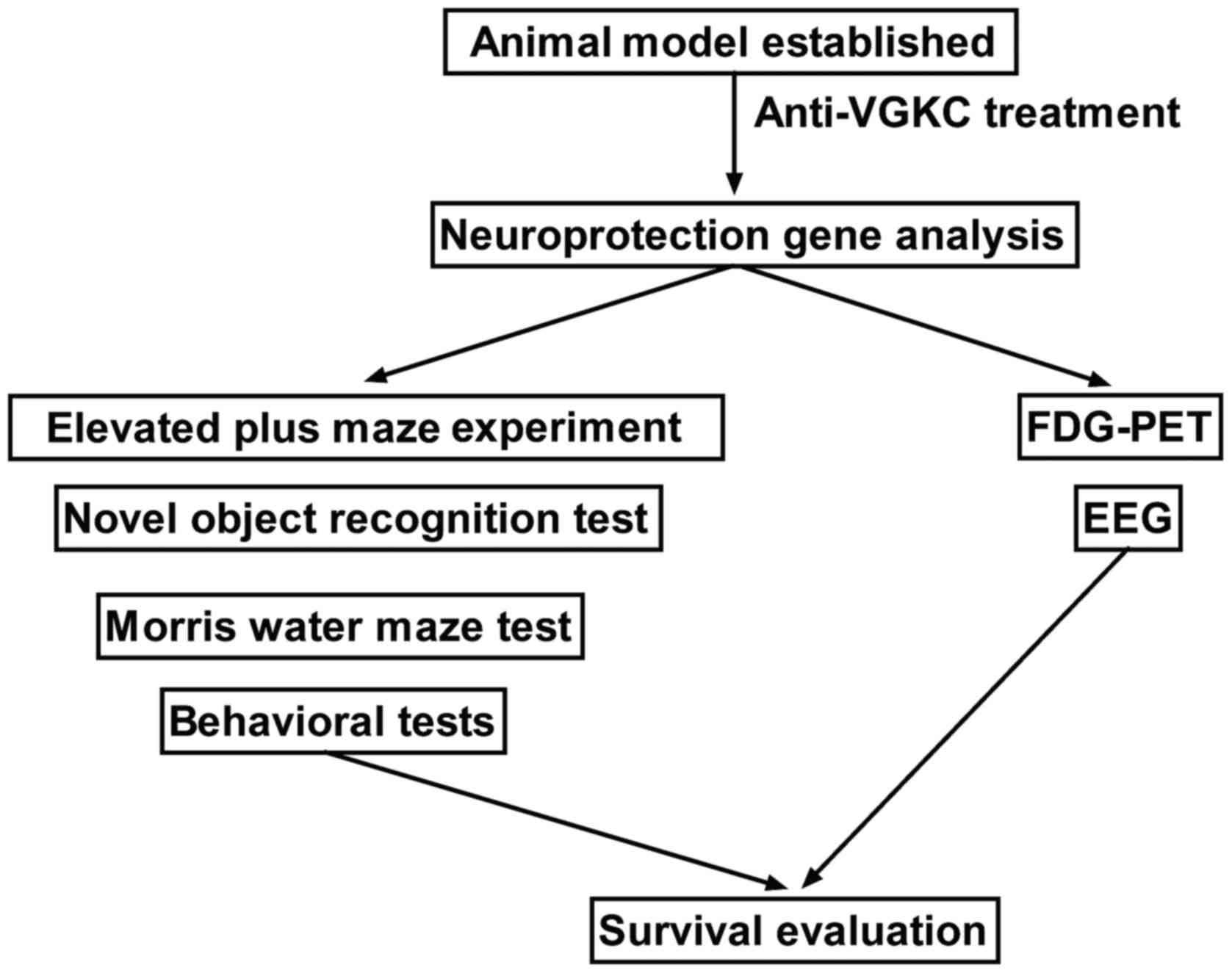
Introduction
Epilepsy is a common neurological disorder disease,
which affects more than 50 million populations in the developing
country (1,2). Though several new antiepileptic drugs
have emerged over the past years, the incidence of refractory
epilepsy remains high and seizure freedom eludes many patients with
epilepsy (3–5). Epidemiological data has indicated
that more than twenty percent of newly diagnosed patients are
translating into refractory epilepsy every year (6,7).
Many factors affect the treatment of epilepsy including
environmental factors and genetic factors (8,9). In
addition, patients with refractory epilepsy present neuronal loss
in hippocampus, which is a common pathogenetic condition (10). Therefore, target therapy for
molecular in hippocampus may be beneficial for the treatment of
patient with refractory epilepsy (11–13).
Furthermore, previous reports have made endeavor to understand the
potential molecular mechanism of refractory epilepsy (13–15).
However, exploring more and more target drug therapies are still
required for refractory epilepsy patients.
Currently, various antibodies targeting of neuronal
proteins have presented therapeutic effects for epilepsy patients
(16,17). Voltage gated potassium channel
complex (VGKC) involves in epilepsy and is associated with memory
impairment, anxiety, visual attention and inhibitory control in
nerve area (18,19). Study has reported that VGKC complex
nerve hyperexcitability and limbic encephalitis was associated with
the spectrum of immunotherapy-responsive clinical features in
patients with epilepsy (20). VGKC
antibody-associated encephalopathy was reported and conclusion
exhibited psychotic disorder cause by VGKC (21). Although substantial evidences upon
recovery are investigated after immunotherapy, residual amnestic
deficits is frequently occurred in patients with epilepsy (22).
Many studies have shown that VGKC antibodies
directly neutralized neuronal antigens on cell surface in patients
with epilepsy, suggesting Anti-VGKC is specificity for molecules in
epilepsy. Some patients with epilepsy exhibited higher
autoantibodies of VGKC-complex (23,24).
Therefore, this study investigated the efficacy of Anti-VGKC on
motor attention impairment, cognitive competence, impulsivity,
compulsivity and seizures in a subpopulation of neuroprotected
mice. A recent study of the therapeutic effects of Anti-VGKC
immunotherapy on cognitive improvement and neuronal loss has
indicated that Anti-VGKC was beneficial for faciobrachial dystonic
seizures, which contributed to motor attention impairment and
potentially treatable disorder (25). However, these reports only studied
benefits of VGKC-complex antibody for limbic encephalitis, but not
analyzed the anxiety, visual attention and inhibitory control.
Also, few preclinical studied the fundamental mechanism and
therapeutic effects of Anti-VGKC on epilepsy and cognitive
competence, impulsivity, compulsivity and seizures in a
subpopulation of neuroprotected rats were limited to the few
preceding reported (26).
In the present study, the effects of Anti-VGKC on
the anticonvulsant activity of seizure control and improvements of
cognitive function have been evaluated in models of epilepsy. A
series of experiments were performed to analyze the therapeutic
effects of Anti-VGKC for models of epilepsy mice (Fig. 1). Our data presented that cognitive
impairment was relieved after immunotherapy of Anti-VGKC. In
addition, although mice brain PET has shown that evidence of
hippocampal atrophy was associated with progress to epilepsy
observed in mice with epilepsy, the primary of hippocampal signal
changes seldom reported in previous study. In this study, we
undertook experiments to investigate the role of Anti-VGKC in mice
model of lithium-pilocarpine temporal lobe epilepsy. Further
evaluation of more preclinical data is essential to understand the
potential impact of Anti-VGKC interactions on efficacy,
tolerability, and dosing of new antiepileptic drug.
Materials and methods
Ethical approval and participant
consent
The present study was approved by the Ethics
Committee of Provincial Hospital affiliated to Shandong University
(ref. 11/H1011/102). All surgery and euthanasia of experimental
mice were performed under sodium pentobarbital anesthesia to make
minimize suffering.
Animals
A total of 20 J20 mice (eight weeks old, 24–32 g
body weight) were purchased from the Chinese Academy of Sciences
Institute of Biophysics (Beijing, China). All mice were housed at
preference temperature (22–24°C) under a 12 h light-dark cycle and
free accessed food and water. Mice were divided into two groups
(n=10 in each group). The epileptic mice were treated with
intravenous injection for 30 days of 0.24 mg/kg Anti-VGKC or
vehicle of PBS once a day for a total of 30 days.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was obtained from peripheral blood
mononuclear cells and hippocampus cells by using RNAeasy mini kit
(Qiagen Sciences, Inc., Gaithersburg, MD, USA). A total of 1 µg
total RNA was reverse transcribed into cDNA. One-tenth of the cDNA
was subjected to qPCR using an iQ SYBR-Green system. All the
primers were synthesized by Invitrogen; Thermo Fisher Scientific,
Inc. (Waltham, MA, USA). Primers sequences were as follow: VGKC,
forward, 5′-CGCAGAACTTTCATTCTTTGGAC-3′; reverse,
5′-CTGGGCAAGTTTCAATAGGAGA-3′; Foxp2, forward,
5′-AACAGAGACCACTGCAGGTGCC-3′; reverse, 5′-TCCCTGACGCTGAAGGCTGAG-3′;
SxIP, forward, 5′-GTGCAGTTGAGGTCCTTTCG-3′; reverse,
5′-GTCAGGAACAAACCCAGCTG-3′; EB, forward,
5′-GGTACAGGTGTGGTTCCAGA-3′; reverse, 5′-CTGGAGGGTGTCTGGAAGAG-3′;
β-actin, forward, 5′-CGGAGTCAACGGATTTGGTC-3′; reverse,
5′-AGCCTTCTCCATGGTCGTGA-3′. PCR following thermocycling conditions
were performed: 40 amplification cycles consisting of denaturation
at 95°C for 5 min, primer annealing at 55°C for 20 sec with
touchdown to 56°C for 20 sec, and applicant extension at 72°C for 5
min. Relative mRNA expression changes were calculated by
2−ΔΔCq (27). The
results are expressed as the n-fold way compared to β-actin.
Enzyme Linked Immunosorbent Assay
(ELISA) analysis
VGKC proteins ELISA kits (cat. no. CSB-E13512h;
Cusabio Biotech Co., Ltd., Houston, USA) were used to determine
serum and cerebrospinal fluid concentration levels of the VGKC. The
operating steps were conducted according to the manufacturer's
instructions. The results were analyzed using an ELISA reader
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Electroencephalography (EEG)
All mice treatment with VGKC or PBS underwent
conventional 19-channel scalp EEG examination. On day 30,
experimental mice were anesthetized to during the measurement. EEG
was measured starting from the recovery of movement and data were
recorded continuously for 30 min using the 10–20 system (EGI, Inc.,
Eugene, OR, USA) according to manufacturer's instructions.
Positron emission tomography imaging
(FDG-PET)
FDG-PET was used to analyze the discharge sites by
using statistical parametric mapping (SPM) software (SPM v2) and
identified significant regions of cerebral neurons. FDG-PET images
were spatially normalized onto the MNI (Montreal Neurological
Institute, McGill University, Montreal, Canada) PET brain template
which defined regions of interest. Normalized images were smoothed
by convolution with a 10 mm FWHM Gaussian kernel to increase the
signal to noise ratio. Detailed procedures for FDG-PET acquisition
and image processing have been described in previous study
(28).
Behavioral tests
On day 30, cognitive competence of mice was
determined by the open field activity levels that a 60×60×25 cm
black plexiglas box (length × width × height) was used to analyzed
therapeutic effects of Anti-VGKC for epilepsy mice. Experimental
mice were placed to the open black box 15 min and the behaviors
were monitored and evaluated using an auto-tracking system (v3.2;
SmarTrack; Smart Solutions, Inc., Madison, WI, USA). The 5-choice
serial reaction time task (5-CSRTT) was used to evaluate a mice who
exhibited beneficial performance according to previous study
(29).
Morris water maze test
The path efficacy was analyzed using Morris water
maze test at prior and post treatment of Anti-VGKC and control.
Briefly, experimental mice were placed a circular stainless-steel
tank with 155×60 cm (diameter × depth). Tank was filled with 40 cm
water and skim milk at 27.0±1.0°C. Subsequently, the path efficacy
in learned to find a hidden circular platform was observed in
experimental mice.
Novel object recognition test
The novel object recognition test was performed in a
50×50×49 cm (length × width × height) white plexiglass box prior
and post treatments. On day 30, experimental mice (n=4) in
Anti-VGKC and control groups were subjected to a training program
that all mice were exposed to two of the same objects. We recorded
the exploration time for all mice. Twenty-four hours later, mice
were placed back in white plexiglass box to recognize a novel
object in the same place. The times in searching the novel objects
were measured and recorded for accessing the spatial cognitive
ability of the mice.
Experiment of elevated plus maze
Coordinating behavior, spiking frequency and escape
latency of mice were evaluated using an elevated plus maze trial
based on the hypothesis that mice exhibited fear of open field. The
details of the elevated plus maze trial were as described
previously (30), with the space
the mice were subjected to increased three times to a size of
100×20×80 (length × width × height) cm. The experimental mice with
schizophrenia were placed into the center of the elevated plus
maze. The mice with schizophrenia were made to face the open arm
for a total of 5 min. The time spent in the open and closed arms
was recorded and calculated by employing the formula: D2=(B-A)/(B +
A). A, represented the time spent in the open arm; B, represented
the time spent in the closed arm; and D2, represented the
discrimination index. Anxious behavior was measured using the
aforementioned formula.
Rankin score and Status Epilepticus
Severity Score (STESS)
The ability of Rankin score and STESS was evaluated
as described previously (31,32).
STESS' ability was used to predefine outcome of Anti-VGKC for mice
with epilepsy. Rankin score was used to analyze the degree of
nervous functional defects of epilepsy mice.
Histologic analysis
Hippocampus was isolated from experimental mice as
described previously (33).
Paraffin-embedded tissue sections were prepared and epitope
retrieval was performed for further analysis. The paraffin sections
were incubated with hydrogen peroxide (3%) for 15 min at 37°C.
Tissue sections were stained with hematoxylin and eosin (5%) for 1
h at 37°C. After PBST three washes, tissue sections observed using
a light microscope (Olympus BX51; Olympus Corporation, Tokyo,
Japan).
Immunofluorescence
Hippocampal tissue was cut into 4 µm thick sections
and mounted on glass slides. The paraffinized sections were heated
in an oven at 65°C for 24 h, dewaxed to water and rinsed with PBS
three times. The washed sections were placed in EDTA buffer (Beinuo
Bioscience Inc., Shanghai, China), and then boiled at a low heat
following an interval of 10 min at 65°C for a total of three
intervals. Following natural cooling, the sections were washed with
PBS three times, and were placed into 3% hydrogen peroxide solution
(Beina Bioscience Inc.) and washed with PBS three times. Tissue
sections were incubated with rabbit anti-mouse monoclonal
antibodies: Transcripts of forkhead-BOX P2 (Foxp-2) (ABE73), SxIP
(ABE86) and microtubule end binding (EB) (ABC467, all 1:2,000
dilutions; all EMD Millipore; Billerica, MA, USA) at 4°C for 12 h.
After rinsing, sections were incubated a with DyLight488 or
DyLight650-conjugated secondary antibody (1:5,000; Pierce
Biotechnology; Thermo Fisher Scientific, Inc.) at 37°C for 2 h. The
sections were then washed with PBS and observed by fluorescent
video microscopy (BZ-9,000; Keyence Corporation, Osaka, Japan).
Statistical analysis
All data were expressed as mean ± standard
deviation. All experiments were repeated at least three times.
Statistical analysis was performed using GraphPad software 5.0
(GraphPad Software, Inc., La Jolla, CA, USA). Statistical
differences were analyzed by using student t tests or one-way
analysis of variance followed by Tukey HSD test. *P<0.05,
**P<0.01 were considered to indicate a statistically significant
difference.
Results
In vivo therapeutic effects of
Anti-VGKC in mice model of epilepsy
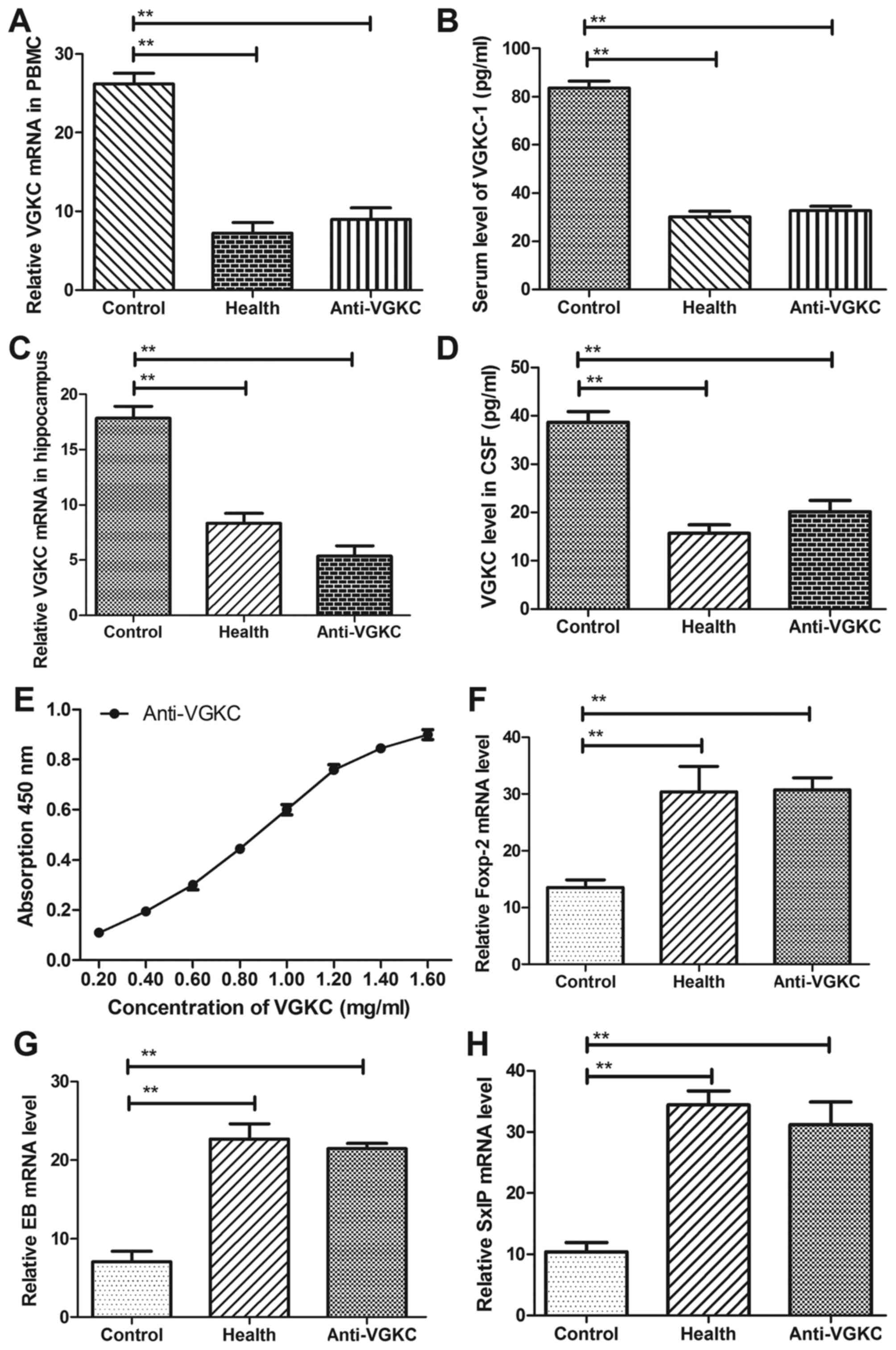
In order to investigate the expression of VGKC in
mice model of epilepsy, we analyze the concentration level of VGKC
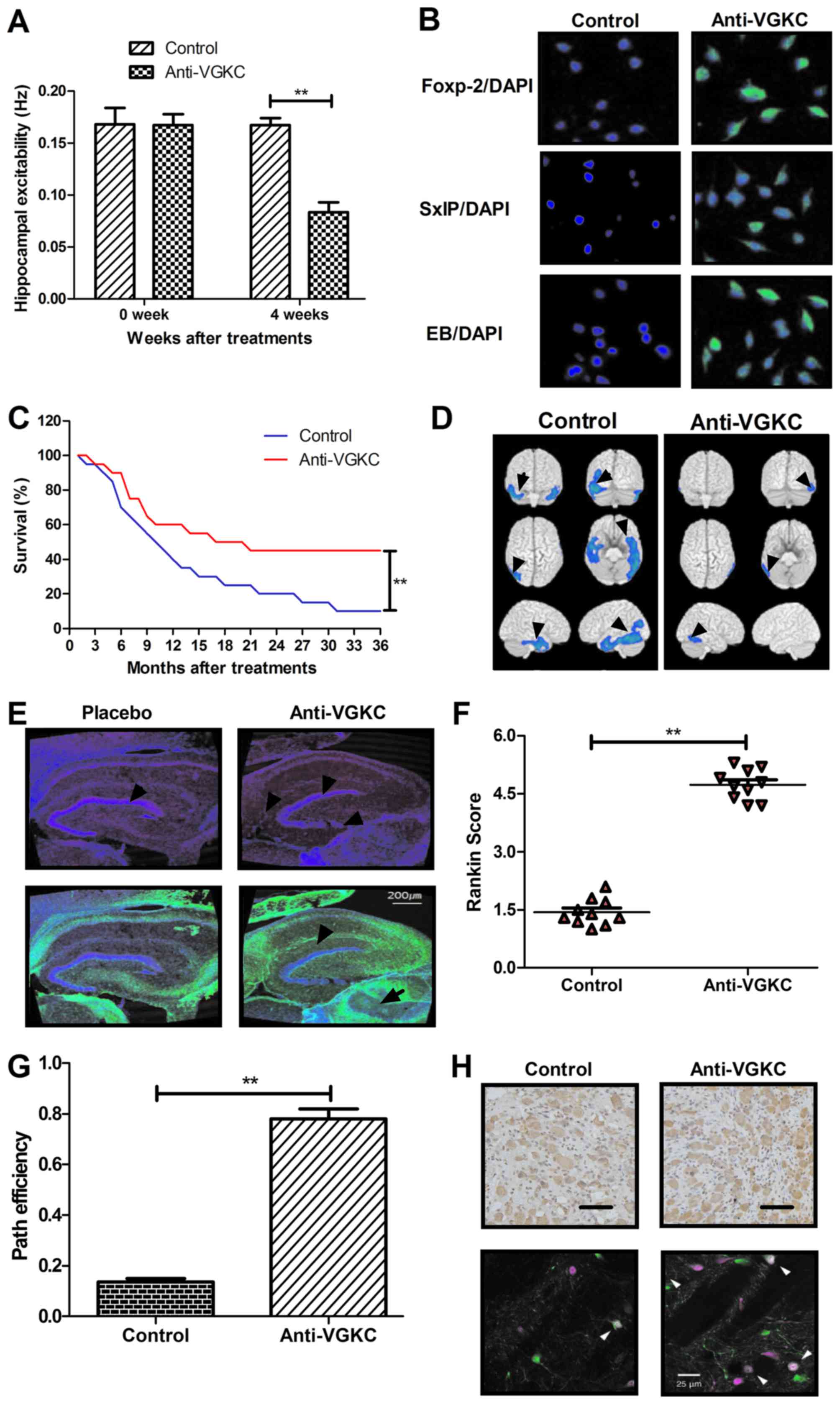
in cerebrospinal fluid (CSF) and serum. The results in Fig. 2A and B, we showed that VGKC was
up-regulated and Anti-VGKC decreased mRNA level of VGKC in
peripheral blood mononuclear cells (PBMC) and serum. We
demonstrated that Anti-VGKC down-regulated VGKC mRNA levels in
hippocampus cells and cerebrospinal fluid (CSF) (Fig. 2C and D). Our data in Fig. 2E exhibited that Anti-VGKC presented
a higher affinity with VGKC, which indicated Anti-VGKC could
decrease the concentration level of VGKC in mice with epilepsy. In
addition, several proteins related cognitive competences were
evaluated in hippocampus cells in mice with epilepsy. The results
in Fig. 2F-H showed that mRNA of
Foxp-2, SxIP and EB were down-regulated in hippocampus cells in
mice with epilepsy. These data suggested VGKC were up-regulated and
neuroprotective gene were down-regulated in mice subjected
epilepsy.
Performance of epilepsy mice after
treatment with Anti-VGKC
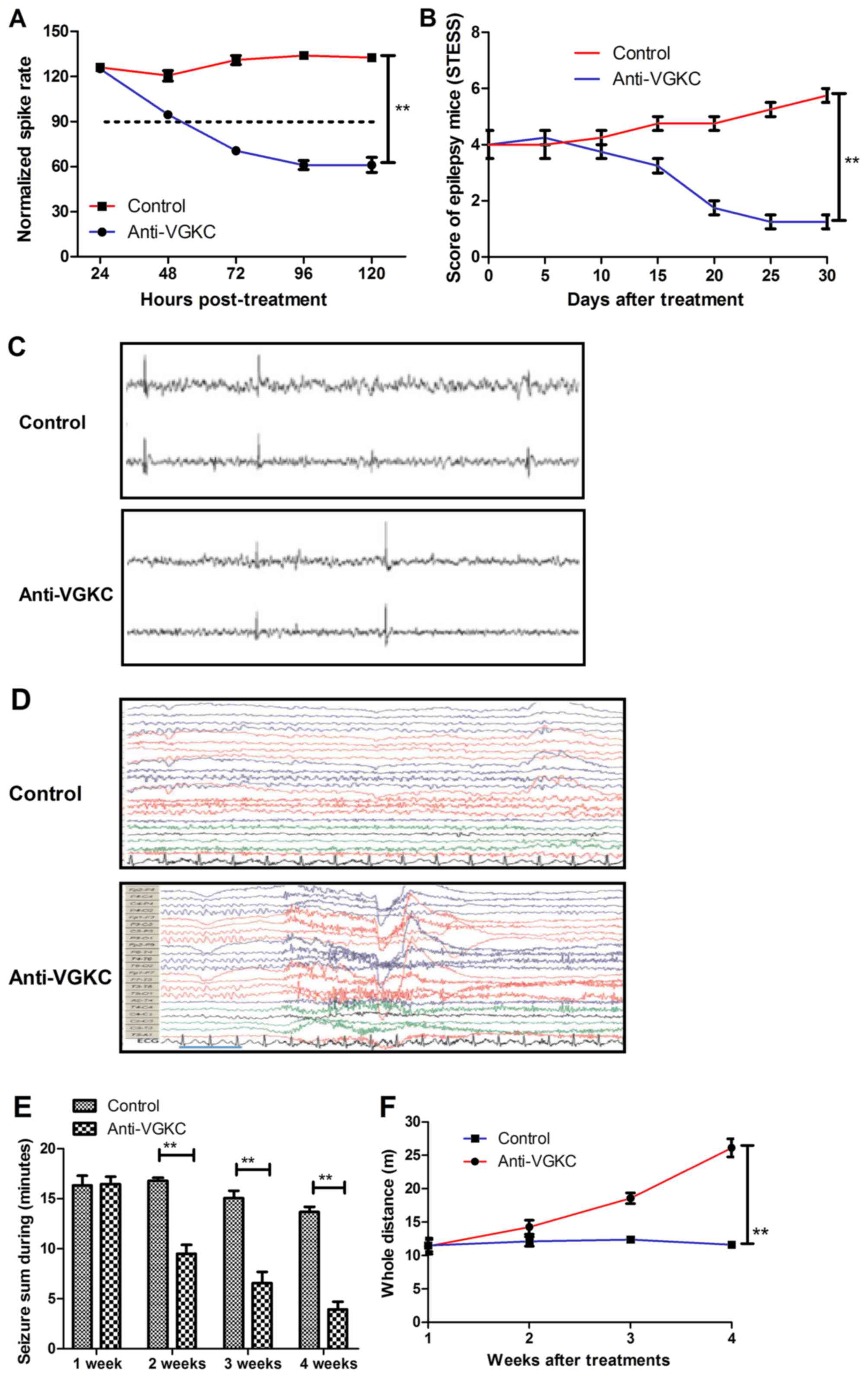
We further analyzed the short therapeutic effects of
Anti-VGKC in epilepsy mice in a four-week treatment period. Our
data in Fig. 3A showed that
Anti-VGKC markedly decreased the spike discharge rate compared to
control mice. The 5-choice serial reaction time task (5-CSRTT) was
used to evaluate the mice who exhibited beneficial performance
corresponding to standard testing conditions. The results in
Fig. 3B showed that Anti-VGKC
treatment improved behaviors of mice with epilepsy scored by STESS.
As shown in Fig. 3C, Anti-VGKC
treatment improved cortical spiking of EEG compared to control. We
demonstrated that time-frequency (Morlet wavelets) power spectra in
epilepsy mice were markedly improved by Anti-VGKC treatment
(Fig. 3D). We also showed that
Anti-VGKC treatment decreased the total seizure duration and
increased the whole traveled distance in the open field activity
test compared to control (Fig. 3E and
F). These results indicate that Anti-VGKC can improve behaviors
and discharge activity of epilepsy mice.
Effect of Anti-VGKC on cortical
epileptiform spiking and neuronal remission in vivo in mice model
of epilepsy
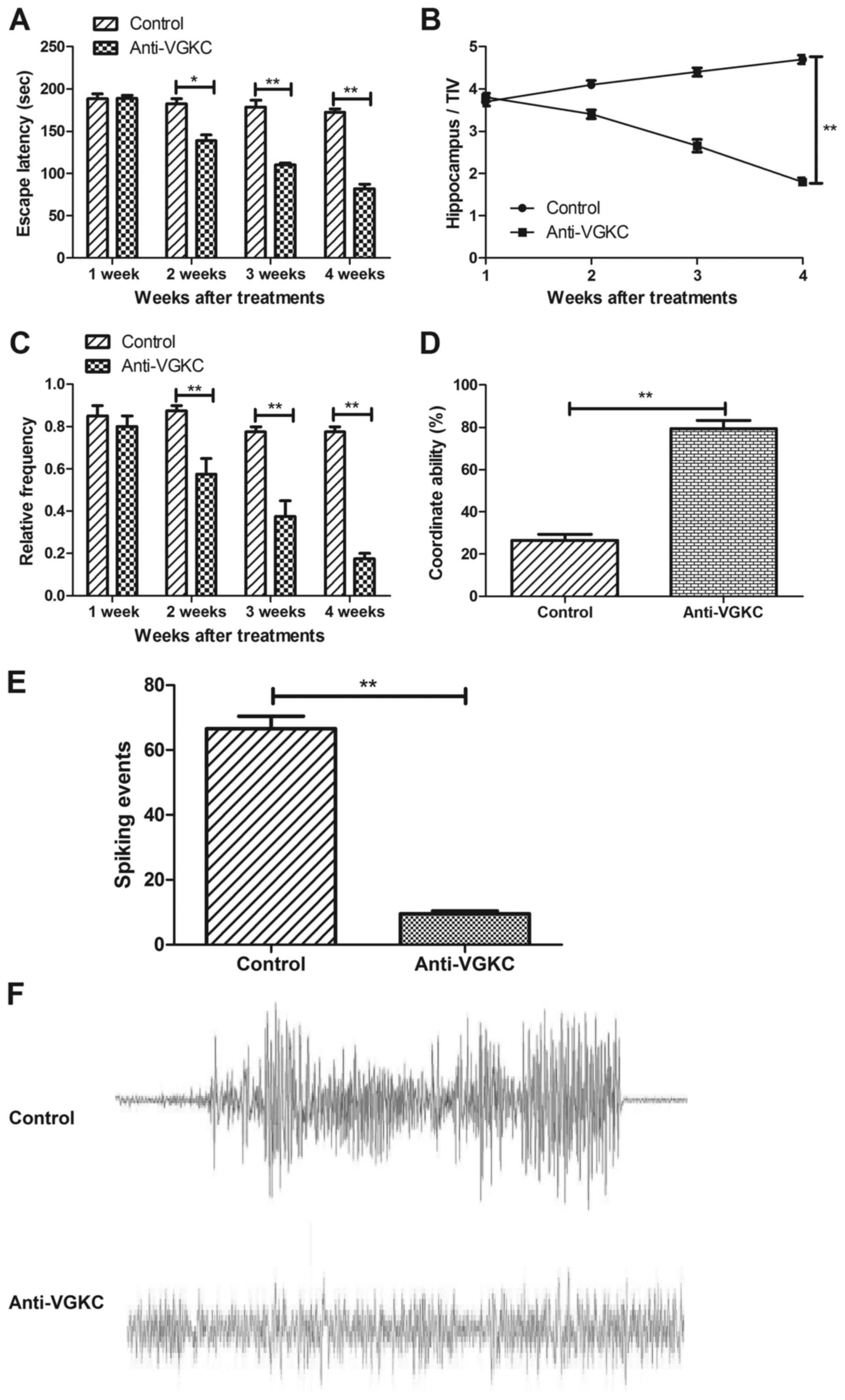
In order to determine whether the effects of
Anti-VGKC were beneficial on cortical epileptiform spiking and
neuronal remission in vivo in mice model of epilepsy,
interictal spike rate and neuronal remission were assessed in the
presence of Anti-VGKC in epilepsy mice model. Our data in Fig. 4A presented that the Anti-VGKC
improved pronounced epileptic phenotype by observation of water
maze path efficiency data. Anti-VGKC treatment significantly
reduced the spike discharge rate compared to control in mice with
epilepsy (Fig. 4B, Bonferroni
post-hoc, P<0.01). The frequency of seizures in mice treated
with Anti-VGKC was significantly reduced from 1.46±0.35 seizures
for vehicle to 0.31±0.08 seizures for Anti-VGKC (P<0.01, t-test,
Fig. 4C). We also found that mice
treated with Anti-VGKC exhibited significant difference in
coordinating behavior and spiking frequency of hippocampus between
Anti-VGKC and control group (Fig. 4D
and E). Also, the results in Fig.
4F indicated that the coordinate ability was significantly
improved by Anti-VGKC compared to control (One-way ANOVA,
P<0.01). These data indicated that Anti-VGKC was beneficial for
epileptiform spiking and neuronal remission in vivo in mice
model of epilepsy.
Anti-VGKC exhibits efficient effects
on cognitive competence
To examine the efficacy of Anti-VGKC on network
excitability, hippocampal slices from experimental mice were
investigated in vitro. We demonstrated that Anti-VGKC
treatment decreased the spike rate compared to control group
(Fig. 5A). The immunohistochemical
analysis of temporal lobe demonstrated that Foxp-2, SxIP and EB
expression levels were up-regulated in brain in Anti-VGKC-treated
mice (Fig. 5B). To investigate the
long circulating carrier of Anti-VGKC, 36-month observation was
conducted and survival rate was also recorded in this work. We
showed that survival rate was relative higher in Anti-VGKC-treated
mice with epilepsy as vehicle-treated as control (Fig. 5C). In addition, the discharge sites
were analyzed by using FDG-PET at pre-surgery and post-surgery
(Fig. 5D). Furthermore, thionin
staining of hippocampi area in Anti-VGKC and control groups
demonstrated significantly difference in dispersion of the
pyramidal cell layer (Fig. 5E). At
last, the cognitive competence and anxiety were evaluated after
Anti-VGKC treatment in mice with epilepsy. As shown in Fig. 4F, results indicated Anti-VGKC
treatment improved cognitive competences determined by Rankin
score. We demonstrated that Anti-VGKC treatment significantly
improved cognitive competence analyzing by path efficiency assay
(Fig. 5F). Anti-VGKC treatment
improved path efficiency of experimental mice compared to control
(Fig. 5G). Anti-VGKC treatment
also decreased the number of damaged neurons in hippocampus
analyzed by histology and immunofluorescence (Fig. 5H). Taken together, the beneficial
efficacy of Anti-VGKC showed significant and rapid effects on
circuit excitability, neuroprotective protein and cognitive
competences in vitro and in vivo, which also
presented the action of Anti-VGKC for inhibition in neuronal
hyperexcitability.
Discussion
Currently, drug therapies for preventing or treating
epilepsy have been extensively used in clinic (34). Numerous factors contribute to the
occurrence of epilepsy. As the worse environment pollution, more
and more epilepsy patients occurred and appeared drug resistance
(35). Therefore, comprehensive
treatment for patients with epilepsy is critical in clinical
therapy. This study demonstrates that the presence of Anti-VGKC
(0.24 mg) showed an efficient therapeutic approach in epileptic
mice by intravenous injection, which is consistent with previous
clinical reports and demonstrates better curative effects (20,36).
In addition, target therapy and immunotherapy of Anti-VGKC are more
compatible used in treatment of patients with epilepsy more than
other antiepileptic drugs that show more efficient for patients
with epilepsy (22,35,37).
The present study showed that mice with epilepsy treatment with
Anti-VGKC led to the therapeutic effects in preventing cognitive
impairment and decreasing of neuronal loss in hippocampus.
Furthermore, in this study, we not only demonstrated the impact of
antiepileptic drug of Anti-VGKC on the efficacy and safety, but
also provided a therapeutic schedule for clinic treatment of
epilepsy. These are consistent intellectual efficacy, which have
VGKC excessive expression in patients with epilepsy (38).
Previous study has reported that VGKC-complex
antibodies were presented in patients with peripheral nerve
Morvan's syndrome, hyperexcitability, epilepsy and limbic
encephalitis (18,39). Although Radja et al
(37) has showed that limbic
encephalitis is usually caused by autoimmune neuropsychiatric
condition. In particular, published study has also reported
variable responses to immunosuppressive therapy in VGKC-complex
antibody and their results that suggested VGKC-complex antibody
showed significant improvements of epilepsy (37).
Here, our study found that the most significant
improvements of Anti-VGKC treatment were demonstrated by epileptic
mice presenting with cognitive impairment, neuron increasing and
consistent neuroradiological changes. These observations
illustrated the importance of Anti-VGKC for seizures frequency and
improved epileptic phenomena in a 30-day treatment period. In
addition, the inhibitory activity for hyperexcitability of neuron
in mice model of epilepsy has not well understood (40,41).
This study clarified that hyperexcitability of neuron was aberrant.
Furthermore, recent evidences in published studies have identified
an essential role for impaired cortical interneurons and seizure
localization of epilepsy was authenticated in mice model of
epilepsy (42–44). Localization-related epilepsy was
presumed to occur in a discrete cortical area in brain. Though
identify the localization-related epilepsy is slight significance
for control of seizures by epilepsy surgical operation, it was
essential for doctors and clinicians in research and development of
drug target therapy (45,46). In this study, we have investigated
the localization-related epilepsy in mice model of J20 and
evaluated the efficacy of Anti-VGKC. We also indicated that
discharge sites in hippocampus was related the seizures
frequency.
Currently, response-element binding
protein-dependent genes expression is significant difference
between in temporal lobe epileptic mice hippocampus and healthy
mice (47). Foxp-2, SxIP and EB
expression levels that owned neuroprotection has investigated and
showed a decreasing trend in mice with epilepsy in previous study
(48,49). In addition, a study has showed that
the NAP (NAPVSIPQ) sequence of activity-dependent neuroprotective
protein (ADNP) contains the SxIP motif, EB protein target, which is
critical for microtubule dynamics leading to synaptic plasticity
and neuroprotection in a schizophrenia mouse model (30). In this work, our design
investigated Foxp-2, SxIP and EB expression levels prior and post
treatment of Anti-VGKC. Though basal mRNA and protein owned
neuroprotective protein are surprisingly frequently observed in
epileptic mice, the changes of these neuroprotective protein did
not studied in temporal lobe epilepsy of mice (50). Importantly, we studied changes of
Foxp-2, SxIP and EB expression levels that owned neuroprotection in
epileptic mice prior and post treatment with Anti-VGKC. These
neuroprotective protein affected seizure frequency, cognitive
impairment, anxiety and EEG observed in the most of events
(51). These observations were
most consistent with hippocampus seizure involvement and EEG.
Interestingly, Anti-VGKC was not only significantly relieved
hippocampus excitability and prolonged survival of epilepsy-bearing
mice, but also presents a potential anti-epilepsy efficacy in path
efficiency (52). Our data
confirmed these therapeutic effects of Anti-VGKC in epilepsy
mice.
In conclusion, the finding in the present study
demonstrated that Anti-VGKC treatment not only improved cognitive
impairment, but also repaired impaired neurons. We further
investigated VGKC expression in serum and CSF. We showed that
improvement of cognitive effects on mice with a model of
intractable epilepsy after Anti-VGKC treatment. However,
significant survival period, cognitive and behavior improvements
were shown, which are major indexes for mice with intractable
epilepsy. It is therefore important that plerosis of injured
midbrain neuron, cognitive impairment, decreasing of seizures of
Anti-VGKC, are investigated so that more trails upon Anti-VGKC
treatments should be explore to explain mechanism of these
observations.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analysed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
SY designed the present study. ZlF and XF performed
the experiments, and ZgF and XZ analysed the experimental data. The
final version of the manuscript was read and approved by all
authors.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Provincial Hospital Affiliated to Shandong University
(Shandong, China; ref. 11/H1011/102).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Allers K, Essue BM, Hackett ML, Muhunthan
J, Anderson CS, Pickles K, Scheibe F and Jan S: The economic impact
of epilepsy: A systematic review. BMC Neurol. 15:2452015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sauro KM, Wiebe S, Dunkley C, Janszky J,
Kumlien E, Moshé S, Nakasato N, Pedley TA, Perucca E, Senties H, et
al: The current state of epilepsy guidelines: A systematic review.
Epilepsia. 57:13–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rathore C and Paterson R: Stopping
antiepileptic drugs in patients with epilepsy in remission: Why,
when and how? Neurol India. 62:3–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Markoula S, Teotonio R, Ratnaraj N, Duncan
JS, Sander JW and Patsalos PN: Lacosamide serum concentrations in
adult patients with epilepsy: The influence of gender, age, dose,
and concomitant antiepileptic drugs. Ther Drug Monit. 36:494–498.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Braun KP and Schmidt D: Stopping
antiepileptic drugs in seizure-free patients. Curr Opin Neurol.
27:219–226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stewart E, Catroppa C and Lah S: Theory of
mind in patients with epilepsy: A systematic review and
meta-analysis. Neuropsychol Rev. 26:3–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Xing DG, Ma EM, Qiu B, Ou SW and
Wang YJ: Microsurgical outcomes of secondary epilepsy from
hippocampal lesions: A report of 56 cases and literature review.
Turk Neurosurg. 26:29–38. 2016.PubMed/NCBI
|
|
8
|
Verrotti A, Matricardi S, Rinaldi VE,
Prezioso G and Coppola G: Neuropsychological impairment in
childhood absence epilepsy: Review of the literature. J Neurol Sci.
359:59–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Buckmaster PS and Haney MM: Factors
affecting outcomes of pilocarpine treatment in a mouse model of
temporal lobe epilepsy. Epilepsy Res. 102:153–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sanabria-Castro A, Henriquez-Varela F,
Lara-Maier S, Monge-Bonilla C and Sittenfeld-Appel M:
Characteristics of patients with refractory epilepsy attended in a
tertiary referral center in Costa Rica. Rev Neurol. 63:58–64.
2016.(In Spanish). PubMed/NCBI
|
|
11
|
Horvath GA, Demos M, Shyr C, Matthews A,
Zhang L, Race S, Stockler-Ipsiroglu S, Van Allen MI, Mancarci O,
Toker L, et al: Secondary neurotransmitter deficiencies in epilepsy
caused by voltage-gated sodium channelopathies: A potential
treatment target? Mol Genet Metab. 117:42–48. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen Y, Liao Y, Feng G, Gu X and Wan S:
Uterine artery embolization for hemorrhage resulting from
second-trimester abortion in women with scarred uterus: Report of
two cases. Int J Clin Exp Med. 8:14196–14202. 2015.PubMed/NCBI
|
|
13
|
Liu J, Liu Z, Ding H and Yang X: Adherence
to treatment and influencing factors in a sample of Chinese
epilepsy patients. Epileptic Disord. 15:289–294. 2013.PubMed/NCBI
|
|
14
|
Möttönen T, Katisko J, Haapasalo J,
Tähtinen T, Saastamoinen A, Peltola J, Öhman J and Lehtimäki K: The
correlation between intraoperative microelectrode recording and
3-Tesla MRI in patients undergoing ANT-DBS for refractory epilepsy.
Stereotact Funct Neurosurg. 94:86–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lambrechts DA, Brandt-Wouters E,
Verschuure P, Vles HS and Majoie MJ: A prospective study on changes
in blood levels of cholecystokinin-8 and leptin in patients with
refractory epilepsy treated with the ketogenic diet. Epilepsy Res.
127:87–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu YX, Wang HQ, Yan J, Sun XB, Guo JC and
Zhu CQ: Antibody binding to cell surface amyloid precursor protein
induces neuronal injury by deregulating the phosphorylation of
focal adhesion signaling related proteins. Neurosci Lett.
465:276–281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bès C, Troadec S, Chentouf M, Breton H,
Lajoix AD, Heitz F, Gross R, Plückthun A and Chardès T: PIN-bodies:
A new class of antibody-like proteins with CD4 specificity derived
from the protein inhibitor of neuronal nitric oxide synthase.
Biochem Biophys Res Commun. 343:334–344. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liimatainen S, Peltola J, Hietaharju A,
Sabater L and Lang B: Lack of antibodies to NMDAR or VGKC-complex
in GAD and cardiolipin antibody-positive refractory epilepsy.
Epilepsy Res. 108:592–596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Watanabe O: Voltage-gated potassium
channel-complex antibodies associated encephalopathy and related
diseases. Brain Nerve. 68:1011–1023. 2016.(In Japanese). PubMed/NCBI
|
|
20
|
Lilleker JB, Jones MS and Mohanraj R: VGKC
complex antibodies in epilepsy: Diagnostic yield and therapeutic
implications. Seizure. 22:776–779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van Elst LT, Klöppel S and Rauer S:
Voltage-gated potassium channel/LGI1 antibody-associated
encephalopathy may cause brief psychotic disorder. J Clin
Psychiatry. 72:722–723. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lang B, Makuch M, Moloney T, Dettmann I,
Mindorf S, Probst C, Stoecker W, Buckley C, Newton CR, Leite MI, et
al: Intracellular and non-neuronal targets of voltage-gated
potassium channel complex antibodies. J Neurol Neurosurg
Psychiatry. 88:353–361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Somers KJ and Sola CL: Voltage-gated
potassium channel-complex antibody-associated limbic encephalitis.
Psychosomatics. 52:78–81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aradillas E and Schwartzman RJ:
Kinesigenic dyskinesia in a case of voltage-gated potassium
channel-complex protein antibody encephalitis. Arch Neurol.
68:529–532. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Irani SR, Stagg CJ, Schott JM, Rosenthal
CR, Schneider SA, Pettingill P, Pettingill R, Waters P, Thomas A,
Voets NL, et al: Faciobrachial dystonic seizures: The influence of
immunotherapy on seizure control and prevention of cognitive
impairment in a broadening phenotype. Brain. 136:3151–3162. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
González Otárula KA, Ugarnes G, Suárez
Fernández M and D'Giano C: Faciobrachial dystonic seizures.
Semiologic diagnosis in limbic encephalitis. Medicina (B Aires).
75:407–409. 2015.(In Spanish). PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Teoh EJ, McGowan DR, Bradley KM, Belcher
E, Black E, Moore A, Sykes A and Gleeson FV: 18F-FDG PET/CT
assessment of histopathologically confirmed mediastinal lymph nodes
in non-small cell lung cancer using a penalised likelihood
reconstruction. Eur Radiol. 26:4098–4106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bharmal AV, Kent BA, Bussey TJ and Saksida
LM: Performance of transgenic TgTau-P301L mice in a 5-choice serial
reaction time task (5-CSRTT) as a model of Alzheimer's disease.
Psychiatr Danub. 27 Suppl 1:S515–S525. 2015.PubMed/NCBI
|
|
30
|
Vaisburd S, Shemer Z, Yeheskel A, Giladi E
and Gozes I: Risperidone and NAP protect cognition and normalize
gene expression in a schizophrenia mouse model. Sci Rep.
5:163002015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dennis M, Mead G, Doubal F and Graham C:
Determining the modified Rankin score after stroke by postal and
telephone questionnaires. Stroke. 43:851–853. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rossetti AO, Logroscino G, Milligan TA,
Michaelides C, Ruffieux C and Bromfield EB: Status epilepticus
severity score (STESS): A tool to orient early treatment strategy.
J Neurol. 255:1561–1566. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Khodaie B, Lotfinia AA, Ahmadi M, Lotfinia
M, Jafarian M, Karimzadeh F, Coulon P and Gorji A: Structural and
functional effects of social isolation on the hippocampus of rats
with traumatic brain injury. Behav Brain Res. 278:55–65. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Arida RM, de Almeida AC, Cavalheiro EA and
Scorza FA: Experimental and clinical findings from physical
exercise as complementary therapy for epilepsy. Epilepsy Behav.
26:273–278. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sandow N, Kim S, Raue C, Päsler D, Klaft
ZJ, Antonio LL, Hollnagel JO, Kovacs R, Kann O, Horn P, et al: Drug
resistance in cortical and hippocampal slices from resected tissue
of epilepsy patients: No significant impact of p-glycoprotein and
multidrug resistance-associated proteins. Front Neurol. 6:302015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huda S, Wong SH, Pettingill P, O'Connell
D, Vincent A and Steiger M: An 11-year retrospective experience of
antibodies against the voltage-gated potassium channel (VGKC)
complex from a tertiary neurological centre. J Neurol. 262:418–424.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Radja GK and Cavanna AE: Treatment of VGKC
complex antibody-associated limbic encephalitis: A systematic
review. J Neuropsychiatry Clin Neurosci. 25:264–271. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park SB: The clinical relevance of voltage
gated potassium channel (VGKC)-complex antibodies: The story is
still unfolding. J Neurol Neurosurg Psychiatry. 85:5962014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Watanabe O: Anti-VGKC complex antibody
associated limbic encephalitis. Nihon Rinsho. 73 Suppl 7:S613–S618.
2015.(In Japanese).
|
|
40
|
Menon P, Kiernan MC and Vucic S: Cortical
hyperexcitability precedes lower motor neuron dysfunction in ALS.
Clin Neurophysiol. 126:803–809. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nuwer MO, Picchione KE and Bhattacharjee
A: PKA-induced internalization of slack KNa channels produces
dorsal root ganglion neuron hyperexcitability. J Neurosci.
30:14165–14172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sotgiu ML and Biella G: Differential
effects of MK-801, a N-methyl-D-aspartate non-competitive
antagonist, on the dorsal horn neuron hyperactivity and
hyperexcitability in neuropathic rats. Neurosci Lett. 283:153–156.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Murro AM, Park YD, King DW, Gallagher BB,
Smith JR, Yaghmai F, Toro V, Figueroa RE, Loring DW and Littleton
W: Seizure localization in temporal lobe epilepsy: A comparison of
scalp-sphenoidal EEG and volumetric MRI. Neurology. 43:2531–2533.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Englot DJ, Nagarajan SS, Imber BS, Raygor
KP, Honma SM, Mizuiri D, Mantle M, Knowlton RC, Kirsch HE and Chang
EF: Epileptogenic zone localization using magnetoencephalography
predicts seizure freedom in epilepsy surgery. Epilepsia.
56:949–958. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Marson AG, Hutton JL, Leach JP, Castillo
S, Schmidt D, White S, Chaisewikul R, Privitera M and Chadwick DW:
Levetiracetam, oxcarbazepine, remacemide and zonisamide for drug
resistant localization-related epilepsy: A systematic review.
Epilepsy Res. 46:259–270. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chaisewikul R, Privitera MD, Hutton JL and
Marson AG: Levetiracetam add-on for drug-resistant localization
related (partial) epilepsy. Cochrane Database Syst Rev: CD001901.
2001. View Article : Google Scholar
|
|
47
|
Dubey D and Porter BE: CRTC1 nuclear
localization in the hippocampus of the pilocarpine-induced status
epilepticus model of temporal lobe epilepsy. Neuroscience.
320:57–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bourque M, Morissette M and Di Paolo T:
Neuroprotection in Parkinsonian-treated mice via estrogen receptor
α activation requires G protein-coupled estrogen receptor 1.
Neuropharmacology. 95:343–352. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Loebel A and Citrome L: Lurasidone: A
novel antipsychotic agent for the treatment of schizophrenia and
bipolar depression. BJPsych Bull. 39:237–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Loebel A, Brams M, Goldman RS, Silva R,
Hernandez D, Deng L, Mankoski R and Findling RL: Lurasidone for the
treatment of irritability associated with autistic disorder. J
Autism Dev Disord. 46:1153–1163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Calabrese J, Rajagopalan K, Ng-Mak D,
Bacci ED, Wyrwich K, Pikalov A and Loebel A: Effect of lurasidone
on meaningful change in health-related quality of life in patients
with bipolar depression. Int Clin Psychopharmacol. 31:147–154.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Enomoto T, Ishibashi T, Tokuda K, Ishiyama
T, Toma S and Ito A: Lurasidone reverses MK-801-induced impairment
of learning and memory in the Morris water maze and radial-arm maze
tests in rats. Behav Brain Res. 186:197–207. 2008. View Article : Google Scholar : PubMed/NCBI
|