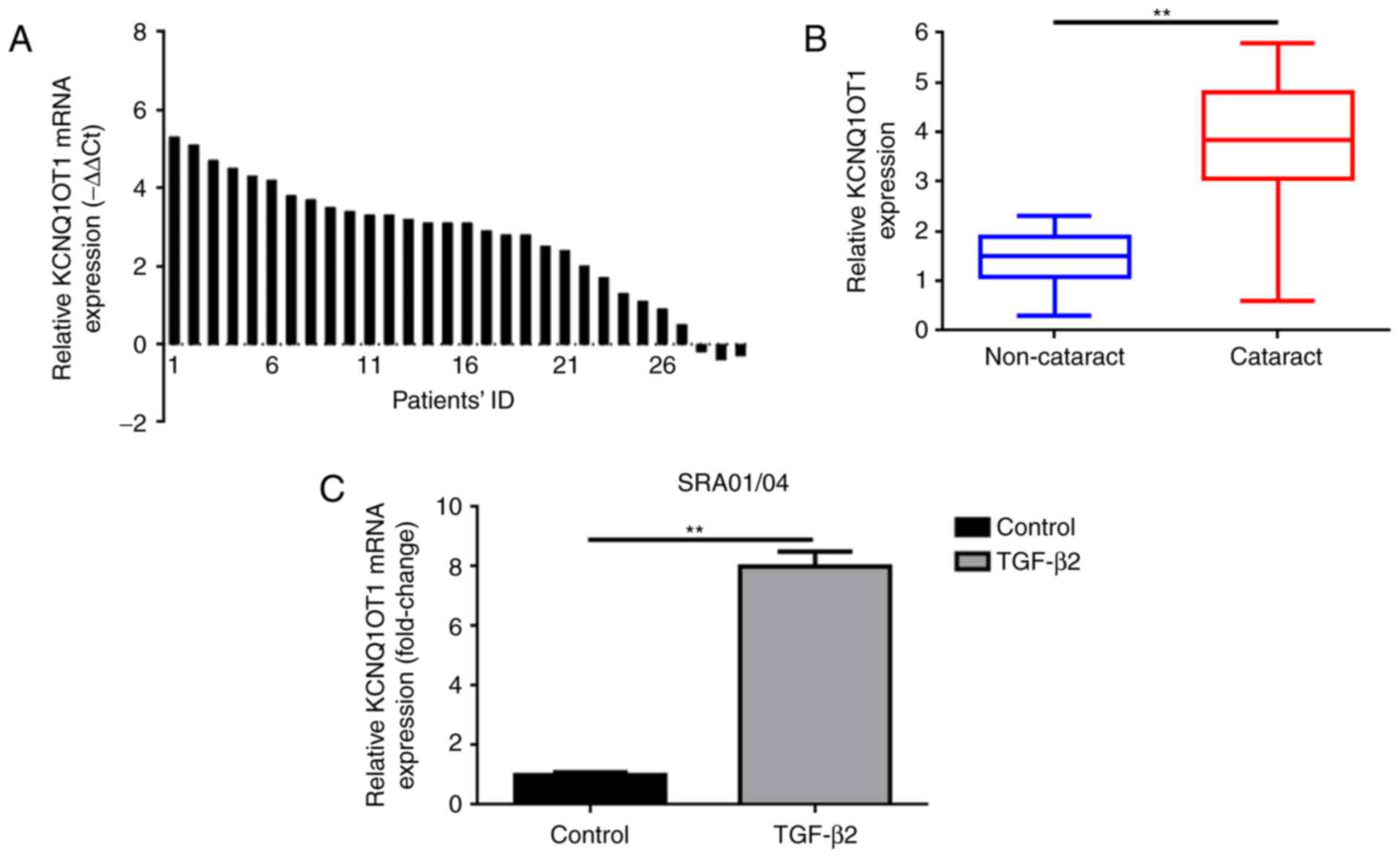
The present study investigated the expression levels
of KCNQ1OT1 in 30 fresh specimens of posterior lens capsules with
age-associated cataract and 30 paired posterior lens capsules
specimens without age-associated cataracts via RT-qPCR analysis. As
presented in Fig. 1A and B,
KCNQ1OT1 was upregulated in 90% (27/30) specimens of posterior lens
capsules with age-associated cataracts compared with in adjacent
non-cataract specimens.
TGF-β2 was administered to SRA01/04 cells to induce
a cataract cell model as previously reported (9). RT-qPCR was also applied to measure
the expression of KCNQ1OT1 in the human LEC line SRA01/04 treated
with or without TGF-β2. As presented in Fig.1C, significantly increased expression
of KCNQ1OT1 was confirmed in SRA01/04 cells treated TGF-β2, however
not in SRA01/04 cells incubated without TGF-β2.
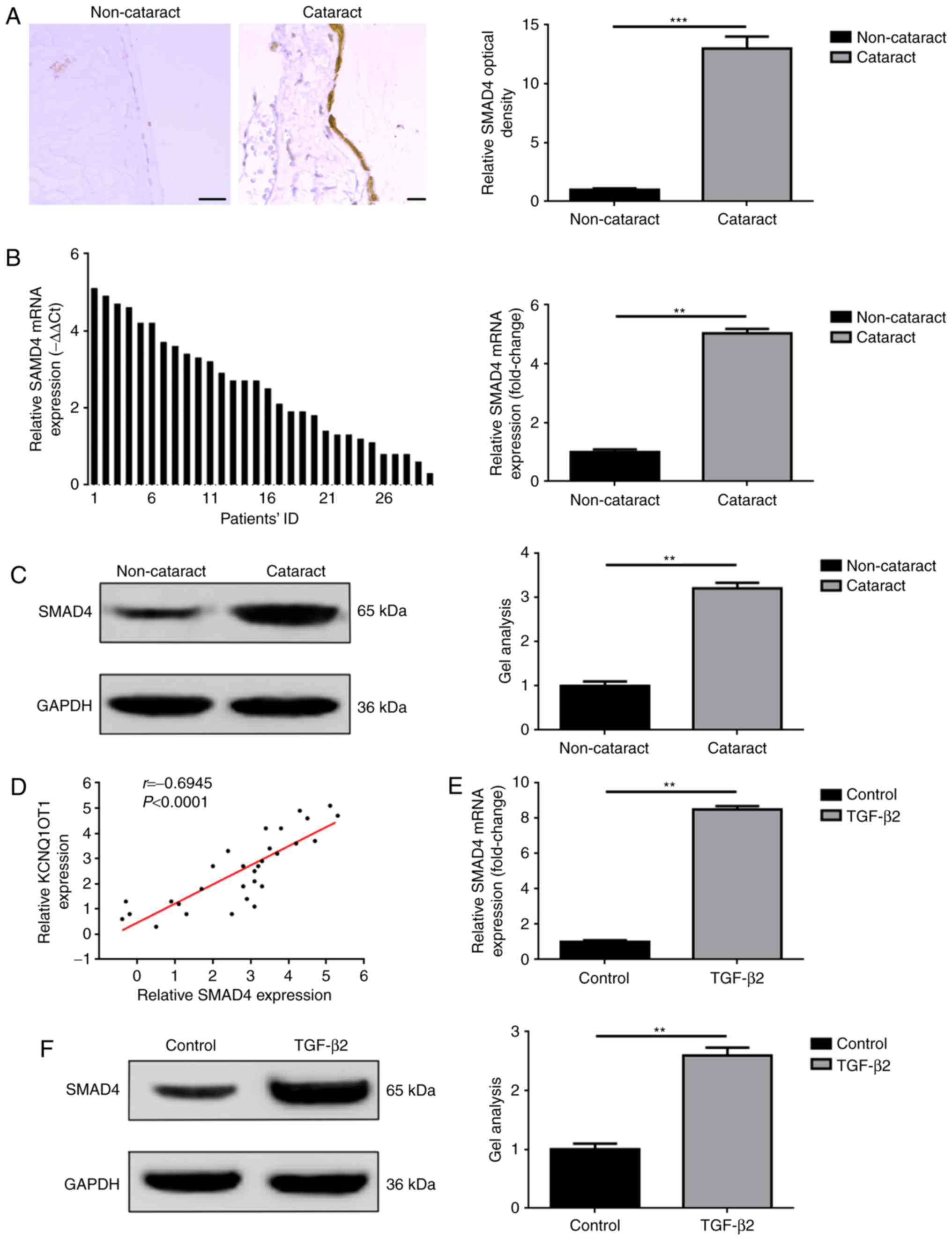
SMAD4 is overexpressed in human
cataract lens posterior capsular samples and in TGF-β2 treated
SRA01/04 cells
The associated effects of SMAD4, including the
mediation of proliferation and EMT have been widely reported in a
variety of diseases, such as cataracts (15–17).
In the present study, the expression levels of SMAD4 in collected
tissue specimens and in cataract cell models induced by TGF-β2 were
investigated. The results were in accordance with previous studies
(17). As presented in Fig. 2A-C, the overexpression of SMAD4 was
detected only in tissue specimens with cataract disease, confirmed
by IHC, RT-qPCR and western blot analyses. In addition, a positive
correlation between KCNQ1OT1 and SMAD4 expression in cataract
tissue specimens was determined by Spearman correlation analysis
(Fig. 2D). Furthermore,
significant upregulation of SMAD4 expression levels were detected
within SRA01/04 cells treated with TGF-β2 compared with in cells
not treated with TGF-β2, as determined via RT-qPCR and western blot
assays (Fig. 2E and F).
Knockdown of KCNQ1OT1 suppresses the
proliferation and EMT of TGF-β2-treated SRA01/04 cells
As previously mentioned, the expression of KCNQ1OT1
in tissue and at the cellular levels were analyzed. The present
study also investigated the effects of KCNQ1OT1 on LEC
proliferation and EMT. Firstly, KCNQ1OT1 in TGF-β2 treated SRA01/04
cells was suppressed via transfection with KCNQ1OT1 shRNA, which
was confirmed by RT-qPCR (Fig.
3A). Secondly, CCK-8 and EDU assays were performed to determine
alterations in proliferative ability following the suppression of
KCNQ1OT1 expression. As demonstrated in Fig. 3B and C, the downregulation of
KCNQ1OT1 significantly suppressed SRA01/04 cell proliferation in
the presence of TGF-β2. Lastly, the effects of KCNQ1OT1 on SRA01/04
cells EMT with TGF-β2 treatment were investigated. As presented in
Fig. 3D, TGF-β2 induced the
increase of fibronectin but the suppression of E-cadherin
expression levels in SRA01/04 cells. This phenomenon was reversed
by knockdown of KCNQ1OT1 (transfection of KCNQ1OT1 shRNA),
suggesting that the suppression of KCNQ1OT1 expression inhibited
EMT in TGF-β2-induced cataract cell models.
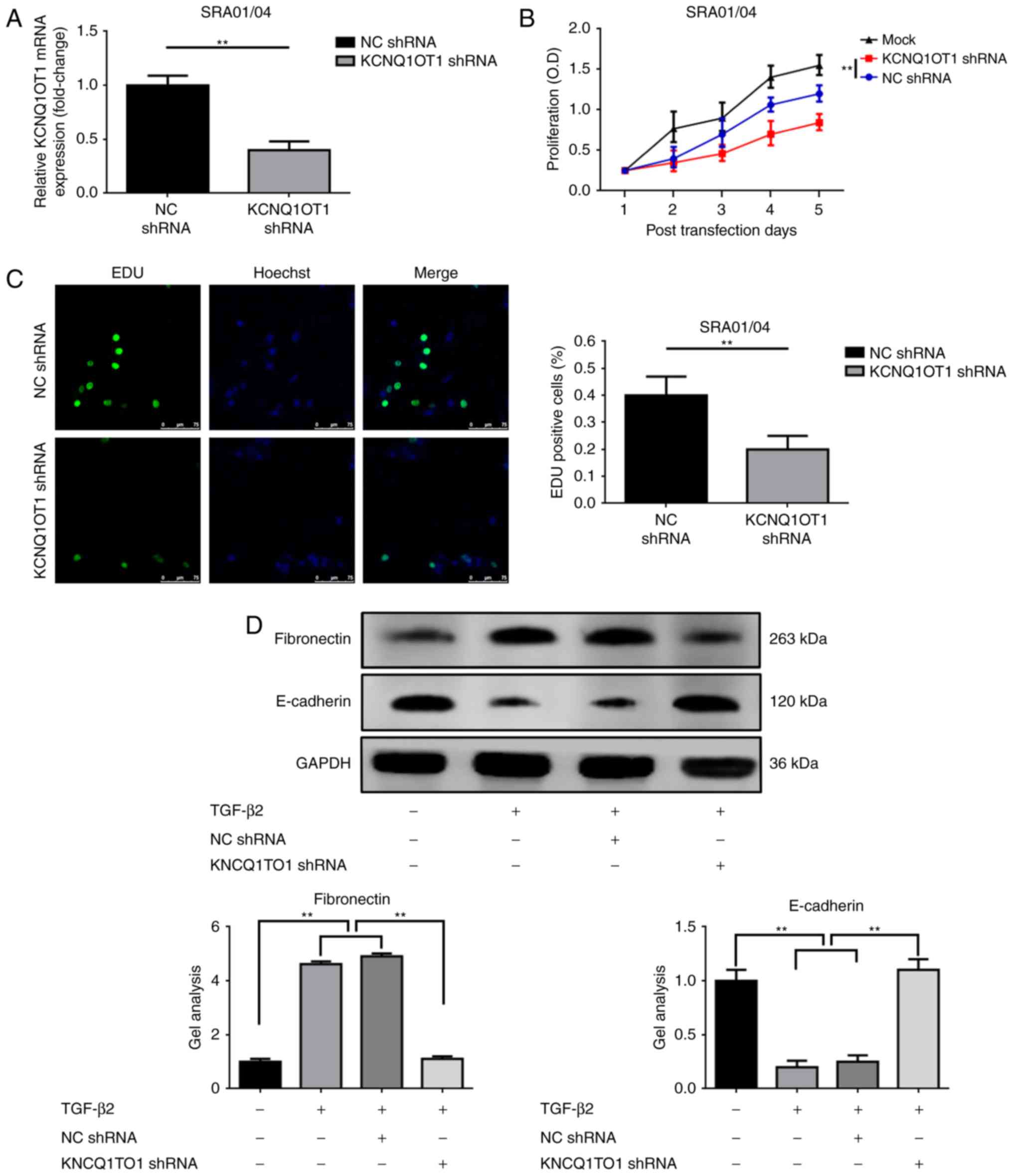 | Figure 3.Downregulation of KCNQ1OT1 suppresses
the proliferation and EMT of TGF-β2-treated SRA01/04 cells. (A)
KCNQ1OT1 expression was suppressed by transfection with KCNQ1OT1
shRNA as confirmed by reverse transcription-quantitative polymerase
chain reaction, **P<0.01 vs. NC shRNA group. Suppression of
KCNQ1OT1 expression inhibited SRA01/04 cell proliferation as
determined by (B) Cell Counting Kit-8 and (C) EdU assays,
**P<0.01 vs. NC shRNA group. (D) TGF-β2 induced an elevation of
fibronectin but a decrease in E-cadherin expression levels
(promotion of EMT), and the tendency was reversed by the
suppression of KCNQ1OT1, as detected by western blotting,
**P<0.01. All data were present as the mean ± standard deviation
from three independent experiments. EdU, 5-Ethynyl-20-deoxyuridine;
KCNQ1OT1, potassium voltage-gated channel subfamily Q member 1
opposite strand/antisense transcript 1; NC, negative control;
shRNA, short hairpin RNA; TGF-β2, transforming growth
factor-β2. |
KCNQ1OT1 promotes proliferation and
EMT via upregulation of SMAD4
As both KCNQ1OT1 and SMAD4 have been revealed to be
involved in LEC proliferation and EMT, the association between
KCNQ1OT1 and SMAD4 was investigated in the present study. The
results revealed that upregulated and downregulated KCNQ1OT1 may
increase or reduce the expression levels of SMAD4, respectively
(Fig. 4A-C). Subsequently, RNAi
was employed to determine the function of SMAD4, which may be
involved in KCNQ1OT1-induced promotion of proliferation and EMT.
The silencing effect of SMAD4 shRNA was firstly confirmed by
RT-qPCR and western blot analyses (Fig. 4D and E). As expected, the results
of the present study revealed that KCNQ1OT1-induced proliferation
of SRA01/04 cells in the presence of TGF-β2 was reversed by
silencing SMAD4 (co-transfection of pcDNA-KCNQ1OT1 and SMAD4 shRNA;
Fig. 4F). Furthermore, the same
trend was observed in the analysis of EMT, characterized by
fibronectin and E-cadherin expression within SRA01/04 cells in the
presence of TGF-β2 (Fig. 4G).
These findings suggested that KCNQ1OT1 enhanced SRA01/04 cell
proliferation and EMT via the SMAD4 signaling pathway.
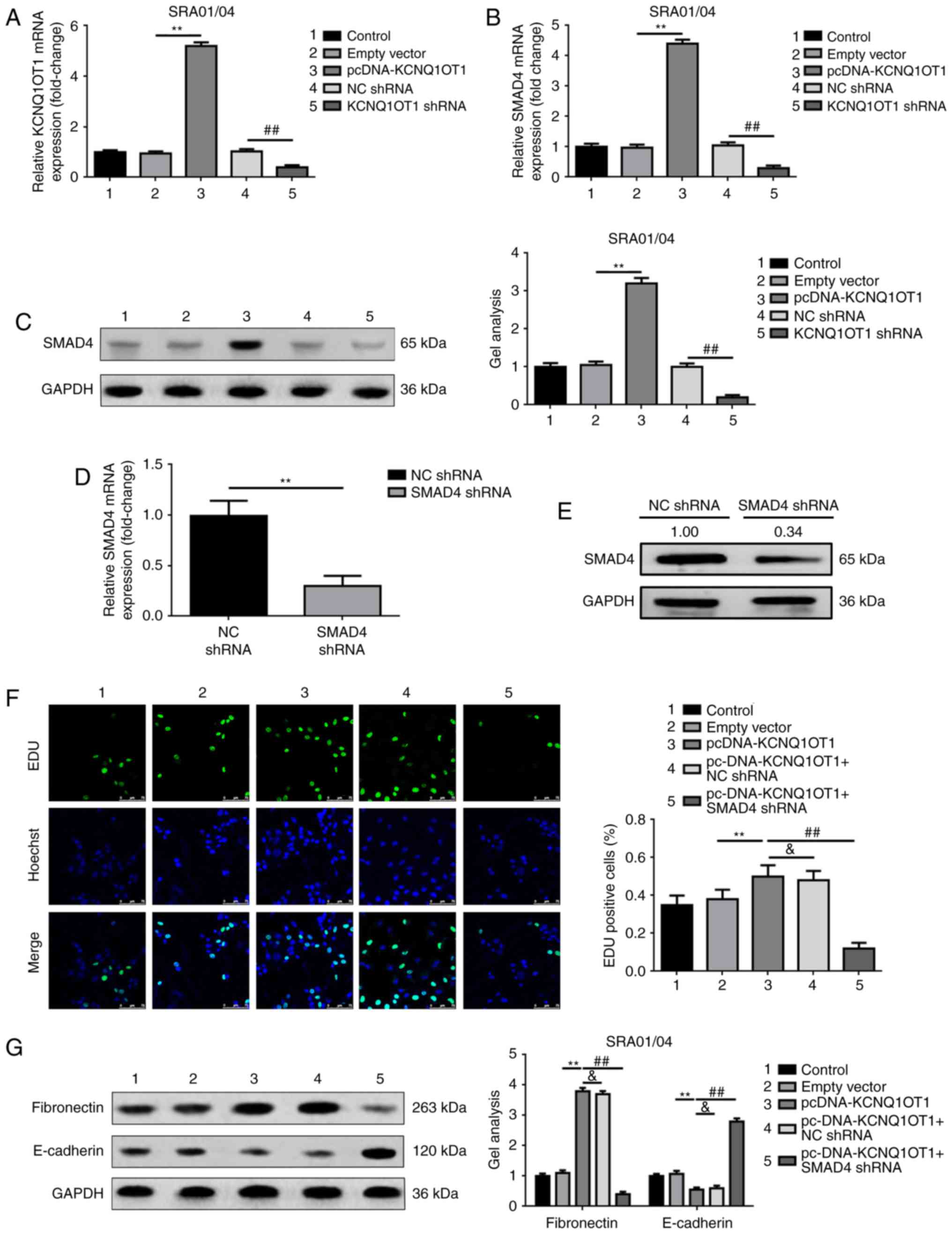 | Figure 4.KCNQ1OT1 promotes proliferation and
EMT via the upregulation of SMAD4. (A) Elevation and suppression of
KCNQ1OT1 was confirmed by RT-qPCR, **P<0.01 vs. empty vector
group, ##P<0.01 vs. NC shRNA group. Elevation and
suppression of KCNQ1OT1 positively regulated SMAD4 mRNA and protein
expression levels as determined by (B) RT-qPCR and (C) western
blotting, respectively. **P<0.01 vs. empty vector group,
##P<0.01 vs. NC shRNA group. SMAD4 expression levels
were significantly knocked down by transfection of SMAD4 shRNA, as
confirmed by (D) RT-qPCR (**P<0.01 vs. NC shRNA group) and (E)
western blot analyses (Relative SMAD4 protein expression in SMAD4
shRNA group was 0.34 comparing with NC shRNA group, which was
1.00). (F) Upregulation of KCNQ1OT1 (pcDNA-KCNQ1OT1 group) promoted
SRA01/04 cell proliferation, this effect was reduced by silencing
SMAD4 expression (pcDNA-KCNQ1OT1+SMAD4 shRNA group) as detected by
EdU, **P<0.01, ##P<0.01 vs. pcDNA-KCNQ1OT1 group,
&P>0.05. (G) Elevation of KCNQ1OT1 (pcDNA-KCNQ1OT1 group)
promoted the progress of EMT (elevation of fibronectin, but
suppression of E-cadherin expression levels), however the
facilitative effect was decreased by silencing SMAD4 expression
(pcDNA-KCNQ1OT1+SMAD4 shRNA group) as detected by western blotting,
**P<0.01, ##P<0.01 vs. pcDNA-KCNQ1OT1 group,
&P>0.05. 1, control group; 2, empty vector group; 3,
pcDNA-KCNQ1OT1 group; 4, NC shRNA group; and 5, KCNQ1OT1 shRNA
group. All data were presented as the mean ± standard deviation
from three independent experiments. EdU, 5-Ethynyl-20-deoxyuridine;
KCNQ1OT1, potassium voltage-gated channel subfamily Q member 1
opposite strand/antisense transcript 1; NC, negative control;
shRNA, short hairpin RNA; SMAD4, mothers against decapentaplegic
homolog 4; TGF-β2, transforming growth factor-β2. |
Discussion
Cataracts are generally classified into two types of
disease: Anterior subcapsular cataract (ASC) and posterior capsule
opacification (PCO). ASC is a primary cataract disease
characterized by star-shaped or irregular fibrotic plaques beneath
the anterior capsule, resulting in notable reduction in vision due
to visual axis involvement (18).
PCO, known as a secondary lens opacification, is usually caused by
aberrant growth of lens epithelial cells that remain in the
capsular bag following cataract surgery (19). The cellular mechanism of ASC and
PCO involves the proliferation, migration and EMT of LECs, leading
to the transition from epithelial cells to fibroblasts, and the
production of extracellular matrix proteins (collagens I, IV and
fibronectin), which finally contributes to the formation of
subcapsular plaques beneath the lens anterior or posterior capsule
(20). At present, increasing
evidence indicates that numerous factors and pathways mediate the
proliferation, migration and EMT of residual LECs, and may
contribute to the pathology of age-associated cataracts (21–23).
Liu and Xiao (24) reported that
hypoxia promotes hypoxia inducible factor-1α and facilitates EMT
via the Notch homolog 1, translocation-associated
(Drosophila)/snail family transcriptional repressor
1/E-cadherin pathway in SRA01/04 cells. Zhang et al
(25) revealed that the silencing
of mammalian target of rapamycin significantly inhibits the
proliferation and migration of LEC. In the present study, the
proliferation and EMT process of cataracts were investigated at the
cellular level via a TGF-β2-induced cataract cell model; KCNQ1OT1
overexpression and suppression may affect the proliferation and EMT
in SRA01/04 cells in the presence of TGF-β2.
LncRNA KCNQ1OT1 is located at human chromosome
11p15.5 and is generally reported as an oncogene in a variety of
cancers types, including lung adenocarcinoma, hepatocellular
carcinoma and breast cancer (25–27).
Aberration of KCNQ1OT1 transcription is observed at a high
frequency in patients with colorectal cancers (28). Gong et al (29) demonstrated that the knockdown of
KCNQ1OT1 inhibits the proliferation and migration/invasive
abilities of glioma cells via microRNA-370/cyclin E2 pathway. Jin
et al (10) revealed that
KCNQ1OT1 expression levels are elevated in human cataract lens
anterior capsular samples and in SRA01/04 cell lines treated with
H2O2. In the present study, the expression
levels of KCNQ1OT1 were measured in the collected cataract tissue
specimens. A previous report demonstrated an elevation in KCNQ1OT1
expression levels in cataract tissue specimens (30). Ectogenic TGF-β2 is widely used in
cataract-associated research, particularly in LEC-associated EMT
(16,31,32).
In the same manner, the present study employed TGF-β2-treated
SRA01/04 cells to generate a cataract cell model. The expression
levels of KCNQ1OT1 were upregulated in the induced cataract cell
models, as reported in the present study, which indicated that
KCNQ1OT1 may function as an initiator in the formation of
cataracts. Additionally, using a constructed functional experiment,
the downregulation of KCNQ1OT1 inhibits SRA01/04 cell proliferation
and EMT, which are the key phases in cataract formation (19).
SMAD4 belongs to the SMAD family and is a critical
intracellular mediator in the TGF-β/SMAD4 signaling pathway. The
effects of SMAD4 on the mediation of proliferation and EMT have
been reported to be associated with cataractogenesis. Wang et
al (19) suggested that
microRNA-204-5p regulates EMT during human PCO by targeting SMAD4.
In a SMAD4 knockout mouse study, Li et al (33) revealed that loss of SMAD4
suppresses E-cadherin expression, but upregulates N-cadherin
expression, which is associated with congenital cataracts. In a
cataract study, Nahomi et al (18) suggested that LEC cells treated with
TGF-β2 present marked upregulation of SMAD4 and EMT-associated
markers. In the present study, the expression levels of SMAD4 in
cataracts and the function of KCNQ1OT1 on SMAD4 were investigated.
The results of the present study revealed that SMAD4 was
significantly upregulated in cataract tissue specimens and within
induced cataract cell models. Furthermore, the results indicated
that an increase and decrease of KCNQ1OT1 may correspondingly
regulate SMAD4 expression levels. In addition, via antisense
investigations, the present study proposed that SMAD4 was a
downstream target of KCNQ1OT1. The effects of KCNQ1OT1 on cell
proliferation and EMT were achieved via SMAD4 signaling. KCNQ1OT1
may promote cell proliferation and EMT via the regulation of SMAD4
in SRA01/04 cells.
In conclusion, the formation of age-associated
cataracts is an extremely complex issue associated with several
mechanisms and complex networks comprising of numerous molecules.
The findings of the present study provide a novel target and
insight into the pathogenesis of age-associated cataract.
Acknowledgements
The authors would like to thank Dr Xi Zhang from
Institute for Cardiovascular Prevention,
Ludwig-Maximilians-University for helping us prepare the
manuscript.
Funding
The present study was supported by grants from
National Natural Science Foundation of China (grant no. 81502333),
PhD Start-up Research Foundation of Liaoning Province (grant no.
201601225).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BC was responsible for the analysis and
interpretation of data of the manuscript; JM and CL were
responsible for statistical analysis; LZ was responsible for design
and drafting of the manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients for all clinical investigations conducted and the present
study was approved by the Institute Research Medical Ethics
Committee of 4th People's Hospital of Shenyang (Shenyang,
China).
Consent for publication
Written informed consent was obtained.
Conflict of interest
The authors declare that they have no competing
interests.
References
|
1
|
Cruciani F, Amore F, Albanese G and
Anzidei R: Investigation about causes of blindness and low vision
among members of Blind and Visually Impaired Italian Union (UICI).
Clin Ter. 162:e35–e42. 2011.PubMed/NCBI
|
|
2
|
Liu T, Zhang L, Wang Y, Zhang H, Li L and
Bao X: Dickkopf-1 inhibits Wnt3a-induced migration and
epithelial-mesenchymal transition of human lens epithelial cells.
Exp Eye Res. 161:43–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Michael R and Bron AJ: The ageing lens and
cataract: A model of normal and pathological ageing. Philos Trans R
Soc Lond B Biol Sc. 366:1278–1292. 2011. View Article : Google Scholar
|
|
4
|
Zeng K, Feng QG, Lin BT, Ma DH and Liu CM:
Effects of microRNA-211 on proliferation and apoptosis of lens
epithelial cells by targeting SIRT1 gene in diabetic cataract mice.
Biosci Rep. 37:pii: BSR20170695. 2017. View Article : Google Scholar
|
|
5
|
Zhang L, Wang Y, Li W, Tsonis PA, Li Z,
Xie L and Huang Y: MicroRNA-30a regulation of
epithelial-mesenchymal transition in diabetic cataracts through
targeting SNAI1. Sci Rep. 7:11172017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu H, Zhen Q and Fan Y: LncRNA GHET1
promotes esophageal squamous cell carcinoma cells proliferation and
invasion via induction of EMT. Int J Biol Markers. 32:e403–e408.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen Y, Dong LF, Zhou RM, Yao J, Song YC,
Yang H, Jiang Q and Yan B: Role of long non-coding RNA MIAT in
proliferation, apoptosis and migration of lens epithelial cells: A
clinical and in vitro study. J Cell Mol Med. 20:537–548. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Zhang Y, Yang T, Zhao W, Wang N,
Li P, Zeng X and Zhang W: Long non-coding RNA MALAT1 for promoting
metastasis and proliferation by acting as a ceRNA of miR-144-3p in
osteosarcoma cells. Oncotarget. 8:59417–59434. 2017.PubMed/NCBI
|
|
9
|
Ren K, Xu R, Huang J, Zhao J and Shi W:
Knockdown of long non-coding RNA KCNQ1OT1 depressed chemoresistance
to paclitaxel in lung adenocarcinoma. Cancer Chemother Pharmacol.
80:243–250. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin X, Jin H, Shi Y, Guo Y and Zhang H:
Long Non-Coding RNA KCNQ1OT1 Promotes Cataractogenesis via miR-214
and Activation of the Caspase-1 Pathway. Cell Physiol Biochem.
42:295–305. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang B, Chen Y, Qiu M and Ding Z: Long
noncoding RNA expression profile in HLE B-3 cells during TGF-
β2-induced epithelial-mesenchymal transition. BMC
Ophthalmol. 17:692017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Yang T, Zhang Z, Lu M, Zhao W,
Zeng X and Zhang W: Long non-coding RNA TUG1 promotes migration and
invasion by acting as a ceRNA of miR-335-5p in osteosarcoma cells.
Cancer Sci. 108:859–867. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lv J, Fan HX, Zhao XP, Lv P, Fan JY, Zhang
Y, Liu M and Tang H: Long non-coding RNA Unigene56159 promotes
epithelial-mesenchymal transition by acting as a ceRNA of
miR-140-5p in hepatocellular carcinoma cells. Cancer Lett.
382:166–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Yang T, Liu Y, Zhao W, Zhang Z, Lu
M and Zhang W: Decrease of miR-195 promotes chondrocytes
proliferation and maintenance of chondrogenic phenotype via
targeting FGF-18 pathway. Int J Mol Sci. 18:pii: E975. 2017.
|
|
16
|
Nguyen K, Sparks J and Omoruyi FO:
Investigation of the cytotoxicity, antioxidative and
immune-modulatory effects of Ligusticum porteri (Osha) root extract
on human peripheral blood lymphocytes. J Integr Med. 14:465–472.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Iongh RU, Wederell E, Lovicu FJ and
McAvoy JW: Transforming growth factor-beta-induced
epithelial-mesenchymal transition in the lens: A model for cataract
formation. Cells Tissues Organs. 179:43–55. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nahomi RB, Pantcheva MB and Nagaraj RH:
αB-crystallin is essential for the TGF-β2-mediated epithelial to
mesenchymal transition of lens epithelial cells. Biochem J.
473:1455–1469. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Li W, Zang X, Chen N, Liu T,
Tsonis PA and Huang Y: MicroRNA-204-5p regulates
epithelial-to-mesenchymal transition during human posterior capsule
opacification by targeting SMAD4. Invest Ophthalmol Vis Sci.
54:323–332. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lovicu FJ, Schulz MW, Hales AM, Vincent
LN, Overbeek PA, Chamberlain CG and McAvoy JW: TGFbeta induces
morphological and molecular changes similar to human anterior
subcapsular cataract. Br J Ophthalmol. 86:220–226. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lovicu FJ, Shin EH and McAvoy JW: Fibrosis
in the lens. Sprouty regulation of TGFβ-signaling prevents lens EMT
leading to cataract. Exp Eye Res. 142:92–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen X, Xiao W, Chen W, Liu X, Wu M, Bo Q,
Luo Y, Ye S, Cao Y and Liu Y: MicroRNA-26a and −26b inhibit lens
fibrosis and cataract by negatively regulating Jagged-1/Notch
signaling pathway. Cell Death Differ. 24:1431–1442. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu X, Ruan J, Ma B and Luo M: Bit1-a
potential positive regulator of epithelial-mesenchymal transition
in lens epithelial cells. Graefes Arch Clin Exp Ophthalmol.
254:1311–1318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu L and Xiao W: Notch1 signaling induces
epithelial-mesenchymal transition in lens epithelium cells during
hypoxia. BMC ophthalmology. 17:1352017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang C, Liu J, Jin N, Zhang G, Xi Y and
Liu H: SiRNA Targeting mTOR effectively prevents the proliferation
and migration of human lens epithelial cells. PLoS One.
11:e01673492016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiao W, Chen X, Li W, Ye S, Wang W, Luo L
and Liu Y: Quantitative analysis of injury-induced anterior
subcapsular cataract in the mouse: a model of lens epithelial cells
proliferation and epithelial-mesenchymal transition. Sci Rep.
5:83622015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rodriguez BA, Weng YI, Liu TM, Zuo T, Hsu
PY, Lin CH, Cheng AL, Cui H, Yan PS and Huang TH: Estrogen-mediated
epigenetic repression of the imprinted gene cyclin-dependent kinase
inhibitor 1C in breast cancer cells. Carcinogenesis. 32:812–821.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sunamura N, Ohira T, Kataoka M, Inaoka D,
Tanabe H, Nakayama Y, Oshimura M and Kugoh H: Regulation of
functional KCNQ1OT1 lncRNA by β-catenin. Sci Rep. 6:206902016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gong W, Zheng J, Liu X, et al: Knockdown
of Long Non-Coding RNA KCNQ1OT1 Restrained Glioma Cells' Malignancy
by Activating miR-370/CCNE2 Axis. Frontiers in cellular
neuroscience. 11:842017.28. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wan J, Huang M, Zhao H, Wang C, Zhao X,
Jiang X, Bian S, He Y and Gao Y: A novel tetranucleotide repeat
polymorphism within KCNQ1OT1 confers risk for hepatocellular
carcinoma. DNA Cell Biol. 32:628–634. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang KC and Petrash JM: Aldose reductase
mediates transforming growth Factor β2 (TGF-β2)-induced migration
and epithelial-to-mesenchymal transition of lens-derived epithelial
cells. Invest Ophthalmol Vis Sci. 56:4198–4210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shu DY, Wojciechowski MC and Lovicu FJ:
Bone morphogenetic protein-7 suppresses TGFβ2-induced
epithelial-mesenchymal transition in the lens: Implications for
cataract prevention. Invest Ophthalmol Vis Sci. 58:781–796. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li J, Qin Y, Zhao FK, Wu D, He XF, Liu J,
Zhao JY and Zhang JS: Anterior segment dysgenesis correlation with
epithelial-mesenchymal transition in Smad4 knockout mice. Int J
Ophthalmol. 9:943–947. 2016.PubMed/NCBI
|