Introduction
Prostate cancer (PCa) is one of the most prevalent
malignancies in men in the USA and worldwide, and is the second
leading cause of cancer associated mortality in men (1,2).
Each year, more than 913,000 novel cases are diagnosed worldwide,
resulting in the mortalities of >261,000 patients (3). In addition, the incidence and
mortality rate of PCa in China continues to rapidly increase,
particularly in patients suffering from obesity or diabetes
(4,5). Androgen deprivation therapy has been
previously demonstrated to be effective in ~90% of patients with
PCa. However, androgen deprivation therapy may develop into
androgen-independent PCa and eventually into lethal,
castration-resistant PCa (6).
Researchers have developed second generations of hormone therapy,
such as abiraterone and enzalutamide for the treatment of PCa
(7,8). Despite such therapies improving
overall survival, the majority of patients with PCa develop
resistance following initial treatment, and so further
investigation is required to develop novel therapeutic treatment
strategies with a low toxicity.
Biofunctional peptides (2–20 amino acids) are
protein fragments that may exhibit numerous physiological effects,
such as anticancer (9,10), anti-thrombosis (11), antioxidant (12,13),
anti-fatigue (14,15) and antimicrobial (16,17).
Recently, numerous antitumor peptides have been isolated from
marine derived protein hydrolysates to induce PCa cell apoptosis
(18–20). Huang et al (18) demonstrated that a tripeptide
(Gln-Pro-Lys; QPK) isolated from sepia ink inhibits the
proliferation of numerous human PCa lines (DU-145, PC-3 and LNCaP);
whereas Song et al (19)
revealed that a hexapeptide (Tyr-Ala-Leu-Arg-Ala-His; YALRAH),
obtained from the heated products of protein hydrolysates isolated
from Setipinna taty, inhibits PC-3 cell proliferation.
Furthermore, Kim et al (20) demonstrated that a decapeptide
(Ala-Val-Leu-Val-Asp-Lys-Gln-Cys-Pro-Asp; AVLVDKQCPD), isolated
from the protein hydrolysates of Ruditapes philippinarum,
inhibits the proliferation of PCa cells. Notably, numerous
biofunctional peptides have been purified from fish sources,
whereas only a number of studies have investigated proteins
obtained from crustacean and mollusk sources. Cyclina
sinensis, a bivalve mollusk belonging to the Veneridae family,
has been used in traditional Chinese medicine for the treatment of
inflammation, asthma and dental ulcers (21,22).
Furthermore, it has been revealed that Cyclina sinensis has
a high quantity of protein, polysaccharides and lipids, which may
attribute to its therapeutic effects, such as anticancer,
antioxidant and hepatoprotective activities (21–23).
Jiang et al (22,23)
reported that the polysaccharide fraction of Cyclina
sinensis (CSPS) exhibits significant inhibitory effects against
human gastric cancer BGC-823 cells in vivo. In our previous
study (24), the
anti-proliferative potential of protein hydrolysates isolated from
Cyclina sinensis was determined; however, the active
component in the protein hydrolysates was not investigated further.
Therefore, the present study aimed to isolate the pentapeptide
(Ile-Leu-Tyr-Met-Pro; ILYMP) of interest from Cyclina
sinensis protein hydrolyastes via ultrafiltration as well as
chromatographic methods, and was subsequently named CSP. The effect
of CSP on the PCa cell line DU-145 was investigated using
methylthiazolyldiphenyl-tetrazolium bromide (MTT) assays, acridine
orange/ethidium bromide double staining (AO/EB), scanning electron
microscopy and flow cytometry. Furthermore, western blotting was
performed, and the results demonstrated that the Bcl-2-associated X
(Bax), cleaved caspase-3 and cleaved caspase-9 proteins were
activated in CSP-treated DU-145 cells; whereas B-cell lymphoma-2
(Bcl-2) was suppressed in CSP-treated DU-145 cells. These results
suggest that CSP can inhibit the proliferation of human PCa cells
and may represent a therapeutic nutraceutical agent for the
treatment and prevention of PCa.
Materials and methods
Materials
Cyclina sinensis were purchased from a local
fish market in Zhoushan, China. Trypsin and neutral protease were
purchased from YTHX Biotechnology Co., Ltd. (Beijing, China). MTT
and Annexin V-FITC apoptosis detection kits were purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). The DU-145 PCa cell
lines and NIH-3T3 cell lines were purchased from the Shanghai
Institute of Biochemistry and Cell Biology (Shanghai, China).
Antibodies against β-actin (cat. no. AA132), Bax (cat. no. AB026),
Bcl-2 (cat. no. AB112), caspase-3 (cat. no. AC030) and caspase-9
(cat. no. AC062) were purchased from Beyotime Institute of
Biotechnology (Shanghai, China). Horseradish peroxidase-conjugated
goat-anti-rabbit secondary antibodies (cat. no. A0208) were
purchased from Beyotime Institute of Biotechnology. All other
reagents used were of analytical grade.
Fractionation of protein hydrolysates
by ultrafiltration
Cyclina sinensis were hydrolyzed using
neutral protease under conditions: 1,200 U/g, solid-liquid ratio
1:4, (pH 7.0) at 45°C for 6 h. The protein hydrolysates were then
fractionated using ultrafiltration (Amicon 8400; EMD Millipore,
Billerica, MA, USA) with 3, 5 and 8 kDa molecular weight cut-off
membranes at 0.30 MPa, 20°C. The fractions were then collected as
follows: >8, 8–5, 5-3, and <3 kDa. Fractions were then
lyophilized at −60°C to further investigate whether Cyclina
sinensis exhibits an antitumor effect on DU-145 cells.
Gel filtration chromatography
The elution with the highest antitumor activity
following ultrafiltration was dissolved in 0.1 M Tris-HCl (0.05
g/ml, pH 7.0), 500 µl sample was added to a Superose 12 10/300 GL
(GE Healthcare, Chicago, IL, USA; 10×300 mm) pre-equilibrated with
Tris-HCl (0.1 M, pH 7.0) and then eluted at a flow rate of 1 ml/min
using an AKTA purifier 100 (GE Healthcare) at room temperature.
Fractions were isolated and detected at 280 nm, and the elution
peaks were then isolated and lyophilized at −60°C to investigate
antitumor activity analysis.
High performance liquid chromatography
(HPLC)
The elution with the highest antitumor activity was
further separated using reverse phase (RP)-HPLC (Agilent 1260;
Agilent Technologies, Inc., Santa Clara, CA, USA) on a Agilent
Zorbax SB-C18 (4.6×250 mm; 5 µm) column with a linear gradient of
acetonitrile (0–7%) containing 0.06% trifluoroacetic acid at a flow
rate of 1.0 ml/min. The purification was repeated >20 times at
the same elution conditions and the final purified peptide (CSP)
was collected and subsequently lyophilized at −60°C to determine
its amino acid sequence as well as its antitumor activity against
DU-145 cells.
Determination of amino acid sequence
and molecular mass of CSP
CSP was dissolved in 15 µl 37% CH3CN
(v/v) solution and applied to TFA-treated glass fiber filters
(Shimadzu Corporation, Kyoto, Japan) and then sequenced at the
N-terminus using a PPSQ-31A protein sequencer (Shimadzu
Corporation). A mass spectrometer (Waters ZQ 2000; Waters GmbH,
Eschborn, Germany) combined with an electrospray ionization source
was used to determine the molecular weight of the final purified
peptide. Ionization was carried out in positive ion mode with a
capillary voltage of 3.5 kV, a nebulizer gas (N2)
temperature of 250°C and flow rate of 1.5 l/min.
Anti-proliferative activity against
DU-145 cells
The effects of CSP on cell proliferation were
determined using the MTT colorimetric assay according to the
protocol detailed by Tang et al (25), with a number of modifications.
Briefly, DU-145 cells were seeded in a 96-well plate
(1×104 cells/well) and incubated at 37°C overnight.
Cells were then treated with different concentrations of CSP (0,
3.0, 6.0, 12.0, 18.0 and 22.5 mM) for 24, 48 and 72 h time
intervals at 37°C. Following this, PBS (200 µl) with 10% MTT was
added to each well at room temperature. The medium was then
removed, DMSO (150 µl) was added and the plates were then incubated
at 37°C for a further 4 h. The plates was transferred into a TYZD-I
oscillator (Beijing BILON Co., Ltd., Beijing, China) and incubated
for 15 min at room temperature. The cell proliferation inhibition
rate (%) was calculated using the following equation: Inhibition
percentage
(%)=[(ODcontrol-ODtreated)/(ODcontrol-ODblank)]
×100%
Acridine orange/ethidium bromide
(AO/EB) staining
Cell morphology was investigated using AO/EB double
staining as described by Tang et al (25). To accurately distinguish cells in
different stages of apoptosis, DU-145 cells in exponential phase
were digested using 0.25% trypsin, suspended at a final
concentration of 1×105 cells/well in a 6-well
flat-bottomed plate and then cultured at 37°C in a 5%
CO2 incubator. Cells were then treated with 0, 3.0, 12.0
and 18.0 mM CSP for a further 24 h. Finally, cells were stained
using the AO/EB dye mixture (100 µg/ml) and the morphology of
apoptotic cells was then immediately investigated using a
fluorescent microscope (Leica DM 3000; Leica Microsystems GmbH,
Wetzlar, Germany).
Scanning electron microscope
DU-145 cells were seeded in a 25 ml culture vessel
and treated with 0, 3.0 and 12.0 mM CSP at 37°C. When cells reached
a final concentration of 1×105 cells/ml, they were
subsequently cultured at 37°C in a 5% CO2 incubator for
24 h. The harvested cells were fixed with 2.5% glutaraldehyde for
24 h and 1.5% osmium acid for 2 h at room temperature, dried and
coated with gold by an ion sputtering coating machine. Finally, the
cells were observed using a scanning electron microscope (Hitachi
H-7650; Hitachi, Ltd., Tokyo, Japan).
Cell apoptosis analysis
Cell apoptosis rates were determined using an
Annexin V fluorescein isothiocyanate (FITC)/propidium iodide (PI)
staining assay and flow cytometry (BD Biosciences, Franklin Lakes,
NJ, USA). Briefly, DU-145 cells were cultured in 6-well
flat-bottomed plates at 37°C for 24 h and then treated with 0, 3.0,
12.0 and 18.0 mM CSP for a further 24 h. Harvested cells were then
digested with 0.25% trypsin, resuspended in phosphate buffer and
then collected by centrifugation (1,000 × g, 4°C for 5 min).
Finally, the cells were incubated with 5 µl of Annexin V-FITC and
10 µl of PI for 15 min at room temperature in the dark and then
immediately analyzed by flow cytometry using a flow cytometer.
Western blot analysis
Total protein lysates from the different cell groups
were extracted using RIPA lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China), quantified by using BCA protein
assay kit and equal amounts of protein (50 µg) were loaded per well
on a 10% SDS-PAGE gel and then separated. Following SDS-PAGE,
proteins were transferred to a polyvinylidene difluoride membrane
and the membrane was then blocked using 10% non-fat milk for 1.5 h
at room temperature. The membrane was incubated with specific
primary antibodies (β-actin, Bax, Bcl-2, caspase-3 and caspase-9;
1:1,000) at 4°C overnight and then washed three times using
Tris-HCl with 0.05% Tween-20. Following this, the membrane was
incubated with horseradish peroxidase-conjugated goat-anti-rabbit
secondary antibodies (1:3,000) at room temperature for 2 h. The
intensity of specific bands was visualized using enhanced
chemiluminescence (FluorChem FC3, Protein Simple, Inc., San Jose,
CA, USA) and then quantified using Quantity One software (version
4.62; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
of at least four independent experiments. Data were analyzed by
one-way analysis of variance with Turkey's test using the SPSS 19.0
software (IBM Corps., Armonk, NY, USA). P<0.05 was considered to
indicate a significant difference.
Results and Discussion
Purification of activity peptide and
peptide identification
The target peptide was purified from Cyclina
sinensis protein hydrolysates via ultrafiltration, gel
filtration chromatography and RP-HPLC. The anti-proliferative
activity of the peptide against DU-145 cells was used to monitor
the purification process. The <3 kDa peptide fraction was
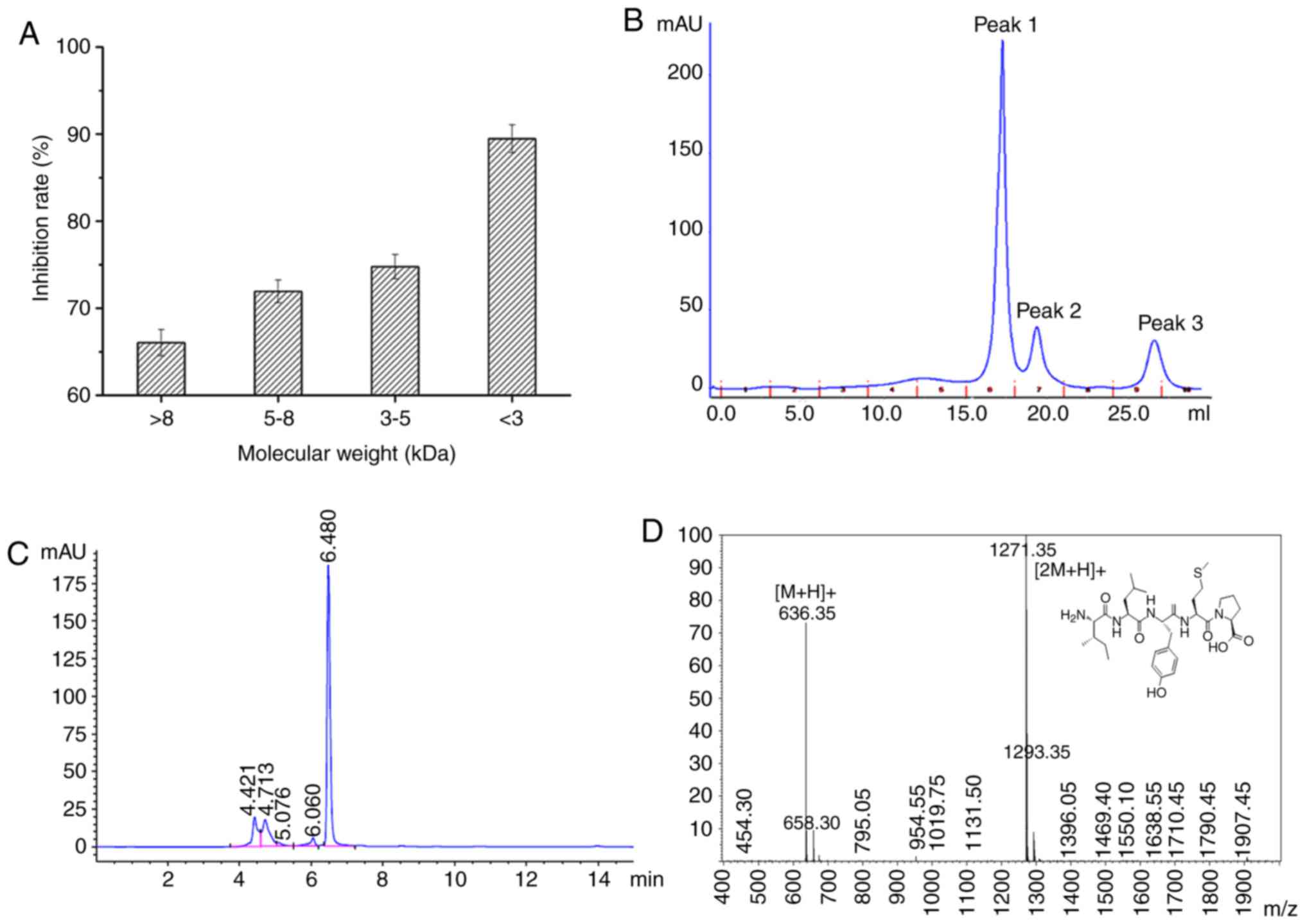
revealed to demonstrate the greatest anti-proliferative activity
towards DU-145 cells (89.46±4.47%; Fig. 1A). Chi et al (9) reported that <1 kDa peptide
fractions isolated from blood clam protein hydrolysates exhibit the
greatest anti-proliferative activity against PC-3, DU-145 and H1299
cells. Similar results were observed in protein hydrolysates
obtained from Setipinna taty and Ruditapes
philippinarum (26,27). The results of the present study
were consistent with previous findings suggesting that short
peptides exhibited greater anticancerous activities, and thus <3
kDa peptide fractions were subsequently collected and lyophilized
for further investigation.
Following this, fractions <3 kDa were separated
into three sub-fractions (peak 1, peak 2 and peak 3). The results
of the MTT assay revealed that peak 3 exhibited the greatest
anti-proliferative activity (12 mM; 59.46±3.67% CSP treatment for
24 h) towards DU-145 cells compared with peak 1 and peak 2
(Fig. 1B). Subsequently, peak 3
was collected, lyophilized and further purified via preparative
RP-HPLC. The peak with a retention time of 6.480 min demonstrated
the greatest inhibitory effect on DU-145 cell proliferation
(Fig. 1C). Following this, this
peak was further purified ~20 times via preparative RP-HPLC and
subsequently subjected to MS and amino acid sequence analysis.
Following analysis using a Shimadzu PPSQ-31A protein sequencer and
electrospray ionization-mass spectrometry, the amino acid sequence
of CSP was determined to be ILYMP (Fig. 1C) with a molecular weight of 635.35
Da [(M+H)+; Fig 1D],
which was consistent with a theoretical mass of CSP (635.71
Da).
CSP exhibits an anti-proliferative
effect against DU-145 cells
Cell proliferation occurs in almost all tissues and
regulates cell proliferation and programmed cell death in order to
ensure tissue and organ integrity. However, uncontrolled cell
division may result in tissue hyperplasia as well as the
development of diseases, such as cancer (10,28).
Thus, inhibition of cell proliferation is considered to represent
an effective therapeutic strategy for the treatment of cancer. In
the present study, MTT assays were used to determine the inhibitory
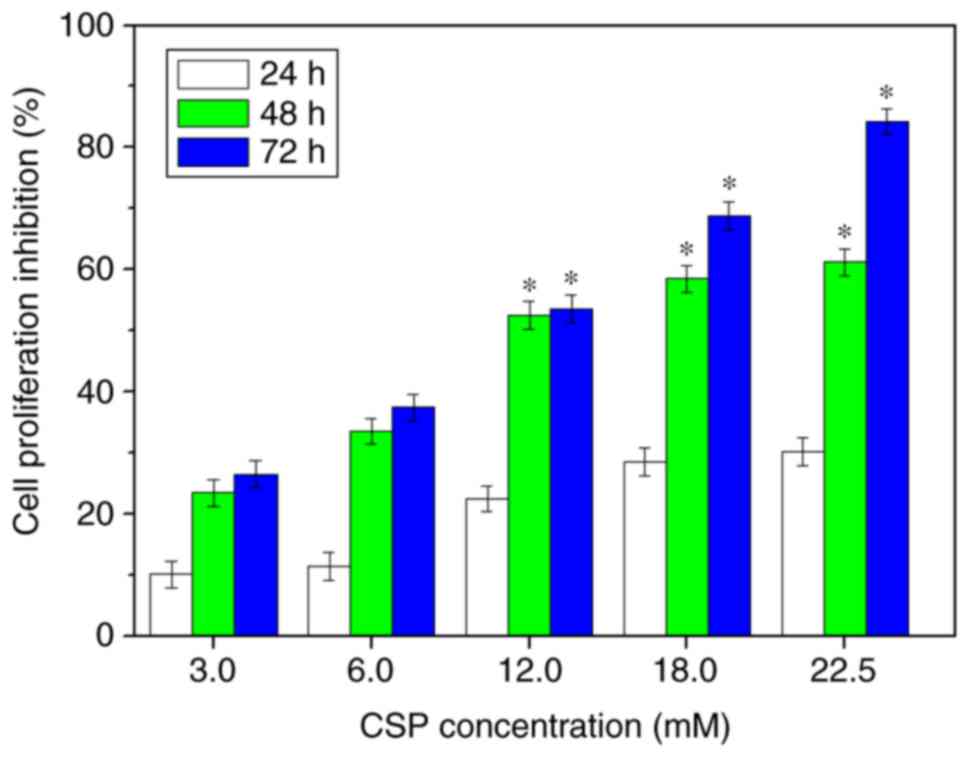
effect of CSP on DU-145 cell proliferation. The results revealed
that CSP exhibited a marked increase in cytotoxicity against DU-145
cells in a dose-dependent manner, with an inhibition rate of
~84.17% at 22.5 mM at the 72 h time interval (Fig. 2). The half-maximal inhibitory
concentration (IC50) of CSP was ~11.25 mM at the 72 h
time interval. Furthermore, CSP did not exhibit any toxicity
towards the normal NIH-3T3 cells (data not shown), which are
frequently used as a healthy control cell line (8). Therefore, the results suggested that
CSP exhibited selective toxicity towards cancer cells compared with
normal cells.
Morphological observations
AO/EB staining has been previously used to
investigate apoptosis in cancer cells (25,29).
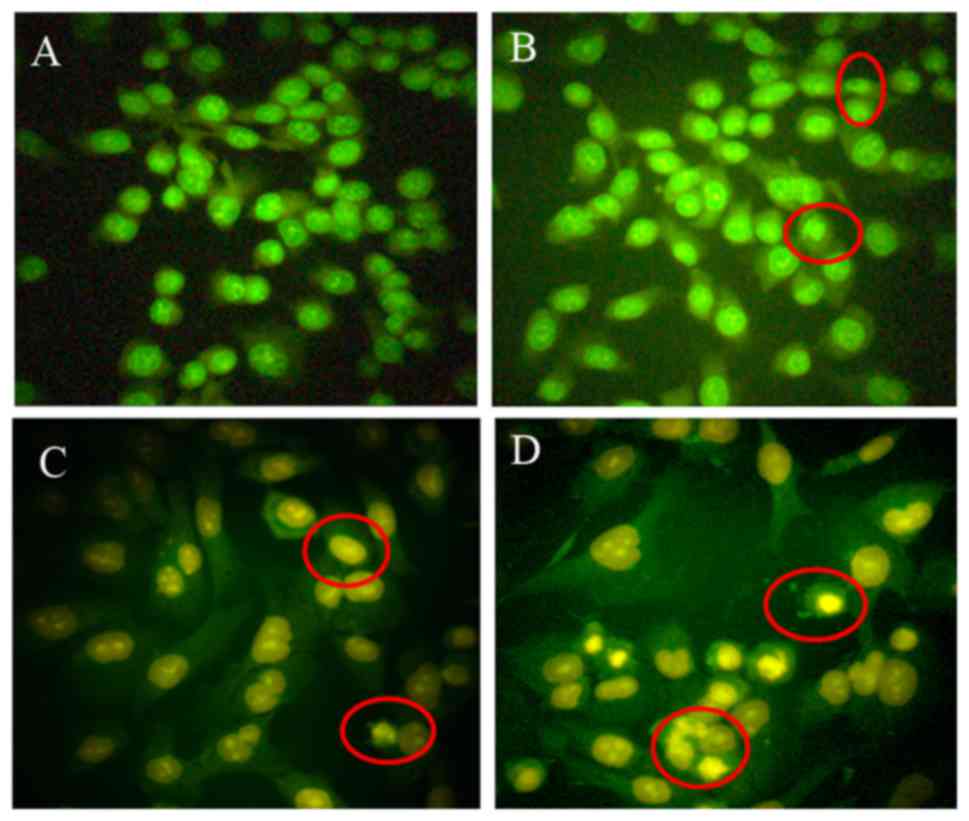
The present study aimed to determine whether CSP-induced inhibition
of cell proliferation occurs via apoptosis by treating DU-145 cells
with 3.0, 12.0 and 18.0 mM CSP and observing changes in cell
morphology using AO/EB staining (Fig.
3). Early-stage apoptotic cells, marked by crescent-shaped or
granular yellow green AO nuclear staining, were observed following
treatment with 3.0 and 12.0 mM CSP for 24 h, which suggested that
DU-145 cells were undergoing early stage apoptosis (Fig. 3A, B and C). Late-stage apoptotic
cells, with concentrated and asymmetrically localized nuclear AO/EB
staining, were observed following treatment with 18.0 mM CSP for 24
h (Fig. 3D). The morphological
characteristics of apoptotic DU-145 cells in the present study were
similar to the results of AO/EB staining in previous studies using
DU-145, PC-3 and LNCaP cells that were treated with sepia ink
oligopeptide QPK (18), HeLa cells
treated with a hexapeptide (Phe-Ile-Met-Gly-Pro-Tyr; FIMGPY)
isolated from Raja porosa cartilage protein hydrolysates
(10) and PC-3 cells treated with
a peptide (Arg-Asp-Gly-Asp-Ser-Cys-Arg-Gly-Gly-Gly-Pro-Val;
RDGDSCRGGGPV) isolated from Bullacta exarata (30).
Scanning electron microscopy was used to further
investigate the effects of CSP on DU-145 cells. Cells in the
control group did not exhibit any typical morphological changes
(microvilli reduction or disappearance, chromatin condensation or
margination) (Fig. 4A and B).
However, typical morphological changes were observed when cells
were treated with 3.0 (Fig. 4C and
D) and 12.0 mM CSP for 24 h (Fig.
4E and F), such as a loss of microvilli structures on the
surface of the cell membrane, chromatin condensation and the
presence of apoptosis bodies. Furthermore, an expansion of the
smooth endoplasmic reticulum, loss of mitochondrial cristae and an
appearance of numerous cytoplasmic vacuoles were observed in
CSP-treated DU-145 cells. In conclusion, these results suggested
that apoptosis is enhanced in cells following treatment with CSP,
and the morphological features observed were similar to those
observed in DU-145 cells following treatment with an oligopeptide
(Asp-Trp-Pro, DWP) isolated from Ruditapes philippinarum
(31).
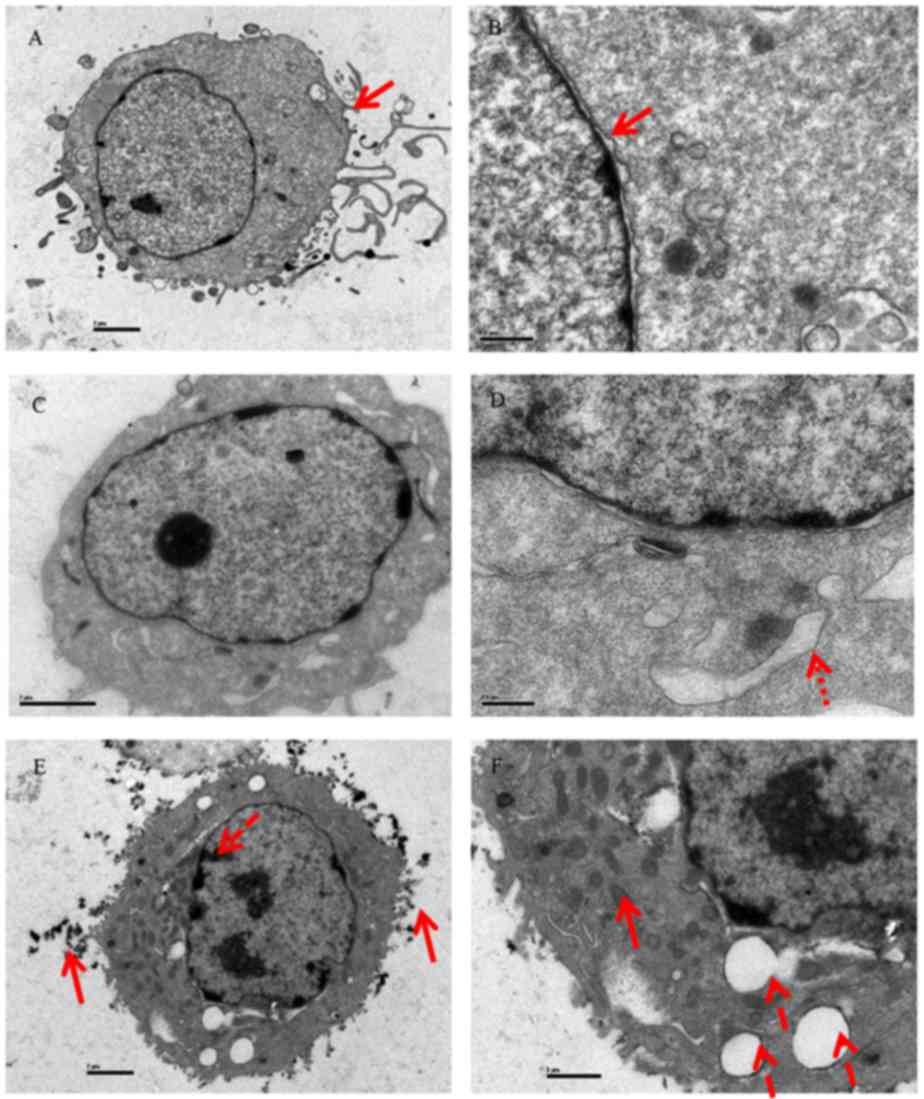 | Figure 4.Morphological observation of DU-145
cells revealed by scanning electron microscopy. Untreated cells did
not exhibit any typical morphological changes at (A) magnification,
×3,700, the red arrow with the solid line in (A) indicates abundant
microvilli are observed on the surface and (B) magnification,
×17,500, the red arrow with the solid line in (B) indicates nuclear
membrane was intact. Cells treated with 3.0 mM CSP at (C)
magnification, ×6,200 and (D) magnification, ×17,500, the red arrow
with the small dots in (D) indicates a smooth endoplasmic reticulum
extension. Cells treated with 12.0 mM CSP at (E) magnification,
×3,700 and (F) magnification, ×8,900. The red arrow with the solid
line in (E) indicates heterochromatin aggregation and the red arrow
with long dashes indicates microvilli loss. The red arrow with the
long dash in (F) indicates vacuoles appearing in the cytoplasm and
the red arrow with the solid line indicates mitochondrial ridges
disappearing. |
Cell apoptotic rate is enhanced in
DU-145 cells following treatment with CSP
Cancer is a disease state characterized by
disordered cell proliferation and inhibition of apoptosis. Previous
therapeutic strategies for the treatment of tumors have focused on
the inhibition of cell proliferation and the induction of
apoptosis. In order to further investigate the induction of
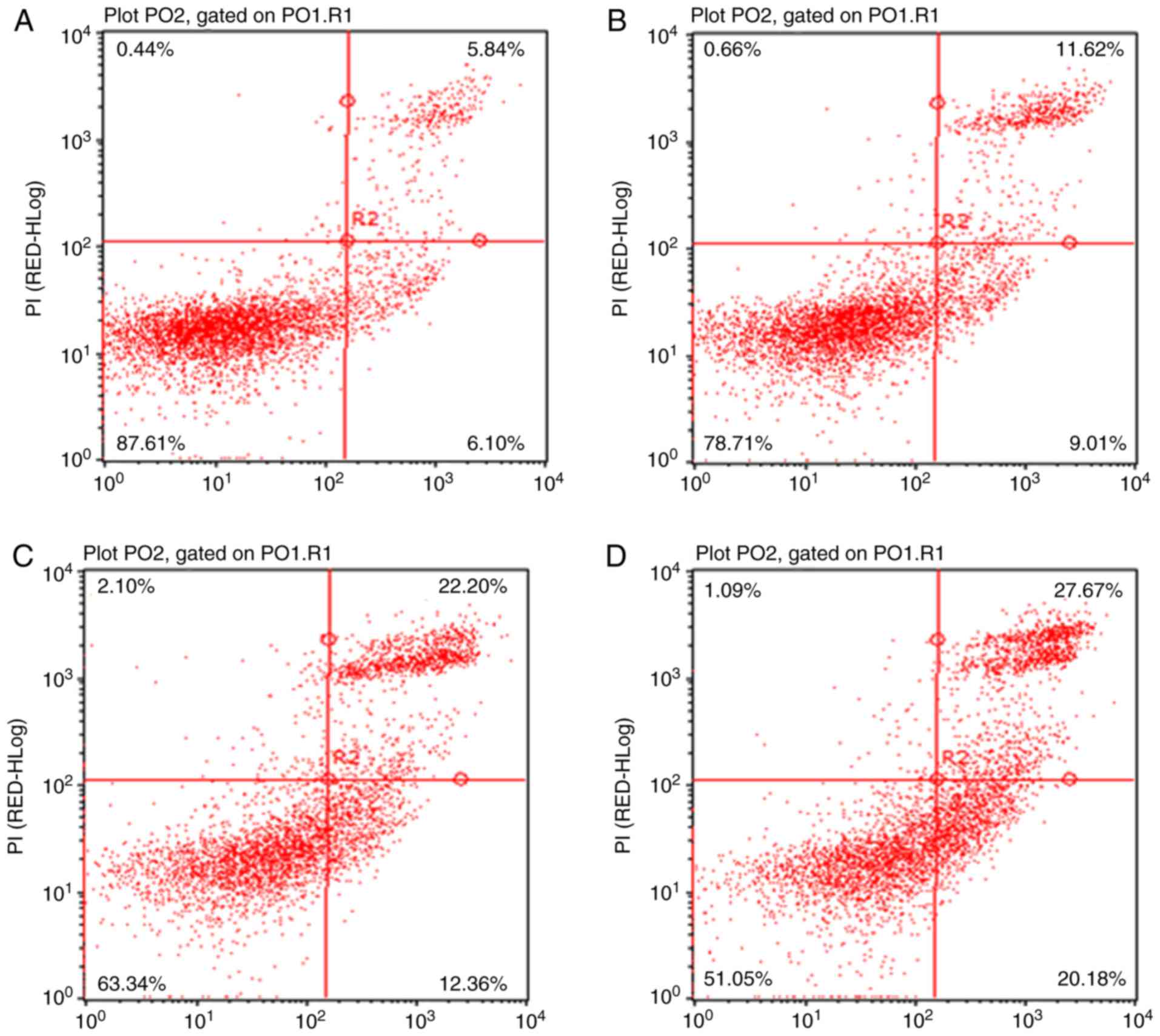
apoptosis by CSP, flow cytometric analysis was performed and the
rate of cellular apoptosis was determined using Annexin V-FITC/PI
double staining. The results revealed that the percentage of
Annexin V-FITC stained DU-145 cells in the control group was
6.10±1.1% (Fig. 5A). Following 24
h exposure to 3.0, 12.0, and 18.0 mM CSP, the percentage of
apoptotic cells increased to 9.01±1.3% (Fig. 5B), 12.36±1.8% (Fig. 5C) and 20.18±1.9% (Fig. 5D), respectively; thus demonstrating
a marked increase in apoptosis rates following treatment with CSP
in a dose-dependent manner. Therefore, the results suggested that
CSP induced apoptosis in DU-145 cells.
Expression levels of apoptosis
associated proteins in DU-145 cells are increased following
treatment with CSP
The results of the flow cytometry analysis suggested
that apoptosis was increased in DU-145 cells following treatment
with CSP in a dose-dependent manner. In order to investigate the
underlying mechanisms, expression levels of anti- and pro-apoptosis
associated proteins were determined in CSP-treated DU-145 cells via
western blotting. The Bcl-2 protein family plays an important role
in the regulation of apoptosis, and includes pro-apoptotic proteins
(Bax, Bcl-2 associated agonist of cell death and Bcl-X) as well as
anti-apoptotic proteins (Bcl-2, Bcl-extra large and Bcl-2-like
protein) (32). Thus, the ratio
between Bax and Bcl-2 protein expression is frequently used as an
apoptotic index (10,18,25).
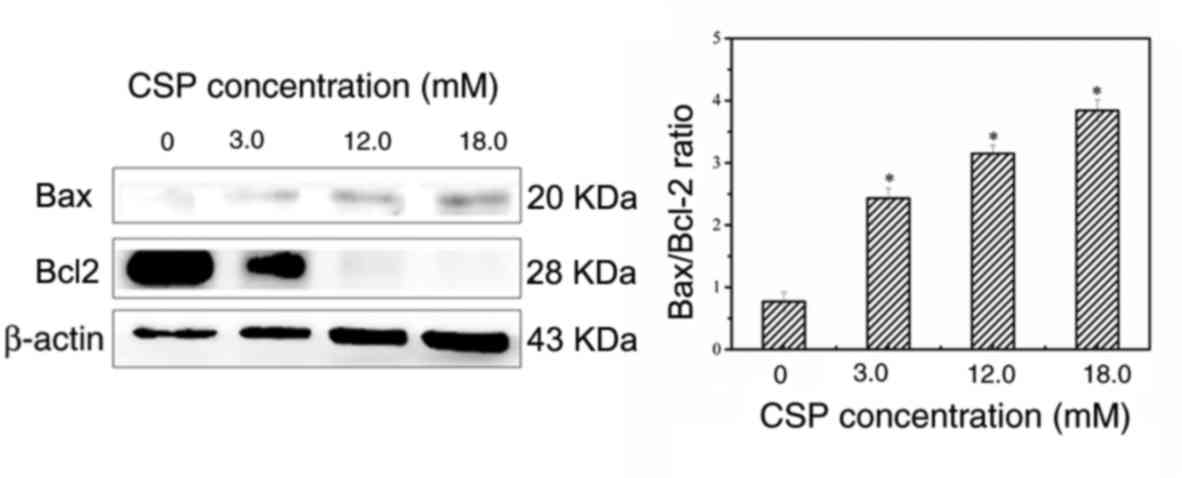
The western blotting results demonstrated that Bcl-2 expression was
significantly decreased, whereas the expression of Bax was
significantly increased following treatment with CSP in a
dose-dependent manner, thus resulting in a dose-dependent increase
in the Bax/Bcl-2 ratio in CSP-treated DU-145 cells (Fig. 6). These results therefore suggested
that treatment with CSP significantly enhanced apoptosis via
upregulation of the Bax/Bcl-2 ratio in DU-145 cells.
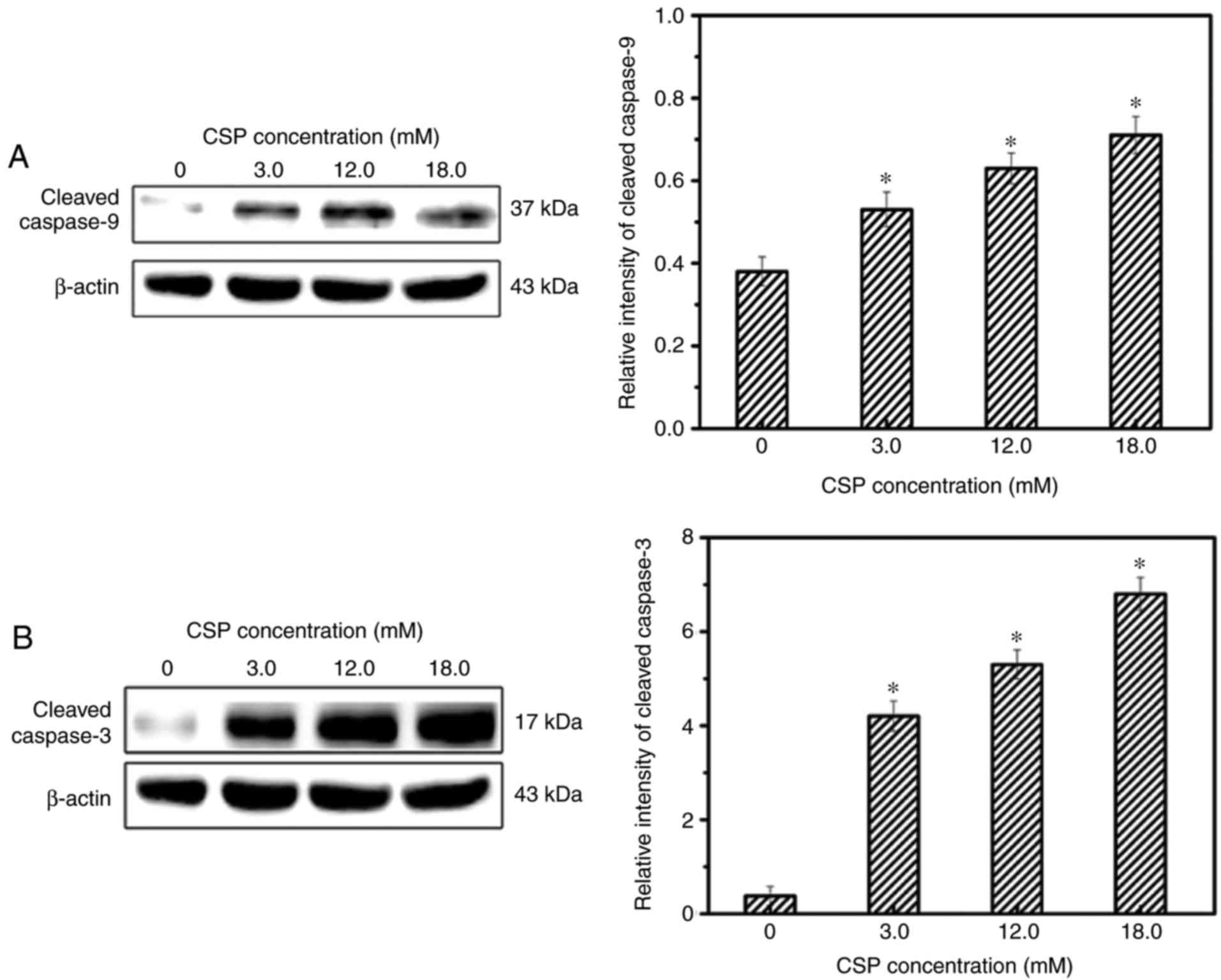
In addition, caspase proteins are important for the
maintenance of cellular homeostasis via the regulation of cell
death and inflammation, and may be classified as initiator caspases
(caspase-8/9) or executioner caspases (caspase-3/6/7) (33). Caspase-9 protein is an important
component of the mitochondrial death pathway and caspase-3 is the
predominant downstream effector caspase responsible for cleaving
the majority of the cellular substrates in apoptotic cells
(34). Western blotting analysis
revealed that the protein expression of cleavedcaspase-3 and
cleavedcaspase-9 were significantly increased following treatment
with CSP in a dose-dependent manner (Fig. 7). Therefore, the results suggest
that CSP-induced apoptosis is associated with the
mitochondrial-mediated death pathway in DU-145 cells.
CSP exhibits anticancer activity
It has been previously established that the
hydrophobic properties of amino acids affect their anticancer
activities. For example, the A and L residues in the YALPAH peptide
isolated from Setipinna taty peptide hydrolysates were
revealed to be associated with its anti-proliferative activities
against PC-3 cells (26). F, I, M,
P and Y residues in the sequence of the FIMGPY hexapeptide isolated
from Raja porosa cartilage were also demonstrated to be
associated with its anticancer activities against HeLa cells
(10). Therefore, it can be
suggested that the amino acid residues in the CSP sequence (ILYMP)
may contribute to its anticancer activities. The CSP isolated from
Cyclina sinensis exhibited anticancer activity to DU-145
cells, with lower toxicity to normal cells. The properties of this
pentapeptide make it a promising therapeutic agent for PCa
prevention or treatment. Further investigation in future studies is
required to fully characterize CSP activity both in vitro
and in vivo.
In conclusion, an anti-proliferation pentapeptide
(ILYMP) named CSP was obtained from protein hydrolysates isolated
from Cyclina sinensis via ultrafiltration and
chromatographic methods. The results revealed that CSP inhibited
the proliferation of DU-145 cells with an IC50 of 11.25
mM at 72 h. Furthermore, AO/EB staining, scanning electron
microscopy and flow cytometry analyses demonstrated that CSP
suppresses DU-145 cell proliferation via the induction of
apoptosis. Enhanced expression of Bax and cleaved caspase-3/9 as
well as suppression of Bcl-2 expression was observed in CSP-treated
DU-145 cells. To the best of the authors' knowledge, this is the
first study to investigate the effects of an antiproliferative
peptide on DU-145 cells derived from Cyclina sinensis, and
the results revealed that the Cyclina sinensis extract may
have a therapeutic effect on PCa. However, the underlying mechanism
of CSP-induced apoptosis was not fully determined, and our future
studies will focus on revealing the molecular and proteomic
mechanisms associated with this effect, using in vitro and
in vivo studies.
Acknowledgements
The authors would like to thank Dr. Xiaojun Zhang
from Aquatic Processing Department, Zhejiang Marine Fisheries
Research Institution for measuring the molecular weight of the
CSP.
Funding
This research was supported by the Natural Science
Foundation of Zhejiang Province (grant nos. LQ16H300001, LY12C20005
and LY12C20008), the Foundation of Zhejiang Educational Committee
(grant no. Y201534400) and the Public Welfare Program of Zhoushan
(grant no. 2015C31012).
Availability of data and materials
The data used in the current study are available
from the corresponding author on reasonable request.
Authors' contributions
ZY and FY conceived and designed the experiments.
YZ, LY and YT performed the experiments. GD and XZ conducted data
analysis. All authors read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choo GS, Lee HN, Shin SA, Kim HJ and Jung
JY: Anticancer effect of fucoidan on DU-145 prostate cancer cells
through inhibition of PI3K/Akt and MAPK pathway expression. Mar
Drugs. 14:E1262016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou Y, Ji Z, Yan W, Zhou Z and Li H: The
biological functions and mechanism of miR-212 in prostate cancer
proliferation, migration and invasion via targeting Engrailed-2.
Oncol Rep. 38:1411–1419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu MB, Bai PD, Wu YS, Zhang LM, Xu H, Na
R, Jiang HW and Ding Q: Higher body mass index increases the risk
for biopsy-mediated detection of prostate cancer in Chinese men.
PloS One. 10:e01246682015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo L, Min Y, Zhong J, Wu H, Pan J, Gong
W, Wang M, Fei F and Hu R: Stroke risk among patients with Type 2
diabetes mellitus in Zhejiang: A population-based prospective study
in China. Int J Endocrinol. 2016:63806202016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karantanos T, Corn PG and Thompson TC:
Prostate cancer progression after androgen deprivation therapy:
Mechanisms of castrate resistance and novel therapeutic approaches.
Oncogene. 32:5501–5511. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fizazi K, Scher HI, Molina A, Logothetis
CJ, Chi KN, Jones RJ, Staffurth JN, North S, Vogelzang NJ, Saad F,
et al: Abiraterone acetate for treatment of metastatic
castration-resistant prostate cancer: Final overall survival
analysis of the COU-AA-301 randomised, double-blind,
placebo-controlled phase 3 study. Lancet Oncol. 13:983–992. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schrader AJ, Boegemann M, Ohlmann CH,
Schnoeller TJ, Krabbe LM, Hajili T, Jentzmik F, Stoeckle M,
Schrader M, Herrmann E and Cronauer MV: Enzalutamide in
castration-resistant prostate cancer patients progressing after
docetaxel and abiraterone. Eur Urol. 65:30–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chi CF, Hu FY, Wang B, Li T and Ding GF:
Antioxidant and anticancer peptides from the protein hydrolysate of
blood clam (Tegillarca granosa) muscle. J Funct Foods.
15:301–313. 2015. View Article : Google Scholar
|
|
10
|
Pan X, Zhao YQ, Hu FY, Chi CF and Wang B:
Anticancer activity of a hexapeptide from Skate (Raja
porosa) cartilage protein hydrolysate in HeLa Cells. Mar Drugs.
14:E1532016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Z, Gao L, Shen C, Rong M, Yan X and
Lai R: A potent anti-thrombosis peptide (vasotab TY) from horsefly
salivary glands. Int J Biochem Cell Biol. 54:83–88. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang B, Wang YM, Chi CF, Luo HY, Deng SG
and Ma JY: Isolation and characterization of collagen and
antioxidant collagen peptides from scales of Croceine Croaker
(Pseudosciaena crocea). Mar Drugs. 11:4641–4661. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pan X, Zhao YQ, Hu FY and Wang B:
Preparation and identification of antioxidant peptides from protein
hydrolysate of skate (Raja porosa) cartilage. J Funct Foods.
25:220–230. 2016. View Article : Google Scholar
|
|
14
|
Wang X, Xing R, Chen Z, Yu H, Li R and Li
P: Effect and mechanism of mackerel (Pneumatophorus
japonicus) peptides for anti-fatigue. Food Funct. 5:2113–2119.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao YQ, Zeng L, Yang ZS, Huang FF, Ding
GF and Wang B: Anti-fatigue effect by peptide fraction from protein
hydrolysate of Croceine Croaker (Pseudosciaena crocea) swim
bladder through inhibiting the oxidative reactions including DNA
damage. Mar Drugs. 14:2212016. View Article : Google Scholar :
|
|
16
|
Ovchinnikova TV, Aleshina GM, Balandin SV,
Krasnosdembskaya AD, Markelov ML, Frolova EI, Leonova YF, Tagaev
AA, Krasnodembsky EG and Kokryakov VN: Purification and primary
structure of two isoforms of arenicin, a novel antimicrobial
peptide from marine polychaeta Arenicola marina. FEBS Lett.
577:209–214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sperstad SV, Haug T, Blencke HM, Styrvold
OB, Li C and Stensvåg K: Antimicrobial peptides from marine
invertebrates: Challenges and perspectives in marine antimicrobial
peptide discovery. Biotechnol Adv. 29:519–530. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang F, Yang ZS, Yu D, Wang J, Li R and
Ding G: Sepia ink oligopeptide induces apoptosis in prostate cancer
cell lines via caspase-3 activation and elevation of Bax/Bcl-2
ratio. Mar Drugs. 10:2153–2165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song R, Wei R, Zhang B, Yang Z and Wang D:
Antioxidant and antiproliferative activities of heated sterilized
pepsin hydrolysate derived from half-fin anchovy (Setipinna
taty). Mar Drugs. 9:1142–1156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim EK, Hwang JW, Kim YS, Ahn CB, Jeon YJ,
Kweon HJ, Bahk YY, Moon SH, Jeon BT and Park PJ: A novel bioactive
peptide derived from enzymatic hydrolysis of Ruditapes
philippinarum: Purification and investigation of its
free-radical quenching potential. Process Biochem. 48:325–330.
2013. View Article : Google Scholar
|
|
21
|
Jiang C, Xiong Q, Gan D, Jiao Y, Liu J, Ma
L and Zeng X: Antioxidant activity and potential hepatoprotective
effect of polysaccharides from Cyclina sinensis. Carbohyd
Polym. 91:262–268. 2013. View Article : Google Scholar
|
|
22
|
Jiang C, Xiong Q, Li S, Zhao X and Zeng X:
Structural characterization, sulfation and antitumor activity of a
polysaccharide fraction from Cyclina sinensis. Carbohyd
Polym. 115:200–206. 2015. View Article : Google Scholar
|
|
23
|
Jiang C, Wang M, Liu J, Gan D and Zeng X:
Extraction, preliminary characterization, antioxidant and
anticancer activities in vitro of polysaccharides from Cyclina
sinensis. Carbohyd Polym. 84:851–857. 2011. View Article : Google Scholar
|
|
24
|
Yan HQ, Teng FF, Liu ZX, Huang FF, Yang ZS
and Ding GF: Anticancer activity of peptides isolated from
hydrolysates of Cylcina sinensis. J Anhui Agri Sci.
42:3576–3577. 2014.
|
|
25
|
Tang Y, Yu F, Zhang G, Yang Z, Huang F and
Ding G: A purified serine protease from Nereis virens and its
impaction of apoptosis on human lung cancer cells. Molecules.
22:E11232017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song R, Wei RB, Luo HY and Yang ZS:
Isolation and identification of an antiproliferative peptide
derived from heated products of peptic hydrolysates of half-fin
anchovy (Setipinna taty). J Funct Foods. 10:104–111. 2014.
View Article : Google Scholar
|
|
27
|
Kim EK, Kim YS, Hwang JW, Lee JS, Moon SH,
Jeon BT and Park PJ: Purification and characterization of a novel
anticancer peptide derived from Ruditapes philippinarum.
Process Biochem. 48:1086–1090. 2013. View Article : Google Scholar
|
|
28
|
Ibrahim B, Sowemimo A, Spies L, Koekomoer
T, Venter MVD and Odukoya OA: Antiproliferative and apoptosis
inducing activity of Markhamia tomentosa leaf extract on HeLa
cells. J Ethnopharmacol. 149:745–749. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deng X, Qiu Q, Yang B, Wang X, Huang W and
Qian H: Design, synthesis and biological evaluation of novel
peptides with anti-cancer and drug resistance-reversing activities.
Eur J Med Chem. 89:540–548. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma J, Huang F, Lin H and Wang X: Isolation
and purification of a peptide from Bullacta exarata and its
impaction of apoptosis on prostate cancer cell. Mar Drugs.
11:266–273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang Z, Zhao Y, Yan H, Xu L, Ding G, Yu D
and Sun Y: Isolation and purification of oligopeptides from
Ruditapes philippinarum and its inhibition on the growth of
DU-145 cells in vitro. Mol Med Rep. 11:1063–1068. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuwana T and Newmeyer DD: Bcl-2-family
proteins and the role of mitochondria in apoptosis. Curr Opin Cell
Biol. 15:691–699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mcilwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 5:a0086562013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kumar S: Caspase function in programmed
cell death. Cell Death Differ. 14:32–43. 2007. View Article : Google Scholar : PubMed/NCBI
|