Introduction
Goblet cells, which secret mucins, are expressed
throughout mammalian airway tracts, where they function in the
hydration, lubrication and clearance of particles and pathogens
from the underlying airway epithelium (1). Goblet cells in the lung are the
primary secretory cells in the superficial epithelium. Under normal
conditions, the secretion of airway mucus maintains airway
hydration and traps particles and pathogenic microorganisms
(2); however, mucus hypersecretion
is a hallmark of various pulmonary inflammatory diseases, including
chronic obstructive pulmonary disease (COPD), asthma and cystic
fibrosis (2). The major
gel-forming mucin in the human airway, mucin5AC (MUC5AC), is
primarily synthesized by goblet cells. Excessive secretion of
gel-forming mucins, MUC5AC in particular, has been observed in
mortalities that were a result of a severe asthma attack or acute
exacerbation of COPD (3).
The process of MUC5AC secretion into the airway
lumen is strictly controlled and occurs via calcium-dependent
exocytosis of MUC5AC-containing granules from epithelial goblet or
mucous cells. Dysregulation of mucin secretion leads to adverse
alterations in the mucociliary clearance activity and the
subsequent development of obstructive pulmonary disease. Mucin
secretion is a highly regulated process that requires certain
proteins involved in the exocytosis of mucin granules, such as
exocyst family members and ezrin (4,5), to
be recruited. The fusion of mucin granules and target cellular
membranes depends on the formation of soluble
N-ethylmaleimide-sensitive factor attachment protein receptor
(SNARE) compounds (6). The core
SNAREs, namely vesicle-associated membrane protein 8 (VAMP8), which
is a vesicular SNARE protein present on secretory vesicles
(v-SNAREs), and synaptosome-associated protein 23 (SNAP23), which
is a SNARE protein present on target membranes (t-SNAREs), in
airway goblet cells are responsible for the exocytosis of MUC5AC
granules (2,7). In calcium-dependent exocytosis,
granule activation is a key rate-limiting step; when MUC5AC
granules are activated, they acquire the ability to fuse with
target membranes, a process that depends on the presence of
calcium, diacylglycerol (DAG), syntaxin, synaptotagmin and other
factors (8).
Members of the Munc13 protein family act as
important activators of granule secretion in a wide variety of
mammalian cells. To date, four subtypes of Munc13, termed unc13
homologs A to D (Munc13-1 to Munc13-4), have been isolated from
mammalian cells. All of the Munc13 subtypes contain numerous C2
domains, some of which bind calcium and phospholipids, while others
appear to be specialized for protein-protein interactions (9,10).
Additionally, a C1 domain that is able to bind DAG is present in
all Munc13 subtypes, excluding Munc13-4; this site is also the
major pharmacological target of phorbol esters, which stimulate
synaptic transmission (11).
Munc13 proteins also contain a MUN domain, which is an α-helical
region that allows the direct or indirect interaction of Munc13
proteins with syntaxin. This interaction is essential in the
ability of the Munc13 family members to regulate granule exocytosis
(11). In neurons, Munc13-1
activation by DAG leads to the translocation of Munc13-1 to the
plasma membrane, where it subsequently forms a tripartite complex
with Rab3a on tethered vesicles and Rab3a-interacting molecule on
the plasma membrane; however, only Munc13-2 and Munc13-4 are
expressed in the human airway (12). Analysis in mice has demonstrated
that Munc13-2 is a mucin granule activator that aids the regulation
of baseline secretion (13).
Additionally, upon stimulation with extracellular ATP,
intracellular mucin release was maintained in Munc13-2-mutant mice,
indicating that other mechanisms may support agonist-regulated
mucin secretion (13). It has been
revealed that Munc13 may interact with distinct SNARE proteins,
such as those of the syntaxin family (14); however, whether Munc13-4, a
recently discovered Munc13 family member, participates in
agonist-stimulated MUC5AC granule secretion, and the associated
underlying mechanisms, requires further investigation (15).
Materials and methods
Cells, reagents and antibodies
SV40-immortalized human bronchial epithelial cells
(BEAS-2B) and a human lung adenocarcinoma cell line (Calu-3) were
purchased from the American Type Culture Collection (Manassas, VA,
USA). Fetal bovine serum (FBS) was purchased from Invitrogen
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Human
neutrophil elastase (hNE) and Dulbecco's modified Eagle's medium
(DMEM) were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). Primary antibodies, including rabbit anti-human syntaxin
2 (ab12369), rabbit anti-human Munc13-2 (ab97924), rabbit
anti-human Munc13-4 (ab109113) and mouse anti-human MUC5AC
antibodies (ab218466) were purchased from Abcam (Cambridge, MA,
USA). An internal reference β-actin antibody (TA890010) and
secondary antibodies, including horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG (TA130023), fluorescein
isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (TA130022),
and HRP-conjugated goat anti-mouse IgG (TA130005) were purchased
from OriGene Technologies, Inc. (Beijing, China).
Tetramethylrhodamine (TRITC)-conjugated goat anti-mouse IgG
(BA1089-0.5) was purchased from Wuhan Boster Biological Technology,
Ltd., Wuhan, China.
Cell culture
The BEAS-2B and Calu-3 cell lines were cultured in
DMEM with 10% FBS, penicillin (100 IU/ml) and streptomycin (100
IU/ml) at 37°C in a 5% CO2 incubator and were passaged
when cells were 80–90% confluent. Cells at the 3rd passage were
used for subsequent analysis.
MTT
[3-(4,5-dimethylthiazol-2-ul)-2,5-diphenyltetrazolium bromide]
assay
Cells treated with 100 nM hNE according to the
experimental requirements were plated at 96-well plates at a
density of 5,000 cells/well. The MTT reagent, with a finial
concentration of 5 mg/ml, (E606334-0500; Sangon Biotech Co., Ltd.,
Shanghai, China) was added to each well at the indicated time
points (0, 20, 40, 60 and 80 min) and incubated for 4 h at 37°C.
The optical density (OD) was read at 570 nm on a microplate
spectrophotometer. Each group had 5 repeat wells to ensure the
accuracy of the experiment.
Small interfering RNA (siRNA)
preparation and transfection
A human Munc13-2-specific siRNA plasmid, a human
Munc13-4-specific siRNA plasmid and control siRNA plasmids, were
transfected into cells. The vector pGC-silencer-U6/Neo/GFP was
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
siRNA sequences used were Munc13-2 5′-GGCCUGCUUGAACUCUACAUAUGAA-3′,
and Munc13-4 5′-CCAGCCCAGCUACACUGUACACUUU-3′. Munc13-2 and Munc13-4
control siRNAs were scrambled siRNAs containing the similar GU
content as Munc13-2 siRNA and Munc13-4 siRNA, respectively. Prior
to transfection, cells in the exponential growth phase were plated
in 6-well cell culture plates and incubated at 37°C for 12 h. Each
well had a basal area of 9.6 cm2 and contained
~4×106 cells. Following washing with PBS three times to
avoid any interference caused by antibiotics or serum, the cells
were transfected using FuGENE® HD reagent (E2311;
Promega Corporation, Madison, WI, USA) with Munc13-2-specific
siRNA, Munc13-4-specific siRNA or control siRNA (20 µg DNA: 60 µl
transfection reagent) at 22°C for 15 min according to the
manufacturer's protocol. Following transfection, cells were washed
with PBS three times. Cells were incubated in the full culture
medium for 24 h at 37°C, prior to western blotting, RT-qPCR, ELISA
and immunofluorescence).
Co-immunoprecipitation (CoIP)
CoIP was performed using a Co-Immunoprecipitation
kit (26149; Pierce; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. Cells were washed three times with PBS
and lysed on ice for 30 min using IP lysis buffer (Beyotime
Institute of Biotechnology, Shanghai, China) with protease
inhibitors (Thermo Fisher Scientific, Inc.). To remove nuclei and
intact cells, the lysates were centrifuged at 20,000 × g for 15 min
at 4°C. Protein A agarose was washed, diluted 50% with PBS and
added to the protein at a 1:10 (v/v) ratio. The mixture was
agitated on a shaking table for 30 min at 4°C and subsequently
centrifuged at 20,000 × g for 15 min at 4°C for supernatant
collection. The supernatants were standardized at 5 µg/µl for equal
protein loading via a Bicinchoninic Acid Protein assay kit
(Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. Subsequently, 5 µg rabbit anti-human
syntaxin 2 (1:200 dilution) was added to an Eppendorf tube
containing 500 µl cell lysate and the mixture was agitated on a
shaking table at 4°C overnight. The antigen-antibody complexes were
captured by adding 100 µl Protein A agarose and incubating at room
temperature for 90 min. Following centrifugation at 20,000 × g 4°C
for 1 min, the Protein A agarose bound to the antigen-antibody
complexes was washed three times with ice-cold PBS. The
precipitates were mixed with 5X western blot loading buffer and
boiled for 5 min. Following centrifugation at 20,000 × g 4°C for 15
min, the supernatants were separated via SDS-PAGE as described in
the following ‘western blotting’ section.
Western blotting
The expression levels of the proteins of interest in
the cellular lysate supernatants were detected via western
blotting. Generally, cells were washed with PBS three times and
lysed on ice for 20 min using an IPlysis buffer (P0013; Beyotime
Institute of Biotechnology). To remove nuclei and intact cells, the
lysates were centrifuged at 20,000 × g for 15 min at 4°C.
Supernatants were standardized for equal protein concentration (5
µg/µl) using a Bicinchoninic Acid Protein Assay kit (Beyotime
Institute of Biotechnology). Following separation by SDS-PAGE (6%
SDS separation gel for Munc13-2 and Munc13-4, 10% SDS separation
gel for syntaxin2 and β-actin), proteins were transferred onto
polyvinylidene difluoride (PVDF) membranes, which were blocked with
5% skimmed milk for 1 h at a room temperature (22°C) and incubated
with anti-Munc13-2 antibody (1:1,000 dilution), anti-Munc13-4
antibody (1:5,000 dilution), anti-human syntaxin 2 antibody
(1:1,000 dilution) and anti-β-actin antibody (1:200 dilution) at
4°C overnight. Following three washes with PBS with Tween-20
(0.05%) for 15 min each, the PVDF membranes were incubated with
HRP-conjugated goat anti-rabbit IgG at a 1:2,000 dilution for 2 h
at room temperature (22°C). The blots were visualized using
enhanced chemiluminescence according to the protocol of the
New-SUPER ECL kit (KGP1127; Nanjing KeyGen Biotech Co., Ltd.,
Nanjing, China). The intensity of each band was measured using a
Fluor-S Multi Imager and Quantity One version 4.6.2 software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The expression
levels of the proteins of interest were normalized to that of
β-actin.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from cells using TRIzol
(Thermo Fisher Scientific, Inc.). The extraction was confirmed by
RNA electrophoresis on a 1.5% agarose gel and an absorbance
(A260/280) value of 1.8–2.0 was deemed acceptable. RT
was performed using an iScript cDNA Synthesis kit (1708891; Bio-Rad
Laboratories, Inc.) according to the manufacturer's protocol. The
reaction conditions for RT were 5 min at 25°C, 30 min at 42°C, 5
min at 85°C, and hold at 4°C. Synthesized cDNA (0.1 µg/µl) was
prepared for qPCR, which was performed using iQ SYBR Green Supermix
(Bio-Rad Laboratories, Inc.) with qPCR primers in an iCycler
thermal cycler (Bio-Rad Laboratories, Inc.). Generally, qPCR
conditions were pre-denaturation at 95°C for 3 min followed by 35
cycles of denaturation at 95°C for 10 sec, annealing and elongation
at 55°C for 40 sec. To quantify the expression levels of MUC5AC,
Munc13-2 and Munc13-4 mRNA, GAPDH mRNA was used as an internal
control. All of the primers used for the qPCR experiments are
listed in Table I. The qPCR curves
were analyzed using CFX Manager™ software version 3.1 (Bio-Rad
Laboratories, Inc.) to obtain quantification cycle (Cq) values for
each sample (16). mRNA expression
was calculated based on the generated standard curve.
 | Table I.Reverse transcription-quantitative
polymerase chain reaction primers. |
Table I.
Reverse transcription-quantitative
polymerase chain reaction primers.
| Target gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| Munc13-2 |
CTTCCTACTCCTGTAAGCAGGG |
TGTTGACTGGCGCATTGTGG |
| Munc13-4 |
AGGGAAGCCCTTCATCCTGT |
TCTGTAGACAGCCAAACTCC |
| MUC5AC |
ACTTTGATGCTGAGCGGGATG |
CGAAGGCAATATCCTGTCTCTGTG |
| GAPDH |
AGAAGGCTGGGGCTCATTTG |
AGGGGCCATCCACAGTCTTC |
Detection of MUC5AC concentrations in
cell supernatants by ELISA
Secreted MUC5AC in cell culture supernatants was
assessed via ELISA assays. Then, 24 h following transfection, the
culture supernatants (50 µl/well) were added to a 96-well plate and
incubated at 40°C until dry. Following the washing of the wells and
blocking with 2% FBS (Gibco; Thermo Fisher Scientific, Inc.) for 1
h at room temperature, a mouse monoclonal antibody against MUC5AC
(1:200 dilution) was incubated in the wells for 1 h at room
temperature. The plates were washed three times with PBS and
incubated with 100 µl/well of HRP-conjugated goat anti-mouse IgG at
1:5,000 dilution. After 1 h incubation at room temperature, the
plates were washed three times with PBS.
3,3′,5,5′-tetramethylbenzidineperoxidase solution (P0209; Beyotime
Institute of Biotechnology) was used to produce a color reaction,
which was terminated upon the addition of
H2SO4. The absorbance was read at 450 nm and
the results were expressed as the ratio of MUC5AC to the
untransfected cells without hNE treatment (the CTL NT group).
Immunofluorescence staining and laser
confocal microscopy
Direct visual observation of Munc13 protein family
members and intracellular MUC5AC protein was performed by
immunofluorescence staining and laser confocal microscopy. Cells
were plated at a density of 2×105/ml on a glass
coverslip in each well of 24-well plates. Following three washes
with PBS, the cells were fixed with a formaldehyde solution freshly
prepared from 4% paraformaldehyde (dissolved in PBS) for 10 min at
room temperature and were washed again with PBS. The fixed cells
were permeabilized with 0.1% Triton X-100 in PBS for 3 min at room
temperature and washed three times with PBS. The cells were
subsequently blocked in 5% goat serum (AR0009; Wuhan Boster
Biological Technology, Ltd.) for 60 min and incubated with mouse
anti-MUC5AC antibody (1:500 dilution), rabbit anti-Munc13-2
antibody (1:100 dilution) or rabbit anti-Munc13-4 antibody (1:100
dilution) at 4°C overnight. Following three washes with PBS, the
slides were incubated with TRITC-linked goat anti-mouse IgG (1:200
dilution) or FITC-conjugated goat anti-rabbit IgG (1:200 dilution)
for 60 min at room temperature. The cells were then washed 3 times
with PBS and incubated with 100 ng/ml
4′,6-Diamidino-2-Phenylindole, Dihydrochloride (DAPI; C1002;
Beyotime Institute of Biotechnology) for 3 min at room temperature.
Following washing 5 times with PBS, the cells were mounted in 50%
glycerol and visualized using a confocal laser microscope (TCSSP2;
Leica Microsystems GmbH, Wetzlar, Germany). Representative images
were captured and processed with Adobe Photoshop version 7.0 (Adobe
Systems, Inc., San Jose, CA, USA). A total of 20 cells on each
slide were evaluated for each condition. The fluorescence intensity
(original magnification, ×400) and line scan (magnification, ×800)
analysis (17) were recorded and
calculated using Leica Microsystem (LeicaTCS SP2).
Statistical analysis
Data are presented as the mean ± standard deviation
of six independent experiments. All data were analyzed using the
SPSS 17.0 statistical package (SPSS, Inc., Chicago, IL, USA).
One-way analysis of variance followed by a Student-Newman-Keuls
test was performed to compare differences between groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
Cell viability and the effect of hNE
stimulation on Munc13 synthesis
Cell viability was assessed using a conventional MTT
reduction assay. The results revealed no significant decreases in
the viability of BEAS-2B or Calu-3 cells following exposure to 100
nM hNE for up to 1 h (Fig. 1).
Thus, 1 h was selected as the appropriate exposure time for
subsequent experiments.
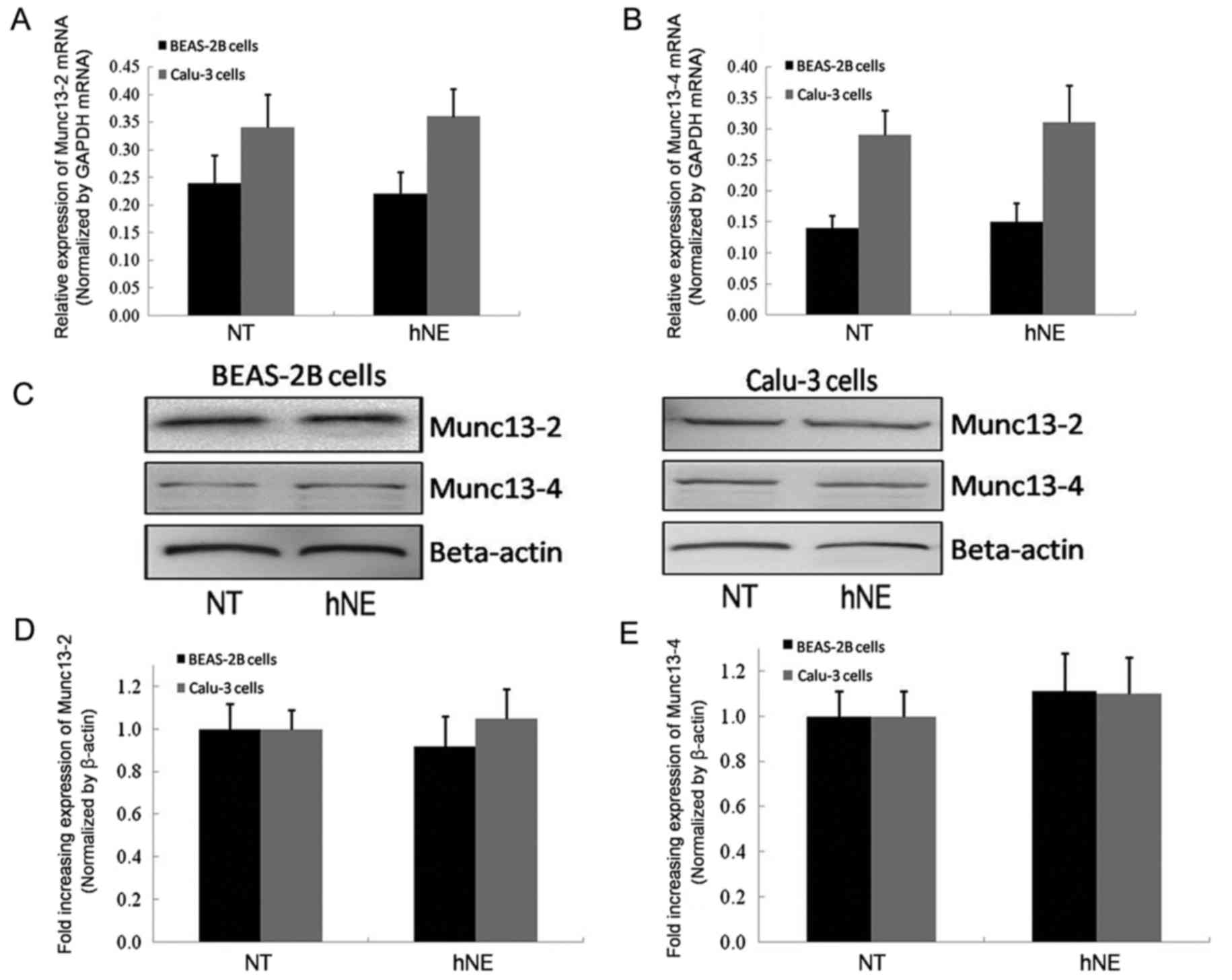
Subsequently, the present study investigated whether
hNE influenced the synthesis of Munc13-2 or Munc13-4 in human
airway epithelial cell lines. The results of RT-qPCR and western
blotting demonstrated stable and unaltered mRNA and protein
expression of Munc13-2 and Munc13-4 prior to and following
stimulation with hNE in both cell lines (Fig. 2). To examine the cellular
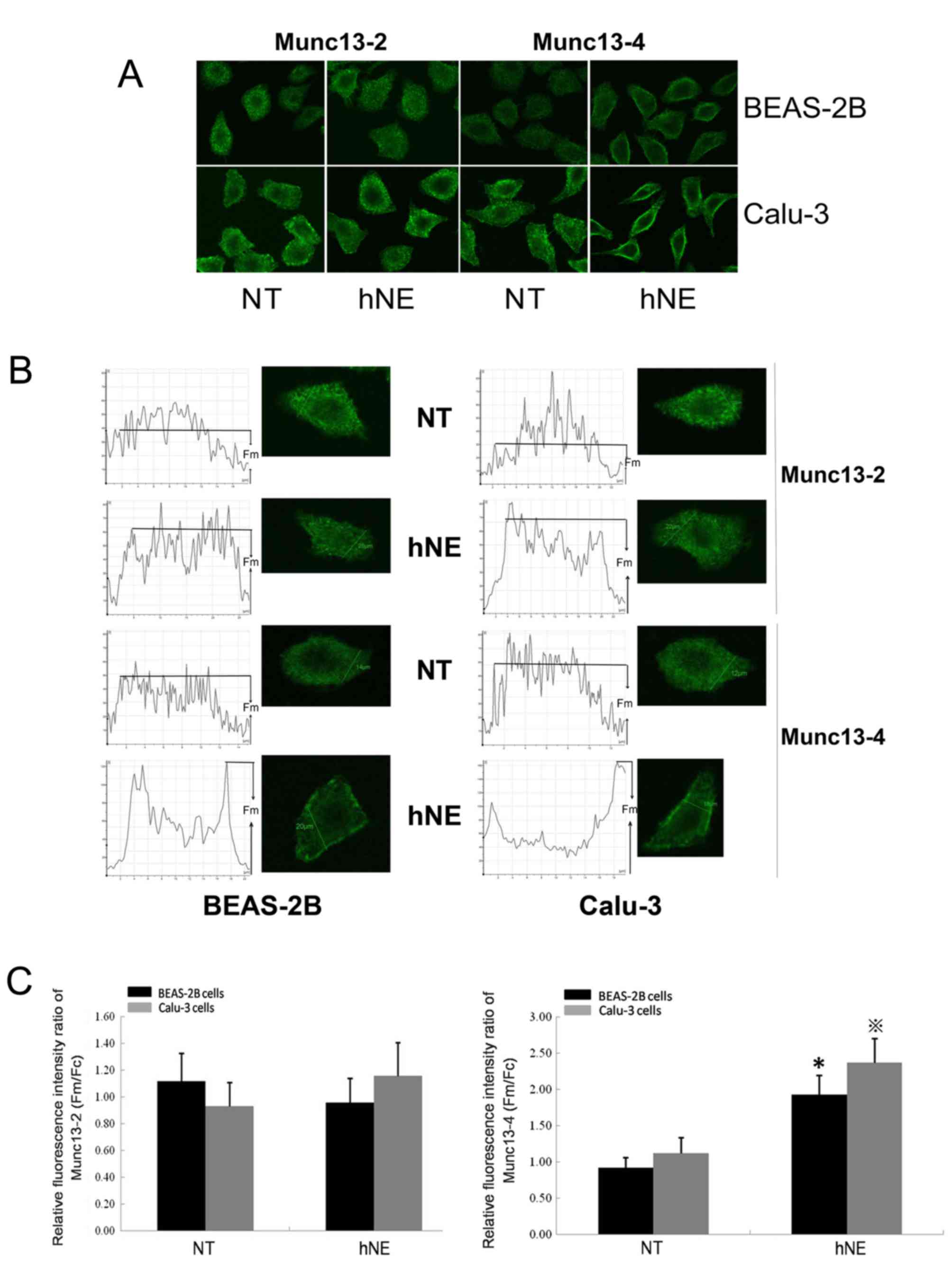
distribution of Munc13-2 and Munc13-4 in cells induced to
hypersecrete MUC5AC by hNE, the present study conducted cell
immunofluorescence staining and laser confocal microscopy of
BEAS-2B and Calu-3 cells (Fig.
3A). Upon hNE application, the plasma membrane fluorescence
intensity/cellular mean fluorescence intensity ratios of Munc13-4
were significantly increased compared with in the respective
control groups, indicating the recruitment of Munc13-4 to the
cellular membrane (Fig. 3). Prior
to hNE stimulation, Munc13-4 exhibited cytoplasmic distribution
(Fig. 3). Conversely, no marked
alterations in the cellular distribution of Munc13-2 were observed
upon hNE application (Fig. 3).
hNE increases the binding of Munc13-4
to syntaxin2 during MUC5AC hypersecretion
Previous in vitro studies using
membrane-integrated SNARE proteins to reveal the SNARE-binding
properties of the Munc13 protein family members have reported that
an interaction exists between the N-terminus of Munc13 and syntaxin
(14,18,19).
Syntaxin is a crucial SNARE component whose C-terminus binds to the
plasma membrane. Syntaxin 1, syntaxin 2 and syntaxin 3 are all
present in human airway epithelial cells (20,21)
and syntaxin 2 serves a crucial role in regulating the exocytosis
of mucin granules (22). In
mammalian endocrine cells, Munc13 was reported to competitively
bind to syntaxin and release Munc18 during the exocytosis of
secretory granules (SGs) (14,23).
In the present study, CoIP assays demonstrated that Munc13-2 binds
to syntaxin2 with or without hNE pretreatment in BEAS-2B and Calu-3
cells (Fig. 4). Conversely,
Munc13-4 demonstrated limited levels of binding to syntaxin2 in the
absence of hNE pretreatment, with enhanced binding observed
following pretreatment with hNE (Fig.
4).
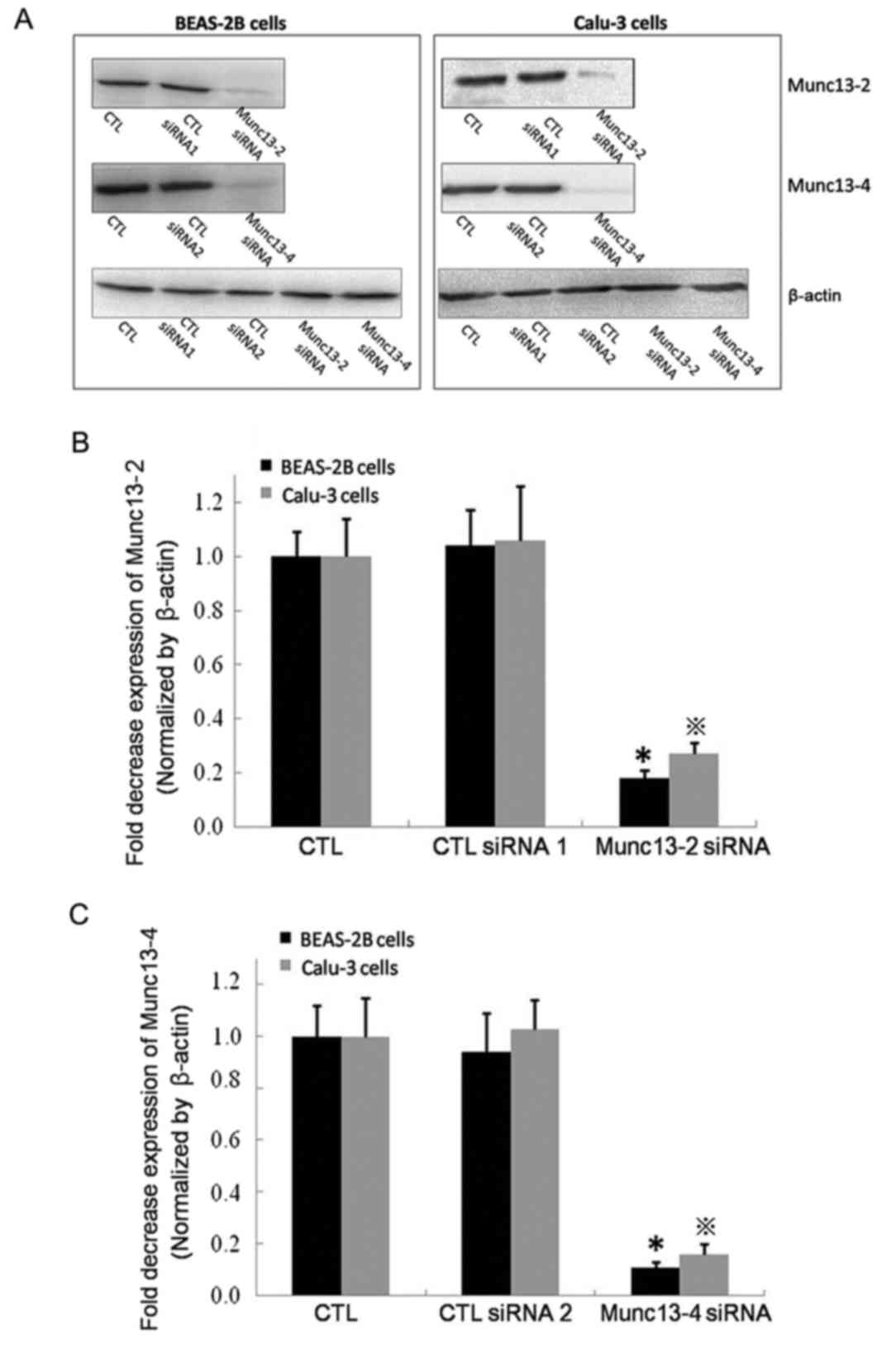
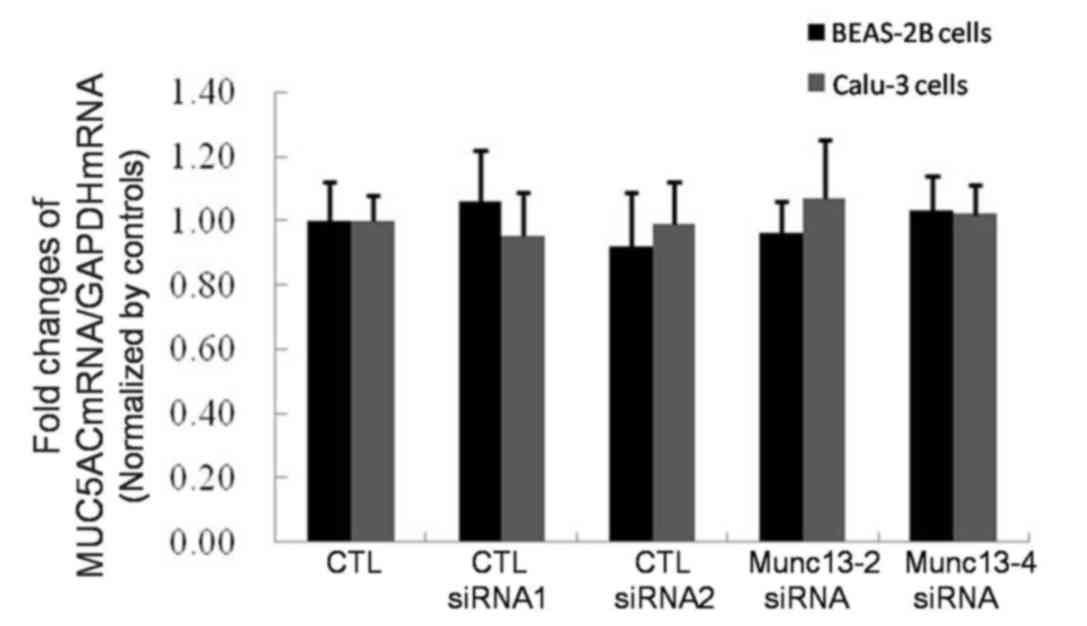
siRNA-mediated downregulation of
Munc13-2 or Munc13-4 does not affect MUC5AC mRNA expression
siRNA was used to inhibit Munc13-2 or Munc13-4
expression, and the downregulation of expression was verified by
western blotting (Fig. 5). MUC5AC
mRNA expression levels were investigated in untransfected cells and
Munc13-knockdown cells using RT-qPCR analysis. The untransfected
cells served as a negative control. The MUC5AC mRNA expression
levels were normalized to that of GAPDH and the results were
expressed as fold changes compared with the untransfected cells.
The findings of the present study revealed no significant
differences in MUC5AC mRNA expression among the Munc13-2 knockdown
cells, Munc13-4 knockdown cells, control siRNA-transfected cells
and untransfected cells (Fig.
6).
Munc13-4 participates in the MUC5AC
hypersecretion induced by hNE
As Munc13-4 is recruited to the plasma membrane and
interacts with syntaxin 2, which has been demonstrated to serve a
crucial role in MUC5AC granule exocytosis (22), the present study employed siRNA to
downregulate Munc13-2 or Munc13-4 in the two cell lines to
determine whether either of these proteins are necessary for the
hypersecretion of MUC5AC.
Compared with cells that did not receive hNE
stimulation, the quantity of retained intracellular MUC5AC protein
was marginally increased in the control untransfected cells and
control siRNA-transfected cells (CTL siRNA1 and CTL siRNA2)
following hNE stimulation (Fig.
7). In particular, the levels of retained intracellular MUC5AC
increased by ~1.54 and 1.29-fold in Munc13-2 siRNA-transfected
BEAS-2B and Calu-3 cells without hNE treatment, respectively,
compared with in wild-type cells without hNE treatment (Fig. 7). However, following stimulation
with hNE, there was no significant difference in intracellular red
fluorescence intensity between Munc13-2 siRNA-transfected and
wild-type cells (Fig. 7).
Furthermore, no significant differences in intracellular
fluorescence intensity were detected between Munc13-4-knockdown
cells and untransfected cells without hNE stimulation; however, a
~3-fold increase in intracellular fluorescence intensity was
detected in Munc13-4-knockdown BEAS-2B and Calu-3 cells compared
with in wild-type cells following stimulation with hNE (Fig. 7).
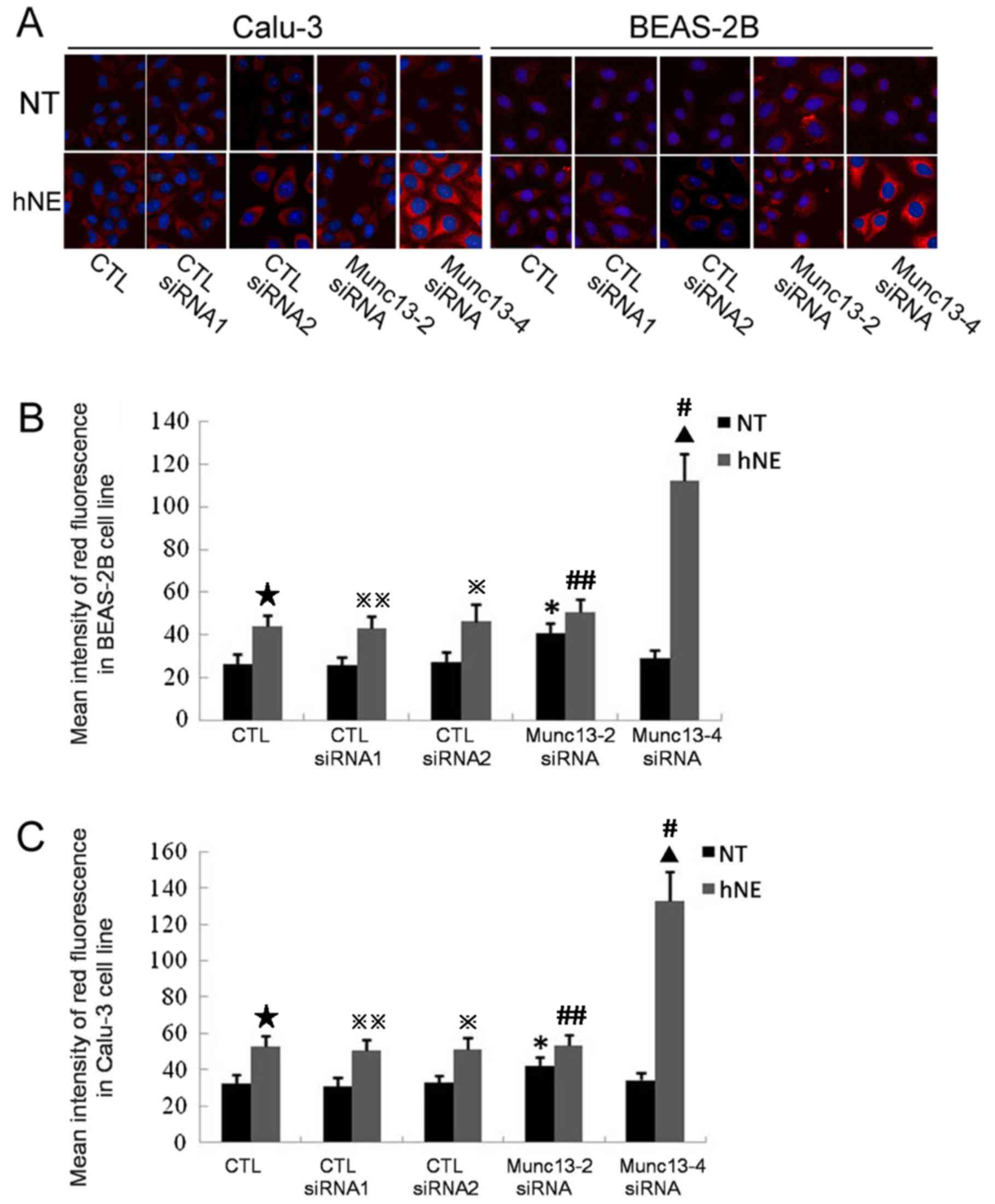 | Figure 7.Effects of Munc13-2 or Munc13-4 knock
down on the expression levels of retained intracellular MUC5AC.
Untransfected cells were employed as controls. Cells were
transfected with Munc13-2 siRNA, Munc13-4 siRNA or CTL siRNAs. CTL
siRNA1 exhibited a similar GC content to the Munc13-2-specific
siRNA, while CTL siRNA2 exhibited a similar GC content to the
Munc13-4-specific siRNA. Intracellular MUC5AC protein (red
fluorescence) was visualized with a tetramethylrhodamine-conjugated
secondary antibody bound to a MUC5AC primary antibody. Nuclei were
labeled with DAPI (blue fluorescence). hNE-treated cells were
exposed to a 100 nM hNE solution for 1 h. (A) Images were captured
using a laser confocal microscope at an original magnification of
×400 in six independent experiments. Quantification and statistical
analysis of intracellular MUC5AC in (B) BEAS-2B cells and (C)
Calu-3 cells. The red fluorescence intensity of 20 cells on each
slide was measured. The results were recorded and calculated using
Leica Microsystem software. *P<0.05 vs. CTL, CTL
siRNA1-transfected cells or CTL siRNA2-transfected cells with NT;
▲P<0.05 vs. CTL, CTL siRNA1-transfected cells or CTL
siRNA2-transfected cells with hNE treatment. ⋆P<0.05
vs. CTL with NT, ※P<0.05 vs. CTLsiRNA2-transfected
cells with NT, ※※P<0.05 vs. CTL siRNA1-transfected
cells with NT, #P<0.05 vs. Munc13-4-transfected cells
with NT, ##P<0.05 vs. Munc13-2-transfected cells with
NT. Munc13-2, unc-13 homolog B; Munc13-4, unc-13 homolog D; MUC5AC,
mucin 5AC; siRNA, small interfering RNA; CTL siRNA, control siRNA;
hNE, human neutrophil elastase; CTL group, untransfected cells; NT,
no hNE treatment. |
ELISAs were used to detect MUC5AC in cell
supernatants and the results indicated that Munc13-4 may serve a
major role in hNE-induced MUC5AC exocytosis, concordant with the
laser confocal microscopy analysis results of retained
intracellular MUC5AC. The concentrations of MUC5AC protein in cell
culture supernatants were presented as fold changes compared with
in untransfected cells without hNE stimulation (Fig. 8). Generally, neither the CTL siRNA
for Munc13-2 or Munc13-4 (CTL siRNA1 and 2, respectively)
significantly affected the MUC5AC protein concentration in the cell
culture supernatants prior to or following hNE stimulation
(Fig. 8). The levels of secreted
MUC5AC protein in the culture supernatants of Munc13-2-knockdown
BEAS-2B cells were decreased by ~0.30-fold compared with the
untransfected BEAS-2B cells without hNE treatment. However, the
levels between these two groups following hNE treatment became no
longer significantly different (Fig.
8A). Similar results were observed for Calu-3 cells (Fig. 8B). These findings are consistent
with analysis conducted within mouse models (13) and indicate that Munc13-2 may be a
key regulator of baseline MUC5AC secretion. The levels of secreted
MUC5AC protein in the culture supernatants of Munc13-4-knockdown
BEAS-2B cells were not significantly different from those in the
supernatants of untransfected BEAS-2B cells without hNE treatment,
whereas Munc13-4-knockdown BEAS-2B cells subjected to hNE
pretreatment exhibited only 60% of the secreted MUC5AC protein
levels of untransfected BEAS-2B cells (Fig. 8A). Similar data were obtained for
the Calu-3 cell line (Fig. 8B).
These results indicate that Munc13-4 may serve a crucial role in
the excess secretion of MUC5AC induced by hNE stimulation but may
not be involved in maintaining baseline secretion levels.
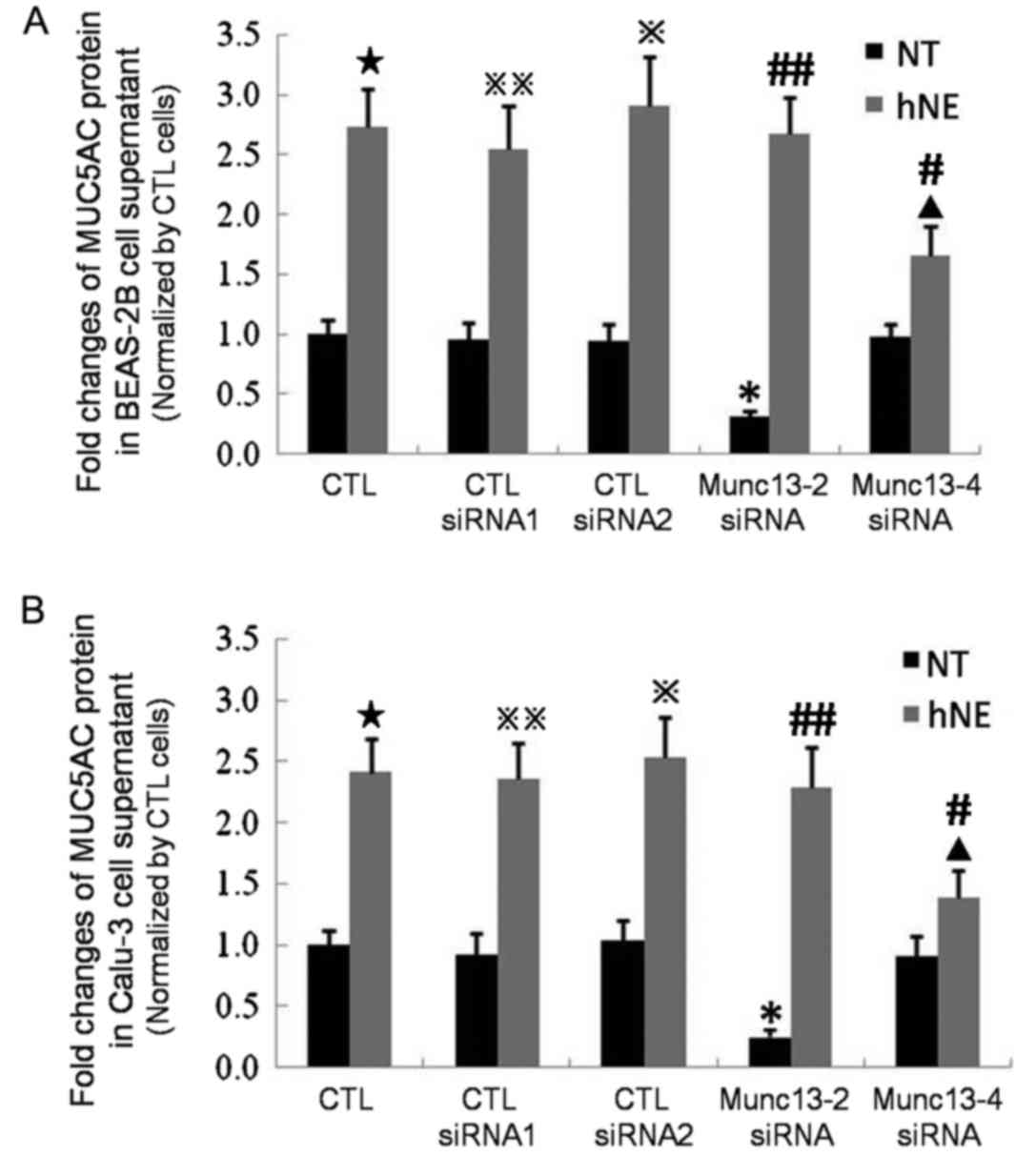 | Figure 8.Secreted MUC5AC protein expression
levels in the cell culture supernatants of Munc13-2- and
Munc13-4-knockdown BEAS-2B and Calu-3 cells were measured by ELISA.
Untransfected cells were employed as controls. Cells were
transfected with Munc13-2 siRNA, Munc13-4 siRNA or CTL siRNA. CTL
siRNA1 exhibited a similar GC content to the Munc13-2-specific
siRNA, while CTL siRNA2 exhibited a similar GC content to the
Munc13-4-specific siRNA. ELISA was performed to determine MUC5AC
protein levels in the cell culture supernatants of (A) BEAS-2B and
(B) Calu-3 cell lines. The results were recorded as fold changes
compared with the CTL group. *P<0.05 vs. CTL, CTL siRNA1 and CTL
siRNA2 NT groups; ▲P<0.05 vs. CTL, CTL siRNA1 and CTL
siRNA2 hNE groups. ⋆P<0.05 vs. CTL with NT,
※P<0.05 vs. CTL siRNA2-transfected cells with NT,
※※P<0.05 vs. CTL siRNA1-transfected cells with NT,
#P<0.05 vs. Munc13-4-transfected cells with NT,
##P<0.05 vs. Munc13-2-transfected cells with NT.
MUC5AC, mucin 5AC; Munc13-2, unc-13 homolog B; Munc13-4, unc-13
homolog D; siRNA, small interfering RNA; CTL siRNA, control siRNA;
CTL group, untransfected cells; hNE, human neutrophil elastase; NT,
no hNE treatment. |
Discussion
In respiratory diseases, various stimuli, including
infectious agents, irritants and allergy-associated stimuli, leads
to increases in mucin synthesis and the activation of the exocytic
pathway, which results in mucus hypersecretion. The process of
mucin secretion involves multiple steps and relies on the
recruitment of particular proteins, such as members of the exocyst
family and ezrin (4,5), that facilitate the activation and
exocytosis of mucin granules. In airway goblet cells, the exocytic
complex functions as essential fusion machinery for vesicle
trafficking during MUC5AC secretion; however, the molecular
composition of this complex and the mechanism of membrane fusion
during MUC5AC granule exocytosis are not fully established at
present.
The final steps of vesicular trafficking mediate the
docking and fusion of SGs with the plasma membrane. Previous
studies have identified the components of the exocytic machinery
involved in this process and have delineated their essential
functions in protists and mammalian nerve cells; however, the
tethering, docking and membrane fusion processes employed during
mucin granule exocytosis in the airway require further
investigation. The central components of this process are soluble
SNARE proteins present on secretory vesicles (v-SNAREs) and their
target membrane proteins (t-SNAREs) (24,25).
In MUC5AC SG exocytosis, the combination of VAMP8 and SNAP23 has
been demonstrated to be essential in the docking and fusion of SGs
and plasma membranes (26). As one
of the most important SG activators, the Munc13 family was
originally investigated in Caenorhabditis elegans. Further
studies in mammalian secretory cells revealed that the Munc13
family not only acts as the crucial activator of SGs in the nervous
system, but is also indispensable for the activation of SGs in
non-nervous system secretory cells (27,28).
The specific mechanisms involved in the promotion of SG activation
by Munc13 have not been fully elucidated. Studies in neuronal cells
have indicated that Munc13 may promote the depolymerization of
syntaxin-Munc18 complexes and maintain the stability of syntaxin,
which may promote the combination of VAMP and SNAP25 (23,29–32).
Previously, syntaxin2 was demonstrated to be localized in human
airway goblet cells and was implicated in regulating mucin SG
exocytosis (33).
In the present study, the functions of the Munc13
family in the BEAS-2B and Calu-3 immortalized human airway
epithelial cell lines were investigated, which are the most
commonly used cell lines for studies on airway mucin SG exocytosis
due to the similarity of their physical characteristics to those of
human airway goblet cells (33,34).
To date, two isoforms of the Munc13 family, Munc13-2 and Munc13-4,
have been isolated from the human airway epithelium (35). According to the in vitro
assay results of the present study, hNE may not influence the
synthesis of Munc13-2 or Munc13-4; however, hNE stimulation
recruited Munc13-4, but not Munc13-2, to the cellular plasma
membrane. The binding of Munc13-2 and Munc13-4 to syntaxin 2 was
also investigated in the present study, which is the major syntaxin
subtype involved in airway mucin SG exocytosis. The present study
reported that Munc13-2 exhibits a stable bond with syntaxin 2 in
response to with or without hNE stimulation; syntaxin2-Munc13-4
complexes were rare in both cell lines, but they increased in
number following stimulation with hNE. These results indicate that
Munc13-4 may be sensitive to hNE stimulation during airway mucin
hypersecretion. To further investigate this hypothesis, siRNA was
employed to individually downregulate Munc13-2 and Munc13-4 within
the two cell lines and analyze the subsequent secretion of MUC5AC
in the present study. No significant differences in MUC5AC mRNA
expression were detected in untransfected, Munc 13-2-knockdown or
Munc13-4-knockdown cells for either the BEAS-2B or Calu-3 cell
lines. Munc13-2-knockdown cells partially lost their baseline
MUC5AC exocytosis ability, where as Munc13-4-defective cells
retained this ability. These data indicate that Munc13-2, but not
Munc13-4, may be an essential regulator of baseline MUC5AC
exocytosis, which is consistent with previous investigation within
a mouse model (13). hNE was
employed in the present study to induce MUC5AC hypersecretion in
both cell lines. Unlike Munc13-4-knockdown cells,
Munc13-2-knockdown cells retained the majority of their sensitivity
to hNE when in MUC5AC hypersecretion mode, indicating that Munc13-4
may be an essential regulator of MUC5AC SG hypersecretion, at least
in the presence of hNE. Conversely, Munc13-2 appears to participate
in baseline MUC5AC exocytosis rather than agonist-induced MUC5AC
hypersecretion. The present study indicated the crucial role of
Munc13-2 in the basal MUC5AC exocytosis and the essential role of
Munc13-4 in hNE-induced MUC5AC hypersecretion mode. Beyond core
complex composed by v-SNARE and t-SNARE, the tethering docking and
releasing of secretory granules (SGs) depend on a series of
multi-subunit protein complexes, including EEA1, the Rab family and
its effector (36). The complex
interrelationships between these proteins and the core complex are
understood in yeast and neuronal SGs. However, they are still
ambiguous in nonneuronal SGs. As a majority of the functional
relationships between the SNARE complex members in the exocytosis
of airway MUC5AC SGs are still unknown, further researches on the
functional bindings of SNARE complex members in the tethering
docking and releasing of MUC5AC SGs will be performed in the
future. In conclusion, Munc13 family members act as important
activators of MUC5AC granule secretion. Munc13-2 may be an
essential regulator of baseline MUC5AC exocytosis, whereas Munc13-4
appears to be sensitive to hNE stimulation during airway MUC5AC
hypersecretion. The present study has extended our current
knowledge about the tethering/docking procedure during MUC5AC
hyper-secretion and provides potential targets for the development
of novel therapy in the management of post-infectious airway mucus
hypersecretion.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Nature Science Foundation of China (grant nos. 81660010,
81500015 and 81611530713) and Russian Foundation for Basic Research
(RFBR, grant no. 17-54-53162).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XZ designed the research and participated in its
coordination. RX carried out the CoIP and western blotting
experiments and participated in drafting the manuscript. QL
performed the RT-qPCR and confocal microscopy analyses. JMP and VPK
prepared the siRNA and cell transfection. JZ performed the
statistical analysis. All authors were involved in the data
interpretation and writing of the paper. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thai P, Loukoianov A, Wachi S and Wu R:
Regulation of airway mucin gene expression. Annu Rev Physiol.
70:405–429. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Turner J and Jones CE: Regulation of mucin
expression in respiratory diseases. Biochem Soc Trans. 37:877–881.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Voynow JA and Rubin BK: Mucins, mucus, and
sputum. Chest. 135:505–512. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kimura RE: Fatty acid metabolism in the
fetus. Semin Perinatol. 13:202–210. 1989.PubMed/NCBI
|
|
5
|
Li Q, Li N, Liu CY, Xu R, Kolosov VP,
Perelman JM and Zhou XD: Ezrin/Exocyst complex regulates mucin 5AC
secretion induced by neutrophil elastase in human airway epithelial
cells. Cell Physiol Biochem. 35:326–338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adler KB, Tuvim MJ and Dickey BF:
Regulated mucin secretion from airway epithelial cells. Front
Endocrinol (Lausanne). 4:1292013.PubMed/NCBI
|
|
7
|
Jones LC, Moussa L, Fulcher ML, Zhu Y,
Hudson EJ, O'Neal WK, Randell SH, Lazarowski ER, Boucher RC and
Kreda SM: VAMP8 is a vesicle SNARE that regulates mucin secretion
in airway goblet cells. J Physiol. 590:545–562. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tuvim MJ, Mospan AR, Burns KA, Chua M,
Mohler PJ, Melicoff E, Adachi R, Ammar-Aouchiche Z, Davis CW and
Dickey BF: Synaptotagmin 2 couples mucin granule exocytosis to Ca2+
signaling from endoplasmic reticulum. J Biol Chem. 284:9781–9787.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feldmann J, Callebaut I, Raposo G, Certain
S, Bacq D, Dumont C, Lambert N, Ouachée-Chardin M, Chedeville G,
Tamary H, et al: Munc13-4 is essential for cytolytic granules
fusion and is mutated in a form of familial hemophagocytic
lymphohistiocytosis (FHL3). Cell. 115:461–473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kabachinski G, Yamaga M, Kielar-Grevstad
DM, Bruinsma S and Martin TF: CAPS and Munc13 utilize distinct
PIP2-linked mechanisms to promote vesicle exocytosis. Mol Biol
Cell. 25:508–521. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rhee JS, Betz A, Pyott S, Reim K,
Varoqueaux F, Augustin I, Hesse D, Südhof TC, Takahashi M,
Rosenmund C and Brose N: Beta phorbol ester- and
diacylglycerol-induced augmentation of transmitter release is
mediated by Munc13s and not by PKCs. Cell. 108:121–133. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Andrews-Zwilling YS, Kawabe H, Reim K,
Varoqueaux F and Brose N: Binding to Rab3A-interacting molecule RIM
regulates the presynaptic recruitment of Munc13-1 and ubMunc13-2. J
Biol Chem. 281:19720–19731. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu Y, Ehre C, Abdullah LH, Sheehan JK,
Roy M, Evans CM, Dickey BF and Davis CW: Munc13-2-/- baseline
secretion defect reveals source of oligomeric mucins in mouse
airways. J Physiol. 586:1977–1992. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boswell KL, James DJ, Esquibel JM,
Bruinsma S, Shirakawa R, Horiuchi H and Martin TF: Munc13-4
reconstitutes calcium-dependent SNARE-mediated membrane fusion. J
Cell Biol. 197:301–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elstak ED, te Loo M, Tesselaar K, van
Kerkhof P, Loeffen J, Grivas D, Hennekam E, Boelens JJ,
Hoogerbrugge PM, van der Sluijs P, et al: A novel Dutch mutation in
UNC13D reveals an essential role of the C2B domain in munc13-4
function. Pediatr Blood Cancer. 58:598–605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li M, Li Q, Yang G, Kolosov VP, Perelman
JM and Zhou XD: Cold temperature induces mucin hypersecretion from
normal human bronchial epithelial cells in vitro through a
transient receptor potential melastatin 8 (TRPM8)-mediated
mechanism. J Allergy Clin Immunol. 128(626–634): e1–e5. 2011.
|
|
18
|
Guan R, Dai H and Rizo J: Binding of the
Munc13-1 MUN domain to membrane-anchored SNARE complexes.
Biochemistry. 47:1474–1481. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Daily NJ, Boswell KL, James DJ and Martin
TF: Novel interactions of CAPS (Ca2+-dependent activator protein
for secretion) with the three neuronal SNARE proteins required for
vesicle fusion. J Biol Chem. 285:35320–35329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kreda SM, Okada SF, van Heusden CA, O'Neal
W, Gabriel S, Abdullah L, Davis CW, Boucher RC and Lazarowski ER:
Coordinated release of nucleotides and mucin from human airway
epithelial Calu-3 cells. J Physiol. 584:245–259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shukla A, Berglund L, Nielsen LP, Nielsen
S, Hoffmann HJ and Dahl R: Regulated exocytosis in immune function:
Are SNARE-proteins involved? Respir Med. 95:773–780. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
ter Beest MB, Chapin SJ, Avrahami D and
Mostov KE: The role of syntaxins in the specificity of vesicle
targeting in polarized epithelial cells. Mol Biol Cell.
16:5784–5792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sassa T, Harada S, Ogawa H, Rand JB,
Maruyama IN and Hosono R: Regulation of the UNC-18-Caenorhabditis
elegans syntaxin complex by UNC-13. J Neurosci. 19:4772–4777. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Davis CW and Dickey BF: Regulated airway
goblet cell mucin secretion. Annu Rev Physiol. 70:487–512. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brunger AT: Structure and function of
SNARE and SNARE-interacting proteins. Q Rev Biophys. 38:1–47. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stackl W, Hasun R and Marberger M:
Intracavernous injection of prostaglandin E1 in impotent men. J
Urol. 140:66–68. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elstak ED, Neeft M, Nehme NT, Voortman J,
Cheung M, Goodarzifard M, Gerritsen HC, van Bergen En, Henegouwen
PM, Callebaut I, de Saint Basile G and van der Sluijs P: The
munc13-4-rab27 complex is specifically required for tethering
secretory lysosomes at the plasma membrane. Blood. 118:1570–1578.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Caviglia S, Brankatschk M, Fischer EJ,
Eaton S and Luschnig S: Staccato/Unc-13-4 controls secretory
lysosome-mediated lumen fusion during epithelial tube anastomosis.
Nat Cell Biol. 18:727–739. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Betz A, Ashery U, Rickmann M, Augustin I,
Neher E, Südhof TC, Rettig J and Brose N: Munc13-1 is a presynaptic
phorbol ester receptor that enhances neurotransmitter release.
Neuron. 21:123–136. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rizo J and Südhof TC: Snares and Munc18 in
synaptic vesicle fusion. Nat Rev Neurosci. 3:641–653. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu D, Xie L, Kang Y, Dolai S, Hansen
Bondo J, Qin T, Xie H, Liang T, Rubin DC, Osborne L and Gaisano HY:
Syntaxin 2 acts as inhibitory SNARE for insulin granule exocytosis.
Diabetes. 66:948–959. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Christie MP, Hu SH, Whitten AE, Rehman A,
Jarrott RJ, King GJ, Collins BM and Martin JL: Revisiting
interaction specificity reveals neuronal and adipocyte Munc18
membrane fusion regulatory proteins differ in their binding
interactions with partner SNARE Syntaxins. PLoS One.
12:e01873022017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ou SK, McDonald C and Patterson PH:
Comparison of two techniques for targeting the production of
monoclonal antibodies against particular antigens. J Immunol
Methods. 145:111–118. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou J, Perelman JM, Kolosov VP and Zhou
X: Neutrophil elastase induces MUC5AC secretion via
protease-activated receptor 2. Mol Cell Biochem. 377:75–85. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Koch H, Hofmann K and Brose N: Definition
of Munc13-homology-domains and characterization of a novel
ubiquitously expressed Munc13 isoform. Biochem J. 349:247–253.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grosshans BL, Ortiz D and Novick P: Rabs
and their effectors: Achieving specificity in membrane traffic.
Proc Natl Acad Sci USA. 103:11821–11827. 2006. View Article : Google Scholar : PubMed/NCBI
|