Introduction
Researchers have paid an increasing amount of
attention to the applications of dental pulp stem cells (DPSCs) in
the field of dental regenerative medicine. DPSCs can differentiate
into dental tissue and bone tissue in vitro (1). Additionally, endothelial cells (ECs)
can secrete a series of bioactive substances, including
endothelin-1 (ET-1) and insulin-like growth factor (IGF). Shi and
Gronthos (2) reported that DPSCs
were present in the micrangium region of dental pulp. Cell staining
also revealed that STRO-1 (a marker of mesenchymal cells), cluster
of differentiation (CD)146 (a marker of endothelial cells) and
α-smooth-muscle actin (a marker of pericytes) were positively
expressed on the surface of perivascular cells (2). Notably, the elevated levels of CD146
expression suggested a perivascular origin of DPSCs. The migration
of pre-odontoblasts to blood vessels may be due to the degradation
reaction of dentin (3). In turn,
ECs may regulate the development of dentine/pulp tissue.
Additionally, ECs may control the proliferation of cells by
maintaining the stabilization of blood vessels and secreting
relevant molecules, and, therefore ECs may be considered to be a
novel resource for tissue regeneration (3). Mathieu et al (4) demonstrated that dental pulp was a
type of vascularized tissue, which may stimulate ECs to secrete
chemokines and signaling molecules upon infection. Subsequently,
Mathieu et al (4) observed
that the inflammation began to promote the secretion of
inflammatory factors and adhesion molecules, which DPSCs require to
accelerate the repair processes within the tissue. Factors
including fibroblast growth factor 2 (FGF-2), secreted by ECs,
participated in angiogenesis and DPSC division (5).
The aforementioned research demonstrated that there
may be an interaction between ECs and DPSCs. Dissanayaka et
al (6) directly co-cultured
ECs and DPSCs and reported that ECs may regulate the odontoblastic
differentiation of DPSCs. Additionally, DPSCs may induce ECs to
generate a vascular-like tissue structure (7). It has been suggested that this
promotion of differentiation and proliferation may be due to ET-1
and IGF (8,9), which are secreted by ECs; however,
the direct co-culture with these two cell types may also be the
reason for the promotion of these processes (3,10,11).
Sueyama et al (12)
implanted mesenchymal stem cells (MSCs) with endothelial cells
(ECs), and observed accelerated pulp tissue regeneration/healing
and induction of dentin bridge formation in a rat model of molar
coronal pulp regeneration.
ETs were originally identified by Yanagisawa in 1988
(13). The main role of ETs is to
maintain vascular homeostasis under physiological conditions, as
well as during nociception and periods of local inflammation
(14–16). There are three different subtypes
of ETs, namely ET-1, ET-2, and ET-3. ET-1 is the most common type
observed in humans (17). ET-1 is
a type of bioactive peptide composed of 21 amino acid residues, and
may be extracted from aortic endothelial cells; ET-1 affects the
proliferation and differentiation of MSCs, and preosteoblasts, as
reported by Sin et al (18). ET-1 can also maintain vascular
tension and stability in the cardiovascular system (19). Furthermore, it serves a significant
role in the development of diseases, including hypertension and
atherosclerosis (20). In the
culture of rat ophthalmic arteries, ET-1 can mediate
vasoconstriction (21). There is
substantial evidence that, in numerous pathophysiologies associated
with endothelial dysfunction, ET-1 may release potent
vasoconstrictors and sustain elevated vascular tone; however, there
is considerably less data to support the role of ET-1 in the
regulation of vascular tone under physiological conditions
(22–24). In addition, ET-1 also serves a role
in osteogenesis and bone remodeling. Sin et al (18) indicated that ET-1 may induce the
differentiation of osteoblasts via the membrane protein ankyrin 43.
In addition, ET-1 may enhance the mRNA expression of osteopontin
and osteocalcin, and stimulate the release of alkaline phosphatase
and secretion of collagenase type I (25); however, compared with its
expression in osteoblasts, ET-1 can be detected on the cell
membrane and in the cytoplasm of osteoclasts by immunostaining
(26). This indicated that
osteoclasts may be a target cell affected by ET-1. Accordingly,
ET-1 may accelerate the resorption of bone by endothelin type A (ET
A) receptor (18). Therefore, ECs
and ET-1 may regulate osteogenesis, remodeling and bone resorption
by controlling the activity of osteoblasts and osteoclasts. These
findings present the advances in bone research regarding ET-1
function. Several studies have investigated odontogenesis with
regards to the potential involvement of ET-1. Warner (27) revealed that ET-1 affected the
concentration of calcium in contractile fiber cells via the ET A
receptor. Additionally, a high concentration of ET-1 during the
process of dental morphogenesis can be observed (28). It has been suggested that
fibronectin (FN) serves an important role in the formation of the
tooth root. A previous study demonstrated that ET-1 may induce the
production of extracellular matrix protein and FN (29). Therefore, the additional function
of ET-1 may be to promote the proliferation and differentiation of
cells in the tooth root. Injection of ET into the dental pulp of
dogs can cause vasoconstriction and decreased blood circulation
(30), demonstrating that
receptors for ET also exist in the dental pulp. When treated with
ET-1, there is a constant release of Ca2+ from DPSCs
(3). Preconditioning of MSCs with
ET-1 exhibits strong cytoprotective effects via the activation of
survival signaling molecules and trophic factors (31).
Despite the aforementioned findings, it is evident
that the mechanism of ECs and ET-1 in histogenesis and tissue
formation requires further investigation. Therefore, the present
study aimed to investigate the effect of human umbilical vein
endothelial cells (HUVECs) and ET-1 on DPSC differentiation in
vitro.
Materials and methods
Primary pulp cell cultures
Human pulp cells were prepared from immature third
molars at the 2/3 root formation stage by the explant outgrowth
method (32). The teeth were
obtained from at least three different donors for each experiment
(n=12; four molars per donor; age, 18–25 years; 1:1 male to
female). Surgeries were performed at the Oral and Maxillofacial
Surgery Department of the Second Affiliated Hospital of Harbin
Medical University (Harbin, China). HUVECs were provided by the
Research and Test Center of the Second Affiliated Hospital of
Harbin Medical University (Harbin, China). The present study was
approved by the Institutional Ethics Committee of the Second
Affiliated Hospital of Harbin Medical University (Harbin, China).
Written informed consent was obtained from all patients. The basic
cell culture medium used consisted of Dulbecco's modified Eagle's
medium/F-12 (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA),
supplemented with 15% fetal bovine serum (Biological Industries,
Kibbutz Beit Haemek, Israel) and 1% penicillin and 1%
streptomycin.
Cell groups and culture
conditions
Cells were cultured in a 37°C incubator with 5%
CO2. Cells (all 8×104) were divided into four
groups: i) a DPSC-only control group; ii) a DPSC with ET-1
(10−8 M) administration group; iii) a DPSC and HUVEC
direct co-culture group (DPSCs:HUVECs, 5:1); and iv) a DPSC and
HUVEC indirect co-culture group using a Transwell system, in which
HUVECs were inoculated into the chamber at a density of
2×104 cells. The odontoblastic differentiation culture
medium consisted of basic cell culture medium with 10 nmol/l
dexamethasone, 5 mmol/l β-glycerophosphate and 50 mg/ml
vitamin-C-phosphate.
Induction of DPSC differentiation into
adipocytes, chondroblasts and osteoblasts
Cells were cultured to the third generation in a
37°C incubator with 5% CO2. All groups of cells were
plated at a density of 5×104 per well. To induce DPSCs
to differentiate into adipocytes, the culture medium was
supplemented with 0.5 µM isobutylmethylxanthine, 50 µM indomethacin
and 0.5 µM dexamethasone for 3 weeks. The adipogenic cultures were
fixed in 4% paraformaldehyde for 30 min at room temperature and
stained with fresh Oil Red O solution for 1 h at room temperature.
The chondroblast induction medium consisted of 1 µM dexamethasone,
37.5 µg/ml vitamin-C-phosphate, 1 mM sodium pyruvate, 10 ng/ml
transforming growth factor-β (TGF-β), 1 ng/ml β-FGF, 1X
Insulin-Transferrin-Selenium premix (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), in which DPSCs were maintained for 3 weeks.
The chondrogenic cultures were fixed in 4% paraformaldehyde for 30
min at room temperature and stained with toluidine blue for 30 min
in a 37°C incubator. The osteoblast induction medium contained 10
nmol/l dexamethasone, 5 mmol/l β-glycerophosphate and 50 mg/ml
vitamin-C-phosphate, in which DPSCs were cultured for 3 weeks. The
osteoblast cultures were fixed in 95% ethanol for 30 min at 37°C
and stained with Alizarin Red S for 30 min in a 37°C incubator.
Cells were observed under a light microscope (original
magnification, ×40).
Cytotoxicity (MTT) assay
This assay employed two control groups: i) a
DPSC-only culture group; and ii) a HUVEC-only culture group. Two
experimental groups were also employed: i) a DPSC plus
10−8 M ET-1 group; and ii) a DPSC and HUVEC (5:1) direct
co-culture group. The present study employed 96-well plates with
1×103 cells/well. Dimethyl sulfoxide (200 µl) was added
to dissolve the formazan crystals. The absorbance was measured with
an ELISA reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
at a wavelength of 490 nm. The cell viability ratio was calculated
using the following formula:
Inhibitory ratio (%) = [optical density (OD) control
- OD treated)/(OD control)] ×100.
Mineralization induction and
quantification
This assay used four groups: i) a DPSC-only control
group; ii) a DPSC with ET-1 administration group; iii) a DPSC and
HUVEC direct co-culture group; and iv) a DPSC and HUVEC indirect
co-culture group using a Transwell system. Cells
(5×104/well) were seeded onto 6-well plates and cultured
for 21 days. Calcium accumulation was detected by fixing the
cultures with 95% ethanol for 30 min at 37°C, followed by staining
with 0.1% Alizarin Red S (Sigma-Aldrich; Merck KGaA) at 37°C for 30
min. To quantify matrix mineralization, the cultures stained with
Alizarin Red S were incubated with 100 mM cetylpyridinium chloride
for 1 h at 37°C to solubilize and release calcium-bound Alizarin
Red into solution. Subsequently, 200 ml aliquots were transferred
onto a 96-well plate and the OD of the solution was measured at a
wavelength of 570 nm using a microplate reader (Tanon Science &
Technology Co., Ltd., Shanghai, China). Mineralized nodule
formation was represented as OD/µg of total cellular protein,
determined using a Bradford protein assay. Experiments were
performed in triplicate wells and were repeated at least three
times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The four groups of cells were cultured in
mineralized solution (in a 37°C incubator with 5% CO2)
and total RNA was extracted on days 4, 7, 14 and 21 to detect the
gene expression of dentin sialoprotein (DSP) and dentin matrix
acidic phosphoprotein 1 (DMP-1). Total RNA was extracted using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Subsequently, the RNA
was converted to cDNA using a Transcriptor First Strand cDNA
Synthesis kit (Roche Diagnostics GmbH, Mannheim, Germany) using the
following temperature protocol: 50°C for 60 min, 85°C for 5 min and
4°C for 10 min. The expression levels of the genes were quantified
using FastStart Universal SYBR-Green Master Rox mix (Roche
Diagnostics GmbH). The PCR thermocycling conditions were as
follows: 95°C for 2 min, followed by 40 cycles of 95°C for 15 sec
and 60°C for 30 sec. PCR results were normalized against the
reference gene GAPDH to correct for non-specific experimental
variation. The method of ΔΔCq was used to determine the relative
quantity of mRNA expression in samples, and fold change was
determined as 2−ΔΔCq (33). The following primers were used: DSP
forward, 5′-TTTCCGCTTGTCATCATCTCC-3′ and reverse,
5′-GGTGTCCTGGCACTACTGCAT; DMP-1 forward,
5′-AAAATTCTTGTGAACTACGGAGG-3′ and reverse,
5′-GAGCACAGGATAATCCCCAA-3′; GAPDH forward,
5′-GACAACTCCCTCAAGATTGTCAG-3′ and reverse,
5′-ATGGCATGGACTGTGGTCATGAG-3′.
Immunofluorescence staining
The four groups of cells were cultured in 6-well
plates (5×104 cells/well) for 2 weeks prior to
immunostaining. Cells were fixed in 4% paraformaldehyde for 30 min
at room temperature and permeabilized in 0.2% Triton X-100 for 15
min. Cells were blocked in 3% bovine serum albumin (BSA,
Sigma-Aldrich; Merck KGaA) for 1 h at room temperature.
Odontoblasts were detected using a specific antibody against DSP at
4°C overnight (cat. no. sc-33586; 1:50; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA). Cells were subsequently washed 3 times in
PBS with 0.3% Tween for 5 min each wash, and incubated for 1 h at
room temperature with Alexa Fluor-conjugated goat anti-rabbit IgG
(cat. no. E031320-01; 1:500; EarthOx Life Sciences, Millbrae, CA,
USA). Cell nuclei were subsequently stained with DAPI (cat. no.
C1005; Beyotime Institute of Biotechnology, Haimen, China) for 5
min at room temperature. Immunofluorescence was detected using a
fluorescence microscope (magnification, ×20; DMI14000B; Leica
Microsystems GmbH, Wetzlar, Germany).
Western blot analysis
Total protein was extracted from the four groups of
cells on day 7 using cell lysis buffer [20 mM Tris (pH 7.5), 150 mM
NaCl, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM EDTA, 1%
Na3CO4, 0.5 µg/ml leupeptin, 1 mM phenylmethanesulfonyl fluoride].
The lysates were collected by scraping from the plates and
subsequently centrifuged at 15,000 × g at 4°C for 5 min. Protein
concentration was quantified using a bicinchoninic acid assay.
Total protein samples (20 µg/lane) were separated by 12% SDS-PAGE
and transferred onto polyvinylidene fluoride membranes. Membranes
were blocked in 1% BSA with 0.05% Tween-20 at room temperature for
2 h. Membranes were incubated overnight at 4°C with the following
antibodies: Rabbit anti-human DSP (cat. no sc-33586; 1:200; Santa
Cruz Biotechnology, Inc.), and β-actin (cat. no. TA346894; 1:500,
Zhongshan Goldenbridge, Beijing, China). The secondary antibody
used were horseradish peroxidase-conjugated AffiniPure goat
anti-rabbit IgG (cat. no. E030120-01; 1:10,000; EarthOx Life
Sciences, Millbrae, CA, USA) and goat anti-mouse IgG (cat. no.
E030110-01; 1:10,000; EarthOx Life Sciences). Bands were visualized
using an enhanced chemiluminescence western blot kit (CWBIO,
Beijing, China) and a Tanon 1000 digital image gel analytical
system (Tanon Science & Technology Co., Ltd., Shanghai, China)
was used for photography and quantification.
Statistical analysis
All values are expressed as the mean ± standard
error of the mean. Statistical analysis was performed by using
one-way analysis of variance followed by Bonferroni multiple
comparisons using SPSS 13.0 software (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
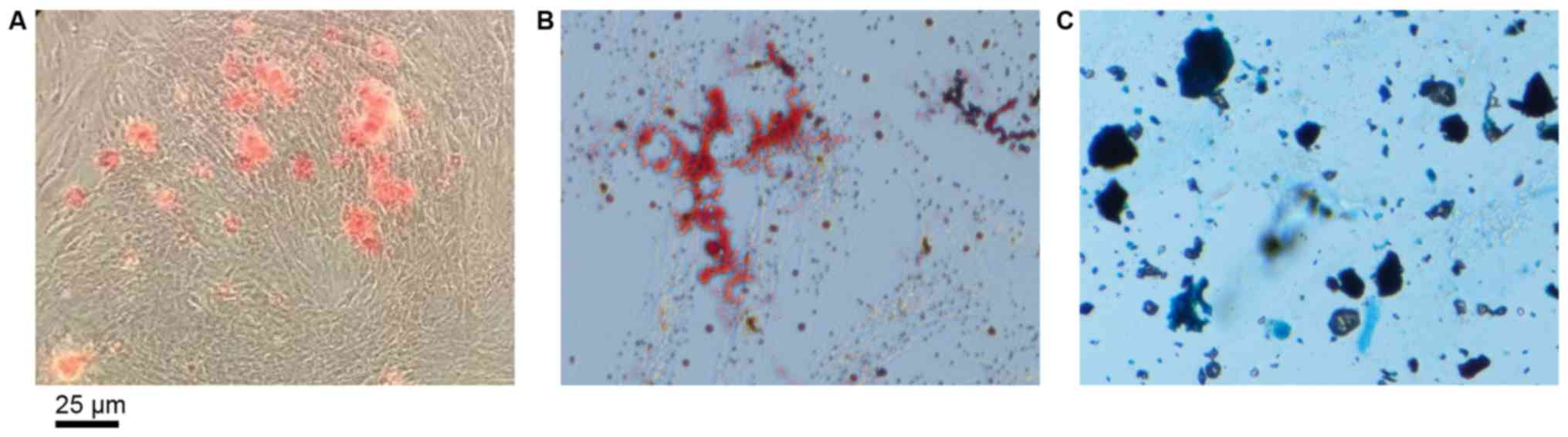
Characterization of the
immunophenotype and differentiation potential of DPSCs in
vitro
The presence of mineralized nodules demonstrated
successful osteogenic induction of DPSCs (Fig. 1A). As presented in Fig. 1B, the formation of neutral lipid
vacuoles also indicated the adipogenic potential of the DPSCs.
Furthermore, the DPSCs were observed to differentiate into
chondroblasts following induction (Fig. 1C).
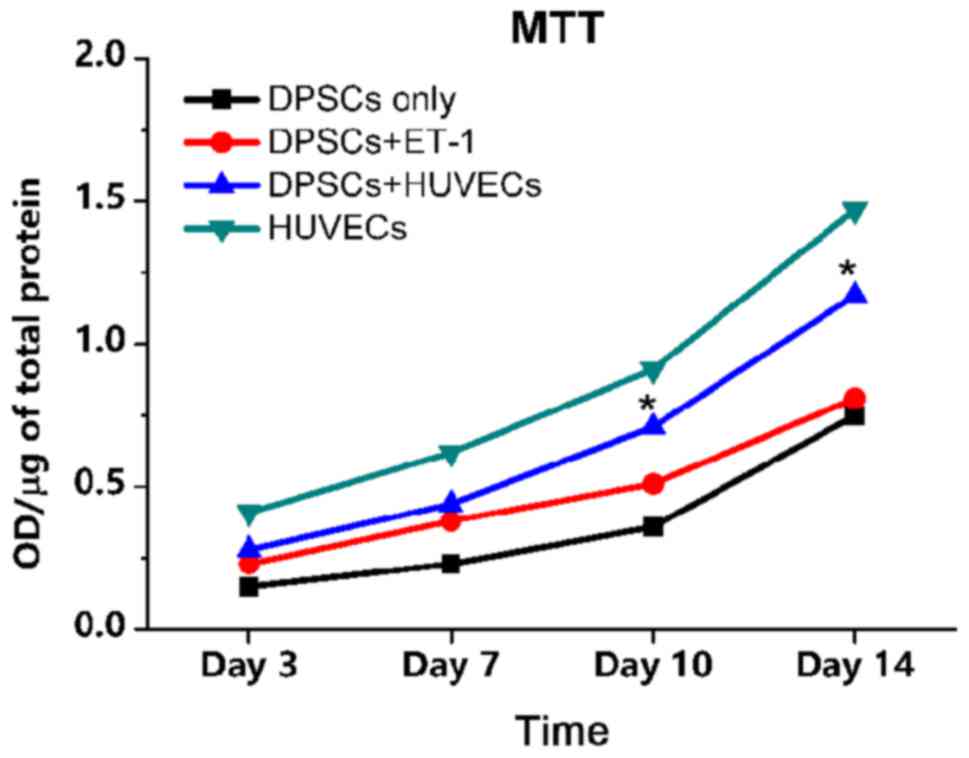
Cytotoxicity (MTT) assay
The data for the HUVEC-only group exhibited the
highest level of cell proliferation among the four groups. DPSC and
HUVEC direct co-culture group revealed that cells underwent notable
proliferation, and proliferation was significantly increased at day
10 and 14, compared with the DPSC + ET-1 and DPSC only groups. The
DPSC + ET-1 and DPSC only groups exhibited almost linear increases
from day 3 to day 14 (Fig. 2).
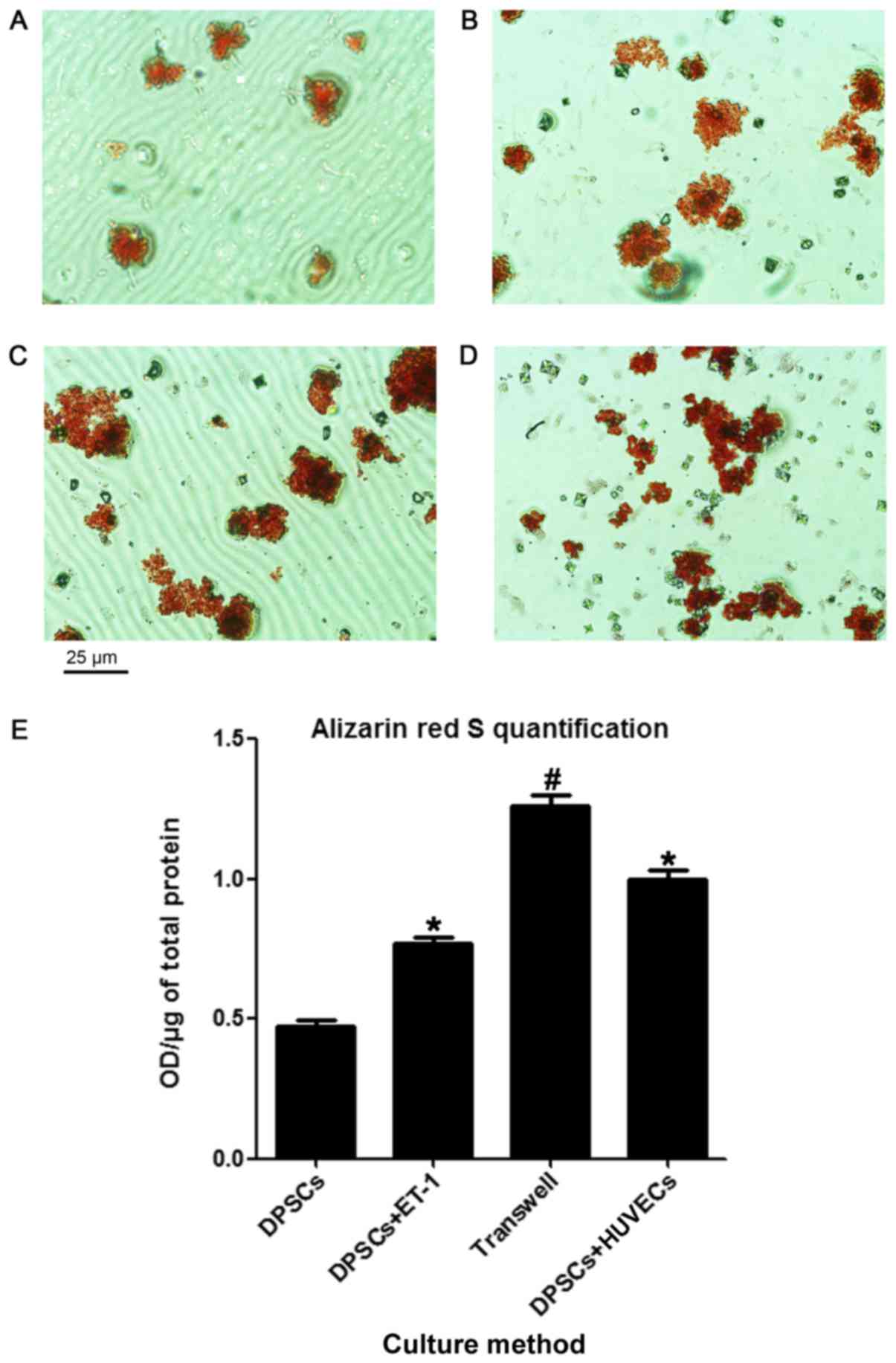
Mineralization assay
The cells adopted an osteoblast-like polygonal
morphology after 21 days of culture. Following the induction of
mineralization, white mottled crystals appeared, which became red
mineralized nodules following staining with Alizarin Red S. As
presented in Fig. 3A, the
mineralized nodules appeared to be smaller and less abundant within
the DPSC-only group compared with in the ET-1 treatment (Fig. 3B), Transwell (Fig. 3C) and co-culture group (Fig. 3D). Nodule formation was observed to
be significantly higher in the DPSC + ET-1, Transwell and DPSC and
HUVEC groups compared with in the control. The Transwell group
generated the highest number of nodules of all four groups
(Fig. 3E).
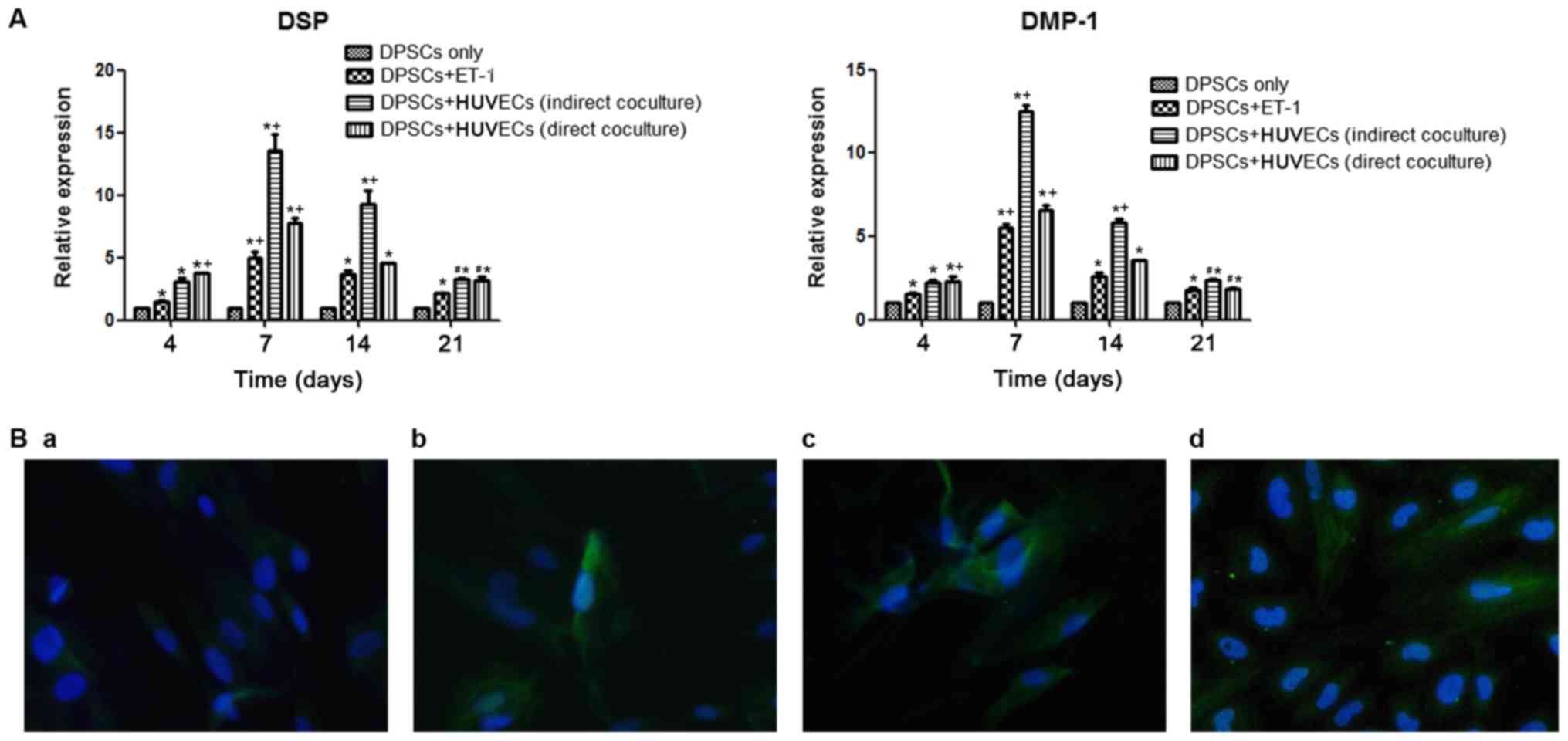
RT-qPCR analysis
Odontogenesis-associated genes were expressed in all
four groups. DSP and DMP-1 were expressed weakly in the DPSC-only
culture group compared with in the other three groups. Over the
experimental period, the expression of DSP and DMP-1 was highest on
day 7, with successively lower levels detected from day 14 to day
21 in all four groups. The expression levels of the two genes were
highest in the DPSC + HUVEC direct co-culture group on day 4, while
being second highest in the Transwell culture group. The two genes
were expressed at the highest level on days 7 and 14 in the
Transwell culture group, with lower levels detected in the direct
co-culture group, the DPSC + ET-1 group and the DPSC-only group,
over this period. The expression of the odontogenesis-associated
genes was notably downregulated on day 21 in the direct co-culture
and Transwell culture groups, but remained significantly higher
compared with the DPSC + ET-1 group (Fig. 4A).
Immunofluorescence staining
Immunofluorescence staining revealed that all four
groups expressed DSP after 2 weeks of culture; however, expression
was weak in the DPSC-only group (Fig.
4B).
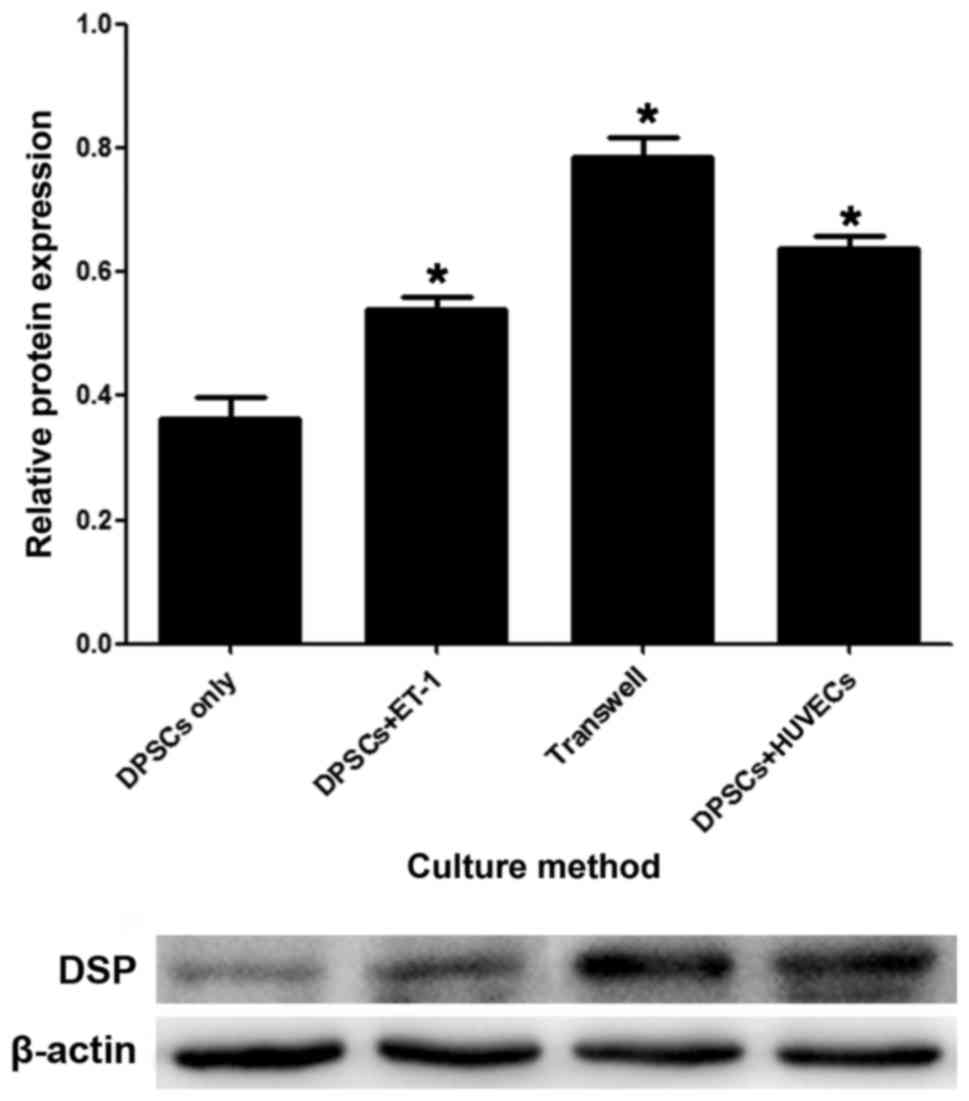
Protein expression during the process
of DPSC differentiation
The western blot assay demonstrated that compared
with the DPSC-only group, the expression levels of DSP protein were
significantly increased in the DPSC + ET-1 group, the direct
co-culture group and the Transwell culture group. The expression of
DSP in the Transwell group was significantly higher than the direct
co-culture group. DSP expression in the DPSC + ET-1 group was lower
than the above two groups (Fig.
5).
Discussion
It is widely acknowledged that further study on
DPSCs may have a positive impact on dental tissue engineering and
tooth regeneration. DPSCs present in their perivascular niche and
ECs present on the surface of blood vessels interact directly or
indirectly within the tumor microenvironment. When caries or
dental-trauma occurs, the direct cell-cell contact may affect the
reparation and regeneration of dental tissue to a certain extent;
however, whether the direct contact or interaction between these
two cell types promotes the secretion of cytokines requires further
investigation. The present study divided DPSCs and HUVECs into
various co-culture groups, in order to compare the different
factors that may impact on the differentiation of dental tissue
(2,34–38).
Researchers have suggested that HUVECs notably affect the function
of DPSCs, which is in accordance with the findings of other studies
(9,34–36,38–40).
Saleh et al (9) reported
that HUVECs may promote the survival, proliferation and aggregation
of bone marrow mesenchymal stem cells. Dissanayaka et al
(6) directly co-cultured HUVECs
and DPSCs, and revealed that HUVECs promoted the proliferation and
differentiation of DPSCs. Therefore, four groups were designed in
the present study, namely the Transwell co-culture, direct
co-culture, DPSC + ET-1 and DPSC-only groups. The Transwell method
enabled the cytokines secreted from HUVECs to migrate via a
semipermeable membrane into the culture below, while preventing
HUVECs from entering the DPSC culture. The detection of
odontogenesis-associated genes indicated that the two co-culture
groups expressed the genes at increased levels compared with the
DPSC-only group on days 4, 7, 14 and 21. The expression levels of
the genes declined from peak levels on day 7. The results of the
present study revealed that the process of co-culture may promote
the differentiation of DPSCs, and that this capability increased on
day 7 and then decreased throughout further cell culture. The
results on day 4 indicated elevated expression levels of the
odontogenic markers in the direct co-culture group compared with in
the Transwell group, which may have been due to the direct contact
of the two cell types initially promoting the differentiation of
the DPSCs. This promotion may be unassociated with cytokine
secretion. The lower expression levels in the Transwell group on
day 4 may support this hypothesis; however, the DPSC + ET-1 and the
DPSC-only groups expressed lower levels of the odontogenic markers
compared with the two co-culture groups on day 4. This result
demonstrated that HUVECs may be advantageous to DPSC
differentiation and the cytokines secreted by the HUVECs may have
promoted the differentiation of the DPSCs. The effect on
differentiation induced by ET-1 alone was not as strong as that in
the co-culture groups. The results of the present study indicated
that ET-1 may be an effective factor promoting the differentiation
of DPSCs. This is in accordance with research on developing rat
teeth (28); however, there may be
other cytokines that accompany ET-1 in promoting the
differentiation of DPSCs, as the results of the co-culture groups
demonstrated in the present study. The results on day 7 and day 14
indicating increased odontogenic gene expression in the Transwell
group may be due to the duration of culture. The direct contact
between the two cell types may serve a stimulatory role; however,
the promotional function may have been limited over time as the
interaction between the two cells was unfavorable to the secretion
of HUVECs, thereby affecting the differentiation of the DPSCs.
Odontogenic gene expression in the Transwell group increased until
day 7, after which the levels were downregulated, which was
consistent with the results of our previous research (32). Our previous research demonstrated
that during the mineralization process of DPSCs, the ability for
odontogenesis decreased over time (32). Alternatively, the effect on
differentiation may be due to increasing cell density. The
measurements on day 21 in the present study revealed that the two
co-culture groups exhibited downregulated expression levels of the
odontogenic markers compared with in the other groups; however, the
differences between the two groups were not significant.
There are two potential reasons for the
downregulated expression, including that the density of the cells
may have affected the differentiation. Additionally, the extended
duration of direct contact may have inhibited differentiation;
however, it is evident that there were numerous potential factors
that may have exerted an effect on the promotional effects of the
HUVECs. For instance, HUVECs can secrete insulin growth factor-1,
ET-1, FGF, platelet-derived growth factor, TGF-β and bone
morphogenic protein-2 (9,10), which may promote the proliferation
and aggregation of progenitor cells. Except for ET-1, researchers
have focused on these factors and investigation has revealed that
IGF-1 may promote the proliferation and differentiation of DPSCs
(41). This stimulatory effect was
associated with the activation of the mechanistic target of
rapamycin (mTOR) signaling pathway (41). Notably, when the mTOR signaling
pathway was inhibited, the inducing effect of IGF-1 was reversed
(41). Research by Zhang et
al (42) demonstrated that
basic FGF and nerve growth factor co-secretion were associated with
increased expression levels of phosphorylated (p)-protein kinase B
(AKT) and p-extracellular signal-regulated kinases (ERK), thereby
suggesting that the ERK and AKT signaling pathway may be involved
in the regulation of DPSC neural differentiation. Few
investigations into the effects of ET-1 on DPSCs have been
conducted; however, researchers have demonstrated that ET-1,
secreted by ECs, may promote the interaction of ECs and DPSCs
(8). Therefore, the present study
aimed to investigate the effects of ET-1 on DPSCs.
The effect of bone dynamic balance and odontogenesis
has been a point of interest. Salama et al (43) added ET-1 into the culture medium of
MSCs, and reported that ET-1 may modulate the proliferation,
migration and differentiation of the MSCs. Research has also
suggested that classic wingless-type mouse mammary tumor virus
(Wnt) signaling pathways may modulate the differentiation of MSCs
into osteoblasts, and modify bone growth via specific gene
expression (44,45). Additionally, osteoblasts and
osteocytes have been suggested to modulate the formation of
osteoclasts via the Wnt/β-catenin signaling pathway (46–48).
These studies indicated the regulatory effect of the Wnt signaling
pathway on bone metabolism. The extracellular molecular mechanism
underlying ET-1-mediated bone formation may involve the autocrine
or paracrine activation of Wnt signaling that is essential for
osteoblast proliferation, differentiation and bone development
(18). Dickkopf homologue 1 (DDK1)
is a selective inhibitor of the Wnt signaling pathway, and its
transcription rate may be suppressed by ET-1 in calvarial organ
culture. Conversely, recombinant DDK1 may also inhibit
ET-1-mediated osteoblast proliferation and new bone formation
(18). The present study
investigated the promotional effect of cytokines secreted by HUVECs
on the differentiation of DPSCs. Furthermore, in order to study the
promotional effects of ET-1 on DPSC differentiation, ET-1 was added
to the culture medium of DPSCs. The findings of the present study
demonstrated that the DPSC + ET-1 group expressed elevated levels
of odontogenic markers compared with the DPSC-only group, which
indicated the function of ET-1 in the process of DPSC
differentiation. However, the function of ET-1 was unclear as in
the co-culture group. Since ET-1 may function in an autocrine or
paracrine manner, ET-1 exerted a stronger effect on DPSC
differentiation compared with in the DPSC-only group, but the
mechanisms of action require further investigation. In addition,
the alteration of DSP expression in the DPSC + ET-1 group was not
as notable as those in the co-culture group, demonstrating that the
mechanisms of cytokines are complex. It is possible that two
cytokines may synergistically promote differentiation, or inhibit
further differentiation following initial promotion. Recently, the
cytokines associated with DPSC differentiation have become a focus
of research (49,50). The results of the present study
suggested that ET-1 may be associated with increases and subsequent
decreases in differentiation, which was similar to the trends of
the DPSC-only group. Further investigation may be conducted into
the maintaining the promotion of differentiation for 21 days.
The small integrin-binding ligand N-linked
glycoprotein protein family, including DSP and DMP-1, exhibit
positive effects in the process of dentinogenesis (51,52).
Dentin sialophosphoprotein (DSPP), a major non-collagenous matrix
protein of odontoblasts, undergoes cleavage to dentin
phosphoprotein and DSP (53). DSPP
is the first putative marker of odontoblastic differentiation and
its upregulation has suggested that DPSCs may acquire the capacity
to secrete mineralizable dentin (54). DMP-1 is an extracellular matrix
glycoprotein important for the mineralization of dentin; during
odontoblast maturation, DMP-1 is phosphorylated and exported to the
extracellular matrix where it organizes the formation of
mineralized matrix (55,56). Research has demonstrated that DMP-1
was initially expressed in mineralized matrix in alveolar bone;
however, the expression of DMP-1 was downregulated to lower levels
compared with DSP (54). In the
present study, the expression levels of DSP were increased compared
with DMP-1 at all time points, which was consistent with the
aforementioned findings.
In conclusion, HUVECs may promote the
differentiation of DPSCs, potentially via a synergetic manner of
cytokines secreted by HUVECs. Therefore, DPSCs and ECs may be
applicable in regenerative pulp therapy in the future.
Acknowledgements
The authors are grateful to the Key Laboratory of
Myocardial Ischemia of the Ministry of Education (the Second
Affiliated Hospital of Harbin Medical University, Harbin, China)
for providing facilities to conduct the investigations of the
present study.
Funding
The present study was supported by the Heilongjiang
Natural Science Foundation of China under contract no. H2013101,
and by the Graduate Science and Technology Innovation Projects
program of Harbin Medical University (grant no.
YJSCX2016-24HYD).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML designed the study, performed the experiments and
wrote the manuscript. LZ performed the experiments and organized
the images. JH and LW participated in this experiment and made
substantial contributions to the acquisition of data. NL analyzed
and interpreted the data. DW and XS organized the images and were
involved in drafting the manuscript. MY performed the surgery to
obtain teeth and was involved in revising the manuscript. WH and XW
helped write the manuscript, and were responsible for the study
funding and design, as well as providing final approval of the
version to be published. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Ethics Committee of the Second Affiliated Hospital of Harbin
Medical University (Harbin, China). Written informed consent was
obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gronthos S, Mankani M, Brahim J, Robey PG
and Shi S: Postnatal human dental pulp stem cells (DPSCs) in vitro
and in vivo. Proc Natl Acad Sci USA. 97:13625–13630. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shi S and Gronthos S: Perivascular niche
of postnatal mesenchymal stem cells in human bone marrow and dental
pulp. J Bone Miner Res. 18:696–704. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spath L, Rotilio V, Alessandrini M,
Gambara G, De Angelis L, Mancini M, Mitsiadis TA, Vivarelli E, Naro
F, Filippini A and Papaccio G: Explant-derived human dental pulp
stem cells enhance differentiation and proliferation potentials. J
Cell Mol Med. 14:1635–1644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mathieu S, El-Battari A, Dejou J and About
I: Role of injured endothelial cells in the recruitment of human
pulp cells. Arch Oral Biol. 50:109–113. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nugent MA and Iozzo RV: Fibroblast growth
factor-2. Int J Biochem Cell Biol. 32:115–120. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dissanayaka WL, Zhan X, Zhang C,
Hargreaves KM, Jin L and Tong EH: Coculture of dental pulp stem
cells with endothelial cells enhances osteo-/odontogenic and
angiogenic potential in vitro. J Endod. 38:454–463. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martin P: Wound healing-aiming for perfect
skin regeneration. Science. 276:75–81. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang DS, Miura M, Demura H and Sato K:
Anabolic effects of 1,25-dihydroxyvitamin D3 on osteoblasts are
enhanced by vascular endothelial growth factor produced by
osteoblasts and by growth factors produced by endothelial cells.
Endocrinology. 138:2953–2962. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saleh FA, Whyte M, Ashton P and Genever
PG: Regulation of mesenchymal stem cell activity by endothelial
cells. Stem Cells Dev. 20:391–403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bouletreau PJ, Warren SM, Spector JA,
Peled ZM, Gerrets RP, Greenwald JA and Longaker MT: Hypoxia and
VEGF up-regulate BMP-2 mRNA and protein expression in microvascular
endothelial cells: Implications for fracture healing. Plast
Reconstr Surg. 109:2384–2397. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu L, Ling J, Wei X, Wu L and Xiao Y:
Stem cell regulatory gene expression in human adult dental pulp and
periodontal ligament cells undergoing odontogenic/osteogenic
differentiation. J Endod. 35:1368–1376. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sueyama Y, Kaneko T, Ito T, Kaneko R and
Okiji T: Implantation of endothelial cells with mesenchymal stem
cells accelerates dental pulp tissue regeneration/healing in
pulpotomized rat molars. J Endod. 43:943–948. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yanagisawa M, Inoue A, Ishikawa T, Kasuya
Y, Kimura S, Kumagaye S, Nakajima K, Watanabe TX, Sakakibara S,
Goto K, et al: Primary structure, synthesis, and biological
activity of rat endothelin, an endothelium-derived vasoconstrictor
peptide. Proc Natl Acad Sci USA. 85:6964–6967. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Griswold DE, Douglas SA, Martin LD, Davis
TG, Davis L, Ao Z, Luttmann MA, Pullen M, Nambi P, Hay DW and
Ohlstein EH: Targeted disruption of the endothelin-B-receptor gene
attenuates inflammatory nociception and cutaneous inflammation in
mice. J Cardiovasc Pharmacol. 36 5 Suppl 1:S78–S81. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pomonis JD, Rogers SD, Peters CM, Ghilardi
JR and Mantyh PW: Expression and localization of endothelin
receptors: Implications for the involvement of peripheral glia in
nociception. J Neurosci. 21:999–1006. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hirata Y and Ishimaru S: Effects of
endothelin receptor antagonists on endothelin-1 and inducible
nitric oxide synthase genes in a rat endotoxic shock model. Clin
Sci (Lond). 103 Suppl 48:332S–335S. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yanagisawa M, Kurihara H, Kimura S, Tomobe
Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K and Masaki T: A novel
potent vasoconstrictor peptide produced by vascular endothelial
cells. Nature. 332:411–415. 1988. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sin A, Tang W, Wen CY, Chung SK and Chiu
KY: The emerging role of endothelin-1 in the pathogenesis of
subchondral bone disturbance and osteoarthritis. Osteoarthritis
Cartilage. 23:516–524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuan W, Zhao MD, Yuan FL, Che W, Duan PG,
Liu Y and Dong J: Association of endothelin-1 expression and
cartilaginous endplate degeneration in humans. PLoS One.
8:e600622013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Inoue A, Yanagisawa M, Kimura S, Kasuya Y,
Miyauchi T, Goto K and Masaki T: The human endothelin family: Three
structurally and pharmacologically distinct isopeptides predicted
by three separate genes. Proc Natl Acad Sci USA. 86:2863–2867.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
W BF, Kristian HA, Ohlsson L, Tolstrup CA,
Warfvinge K and Edvinsson L: Increased endothelin-1-mediated
vasoconstriction after organ culture in rat and pig ocular arteries
can be suppressed with MEK/ERK1/2 inhibitors. Acta Ophthalmol. Jan
25–2018.(Epub ahead of print).
|
|
22
|
Rapoport RM and Merkus D: Endothelin-1
regulation of exercise-induced changes in flow: Dynamic regulation
of vascular tone. Front Pharmacol. 8:5172017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vanhoutte PM: Say No to ET. J Auton Nerv
Syst. 81:271–277. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
De Mey JG and Vanhoutte PM: End o' the
line revisited: Moving on from nitric oxide to CGRP. Life Sci.
118:120–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shioide M and Noda M: Endothelin modulates
osteopontin and osteocalcin messenger ribonucleic acid expression
in rat osteoblastic osteosarcoma cells. J Cell Biochem. 53:176–180.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sasaki T and Hong MH: Localization of
endothelin-1 in the osteoclast. J Electron Microsc (Tokyo).
42:193–196. 1993.PubMed/NCBI
|
|
27
|
Warner TD: Endothelin and its inhibitors.
Springer-Verlag; Berlin: pp. 149–150. 2001
|
|
28
|
Neuhaus SJ and Byers MR: Endothelin
receptors and endothelin-1 in developing rat teeth. Arch Oral Biol.
52:655–662. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khan ZA, Farhangkhoee H, Mahon JL, Bere L,
Gonder JR, Chan BM, Uniyal S and Chakrabarti S: Endothelins:
Regulators of extracellular matrix protein production in diabetes.
Exp Biol Med (Maywood). 231:1022–1029. 2006.PubMed/NCBI
|
|
30
|
Gilbert TM, Pashley DH and Anderson RW:
Response of pulpal blood flow to intra-arterial infusion of
endothelin. J Endod. 18:228–231. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pourjafar M, Saidijam M, Mansouri K, Malih
S, Nejad Ranjbar T, Shabab N and Najafi R: Cytoprotective effects
of endothelin-1 on mesenchymal stem cells: An in vitro study. Clin
Exp Pharmacol Physiol. 43:769–776. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu M, Sun Y, Liu Y, Yuan M, Zhang Z and
Hu W: Modulation of the differentiation of dental pulp stem cells
by different concentrations of β-glycerophosphate. Molecules.
17:1219–1232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hutley LJ, Herington AC, Shurety W, Cheung
C, Vesey DA, Cameron DP and Prins JB: Human adipose tissue
endothelial cells promote preadipocyte proliferation. Am J Physiol
Endocrinol Metab. 281:E1037–E1044. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Villars F, Guillotin B, Amédée T, Dutoya
S, Bordenave L, Bareille R and Amédée J: Effect of HUVEC on human
osteoprogenitor cell differentiation needs heterotypic gap junction
communication. Am J Physiol Cell Physiol. 282:C775–C785. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kaigler D, Krebsbach PH, West ER, Horger
K, Huang YC and Mooney DJ: Endothelial cell modulation of bone
marrow stromal cell osteogenic potential. FASEB J. 19:665–667.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Crisan M, Yap S, Casteilla L, Chen CW,
Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al: A
perivascular origin for mesenchymal stem cells in multiple human
organs. Cell Stem Cell. 3:301–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arai K and Lo EH: An oligovascular niche:
Cerebral endothelial cells promote the survival and proliferation
of oligodendrocyte precursor cells. J Neurosci. 29:4351–4355. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vernon SM, Campos MJ, Haystead T, Thompson
MM, DiCorleto PE and Owens GK: Endothelial cell-conditioned medium
downregulates smooth muscle contractile protein expression. Am J
Physiol. 272:C582–C891. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guillotin B, Bareille R, Bourget C,
Bordenave L and Amédée J: Interaction between human umbilical vein
endothelial cells and human osteoprogenitors triggers pleiotropic
effect that may support osteoblastic function. Bone. 42:1080–1091.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Feng X, Huang D, Lu X, Feng G, Xing J, Lu
J, Xu K, Xia W, Meng Y, Tao T, et al: Insulin-like growth factor 1
can promote proliferation and osteogenic differentiation of human
dental pulp stem cells via mTOR pathway. Dev Growth Differ.
56:615–624. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang J, Lian M, Cao P, Bao G, Xu G, Sun
Y, Wang L, Chen J, Wang Y, Feng G and Cui Z: Effects of nerve
growth factor and basic fibroblast growth factor promote human
dental pulp stem cells to neural differentiation. Neurochem Res.
42:1015–1025. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Salama M, Andrukhova O, Jaksch P, Taghavi
S, Kelpetko W, Dekan G and Aharinejad S: Endothelin-1 governs
proliferation and migration of bronchoalveolar lavage-derived lung
mesenchymal stem cells in bronchiolitis obliterans syndrome.
Transplantation. 92:155–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Boland GM, Perkins G, Hall DJ and Tuan RS:
Wnt 3a promotes proliferation and suppresses osteogenic
differentiation of adult human mesenchymal stem cells. J Cell
Biochem. 93:1210–1230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu G, Vijayakumar S, Grumolato L,
Arroyave R, Qiao H, Akiri G and Aaronson SA: Canonical Wnts
function as potent regulators of osteogenesis by human mesenchymal
stem cells. J Cell Biol. 185:67–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bianco P: Minireview: The stem cell next
door: Skeletal and hematopoietic stem cell ‘niches’ in bone.
Endocrinology. 152:2957–2962. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Long F: Building strong bones: Molecular
regulation of the osteoblast lineage. Nat Rev Mol Cell Biol.
13:27–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tüysüz B, Bursalı A, Alp Z, Suyugül N,
Laine CM and Mäkitie O: Osteoporosis-pseudoglioma syndrome: Three
novel mutations in the LRP5 gene and response to bisphosphonate
treatment. Horm Res Paediatr. 77:115–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nakayama H, Iohara K, Hayashi Y, Okuwa Y,
Kurita K and Nakashima M: Enhanced regeneration potential of
mobilized dental pulp stem cells from immature teeth. Oral Dis.
23:620–628. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
He X, Jiang W, Luo Z, Qu T, Wang Z, Liu N,
Zhang Y, Cooper PR and He W: IFN-γ regulates human dental pulp stem
cells behavior via NF-κB and MAPK signaling. Sci Rep. 7:406812017.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Batouli S, Miura M, Brahim J, Tsutsui TW,
Fisher LW, Gronthos S, Robey PG and Shi S: Comparison of
stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res.
82:976–981. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tye CE, Rattray KR, Warner KJ, Gordon JA,
Sodek J, Hunter GK and Goldberg HA: Delineation of the
hydroxyapatite-nucleating domains of bone sialoprotein. J Biol
Chem. 278:7949–7955. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Qin C, Baba O and Butler WT:
Post-translational modifications of sibling proteins and their
roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med.
15:126–136. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hao J, Ramachandran A and George A:
Temporal and spatial localization of the dentin matrix proteins
during dentin biomineralization. J Histochem Cytochem. 57:227–237.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Aplin HM, Hirst KL, Crosby AH and Dixon
MJ: Mapping of the human dentin matrix acidic phosphoprotein gene
(DMP1) to the dentinogenesis imperfecta type II critical region at
chromosome 4q21. Genomics. 30:347–349. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hirst KL, Simmons D, Feng J, Aplin H,
Dixon MJ and MacDougall M: Elucidation of the sequence and the
genomic organization of the human dentin matrix acidic
phosphoprotein 1 (DMP1) gene: Exclusion of the locus from a
causative role in the pathogenesis of dentinogenesis imperfecta
type II. Genomics. 42:38–45. 1997. View Article : Google Scholar : PubMed/NCBI
|