Introduction
Cardiac function is reduced following myocardial
infarction (MI) due to myocardial injury and changes in the viable
non-ischemic myocardium, a process known as cardiac remodeling.
This response is characterized by the development of cardiac
hypertrophy, altered cardiac chamber geometry, a shift in the
expression of contractile proteins to a fetal pattern, a switch to
glucose metabolism and the induction of fibrosis (1,2).
Current treatments for patients with acute MI (AMI)
reduce infarct size, preserve left ventricular (LV) function and
improve survival. However, these therapies do not prevent
remodeling, which may lead to heart failure (3,4). In
fact, 30–46% of patients fail to exhibit functional recovery until
6 months after AMI, despite the current treatments (4,5).
This requires further understanding of the pathophysiology of
cardiac remodeling in order to comprehend the factors the
contribute to disease progression, and to introduce novel effective
treatments to repair the injured myocardium (6).
It has been well documented that thyroid hormone
(TH) levels decrease following AMI (3,7), in
cardiac surgery (8) and in heart
failure (9). The physiological
significance of this response remains largely unknown and there is
much controversy as to whether low T3 syndrome requires treatment
(10). However, several
observational studies showing an association between changes and
clinical outcome have recently been presented, suggesting that it
may be critical for the myocardial stress response. Indeed,
experimental studies using cell and animal models have shown that
TH receptor (TR) signaling is altered following myocardial ischemia
or mechanical loading with important physiological consequences,
while TH treatment may have beneficial effects (10). Our laboratory and others have shown
that TH can favorably remodel the post-ischemic myocardium
(11,12). The effects of TH on cardiac
remodeling after MI seem to be dose- and time-dependent, and were
preserved in the presence of co-morbidities such as diabetes
(10,13–17).
In rat models of MI, TH treatment promoted physiological remodeling
of the non-ischemic myocardium, characterized by compensatory
growth with favorable changes in the myosin heavy chain (MHC)
expression pattern (decreased β-MHC and increased α-MHC), ellipsoid
reshaping and an improved LV ejection fraction (EF%) (15,16).
Considering these previous findings, the purpose of
the present study was to merge and analyze a large quantity of
prior experimental data produced/published by our laboratory
concerning the coronary artery ligation (CAL) model in rats at
different time points, in order to provide a complete analysis that
would allow us to delineate the process of myocardial remodeling
and the effect of TH treatment. Towards that aim, we have assessed
several myocardial parameters in rat experimental models using
computational methodologies to achieve more in-depth study of the
mechanisms of myocardial remodeling. The results of this analysis
provided insights to better understand the favorable effect of TH
treatment, which may assist efforts in translating this novel
concept into clinical practice. Furthermore, the present study
could be considered a first approach to creating a computational
model to describe the phenomenon of myocardial remodeling under TH
treatment.
Materials and methods
Animals
Animals were used in the present study as previously
reported (15–17). The present study adhered to the
principles of the Declaration of Helsinki regarding the ethical
conduct of animal research, upheld by the World Medical
Association. In addition, all experiments conformed to European
legislation (European Union directive for the protection of animals
used for scientific purposes 609/1986, revised in 2010/63/EU). The
experimental protocols were approved by the Bioethics Committee of
the National and Kapodistrian University of Athens Medical School
(Prot. no. 1516013641/24/3/2010). The animals were administered
humane care and housed in Plexiglas® chambers with ad
libitum access to standard rodent pellet-diet and water at the
Animal House of the Department of Pharmacology, University of
Athens (license no. EL25 BIO 09). The laboratory conditions were
maintained as optimal, in terms of temperature (22–24°C), humidity
(35–65%) and light-to-dark cycles (12/12 h).
Experimental model and induction of
MI
MI procedures were conducted as previously reported
(15–17). In brief, rats were anesthetized
with an intraperitoneal injection of ketamine (70 mg/kg) and
midazolame (0.1 mg/kg), intubated and ventilated via a tracheal
cannula using a constant-volume rodent ventilator (Inspira; 50
breaths/min; 1 ml/100 gr tidal volume; Harvard Apparatus,
Cambridge, MA, USA). Anesthesia was maintained by inhalation of
small doses of sevoflurane (1–2%). Left thoracotomy was performed
at the fourth intercostal space, followed by pericardiotomy. The
left coronary artery was then ligated with a 6-0 silk round-bodied
suture. The heart was quickly returned to the chest cavity, the
chest was closed and the rats were allowed to recover using assist
mode ventilation. The recovery period was around 30–40 min.
Atelectasis was prevented by using positive end-expiratory pressure
at the end of the surgical procedure. A continuous
electrocardiogram (ECG) was used to monitor heart beats and ECG
ischemic changes following CAL. Body temperature was maintained at
37°C with a heating blanket (Harvard Homeothermic Blanket; 50-7061;
Harvard Apparatus). The mortality rate was recorded as 15–20% in
the MI group during the first 24 h following surgery. The animals
were left to recover for 2, 4 or 13 weeks after MI in order to
assess short-term, mid-term and long-term recovery of function. The
same procedure was followed for sham-operated animals, but the
coronary artery was not ligated.
TH administration
Rats subjected to CAL were randomly divided in two
groups 24 h after the operation. The first group received standard
rat chow (designated the CAL group), while the second group
received food containing 0.05% thyroid powder (T1251, with 0.42
µg/mg T3 and 1.7 µg/mg T4; designated the
CALTH group; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), as
previously described (15,16). The mean daily intake of TH per rat
was estimated at 3.0 µg T3 and 12 µg T4.
Sham-operated rats receiving standard rat chow were designated the
SO group.
Echocardiography
Rats were sedated with ketamine hydrochloride (100
mg/kg) and heart function was evaluated by echocardiography, as
previously described (15,16). Short and long-axis images were
acquired using a GE Vivid 7 Pro digital ultrasound system and a GE
I13L probe at 14.0 MHz (GE Healthcare Life Sciences, Chicago, IL,
USA). A large number of consecutive measurements were performed and
subsequently analyzed by two independent operators.
LV internal diameter in the diastolic phase (LVIDd),
LVID in the systolic phase (LVIDs), LV posterior wall thickness in
the diastolic phase (LVPWd), and the EF% were all measured. EF% was
calculated using the Simpson's rule. EF% was used to determine the
global contractile LV function.
Wall tension index (WTI) was defined as the ratio
[(LVIDd/2) × PW thickness], as previously described (16,18).
WTI was measured in order to indirectly assess myocardial wall
stress. In addition, the sphericity index (SI), defined as the
ratio of the maximum long axis (in mm) to the maximum short axis
(in mm) of the LV, was determined in order to assess LV geometry.
All measurements were averaged for ≥3 consecutive cardiac
cycles.
Experimental procedure
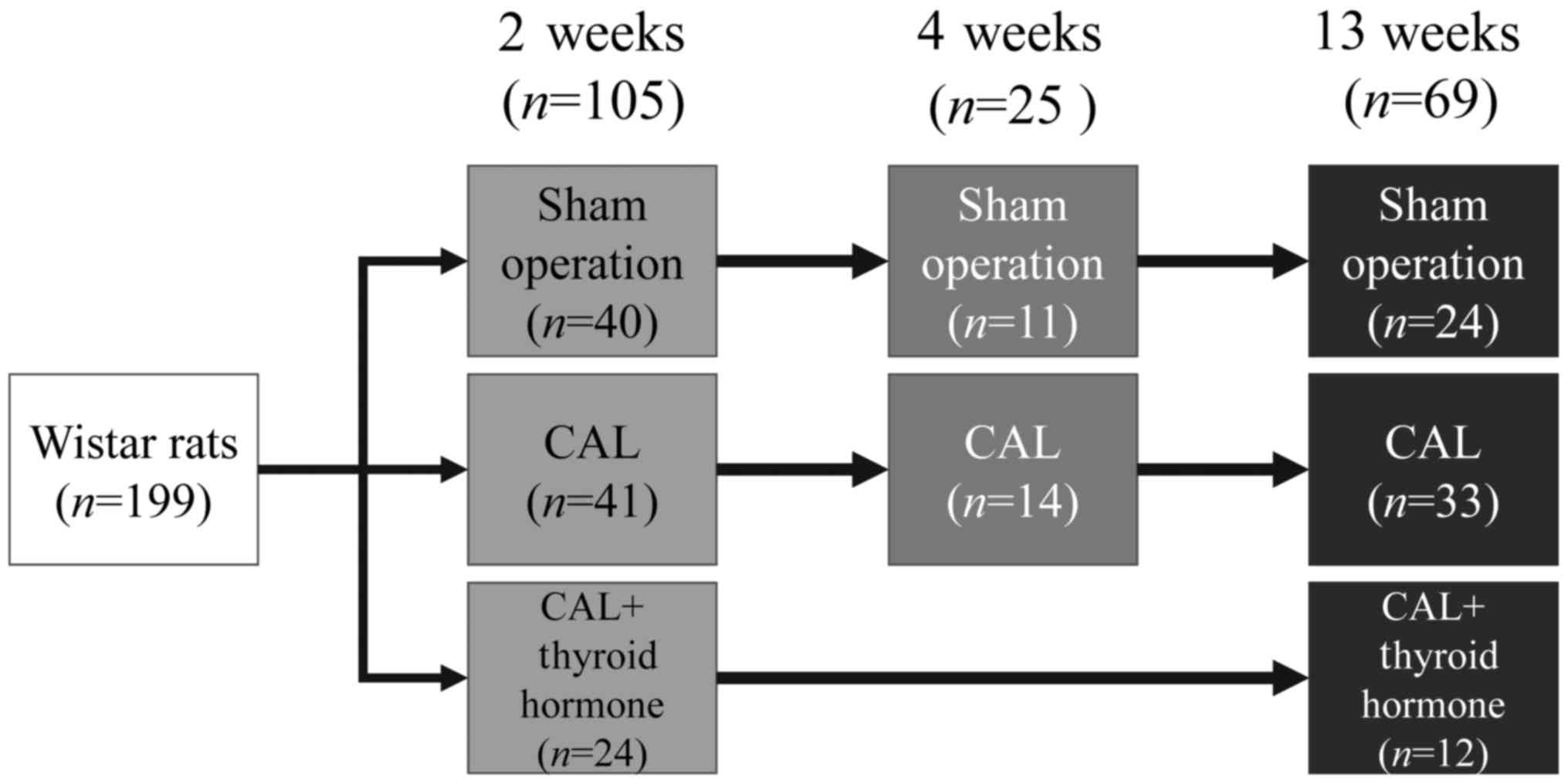
Rats (n=199) were divided into three groups:
Those that underwent a sham-operation (SO; n=75); those that
were subjected to CAL without TH treatment (CAL; n=88); and
those that were subjected to CAL with TH treatment (CALTH;
n=36). Rats in the SO and CAL experimental groups were
monitored for 2 (n=81), 4 (n=25) or 13 (n=69)
weeks. Rats in the CALTH group were monitored for 2 (n=24)
or 13 weeks (n=12). The experimental procedures are
summarized in Fig. 1. At each time
period following MI (2, 4 and 13 weeks), the rats were
anaesthetized with ketamine hydrochloride and midazolam, subjected
to echocardiography analysis and sacrificed while heart and lungs
were removed.
Collected data and variables
Collected variables for each animal included the
following: i) Surgery (intervention); ii) TH treatment; iii)
duration of intervention; iv) LV-end diastolic diameter (cm); v)
LV-end systolic diameter (cm); vi) LVPW thickness (cm); vii) long
axis diameter (cm); viii) LV EF%; ix) systolic velocity (cm/sec);
x) WTI(ratio); xi) SI (ratio); xii) heartbeats (beats per min);
xiii) LV weight (mg); xiv) scar weight (SW) (mg); xv) scar area
(SA) (mm2). LV scar tissue was excised and the SA was
measured in mm2 and the SW in mg. Variables are
summarized in Table I.
 | Table I.Summary of variables estimated in the
present study. |
Table I.
Summary of variables estimated in the
present study.
| Variable | Variable type | Values | Units | Method of
assessment | Variable's
physiological interpretation |
|---|
| Surgery
(intervention) | Nominal | Sham operation
and/or CAL |
Non-dimensional | None | Describes the type
of intervention/operation. |
| Duration of
intervention | Nominal | 2, 4, 13 weeks |
Non-dimensional | None | The time of
intervention and thyroxine, or no treatment prior to
sacrifice. |
| Thyroxine
treatment | Nominal | Yes, No |
Non-dimensional | None | Whether rats
received thyroxine or not. |
| Body weight | Numerical | R | gr | Balance |
|
| LV-end diastolic
diameter | Numerical | R | cm |
Echocardiography | Left ventricle
diameter at end diastolic phase. |
| LV-end systolic
diameter | Numerical | R | cm |
Echocardiography | Left ventricle
diameter at end systolic phase. |
| LV-posterior wall
thickness | Numerical | R | cm |
Echocardiography | Left ventricle
posterior wall thickness. |
| LV-long-axis
diameter | Numerical | R | cm |
Echocardiography | Left ventricle long
axis diameter. |
| LV-ejection
fraction | Numerical | R | % | Echocardiography,
computation | The fraction of
blood ejected following a systolic heart pulse. |
|
|
|
|
| EF=VEjVFilled·100 |
|
| Systolic
velocity | Numerical | R | cm/sec | Echocardiography,
computation | Mean velocity of
posterior wall movement during systole. |
|
|
|
|
| usystolic=dsdt |
|
| Wall tension
index | Numerical | R | Ratio | Echocardiography,
computation | Derived from
Laplace's law. High wall tension index values indicated increased
intra-ventricle pressure, not balanced by cardiac hypertrophy. |
|
|
|
|
| Tindex=PVVVdV |
|
| Sphericity
index | Numerical | R | Ratio | Echocardiography,
computation (87, 88) | Heart geometry.
Index values close to 1 indicate a quasi-spherical heart. Normal
values are between 1.8–2.0, which indicate an ellipsoid. |
|
|
|
|
| SIindex=VLV_End_Diastolic_Long_axisVLV_End_Diastolic_diameter |
|
| Heart beats | Numerical | Z+a | Beats/min | Measurements |
|
| LV weight | Numerical | R | mg | Balance,
measurements | Indicates cardiac
atrophy or hypertrophy. |
| Scar weight | Numerical | R | mg | Balance,
measurements | Scar weight
following CAL. |
| Scar area | Numerical | R | mm2 | Balance,
measurements | Scar area following
CAL. |
Statistical analysis
Multiparameter analyses were performed with
MATLAB® simulation environment (The MathWorks, Inc.,
Natick, MA, USA). One-, two- and three-way analysis of variance
with a Bonferroni post hoc test were used to calculate the mean
differences between groups. Continuous variables are expressed as
the median ± standard deviation, unless otherwise indicated.
Correlations between variables were calculated using the Pearson's
correlation coefficient. Linear regressions were performed using
the y=ax+b form and curves were estimated using a
least-chi-squared approach. K-means, clustered scatter plots and
hierarchical clustering algorithms were implemented using the
MATLAB® simulation environment (The MathWorks, Inc.).
The datasets used and/or analyzed during the current study are
available from the corresponding author upon reasonable
request.
Results
The time-independent effects of CAL
(SO and CAL populations)
CAL appeared to have a significant detrimental
effect on several variables of cardiac function. In particular, LV
EF% (Fig. 2A), LV systolic
velocity (cm/sec) (Fig. 2B), and
SI (Fig. 2C) were significantly
higher in the SO group when compared with those in the CAL group.
In addition, indexes of cardiac remodeling such as LVEDD (cm)
(Fig. 2D), LVESD (cm) (Fig. 2E), WTI (Fig. 2F) and LV weight (mg) (Fig. 2G) manifested as significantly
higher in the CAL group when compared with those in the SO group.
These results were predicted; the results also indicate the effects
of CAL irrespective of time. Thus, it appears that there are
time-independent effects of CAL.
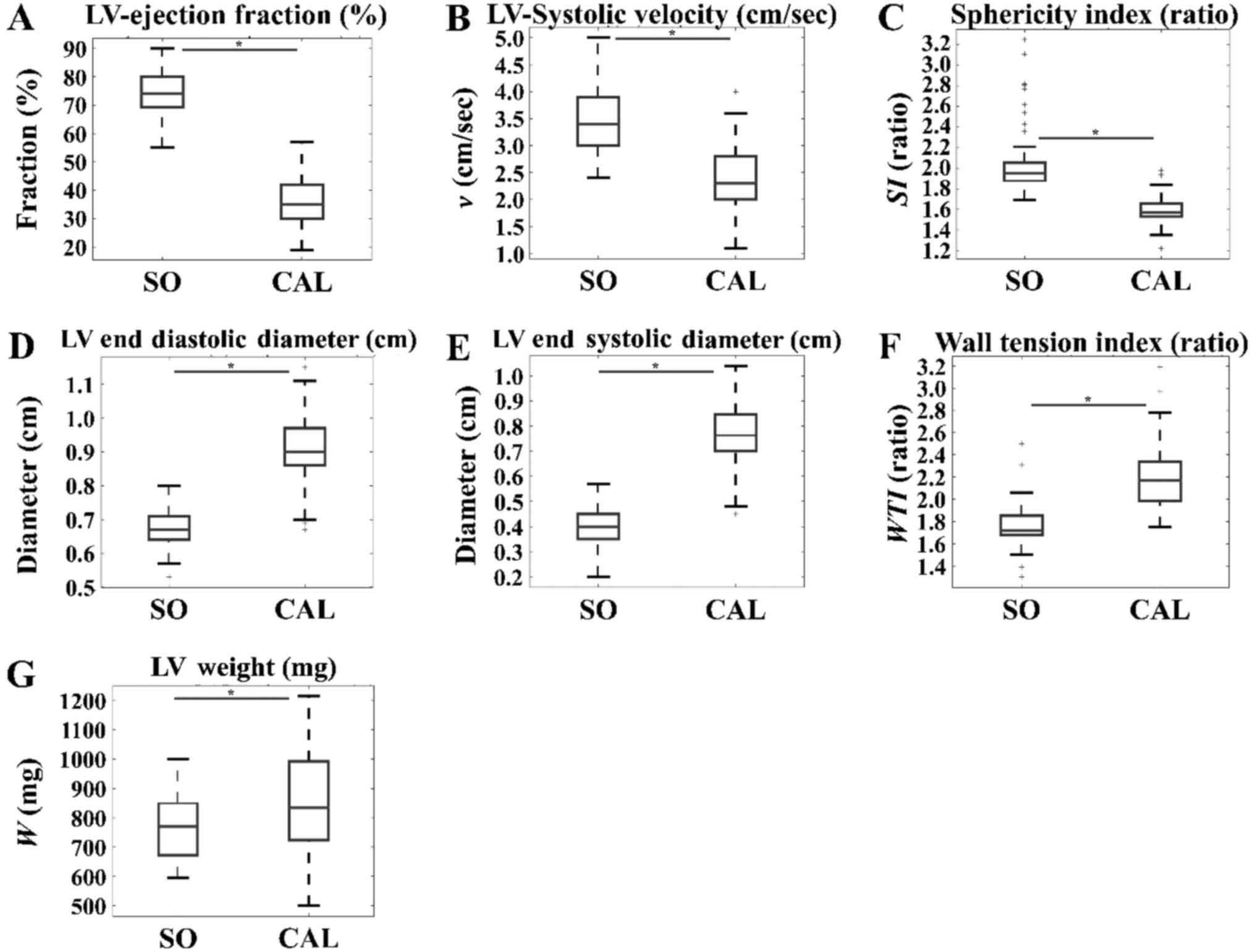 | Figure 2.Effects of intervention in SO and CAL
populations that did not receive thyroid hormone, irrespective of
time. (A) LV ejection fraction (%), (B) LV systolic velocity and
(C) SI manifested to a significantly higher level in the SO group
when compared with the CAL group. On the other hand, (D) LV end
diastolic diameter, (E) LV end systolic diameter, (F) wall tension
index and (G) LV weight were significantly higher in the CAL group
when compared within the SO group. *P<0.05, as indicated. SO,
sham operation; CAL, coronary artery ligation; LV, left
ventricular; SI, sphericity index; SO, sham operation; w,
weight. |
The time-independent effects of ΤΗ
treatment in the CALTH group
The time-independent effects of ΤΗ in the CAL group
exhibited significant differences, being lower in the untreated
group as compared with in the treated group with regard to LV EF%
(Fig. 3A), LVPW thickness
(Fig. 3D), systolic velocity
(Fig. 3E), SI (Fig. 3G) and heart beats (Fig. 3H). Conversely, significantly higher
values were observed in the untreated group as compared with in the
treated group with regard to the LVEDD (Fig. 3B), LVESD (Fig. 3C) and WTI (Fig. 3F).
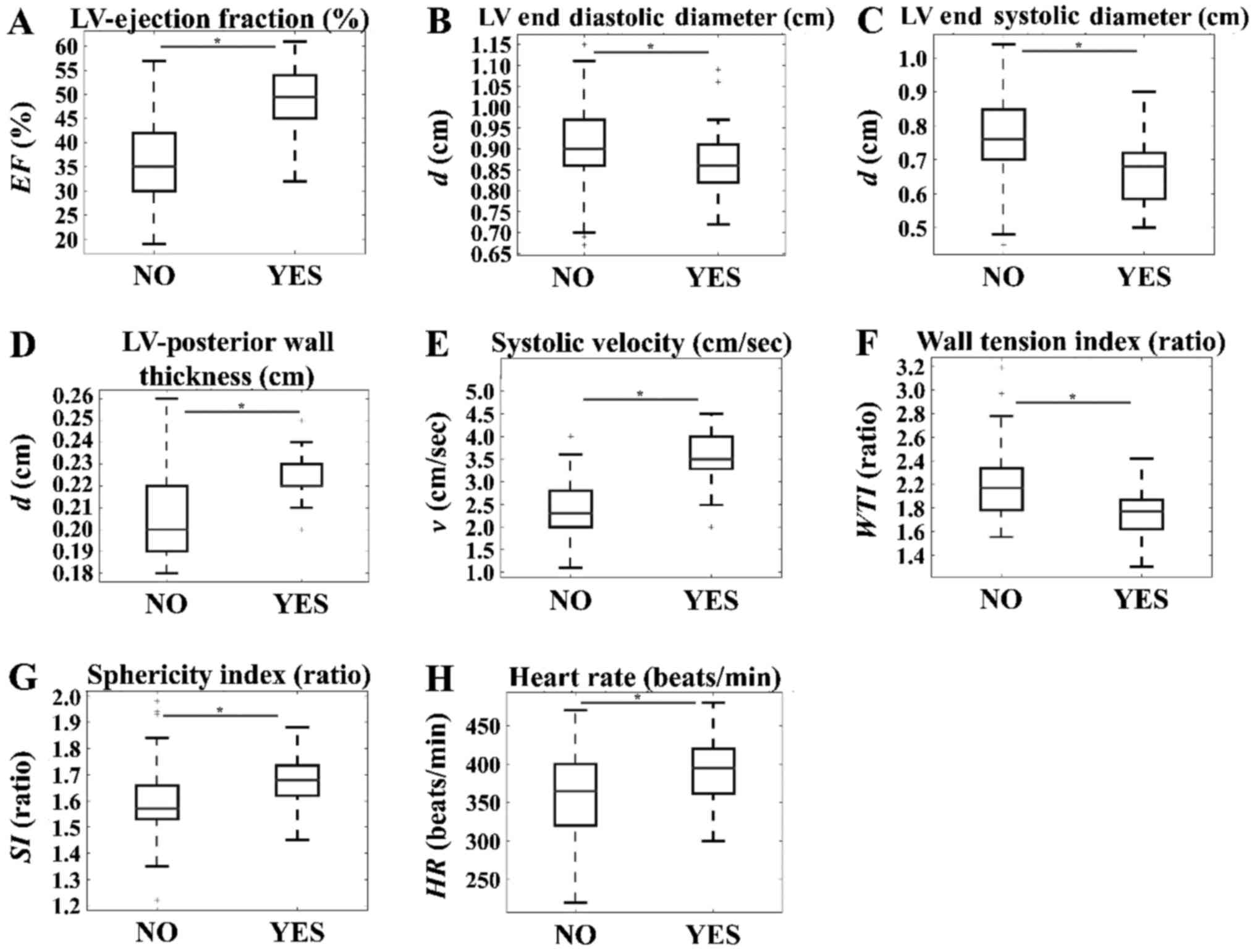 | Figure 3.Time-independent effects of thyroid
hormone in the CAL group. Significant reductions in the untreated
group compared with the treated group were observed for (A) LV
ejection fraction (%), (D) LV posterior wall thickness, (E)
systolic velocity, (G) sphericity index and (H) heart rate. On the
other hand, significantly higher values were observed in the
untreated group when compared with the treated group, for (B) LV
end diastolic diameter, (C) LV end systolic diameter and (F) wall
tension index. *P<0.05, as indicated. SO, sham operation; CAL,
coronary artery ligation; LV, left ventricular; EF, ejection
fraction; d, diameter; v, velocity; WTI, wall tension index; SI,
sphericity index; HR, heart rate; NO, indicates CAL samples not
treated with thyroid hormone; YES, indicates CAL samples that did
receive thyroid hormone. |
The time-dependent effects in the CAL
group
We studied the effect of treatment over time in the
CAL group. LV EF% significantly deteriorated between 2 and 4 weeks
post-treatment, and was further reduced at 13 weeks (Fig. 4A). The LV diastolic diameter and
systolic diameter significantly increased between 2 and 4 weeks,
but not between 4 and 13 weeks at sacrifice (Fig. 4B and C). LVPW thickness
significantly increased only after 13 weeks (Fig. 4D). WTI reached a maximum value at 4
weeks as compared with 2 and 13 weeks (Fig. 4E). SI reached its lowest value at
13 weeks, compared with at 2 and 4 weeks (Fig. 4F). A similar trend was observed for
systolic velocity, with significant differences being observed
between 2 and 4 weeks at sacrifice, and between 4 and 13 weeks at
sacrifice (Fig. 4G). Finally, LV
weight exhibited an increasing linear behavior with respect to
time, with significant differences observed between 2 and 13 weeks
at sacrifice, as well as at 4 and 13 weeks at sacrifice (Fig. 4I).
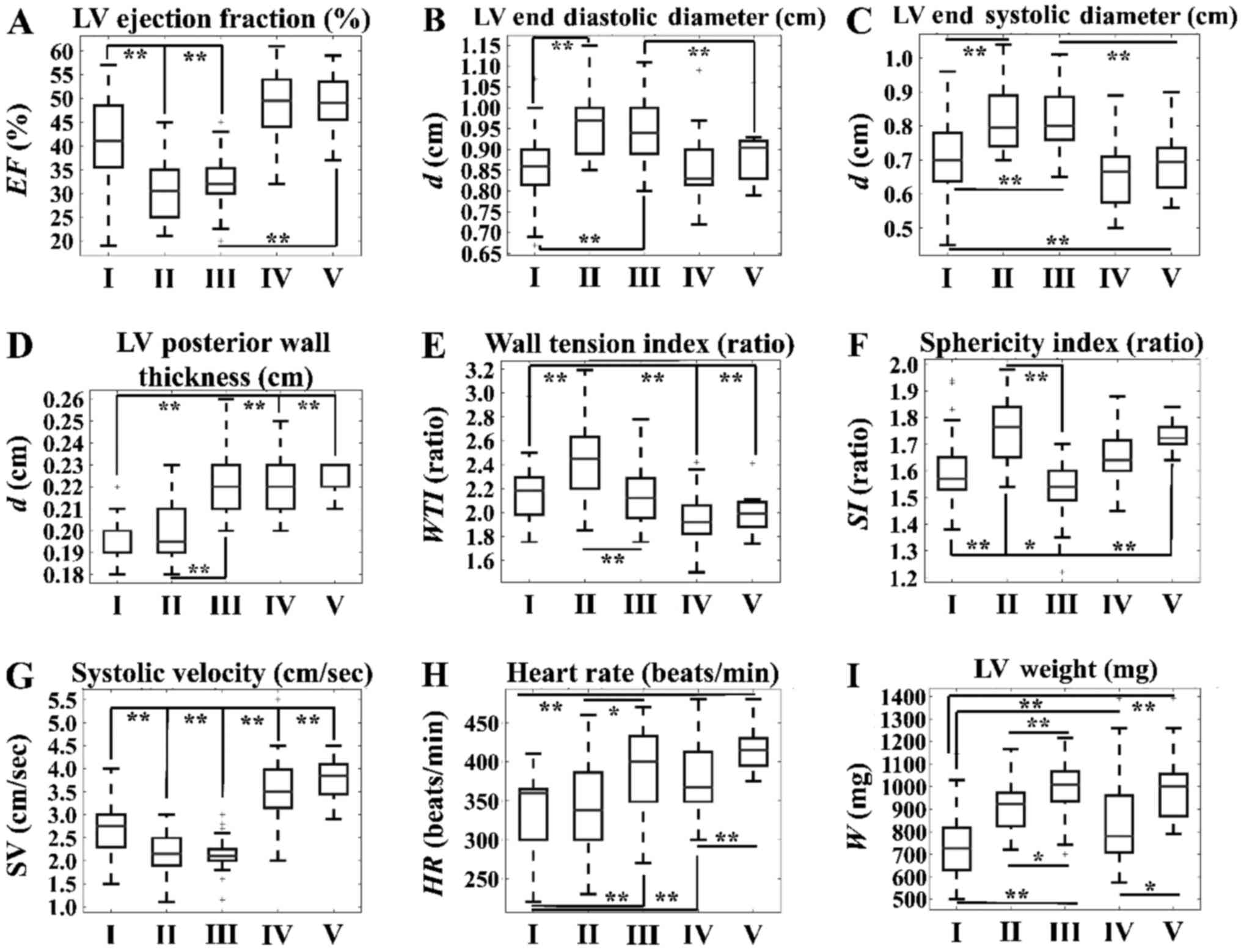 | Figure 4.Time-dependent effects of TH in the
CAL group. Significant differences were observed with respect to
(A) LV ejection fraction, (B) LV end diastolic diameter, (C) LV end
systolic diameter, (D) LV posterior wall thickness, (E) wall
tension index, (F) sphericity index, (G) systolic velocity, (H)
heart rate and (I) LV weight. *P<0.05 and **P<0.01, as
indicated. SV, systolic velocity; CAL, coronary artery ligation;
LV, left ventricular; TH, thyroid hormone; d, diameter; WTI, wall
tension index; w, weight; I, CAL group with no TH treatment at 2
weeks; II, CAL group with no TH treatment at 4 weeks; III, CAL
group with no TH treatment at 13 weeks; IV, CAL group with TH
treatment at 2 weeks; V, CAL group with TH treatment at 13
weeks. |
The time-dependent effects of ΤΗ
treatment in the CAL group
LV EF% appeared to be higher in CALTH rats as
compared within CAL rats without TH treatment. Notably, no
significant difference was observed in the EF% between 2 and 13
weeks in CALTH rats (Fig. 4A).
Similar behavior, as in the case of EF%, was observed with systolic
velocity (Fig. 4G). At 2 weeks
there was no difference in the LV diastolic rats when compared with
that in CAL rats without TH, whereas the LV diastolic diameter at
13 weeks was significantly lower in CALTH rats (Fig. 4B). There was no significant
difference in LV diastolic diameter between 2 and 13 weeks in the
CALTH rats (Fig. 4B). LV systolic
diameter was significantly higher in the CAL group without TH
treatment when compared with that in the CALTH group at both 2 and
13 weeks. As with the diastolic diameter, there was no significant
difference in the LV systolic diameter of CALTH rats between 2 and
13 weeks (Fig. 4C). LVPW thickness
was significantly increased at 2 weeks in CALTH rats, as compared
with in CAL rats without TH treatment, but no difference was
observed at 13 weeks (Fig. 4D).
Furthermore, WTI was significantly lower in the CALTH group
compared within the CAL group without TH treatment at both 2 and 13
weeks (Fig. 4E). Heart beats was
observed to be lower at 2, 4 and 13 weeks following CAL without TH
treatment, as compared with the 2 and 13 weeks in CALTH rats
(Fig. 4H). Finally, at 2 weeks,
the LV weight exhibited a significant increase in the CALTH group
compared within the CAL without TH treatment group, while no
difference was observed at 13 weeks (Fig. 4I).
Notably, we found significant differences with
respect to SW and SA within the CAL and CALTH groups. In
particular, significant increases were observed in both SW and SA
at 13 weeks, as compared with at 2 weeks, in the CAL group without
TH treatment (Fig. 5).
Furthermore, SA and SW were lower in CALTH rats compared within CAL
rats without TH treatment at 13 weeks (Fig. 5).
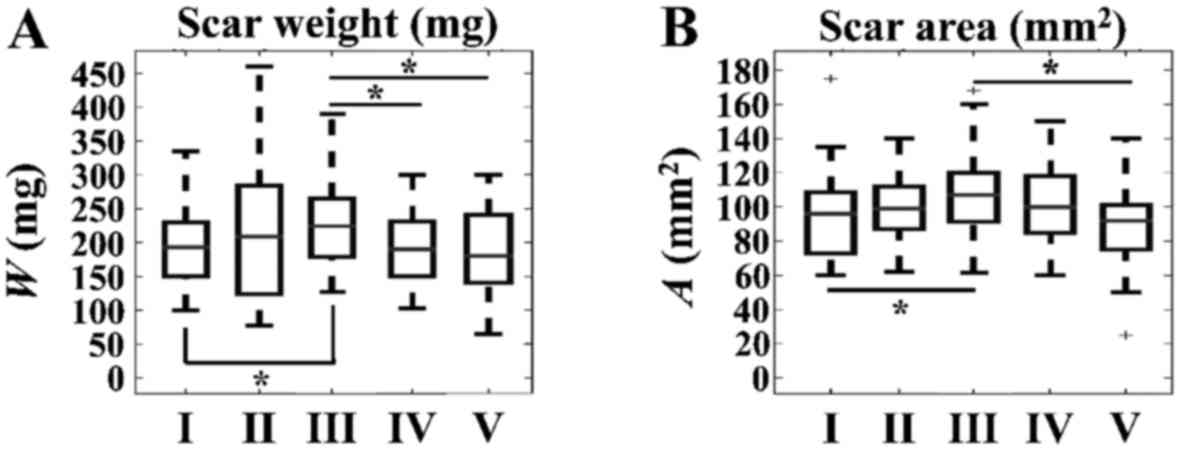 | Figure 5.Time-dependent effects of TH in the
CAL group with respect to scar parameters. Significant differences
were observed with respect to (A) scar weight in the CAL group
without TH treatment between 2 and 13 weeks. Significant
differences were observed between the CAL group without TH
treatment at 13 weeks and the CAL group with TH treatment at 2
weeks, as well as between the CAL group without TH treatment at 13
weeks and the CAL group with TH treatment at 13 weeks. (B)
Significant differences were observed with respect to scar area in
the CAL group without TH treatment between 2 and 13 weeks. In
addition, significant differences were observed between the CAL
group without TH treatment at 13 weeks and the CAL group with TH
treatment at 13 weeks. *P<0.05, as indicated. TH, thyroid
hormone; CAL, coronary artery ligation; w, weight; A, area; I, CAL
group with no TH treatment at 2 weeks; II, CAL group with no TH
treatment at 4 weeks; III, CAL group with no TH treatment at 13
weeks; IV, CAL group with TH treatment at 2 weeks; V, CAL group
with TH treatment at 13 weeks. |
Time-independent Pearson's correlation
of variables in the group without TH treatment
Pearson's correlation analysis revealed parameters
that manifested a rho value of >0.8 within the group that
did not receive TH. In particular, it appeared that a positive
correlation was present between LVSD and LVEDD (rho=0.97),
WTI and LVEDD (rho=0.86), and SA, SW and LVEDD
(rho=0.88 and rho=0.82, respectively), as well as
between SA and SW with respect to LVSD (rho=0.91 and
rho=0.85, respectively). Conversely, it appeared that a
negative correlation (rho<-0.8) was present between the
LV EF% and LVEDD (rho=−0.91), LV EF% and LVSD
(rho=−0.96), and SA and SW with respect to LV EF%
(rho=0.92 and rho=0.86, respectively). Results are
summarized in Table II.
 | Table II.Pearson's correlation of variables
within the group without thyroid hormone treatment. |
Table II.
Pearson's correlation of variables
within the group without thyroid hormone treatment.
| Variable | BW | LVDD | LVSD | LVPWT | LVLAD | LVEF | SV | WTI | SI | HR | LVW | SW | SA |
|---|
| BW | 1 | −0.01092 | −0.08163 | 0.123374 | 0.406542 | 0.12478 | 0.072143 | −0.05072 | 0.248864 | 0.225677 | 0.555866 | −0.1328 | −0.17704 |
| LVDD | −0.01092 | 1 | 0.968114 | 0.453383 | 0.373823 | −0.90591 | −0.72706 | 0.861913 | −0.71073 | −0.03023 | 0.443979 | 0.815996 | 0.881465 |
| LVSD | −0.08163 |
0.968114 | 1 | 0.452522 | 0.311255 | −0.96596 | −0.75527 | 0.823675 | −0.72205 | −0.01684 | 0.411614 | 0.847052 | 0.913934 |
| LVPWT | 0.123374 | 0.453383 | 0.452522 | 1 | 0.086317 | −0.41733 | −0.42096 | −0.04515 | −0.44065 | 0.163385 | 0.507693 | 0.430778 | 0.443461 |
| LVLAD | 0.406542 | 0.373823 | 0.311255 | 0.086317 | 1 | −0.25122 | −0.29975 | 0.380339 | 0.338112 | 0.005855 | 0.293721 | 0.199281 | 0.238772 |
| LVEF | 0.12478 |
-0.90591 | −0.96596 | −0.41733 | −0.25122 | 1 | 0.748729 | −0.77661 | 0.715471 | −0.0032 | −0.34864 | −0.85847 | −0.91975 |
| SV | 0.072143 | −0.72706 | −0.75527 | −0.42096 | −0.29975 | 0.748729 | 1 | −0.58078 | 0.512094 | 0.041901 | −0.32166 | −0.70099 | −0.74491 |
| WTI | −0.05072 |
0.861913 |
0.823675 | −0.04515 | 0.380339 | −0.77661 | −0.58078 | 1 | −0.54656 | −0.1204 | 0.234968 | 0.670458 | 0.732716 |
| SI | 0.248864 | −0.71073 | −0.72205 | −0.44065 | 0.338112 | 0.715471 | 0.512094 | −0.54656 | 1 | 0.043699 | −0.25701 | −0.64719 | −0.681 |
| HR | 0.225677 | −0.03023 | −0.01684 | 0.163385 | 0.005855 | −0.0032 | 0.041901 | −0.1204 | 0.043699 | 1 | 0.307639 | 0.079072 | 0.032759 |
| LVW | 0.555866 | 0.443979 | 0.411614 | 0.507693 | 0.293721 | −0.34864 | −0.32166 | 0.234968 | −0.25701 | 0.307639 | 1 | 0.40708 | 0.353779 |
| SW | −0.1328 |
0.815996 |
0.847052 | 0.430778 | 0.199281 |
-0.85847 | −0.70099 | 0.670458 | −0.64719 | 0.079072 | 0.40708 | 1 |
0.958149 |
| SA | −0.17704 |
0.881465 |
0.913934 | 0.443461 | 0.238772 |
-0.91975 | −0.74491 | 0.732716 | −0.681 | 0.032759 | 0.353779 |
0.958149 | 1 |
Time-independent Pearson's correlation
of variables in the group with TH treatment
Pearson's correlation analysis revealed parameters
that manifested a rho value of >0.8 within the group that
did receive TH. In particular, a positive correlation was present
between LVSD and LVEDD (rho=0.96), WTI and LVEDD
(rho=0.87), and WTI and LVSD (rho=0.81). Conversely,
a negative correlation (rho<-0.8) was present between LV
EF% and LVSD (rho=−0.87). Results are summarized in Table III.
 | Table III.Pearson's correlation of variables
within the group with thyroid hormone treatment. |
Table III.
Pearson's correlation of variables
within the group with thyroid hormone treatment.
| Variable | BW | LVDD | LVSD | LVPWT | LVLAD | LVEF | SV | WTI | SI | HR | LVW | SW | SA |
|---|
| BW | 1 | 0.056403 | 0.0676 | −0.00424 | 0.264712 | 0.148994 | 0.118443 | 0.038453 | 0.310883 | 0.209062 | 0.689122 | 0.07971 | −0.17566 |
| LVDD | 0.056403 | 1 | 0.955092 | 0.080293 | 0.755264 | −0.76796 | −0.53005 | 0.869215 | −0.44544 | −0.24522 | 0.226814 | 0.169746 | 0.65638 |
| LVSD | 0.0676 | 0.955092 | 1 | 0.114573 | 0.72979 | −0.87376 | −0.55627 | 0.81406 | −0.38111 | −0.25119 | 0.201095 | 0.129983 | 0.645036 |
| LVPWT | −0.00424 | 0.080293 | 0.114573 | 1 | 0.104345 | −0.10148 | −0.08778 | −0.41899 | 0.024197 | −0.13488 | −0.1037 | −0.10337 | 0.110662 |
| LVLAD | 0.264712 | 0.755264 | 0.72979 | 0.104345 | 1 | −0.45306 | −0.1958 | 0.634323 | 0.217609 | 0.122681 | 0.485112 | 0.087897 | 0.2646 |
| LVEF | 0.148994 | −0.76796 |
-0.87376 | −0.10148 | −0.45306 | 1 | 0.641915 | −0.65506 | 0.446832 | 0.329945 | 0.03045 | −0.11125 | −0.70099 |
| SV | 0.118443 | −0.53005 | −0.55627 | −0.08778 | −0.1958 | 0.641915 | 1 | −0.42981 | 0.43697 | 0.451114 | −0.02927 | −0.19523 | −0.74585 |
| WTI | 0.038453 |
0.869215 | 0.81406 | −0.41899 | 0.634323 | −0.65506 | −0.42981 | 1 | −0.42164 | −0.15663 | 0.2461 | 0.2027 | 0.541055 |
| SI | 0.310883 | −0.44544 | −0.38111 | 0.024197 | 0.217609 | 0.446832 | 0.43697 | −0.42164 | 1 | 0.505638 | 0.365323 | −0.10123 | −0.59027 |
| HR | 0.209062 | −0.24522 | −0.25119 | −0.13488 | 0.122681 | 0.329945 | 0.451114 | −0.15663 | 0.505638 | 1 | 0.423752 | 0.001773 | −0.50364 |
| LVW | 0.689122 | 0.226814 | 0.201095 | −0.1037 | 0.485112 | 0.03045 | −0.02927 | 0.2461 | 0.365323 | 0.423752 | 1 | 0.309013 | 0.029056 |
| SW | 0.07971 | 0.169746 | 0.129983 | −0.10337 | 0.087897 | −0.11125 | −0.19523 | 0.2027 | −0.10123 | 0.001773 | 0.309013 | 1 | 0.56361 |
| SA | −0.17566 | 0.65638 | 0.645036 | 0.110662 | 0.2646 | −0.70099 | −0.74585 | 0.541055 | −0.59027 | −0.50364 | 0.029056 | 0.56361 | 1 |
Time-independent regressions of
variables in all experimental groups
The time-independent regressions for SA, LV EF% and
LVEDD with respect to the estimated variables in rats without TH
intervention were estimated. The time-independent regression of SA,
with respect to the estimated variables, indicated correlations of
the ultrasound-estimated variables with the surgically estimated
parameters. In particular, we found that SA, LV weight and LV EF%
manifested significant linear correlations with respect to the
following variables: LV EF% vs. SA (R2=0.84)
(Fig. 6A); LVEDD vs. SA
(R2=0.76) (Fig.
6B); and LVESD vs. SA (R2=0.83) (Fig. 5C), irrespective of intervention
(SO, CAL, CALTH) and the time of sacrifice. We also observed that
the LV EF% exhibited significant linear behavior with respect to
the LVEDD (R2=0.82) (Fig. 6D). By contrast, the LV EF% did not
manifest significant linear correlation with respect to LV weight
(R2=0.12) (Fig.
6E), and neither did SI vs. SA (R2=0.46)
(Fig. 6F) or systolic velocity vs.
SA (R2=0.55) (Fig.
6G).
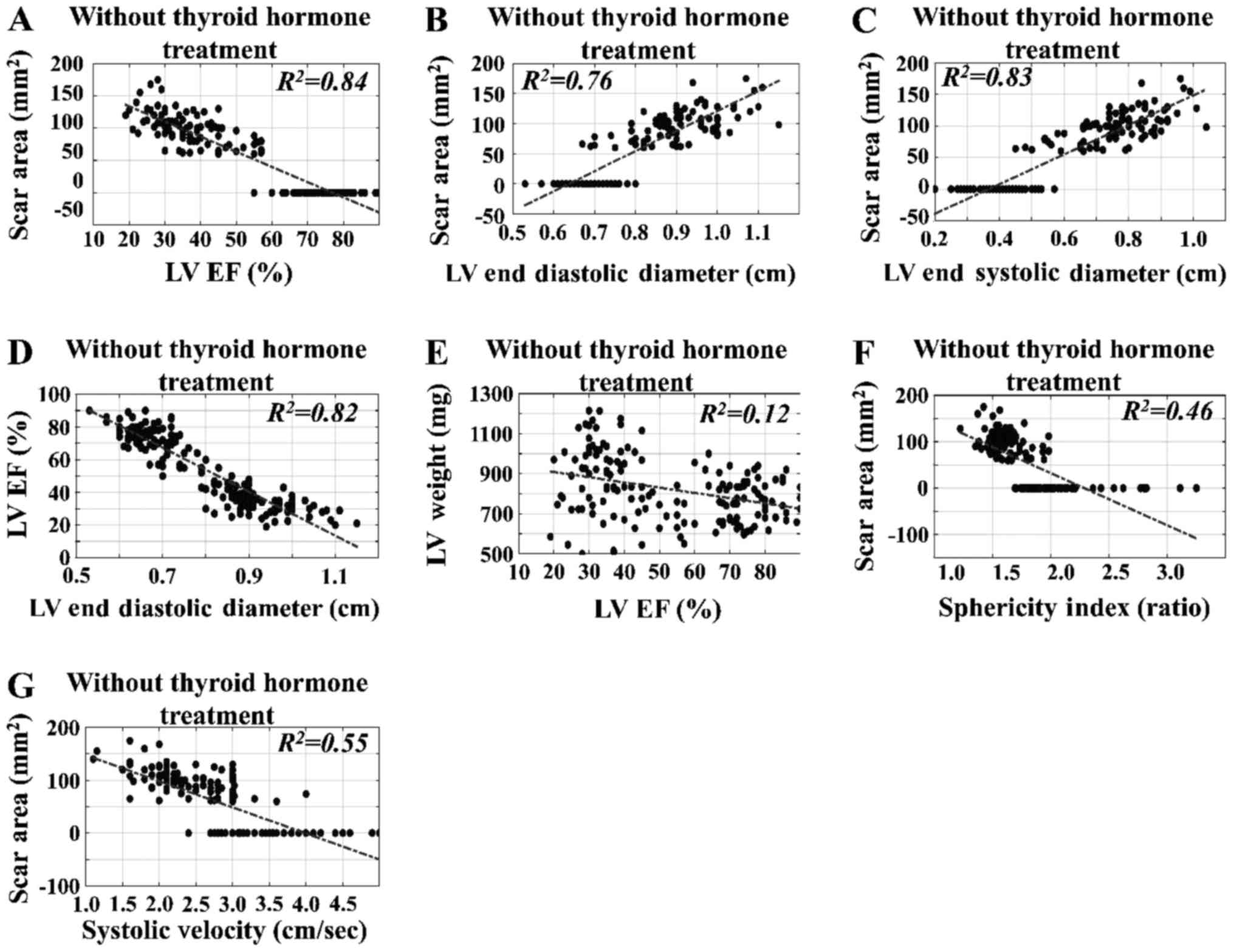 | Figure 6.Time-independent regressions for SA
with respect to estimated variables in rats without thyroid hormone
intervention. The time-independent regression of SA with respect to
the estimated variables indicated correlations between the
ultrasound-estimated variables and the surgically estimated
parameters. In particular, SA, LV weight and LV EF% had markedly
linear behavior with respect to the following variables: (A) LV EF%
vs. SA (R2=0.84), (B) LVEDD vs. SA
(R2=0.76), and (C) LVESD vs. SA
(R2=0.83), irrespective of the intervention and
time of sacrifice. It was also observed that (D) LV EF% manifested
linear behavior with LVEDD (R2=0.82); (E) LV EF%
did not manifest significant linear behavior with respect to LV
weight (R2=0.12); neither did (F) sphericity
index vs. SA (R2=0.46) or (G) systolic velocity
vs. SA (R2=0.55). LV, left ventricular; EF,
ejection fraction; SA, scar area; LVEDD, LV end diastolic diameter;
LVESD, LV end systolic diameter. |
Time-independent regressions of
variables in the CAL experimental groups
The time-independent regressions for SA, LV EF% and
LVEDD with respect to other variables in rats with TH intervention
were estimated. In contrast to the results obtained for rats
without TH treatment, we found that the SA, LV weight and LV EF%
did not exhibit significant linear correlation with respect to the
following variables: LV EF% vs. SA (R2=0.48)
(Fig. 7A); LVEDD vs. SA
(R2=0.41) (Fig.
7B); and LVESD vs. SA (R2=0.40) (Fig. 7C), irrespective of intervention and
the time of sacrifice. We also noted that LV EF% did not manifest
significant linear correlation with respect to the LVEDD
(R2=0.58) (Fig.
7D) or LV weight (R2=0.02) (Fig. 7E), and neither did SI vs. SA
(R2=0.33) (Fig.
7F) or systolic velocity vs. SA (R2=0.54)
(Fig. 7G). Finally, representative
echocardiographic images from the different groups under
investigation are presented in Fig.
8.
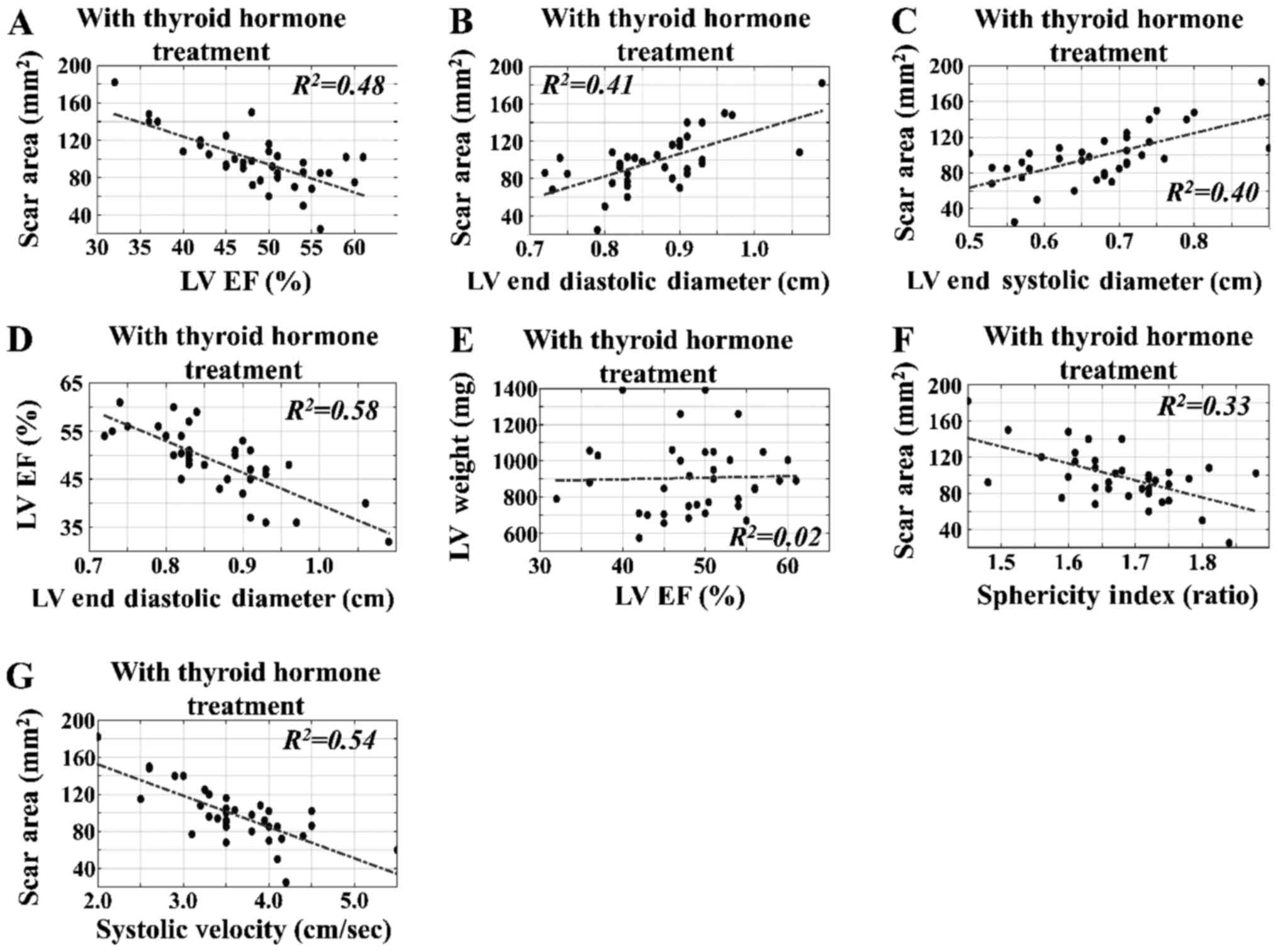 | Figure 7.Time-independent regressions for SA
with respect to estimated variables in rats with TH intervention.
The time-independent regression of SA with respect to the estimated
variables indicated correlations between the ultrasound-estimated
variables and the surgically estimated parameters. In contrast to
the results without TH treatment, SA, LV weight and LV EF% did not
exert significant linear behavior with respect to the following
variables: (A) LV EF% vs. SA (R2=0.48), (B) LVEDD
vs. SA (R2=0.41), and (C) LVESD vs. SA
(R2=0.40), irrespective of the intervention and
time of sacrifice. It was also noted that LV EF% did not have
significant linear behavior with respect to (D) LVEDD
(R2=0.58) or (E) LV weight
(R2=0.02) (E), and (F) neither did sphericity
index vs. SA (R2=0.33) or (G) systolic velocity
vs. SA (R2=0.54). LV, left ventricular; TH,
thyroid hormone; EF, ejection fraction; SA, scar area; LVEDD, LV
end diastolic diameter; LVESD, LV end systolic diameter. |
Discussion
The data included and analyzed in this study were
produced in our laboratory over a period of 10 years, using rats
subjected to CAL-induced AMI to study cardiac remodeling at
different time-points. A number of rats were treated with TH in
order to investigate its effects as a novel therapeutic approach.
Changes in LV morphology and function were evaluated via
echocardiography, and several measurements were included showing
the deterioration of global LV function (EF%), regional myocardial
function (systolic velocity of PW), the development of LV dilation
(LVEDD and LVESD) and hypertrophy (LV weight and PW thickness), as
well as changes in LV geometry (SI) and WTI. We also included
measurements that are considered to be important determinants of
cardiac function, including the degree of cardiac injury (assessed
based on SW and SA) and heart beats.
As expected, CAL resulted in LV dilation, which
manifested as increased LVEDD and LVESD, and as reduced global and
regional LV function. Furthermore, other manifestations of LV
remodeling included the development of cardiac hypertrophy,
increased wall stress and loss of the ellipsoid LV shape in favor
of a spherical shape (manifested as a reduced SI). The
time-dependent analysis of our results indicated a progressive
nature of myocardial remodeling leading to end-stage heart failure.
We found that global and regional LV function after MI
progressively deteriorated over 2, 4 and 13 weeks, indicating that
after the initial acute ischemic injury, the non-infarcted
myocardium progressively deteriorates, leading to end-stage heart
failure. In our experimental model, dilatation of the LV was shown
to develop early, reaching a plateau at 4 weeks and then remaining
stable between 4 and 13 weeks. Accordingly, pronounced LV
dilatation is observed in patients with MI between 4 days and 4
weeks (19). Conversely, our data
also showed that cardiac hypertrophy of the non-infarcted
myocardium developed slowly over time and reached a maximum at 13
weeks post-MI. This mismatch between LV dilatation and LV
hypertrophy may result in increased wall tension, particularly at 4
weeks after MI. Increased mechanical stress due to remodeling is
hypothesized to exacerbate the series of maladaptive events leading
to alterations of the contractile properties of the non-infarct
zone (20). The development of
cardiac hypertrophy at 13 weeks, despite its amelioration of wall
tension, is a maladaptive response due to its delayed onset and
association with unfavorable changes in MHC expression (more β-MHC
and less α-MHC) and calcium cycling proteins (15,16),
leading to a reduction in contractile function. Various approaches,
including cardiac restraint devices and hydrogel injections, have
been developed with the aim of potentially reducing mechanical
stress post-MI (20). Furthermore,
we reported that the LV geometry progressively deteriorated from an
ellipsoid towards a spherical shape over time. Altered geometry
per se can result in nearly a 50% decrease in EF% and is
associated with increased mortality (21), while it has been shown to predict
LV remodeling after MI (22). Our
results also indicate that the scar tissue expands over time
(increased SA and weight at13 weeks compared with at 2 weeks),
which is a characteristic of post-MI LV remodeling (19,20,22–24).
Our large-scale analysis also revealed the favorable effects of TH
treatment after CAL with respect to various parameters of cardiac
function. We found that TH increases global and regional LV
function after MI, and no reduction in functional indices was
observed between 2 and 13 weeks, showing that progressive
deterioration of the non-infarcted myocardium following the initial
acute ischemic injury is halted by TH administration. Furthermore,
in our model, TH was found to allow the initial development of
compensatory LV dilatation at 2 weeks, while inhibiting LV
dilatation between 2 and 13 weeks after CAL. These results indicate
that TH treatment inhibits the progressive development of LV
remodeling that leads to heart failure. Similarly, TH treatment
leads to the early development of cardiac hypertrophy at 2 weeks to
match the early increase in LV dimensions, and normalizes wall
tension as early as 2 weeks after MI, which indicates the
compensatory nature of this hypertrophic response. In addition, no
deterioration in mechanical stress, as measured by WTI, was
observed between 2 and 13 weeks. The quality of this TH-induced
hypertrophic response has been shown to be different, since TH
treatment promotes physiological growth of the non-infarcted
myocardium, as characterized by compensatory hypertrophy with
favorable changes in MHC expression (less β-MHC and increased
α-MHC) and calcium cycling proteins (15,16).
Similarly, in a mouse model of MI, we reported that TH replacement
therapy restored functional recovery, and that this effect was
associated with controlled activation of the pro-survival Akt
signaling pathway, consistent with physiologic growth (10,15,16).
Furthermore, our analysis showed that TH treatment inhibits the
development of unfavorable LV geometry changes that lead to a
spherical ventricle. Thus, TH treatment helps to retain the
ellipsoid shape of the LV over time, which is critical for
contractile performance. This may be of therapeutic relevance, as
the LV geometry appears to be an independent predictor of 10-year
survival in patients with AMI (25).
It is noteworthy that the expansion of the scar
tissue over time was not observed in TH-treated rats, which leads
to a significant reduction in SA at 13 weeks between CAL and CALTH
rats (25).
Regression analysis revealed that the observed
deterioration of myocardial function in CAL rats (reduced EF%), as
well as the extent of LV remodeling depicted by LV dilatation, was
closely associated with the extent of the injury (SA). These
findings are concordant with clinical observations showing that
infarct extension assessed with magnetic resonance imaging has a
strong negative association with myocardial systolic function
(26). Furthermore, changes in the
LV chamber dimensions during the first 6 months following MI in
patients are dependent on the infarct size (27). To the best of our knowledge, our
analysis showed for the first time that myocardial function, as
well as LV dilatation, after CAL and TH treatment is not closely
associated with the extent of injury, indicating that TH
administration may represent a novel therapeutic intervention
capable of modifying favorably the pathophysiology of post-MI
development of heart failure (26,27).
The present study could be considered as a first
step in creating a computational model to describe the phenomenon
of myocardial remodeling under TH treatment. Such modeling
approaches could be developed to enable simulation of the
pathophysiological processes following AMI, and to accurately
predict the effects of novel and/or current treatments that act via
modulation of tissue injury, LV dilation, LV geometry, hypertrophy
and regional contractile function. Efforts have been made to
simulate the post-infarction remodeling processes (28,29).
Successful implementation of such efforts, based on machine
learning depends on a large number of inputs (systems that learn
from data) (30). Data referred in
the present study have been performed by our group using exactly
the same experimental animal model for all reported experiments and
the same equipment. A specific group of scientists has performed
these experiments with a few additions over time. The problem of
using different animals or reagents exists in all types of
long-term experiments. This bias is consistent with the present
measurements. Further on, comparisons reported in the present study
included all measurements and in that sense they included the
experimental bias from all previous experimental setups. Thus,
significant differences observed appeared despite the time- and
handling-related bias. Although the aforementioned factors could be
considered as limitations of the study, our approach has included
the systematic bias and in that sense conclusions could be drawn on
the observed significant differences.
In conclusion, the novel findings from this
large-scale analysis showed that post-AMI TH treatment: i) Improves
LV function, but also inhibits the progressive deterioration over
time of the non-infarcted myocardium and the progressive
development of excessive LV dilatation; ii) inhibits expansion of
the scar tissue over time and leads to smaller infarcts in the
long-term; and iii) changes the pathophysiology of the development
of heart failure, since parameters such as myocardial function and
LV dilatation are not closely associated with the extent of the
injury.
Acknowledgements
The authors would like to thank The National and
Kapodistrian University of Athens, Medical School and The National
Technical University of Athens, School of Electrical and Computer
Engineering, Biomedical Engineering Laboratory (Athens, Greece) for
their support during the present study.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
II collected the data and drafted the manuscript. IM
collected the data, performed the experiments and data analysis,
and drafted the manuscript. GIL performed data analysis and Neural
Networks analysis, created the Matlab code and drafted the
manuscript. DI contributed to the conception and design of the
study, and drafted the manuscript. DDK was involved in the study
conception and design, proof-editing the manuscript and gave final
permission for publication. CP had substantial contribution to the
conception and design of the work, provided the data and funding,
proof-edited the manuscript and gave final permission for
publication.
Ethics approval and consent to
participate
The experimental protocols were approved by the
Bioethics Committee of the National and Kapodistrian University of
Athens Medical School (Athens, Greece; no.
1516013641/24/3/2010).
Consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AMI
|
acute myocardial infarction
|
|
BW
|
body weight
|
|
CAL
|
coronary artery ligation
|
|
CALTH
|
coronary artery ligation with thyroid
hormone treatment
|
|
DI
|
duration of intervention
|
|
ECG
|
electrocardiogram
|
|
LV
|
left ventricular
|
|
LVEF
|
LV-ejection fraction
|
|
LVESD
|
LV-end systolic diameter
|
|
LVLAD
|
LV-long-axis diameter
|
|
LVW
|
left ventricular weight
|
|
MHC
|
myosin heavy chain
|
|
SA
|
scar area
|
|
SI
|
sphericity index
|
|
SV
|
systolic velocity
|
|
SW
|
scar weight
|
|
TH
|
thyroid hormone
|
|
THT
|
thyroid hormone treatment
|
|
TR
|
thyroid hormone receptor
|
|
WTI
|
wall tension index
|
References
|
1
|
Rajabi M, Kassiotis C, Razeghi P and
Taegtmeyer H: Return to the fetal gene program protects the
stressed heart: A strong hypothesis. Heart Fail Rev. 12:331–343.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Swynghedauw B: Molecular mechanisms of
myocardial remodeling. Physiol Rev. 79:215–262. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lymvaios I, Mourouzis I, Cokkinos DV,
Dimopoulos MA, Toumanidis ST and Pantos C: Thyroid hormone and
recovery of cardiac function in patients with acute myocardial
infarction: A strong association? Eur J Endocrinol. 165:107–114.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Springeling T, Kirschbaum SW, Rossi A,
Baks T, Karamermer Y, Schulz C, Ouhlous M, Duncker DJ, Moelker A,
Krestin GP, et al: Late cardiac remodeling after primary
percutaneous coronary intervention-five-year cardiac magnetic
resonance imaging follow-up. Circ J. 77:81–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bolognese L, Neskovic AN, Parodi G,
Cerisano G, Buonamici P, Santoro GM and Antoniucci D: Left
ventricular remodeling after primary coronary angioplasty: Patterns
of left ventricular dilation and long-term prognostic implications.
Circulation. 106:2351–2357. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dargie H: Heart failure post-myocardial
infarction: A review of the issues. Heart. 91 Suppl 2:ii3–6;
discussion ii31, ii43-ii48. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Friberg L, Werner S, Eggertsen G and Ahnve
S: Rapid down-regulation of thyroid hormones in acute myocardial
infarction: Is it cardioprotective in patients with angina? Arch
Int Med. 162:1388–1394. 2002. View Article : Google Scholar
|
|
8
|
Cerillo AG, Storti S, Clerico A and
Iervasi G: Thyroid function and cardiac surgery: What should we
measure and when? Ann Thorac Surg. 89:1010–1012. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pingitore A, Landi P, Taddei MC, Ripoli A,
L'Abbate A and Iervasi G: Triiodothyronine levels for risk
stratification of patients with chronic heart failure. Am J Med.
118:132–136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mourouzis I, Mantzouratou P, Galanopoulos
G, Kostakou E, Roukounakis N, Kokkinos AD, Cokkinos DV and Pantos
C: Dose-dependent effects of thyroid hormone on post-ischemic
cardiac performance: Potential involvement of Akt and ERK
signalings. Mol Cell Biochem. 363:235–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pantos C, Mourouzis I and Cokkinos DV: New
insights into the role of thyroid hormone in cardiac remodeling:
Time to reconsider? Heart Fail Rev. 16:79–96. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pantos C, Mourouzis I and Cokkinos DV:
Rebuilding the post-infarcted myocardium by activating
‘physiologic’ hypertrophic signaling pathways: The thyroid hormone
paradigm. Heart Fail Rev. 15:143–154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kalofoutis C, Mourouzis I, Galanopoulos G,
Dimopoulos A, Perimenis P, Spanou D, Cokkinos DV, Singh J and
Pantos C: Thyroid hormone can favorably remodel the diabetic
myocardium after acute myocardial infarction. Mol Cell Biochem.
345:161–169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mourouzis I, Giagourta I, Galanopoulos G,
Mantzouratou P, Kostakou E, Kokkinos AD, Tentolouris N and Pantos
C: Thyroid hormone improves the mechanical performance of the
post-infarcted diabetic myocardium: A response associated with
up-regulation of Akt/mTOR and AMPK activation. Metabolism.
62:1387–1393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pantos C, Mourouzis I, Markakis K,
Dimopoulos A, Xinaris C, Kokkinos AD, Panagiotou M and Cokkinos DV:
Thyroid hormone attenuates cardiac remodeling and improves
hemodynamics early after acute myocardial infarction in rats. Eur J
Cardiothorac Surg. 32:333–339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pantos C, Mourouzis I, Markakis K,
Tsagoulis N, Panagiotou M and Cokkinos DV: Long-term thyroid
hormone administration reshapes left ventricular chamber and
improves cardiac function after myocardial infarction in rats.
Basic Res Cardiol. 103:308–318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pantos C, Mourouzis I, Tsagoulis N,
Markakis K, Galanopoulos G, Roukounakis N, Perimenis P, Liappas A
and Cokkinos DV: Thyroid hormone at supra-physiological dose
optimizes cardiac geometry and improves cardiac function in rats
with old myocardial infarction. J Physiol Pharmacol. 60:49–56.
2009.PubMed/NCBI
|
|
18
|
Grossman W, Jones D and McLaurin LP: Wall
stress and patterns of hypertrophy in the human left ventricle. J
Clin Invest. 56:56–64. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gaudron P, Eilles C, Kugler I and Ertl G:
Progressive left ventricular dysfunction and remodeling after
myocardial infarction. Potential mechanisms and early predictors.
Circulation. 87:755–763. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Holmes JW, Borg TK and Covell JW:
Structure and mechanics of healing myocardial infarcts. Annu Rev
Biomed Eng. 7:223–253. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sallin EA: Fiber orientation and ejection
fraction in the human left ventricle. Biophys J. 9:954–964. 1969.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li F, Chen YG, Yao GH, Li L, Ge ZM, Zhang
M and Zhang Y: Usefulness of left ventricular conic index measured
by real-time three-dimensional echocardiography to predict left
ventricular remodeling after acute myocardial infarction. Am J
Cardiol. 102:1433–1437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
D'Elia N, D'Hooge J and Marwick TH:
Association between myocardial mechanics and ischemic LV
remodeling. JACC Cardiovasc Imaging. 8:1430–1443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kichula ET, Wang H, Dorsey SM, Szczesny
SE, Elliott DM, Burdick JA and Wenk JF: Experimental and
computational investigation of altered mechanical properties in
myocardium after hydrogel injection. Ann Biomed Eng. 42:1546–1556.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wong SP, French JK, Lydon AM, Manda SO,
Gao W, Ashton NG and White HD: Relation of left ventricular
sphericity to 10-year survival after acute myocardial infarction.
Am J Cardiol. 94:1270–1275. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Palazzuoli A, Beltrami M, Gennari L,
Dastidar AG, Nuti R, McAlindon E, Angelini GD and Bucciarelli-Ducci
C: The impact of infarct size on regional and global left
ventricular systolic function: A cardiac magnetic resonance imaging
study. Int J Cardiovasc Imaging. 31:1037–1044. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Konermann M, Sanner BM, Horstmann E, Grötz
J, Laschewski F, Josephs W, Odenthal HJ and Sturm A: Changes of the
left ventricle after myocardial infarction-estimation with cine
magnetic resonance imaging during the first six months. Clin
Cardiol. 20:201–212. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goktepe S, Abilez OJ, Parker KK and Kuhl
E: A multiscale model for eccentric and concentric cardiac growth
through sarcomerogenesis. J Theor Biol. 265:433–442. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee LC, Wall ST, Genet M, Hinson A and
Guccione JM: Bioinjection treatment: Effects of post-injection
residual stress on left ventricular wall stress. J Biomech.
47:3115–3119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kourou K, Exarchos TP, Exarchos KP,
Karamouzis MV and Fotiadis DI: Machine learning applications in
cancer prognosis and prediction. Comput Struct Biotechnol J.
13:8–17. 2014. View Article : Google Scholar : PubMed/NCBI
|