Introduction
Melanoma is one of the most aggressive metastatic of
skin cancer with resistance to most treatments, which represents
less than 5% of all skin cancers but responsible for a large
majority of skin cancers fatalities (1). This is closely related to resistance
of melanoma cells to the treatment of conventional
chemotherapeutics as well as other biological agents (2,3).
Defects in apoptotic signaling in the malignant melanoma cells are
thought to be one of major contributions to unchecked proliferation
and immortalization of melanoma. Accordingly, developing new
therapeutic approaches targeted at apoptosis induction is a
reasonable and promising strategy in controlling the proliferation
as well as invasiveness of this neoplasm (4).
9,11-dehydroergosterol peroxide [9(11)-DHEP] is the
member of a class of fungal secondary metabolites of 5α,
8α-endoperoxide sterol derivatives. It exits widely in mushrooms.
Studies showed that it had cytotoxic effect on different cancer
cells (5–7) and exhibited anti-inflammatory
activities (8). But the underlying
molecular mechanism of the bioactive steroids on induction of
cancer cell apoptosis is not fully elucidated so far.
Ganoderma lucidum (Leyss. ex Fr.) Karst., a
medicinal fungus called ‘Ling zhi’ in China, is considered to be
not only the dietary supplement that promotes longevity and
maintains the vitality of human beings but also the new medicine
sources for many diseases (9). The
fungus body, dry powders of its body wall as well as a mixture of
extracts from G. lucidum, have been used to prevent and
treat a variety of diseases including cancers for many years
(10). We isolated and purified
9(11)-DHEP from G. lucidum on submerged culture (11). Our results showed that the inducing
apoptosis effects of 9(11)-DHEP on A375 human malignant melanoma
cells involved caspase-dependent and mitochondria-mediated
pathway.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM) medium,
fetal bovine serum (FBS) and antibiotics (penicillin and
streptomycin mixture) were purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). DNase (grade 1) was obtained
from Invitrogen; Thermo Fisher Scientific, Inc. Antibodies for
anti-cleaved PARP (Asp214), PARP, cytochrome c,
Bcl-2-associated X protein (Bax), Bad, Bak, BID, Bmf, Bim, puma,
B-cell lymphoma 2 (Bcl-2), Bcl-xL, myeloid cell leukemia-1 (Mcl-1)
and anti-cleaved caspase-3, and −7, caspase-6, −8, −9, and −10 were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
9(11)-DHEP was prepared and checked according to our previous study
(11). The steroid was dissolved
in ethanol during all the experiments.
Cell culture
Normal skin fibroblast Hs68 cell line was provided
by Dr SM Ngai (Department of Biology, The Chinese University of
Hong Kong, Hong Kong, SAR, China). Human breast adenocarcinoma
MCF-7 cell line was kindly provided by Dr VEC Ooi (Department of
Biology, The Chinese University of Hong Kong); while the human
breast epithelial cell line MCF-10A (cell passage 2) (MCF-10A-2),
human malignant melanoma cell line A375, colorectal adenocarcinoma
cell line Colo201 and SW620 were purchased from American Type
Culture Collection (ATCC; Manassas, VA, USA). MCF-7 cells were
cultured in RPMI-1640 medium with 10% FBS, Colo201 cells were
maintained in RPMI-1640 medium containing 10% FBS, 4.5 mg/ml
glucose, 10 mM HEPES buffer and 1 mM sodium pyruvate. SW620 cells
were cultured in Leibovitz's L-15 medium with 10% FBS. MCF-10A-2
cells were cultured in DMEM/F-12 medium supplemented with 5% horse
serum, 0.5 µg/ml hydrocortisone, 10 µg/ml insulin, 20 ng/ml
epidermal growth factor, 0.1 µg/ml cholera enterotoxin, 2 mM
L-glutamine, and 0.5 µg/ml amphotericin B. Hs68 fibroblast cells
were cultured in DMEM with 1.5 mg/ml sodium bicarbonate, 4.5 mg/ml
glucose, 4 mM L-glutamine and 20% FBS. A375 cells were cultured in
DMEM medium containing 10% FBS. The medium was added 100 units/ml
penicillin, and 100 µg/ml streptomycin in an atmosphere of 5%
CO2 and 95% air at 37°C.
Cell viability
Viability of cells was evaluated by
3-[4,5-dimethylthiazol-2yl]-2,5-diphenyl tetrazolium bromide (MTT)
method (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The
appropriate different cancer cells were plated in 96-well tissue
culture plate and incubated for 24 h, then treated with 0–50 µg/ml
of the steroid for 72 h. The cells were stained with 0.5 mg/ml MTT
for 5 h and then incubated with acidic isopropanol. Optical density
at 570 nm was detected for monitoring the cell viability. Effects
of the steroids on inhibition of cell growth were calculated, and
the cells treated with EtOH at same concentrations as in the drugs
used as controls.
Cell proliferation
DNA synthesis was determined with the Cell
Proliferation ELISA kit, BrdU (Roche Molecular Biochemicals,
Indianapolis, IN, USA). For the BrdU assay, 20 µl BrdU was added to
the cells after treated with the steroids for 72 h and incubated
again for 2 h. Finally, the chemiluminescence readings were
measured by a microliter plate luminometer (ML3000). Results were
the percentage (%) of inhibition in treatment group as compared to
control and were calculated as equation: inhibition
%=(1-Atreatment/Acontrol)×100%, where
Atreatment means the absorbance of treatment group;
Acontrol means the absorbance of control group.
Flow cytometry
Cell cycle was analyzed by flow cytometry. A375
human melanoma cells (2×106 cells/time) were seeded and
after 24 h treated with 0–30 µg/ml samples for 72 h, cells were
fixed with 70% ethanol and then further washed with 1% BSA and
incubated in the dark at 4°C with propidium iodide (PI) staining
mixture overnight, and the fluorescence of individual nuclei
(approximately 10,000 events) was analyzed by flow cytometry (BD
FACScan; BD Biosciences, Franklin Lakes, NJ, USA).
TUNEL assay
Apoptosis morphology of cancer cell was detected by
terminal deoxynucleotidyl transferase-mediated biotinylated UTP
nick end-labeling (TUNEL) assay. Briefly, Cells density of
3×104 cells/chamber were treated with the steroid at
final concentration of 10, 20 and 30 µg/ml. For the control group
(negative control and positive control), 0.05% EtOH (v/v) was used
instead of steroids. After additional 72 h incubation, cells were
fixed with 4% paraformaldehyde, for 1 h and were re-incubated in
0.1% Triton X-100 for 2 min at 2–8°C. Slides were further incubated
with TUNEL reaction mixture containing nucleotide mixture and
terminal deoxynucleotidyl transferase (TdT) for 60 min in a
humidified atmosphere at 37°C in darkness according to
manufacturer's instructions of in situ Cell Death Detection
kit (Roche Molecular Biochemicals). Analysis was performed by
confocal laser scanning microscope (CLSM; Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Annexin V-FLUOS assay
Normal, apoptotic, and necrotic cells were
distinguished by using the Annexin V-PI kit (Roche Molecular
Biochemicals). According to manufacturer's instruction,
3×104 A375 cells was suspended in fresh medium and was
seeded onto chamber slide for pre-incubation at 37°C. Cells were
then treated with different concentration 9(11)-DHEP for 72 h.
Slides were rinsed with PBS (pH 7.4) and were covered with 100
µl/chamber of Annexin V-FLUOS labeling solution (Annexin
V-fluorescein in a Hepes buffer containing PI). Slides were further
incubated for 10–15 min at room temperature. After labeling, slides
were directly analyzed under confocal laser scanning microscope
(CLSM; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Cytochrome c detection
A375 cells (3×106 cells/dish) were
treated with 9(11)-DHEP for 24 h at concentration of 0, 20, and 30
µg/ml. We used the 0.1% EtOH treated cells as a control. Both
adherent and floating cells were collected; cytosolic extracts were
prepared by incubation for 30 min on ice in hypotonic buffer pH
7.5. Then cells were broken in and centrifuged at 1,000 × g for 10
min at 4°C, to remove unbroken cells and nuclei. The homogenates
were then centrifuged twice at 12,000 × g for 30 min at 4°C, and
the mitochondria-free supernatants were frozen at −80°C until
further analysis. The rest pellet was continuously extracted in the
ice-cold lysis buffer (Cell Signaling Technology, Inc.) for 2 h,
and then centrifuged at 14,000 × g for 30 min at 4°C, and the
supernatants were collected as mitochondria fractions for further
analysis (12).
Western blotting
1×107 growing A375 cells were treated
with 9(11)-DHEP for 24 h, cells were harvested and lysed in lysis
buffer (Cell Signaling Technology, Inc.). For tumor protein, the
tumor blocks were homogenized in lysis buffer liquid at 4°C. The
homogenate was centrifuged at 14,000 × g for 30 min at 4°C and the
supernatant was collected as crude protein extract. Total cellular
protein was determined using the BCA assay kit (Sigma-Aldrich;
Merck KGaA). Equal amounts of cell lysates (60–100 µg protein) were
separated by 10–12% Tricine-SDS-PAGE and transferred to a
nitrocellulose membrane. The membrane was probed with specific
primary antibodies at 4°C overnight. The specific protein complex
formed on appropriate secondary antibody treatment was identified
using the LuminGLO substrate reagent. Quantification was performed
by densitometric analysis (Model GS-690 Imaging Densitometer;
Bio-Rad Laboratories, Inc.).
Statistical analysis
All experiments were repeated three times (n=3)
unless otherwise indicated. The results were expressed as means ±
standard deviations (SDs). Statistical analysis was performed using
SPSS statistical package (SPSS 19.0 for Windows; SPSS, Inc.,
Chicago, IL, USA). Linear regression was built to analyze the
IC50 in MTT assay. The difference between two groups was
analyzed by two-tailed Student's t test. The difference between
multiple groups was analyzed by one-way ANOVA, followed by Tukey's
multiple comparison test. P<0.05 was considered to indicate a
statistically significant difference.
Results
9(11)-DHEP inhibited the cell
proliferation of A375 malignant melanoma cells
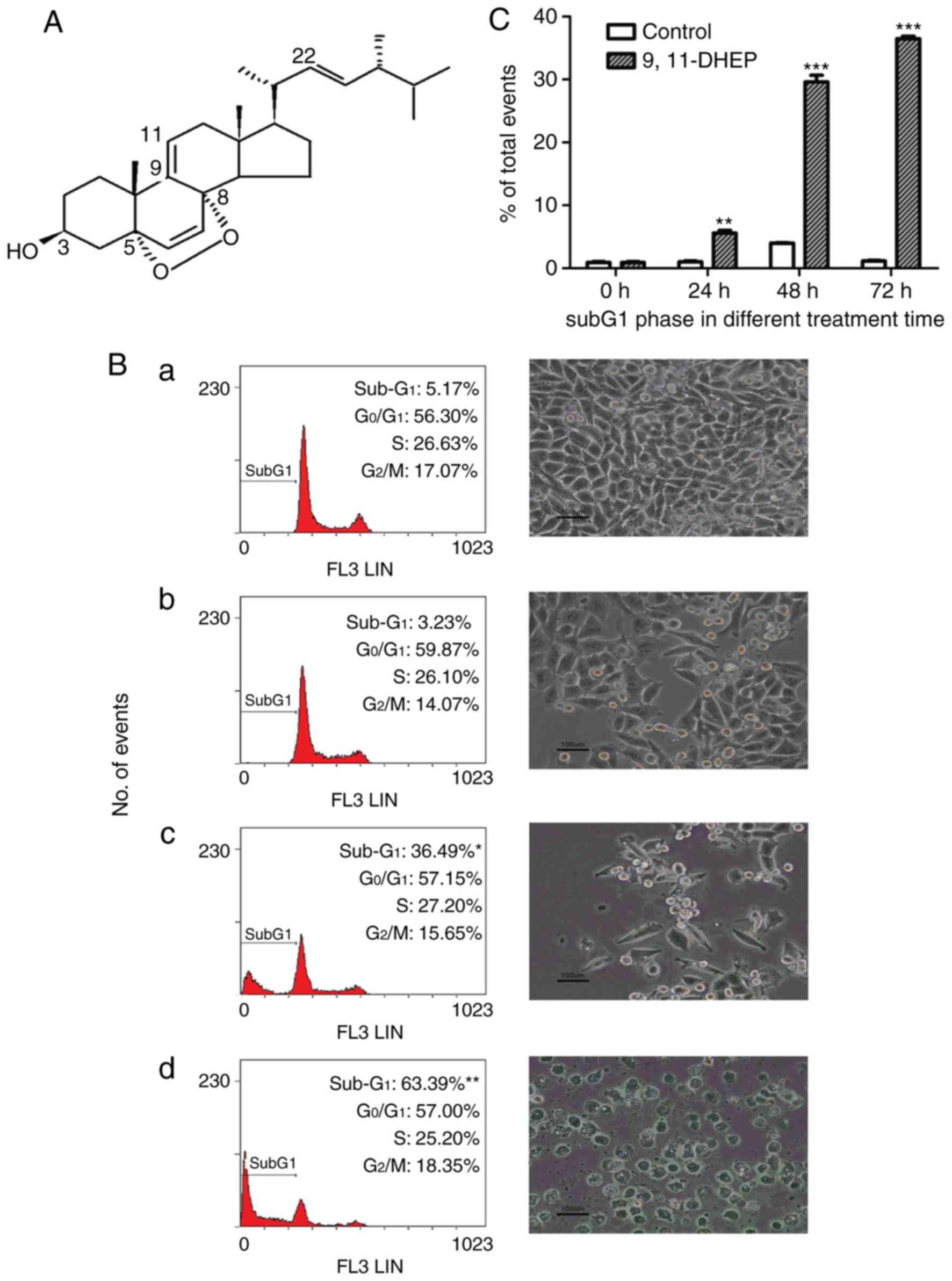
9(11)-DHEP (Fig.
1A) was purified from the submerged culture of G.
lucidum and the purity of the steroid was checked by RP-HPLC
according to the method of Zheng et al (11).
To estimate the antitumor capacity of 9(11)-DHEP,
inhibitory effects of the steroid on cell viability were examined
in different cancer and normal cell lines by MTT assay assay
(Table I). Our studies showed that
9(11)-DHEP suppressed the cell growth of different cancer cells but
not Hs68 and MCF-10A-2 cells (Table
I). A375 malignant melanoma cells were the most sensitive to
the treatment of steroid. The IC50 of A375 cells were
9.147 µg/ml for 9(11)-DHEP (Table
I). The effects of the steroid on the proliferation of A375
cells were investigated by BrdU assay. 9(11)-DHEP inhibited A375
cells proliferation in a dosage-dependent manner. The
IC50 in BrdU assay is about 12.57 µg/ml for
9(11)-DHEP.
 | Table I.Inhibitory effects of 9(11)-DHEP on
cell viability of different cell lines. |
Table I.
Inhibitory effects of 9(11)-DHEP on
cell viability of different cell lines.
| Cell line | IC50
(µg/ml) |
|---|
| A375 | 9.462±1.78 |
| Colo201 | 13.02±0.34 |
| SW620 | 32.87±0.76 |
| MCF-7 | 16.89±1.40 |
| MCF-10A-2 | 67.89±2.64 |
| Hs68 | 40.46±1.39 |
9(11)-DHEP induced apoptosis on A375
malignant melanoma cells
To investigate the mechanism by which the steroid
inhibited cancer cells growth, cell cycle analysis of A375 cells
was performed with flow cytometry. The cells were treated with
different concentration of the steroid for 72 h. As shown in
Fig. 1B, the number of the cells
in subG1 phase increased significantly in a dosage-dependent manner
after treatment with the steroid. These results suggested that the
steroid inhibited the cancer cells growth by inducing apoptosis. 20
µg/ml 9(11)-DHEP treatment resulted in a significantly (P<0.05)
increase in cell population of the subG1 phase from 1.03 to 5.57%
after 24 h incubation (Fig.
1C).
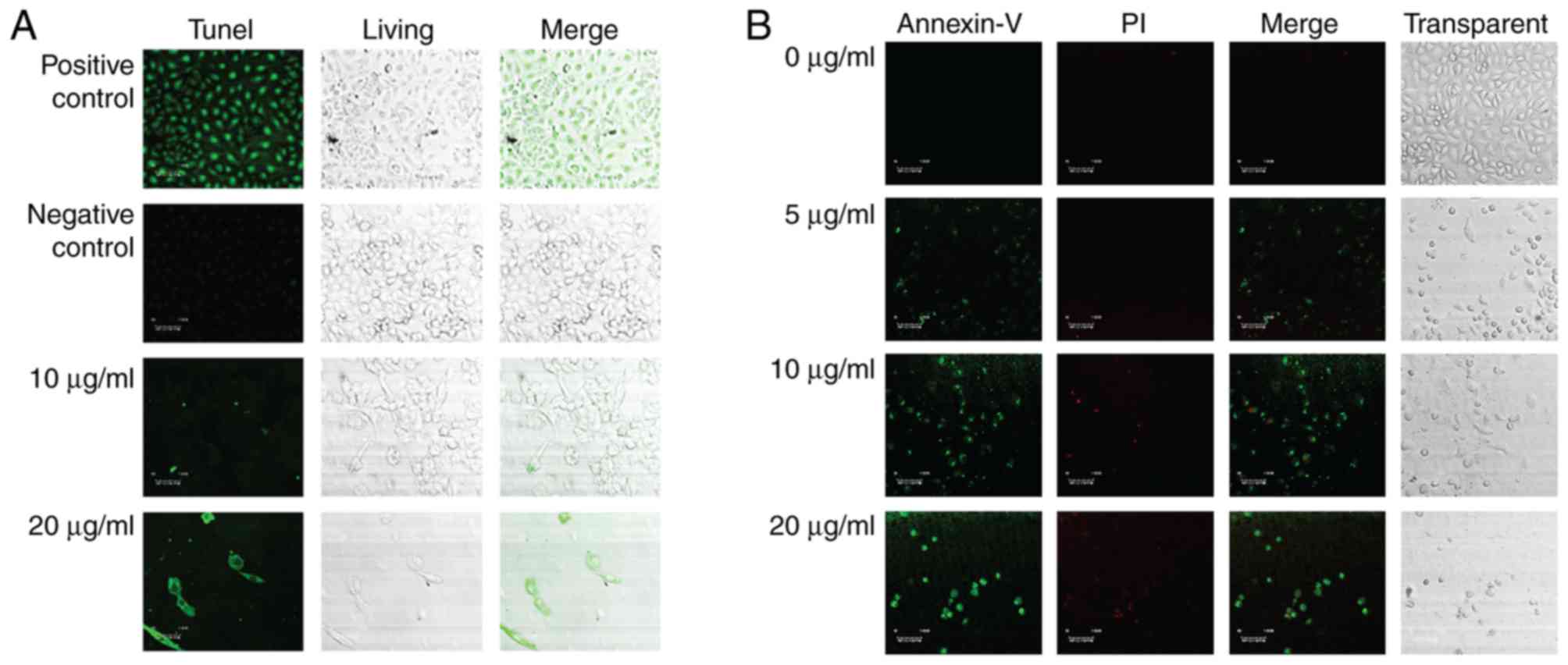
To better the anti-cancer function of the steroid
9(11)-DHEP, we examined the apoptosis inducing potential of the
steroid 9,11-DHEP by TUNEL and Annexin V/PI assay in melanoma cell
line. TUNEL assay is the classical method for detecting the DNA
fragment found in apoptotic cells. Compared with the negative
control, apoptotic bodies were greatly induced in the A375
malignant melanoma cells which were treated with the steroid as the
Fig. 2A showed. The apoptotic
cells intensely stained green in the TUNEL assay were increased in
a dosage-dependent manner. In the Annexin V-PI analysis, the early
phase apoptotic cells with high Annexin V and low PI staining were
clearly differentiated from different sub-popular: Late phase
apoptotic cells with high Annexin V and high PI staining, and
necrotic cells with low Annexin V and high PI staining. As Fig. 2B the results indicated that the
treatment with the steroid decreased the number of A375 cells in a
dosage-dependent manner. The Only 5 µg/ml 9(11)-DHEP treatment
could induce early apoptotic cells. This inducing effect was
dosage-dependent. This characterized further the apoptosis inducing
potential of the steroid 9(11)-DHEP.
Apoptosis of A375 malignant melanoma
cells induced by 9(11)-DHEP was via mitochondria-mediated
pathway
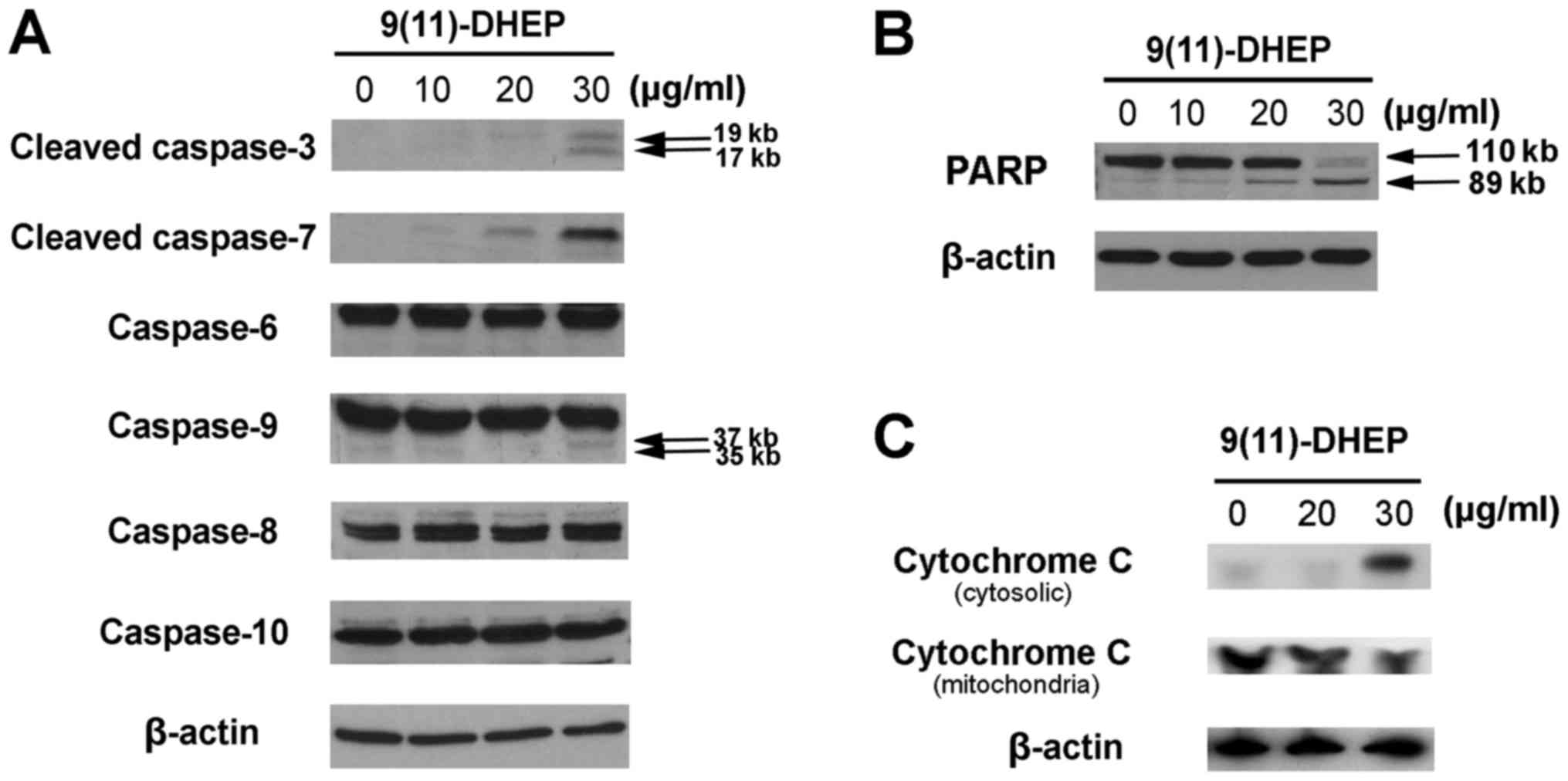
To study how the steroid induces apoptosis of A375
cells, the expressions of cytochrome c, PARP, caspase family
and Bcl-2 family were investigated in our work. Caspases, a family
of cysteine acid proteases, are central regulators of apoptosis.
Several caspase proteins were detected in A375 cells treated by the
steroid. As the results shown in Fig.
3A, both the steroid noticeably stimulated the activities of
caspase-3, −7, −9 in tumor cells in a dosage-dependent manner but
not the caspase-6, −8, −10, which suggest that intrinsic apoptotic
pathway might be involved in the steroid induced apoptosis in the
A375 cells.
PARP, a 116 kDa nuclear poly (ADP-ribose)
polymerase, appears to be involved in DNA repair predominantly in
response to environmental stress. Our results indicated that the
increase expression of cleaved PARP fragments were observed in the
steroid treated cancer cells in a dosage-dependent manner (Fig. 3B), which suggested that the steroid
could induce apoptosis on A375 cells.
Cytochrome c plays an important role in the
mitochondria pathway. Cytochrome c was detected after 48 h
of 9(11)-DHEP treatment. In the cytosolic fraction of 9(11)-DHEP
treated cells, cytochrome c was detected, but not in that of
control cells. While the content of cytochrome c in the
mitochondria fraction decreased a little. Therefore, we concluded
that cytochrome c was released from the mitochondria to the
cytosol after induced by the steroid (Fig. 3C). The results demonstrated damage
to mitochondria membrane further activated the intrinsic pathway of
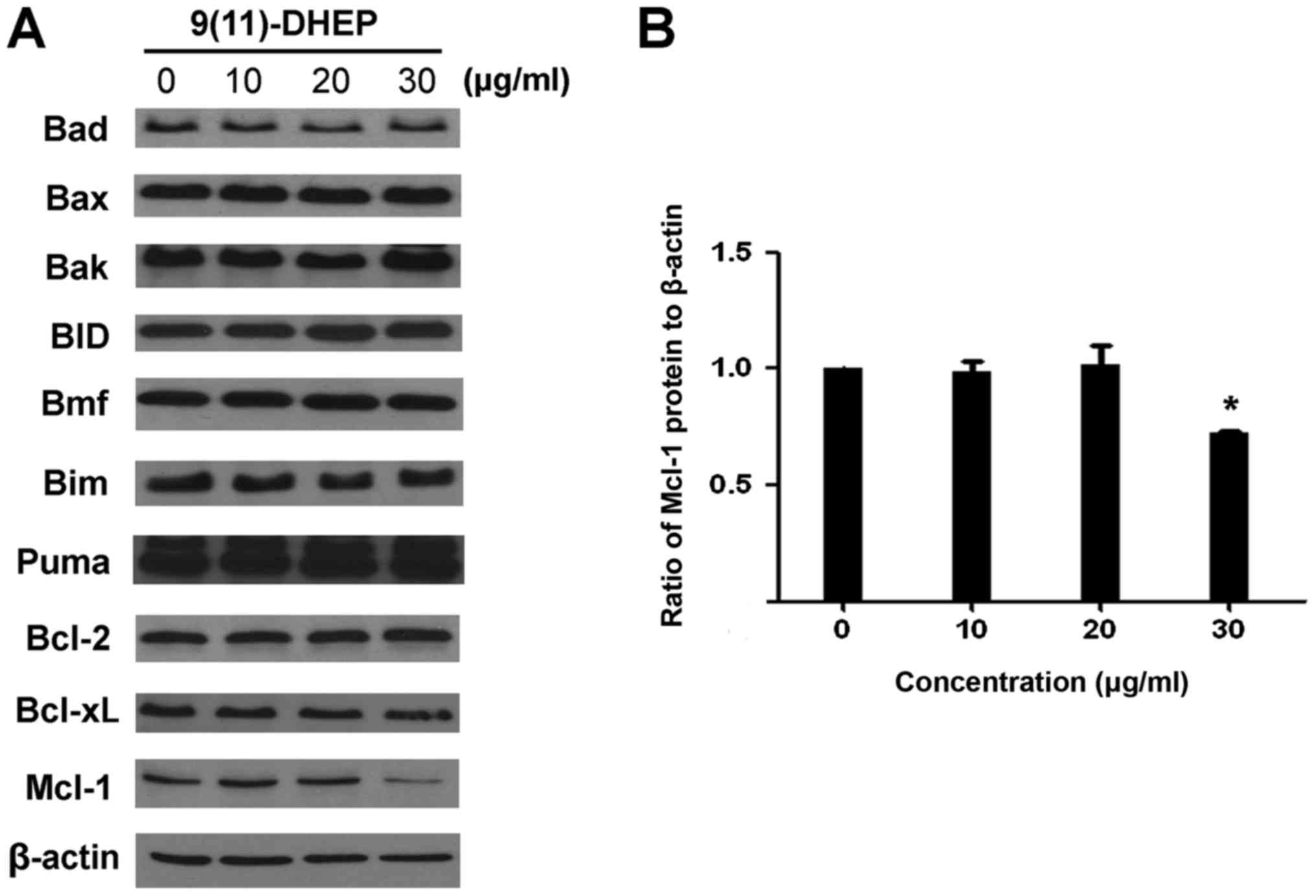
apoptosis. The expressions of the Bcl-2 family of A375 malignant
melanoma cell under the steroid treatment were detected (Fig. 4A). Our result showed that there
were no significant changes on the expression of Bim, Puma, Bax,
BID, Bad and Bcl-2, only the Mcl-1 protein was down-regulated in a
dosage-dependent manner (Fig. 4B).
Treatment of A375 cells with 9(11)-DHEP significantly (P<0.05)
decreased Mcl-1 expression from 1 to 0.86.
Discussion
Ganoderma contains many bioactive natural
components, including triterpenes, polysaccharides, proteins and
steroids. Ganoderma triterpenoids and steroids were reported to be
potential chemopreventive and therapeutic agents for cancer
treatment (13,14). 9(11)-DHEP is a natural product
present in G. lucidum, which has been shown to regulate
various biological process. However, the amount of 9(11)-DHEP,
isolated from fungi, was too little, which was not sufficient to be
used clinically. In this study, we purified 9(11)-DHEP from the
submerged culture of G. lucidum. After confirming the
structure and purity of the chemical, we investigated the molecular
mechanisms by which the cell death of human malignant melanoma
cells was induced.
To investigate the antitumor mechanism of the
steroid 9(11)-DHEP, we actually assay a serial of experiments.
After MTT screening, the tumor cell line most sensitive to the
steroid 9(11)-DHEP was chosen for mechanism study. In the BrdU Cell
Proliferation Assay, the 5-bromo-2′-deoxyuridine (BrdU)
incorporated into cellular DNA during cell proliferation, which was
detected by using an anti-BrdU antibody. The IC50 in
BrdU assay of 9(11)-DHEP confirm the anti-proliferation activity of
the steroid. To learn more about how the steroid inhibit the tumor
cell proliferation, flow cytometry and confocal microscope was used
to detect the cell cycle distribution and morphology change treated
with 9,11-DHEP. Cells undergoing apoptosis show characteristic
morphology and biochemical features. Flow cytometry results
suggested that the A375 malignant melanoma cells treated with the
steroid could be stopped in subG1 phase in a dosage- and
time-dependent manner (Fig. 1). By
using TUNEL assay and Annexin V assay, the apoptosis induction
effect of the steroid which was revealed by cleavage of DNA strands
was identified once again. In our studies, the morphology of cancer
cells treated with the steroid has been observed and these results
suggested that the steroid could inhibit the growth of A375
malignant melanoma cell by inducing apoptosis (Fig. 1). These clearly showed that the
steroid could inhibit A375 cell proliferation through inducing
apoptosis.
Our results indicated that the steroid could
activate the caspase 3, 6 and 7 in a dosage-dependent manner
(Fig. 3A). Caspase 3 cleaved PARP,
which responded to DNA fragmentation, and eventually leaded to A375
cancer cells apoptosis (Fig. 3B).
This suggested that apoptosis induced by the steroid on A375
melanoma cell was caspase-dependent. In addition, the expression of
caspase 8, 9 and 10 were observed. The three caspase were
considered as signaling and key caspase in extrinsic and intrinsic
pathway, respectively. In our case, caspase 9 but not caspase 8 and
caspase 10 was involved in the 9(11)-DHEP-mediated apoptosis of
A375 cancer cells.
In order to elucidate if mitochondria is an
important target for the apoptotic induction by 9(11)-DHEP,
cytochrome c content was detected in both the cytosolic and
mitochondria fraction. Our results showed that the steroid could
induce the release of cytochrome c into the cytosol
(Fig. 3C), which hinted that
apoptosis induced by the steroid was via the mitochondria pathway.
The Bcl-2 family plays a crucial role in apoptosis and they include
both pro- and anti-apoptotic member. In our work, the decrease of
Mcl-1 was observed whereas the expression of the other members
Bcl-2, Bcl-xL, Bax, Bad, Bim, BID, Bmf and puma was not changed
(Fig. 4), which change the ratio
of expression levels of pro- and anti-apoptotic Bcl-2 family
member. Mcl-1, also call myeloid cell leukaemia-1, is an
anti-apoptotic member of Bal-2 family. It is thought to promote
cell survival by involving in the suppression of cytochrome
c release from mitochondria, possibly via heterodimerisation
with and neutralisation of pro-apoptotic Bcl-2 family proteins
(15). While the reduction of
Mcl-1 expression definitely weakens the link between pro- and
anti-apoptotic Bcl-2 family proteins, such as Noxa/Mcl-1 and
Bim/Mcl-1, resulting in the release of pro-apoptotic Bcl-2 family
proteins. Thus Mcl-1 plays a unique role in regulating apoptosis,
as elimination of Mcl-1 is required at an early stage for induction
of apoptosis (16,17). Its expression is controlled by
multiple signaling pathways, including STAT3/5, PI3K/AKT and
MEK/ERK pathways. Studies found that Mcl-1 was frequently
overexpressed in a variety of human cancers thereby providing
protection to the tumor cells from apoptosis (18,19).
It has been identified as an important target in majority of human
cancers (20). Mcl-1 is known to
be critical for survival of melanoma cells under various stress
conditions (16,17). The expression of Mcl-1 is also
known to increase in melanoma with disease progression (21). Overexpression of the Mcl-1 protein
in melanoma has been shown to affect the susceptibility of melanoma
cells to apoptotic stimuli in other system (22). Different from its enhancing
pro-apoptotic protein Bax and PUMA expression in the human
hepatocellular carcinoma cells (23), 9(11)-DHEP only decreased the Mcl-1
expression in human malignant melanoma cell.
In addition, the steroid 9(11)-DHEP exhibited a
dose-dependent inhibitory activity towards IKK-β, which evaluate
their possible inhibitory effects on the NF-κB pathway (8). IKK-β phosphorylate IkB leading to its
ubiquitination and degradation. This results in the subsequent
translocation of the molecule NF-κB to the nucleus. In the nucleus,
NF-kB binds with a consensus sequence of various genes and thus
activates their transcription. NF-κB plays a key role in regulating
the immune response to infection. Incorrect regulation of NF-κB has
been linked to cancer, inflammatory and autoimmune diseases.
Studies showed that novel epigenetic regulation of KPC1 associated
with NF-κB pathway activation, promoting metastatic melanoma
progression (24). These results
suggest that it is possible for the steroid 9(11)-DHEP affect
melanoma progression via its potential immunosuppressive effect by
inhibiting the NF-κB pathway. Our study suggested that 9(11)-DHEP
could be special chemotherapy agent for melanoma treatment by
targeting Mcl-1.
Taken together, our results revealed the anticancer
mechanisms of 9(11)-DHEP involved: i) participation of the Mcl-1
protein deceasing; ii) damage in mitochondrial membrane; iii)
cytochrome-c release; and iv) caspase-9 and −3 activations.
Our work also suggested that the steroid may be natural potential
apoptosis-inducing agent for A375 malignant melanoma treatment,
which represents promising new reagents that can overcome the
immortality of tumor cells.
Acknowledgements
The authors would like to extend their gratitude to
Dr. SM Ngai (Department of Biology, the Chinese University of Hong
Kong) for providing the normal skin fibroblast Hs68 cell line. Many
thanks were also giving to Dr. VEC Ooi (Department of Biology, the
Chinese University of Hong Kong) for providing the human breast
adenocarcinoma MCF-7 cell line.
Funding
The present research was supported by The Chinese
University of Hong Kong, the Science Technology and Innovation
Committee of Shenzhen Municipality grant (grant nos.
JCYJ20150401163247217 and ZDSYS201606081515458) and Medical
Scientific Research Foundation of Guangdong Province (grant no.
A2017402), which was awarded to Dr LZ in part.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LZ and YSW participated in the design of the study,
LZ performed the study and the statistical analysis. MS provided
assistance for confocal figure analysis. SH and FW assisted in
purifying the steroid 9(11)-DHEP. LZ drafted the manuscript. JC was
responsible for the quality control of the steroid 9(11)-DHEP,
funding management and project planning.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Trotter SC, Sroa N, Winkelmann RR, Olencki
T and Bechtel M: A global review of melanoma follow-up guidelines.
J Clin Aesthet Dermatol. 6:18–26. 2013.PubMed/NCBI
|
|
2
|
Soengas MS and Lowe SW: Apoptosis and
melanoma chemoresistance. Oncogene. 22:3138–3151. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wouters J, Stas M, Gremeaux L, Govaere O,
Van den Broeck A, Maes H, Agostinis P, Roskams T, van den Oord JJ
and Vankelecom H: The human melanoma side population displays
molecular and functional characteristics of enriched
chemoresistance and tumorigenesis. PLoS One. 8:e765502013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hersey P, Zhang XD and Mhaidat N:
Overcoming resistance to apoptosis in cancer therapy. Adv Exp Med
Biol. 615:105–126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobori M, Yoshida M, Ohnishi-Kameyama M,
Takei T and Shinmoto H:
5alpha,8alpha-Epidioxy-22E-ergosta-6,9(11),22-trien-3beta-ol from
an edible mushroom suppresses growth of HL60 leukemia and HT29
colon adenocarcinoma cells. Biol Pharm Bull. 29:755–759. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen YK, Kuo YH, Chiang BH, Lo JM and
Sheen LY: Cytotoxic activities of 9,11-dehydroergosterol peroxide
and ergosterol peroxide from the fermentation mycelia of
Ganoderma lucidum cultivated in the medium containing
leguminous plants on Hep 3B cells. J Agric Food Chem. 57:5713–5719.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cui YJ, Guan SH, Feng LX, Song XY, Ma C,
Cheng CR, Wang WB, Wu WY, Yue QX, Liu X and Guo DA: Cytotoxicity of
9,11-dehydroergosterol peroxide isolated from Ganoderma
lucidum and its target-related proteins. Nat Prod Commun.
5:1183–1186. 2010.PubMed/NCBI
|
|
8
|
Parhira S, Zhu GY, Li T, Liu L, Bai LP and
Jiang ZH: Inhibition of IKK-β by epidioxysterols from the flowers
of Calotropis gigantea (Niu jiao gua). Chin Med. 11:92016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sliva D: Ganoderma lucidum (Reishi)
in cancer treatment. Integr Cancer Ther. 2:358–364. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng S and Sliva D: Ganoderma
lucidum for cancer treatment: We are close but still not there.
Integr Cancer Ther. 14:249–257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng L, Si JY and Wong YS: Ergosterol
peroxide and 9,11-dehydroergosterol peroxide from Ling Zhi or
Reishi medicinal mushroom, Ganoderma lucidum (W. Curt.: Fr.)
P. Karst. (Aphyllophoromycetideae)mycelia inhibit the growth of
human breast adenocarcinoma MCF-7 cells. Int J Med Mushroom.
11:249–257. 2009. View Article : Google Scholar
|
|
12
|
Costa MA, Pellerito L, Izzo V, Fiore T,
Pellerito C, Melis M, Musmeci MT and Barbieri G: Diorganotin(IV)
and triorganotin(IV) complexes of
meso-tetra(4-sulfonatophenyl)porphine induce apoptosis in A375
human melanoma cells. Cancer Lett. 238:284–294. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gill BS Navgeet and Kumar S: Ganoderma
lucidum targeting lung cancer signaling: A review. Tumour Biol.
39:10104283177074372017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xia Q, Zhang H, Sun X, Zhao H, Wu L, Zhu
D, Yang G, Shao Y, Zhang X, Mao X, et al: comprehensive review of
the structure elucidation and biological activity of triterpenoids
from Ganoderma spp. Molecules. 19:17478–17535. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Michels J, Johnson PW and Packham G:
Mcl-1. Int J Biochem Cell Biol. 37:267–271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nijhawan D, Fang M, Traer E, Zhong Q, Gao
W, Du F and Wang X: Elimination of Mcl-1 is required for the
initiation of apoptosis following ultraviolet irradiation. Genes
Dev. 17:1475–1486. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Lv J, Cheng Y, Du J, Chen D, Li C
and Zhang J: Apoptosis induced by Ginkgo biloba (EGb761) in
melanoma cells is Mcl-1-dependent. PLoS One. 10:e01248122015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Quinn BA, Dash R, Azab B, Sarkar S, Das
SK, Kumar S, Oyesanya RA, Dasgupta S, Dent P, Grant S, et al:
Targeting Mcl-1 for the therapy of cancer. Expert Opin Investig
Drugs. 20:1397–1411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Akgul C: Mcl-1 is a potential therapeutic
target in multiple types of cancer. Cell Mol Life Sci.
66:1326–1336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mukherjee N, Lu Y, Almeida A, Lambert K,
Shiau CW, Su JC, Luo Y, Fujita M, Robinson WA, Robinson SE, et al:
Use of a MCL-1 inhibitor alone to de-bulk melanoma and in
combination to kill melanoma initiating cells. Oncotarget.
8:46801–46817. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhuang L, Lee CS, Scolyer RA, McCarthy SW,
Zhang XD, Thompson JF and Hersey P: Mcl-1, Bcl-XL and Stat3
expression are associated with progression of melanoma whereas
Bcl-2, AP-2 and MITF levels decrease during progression of
melanoma. Mod Pathol. 20:416–426. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thallinger C, Wolschek MF, Wacheck V,
Maierhofer H, Günsberg P, Polterauer P, Pehamberger H, Monia BP,
Selzer E, Wolff K and Jansen B: Mcl-1 antisense therapy
chemosensitizes human melanoma in a SCID mouse xenotransplantation
model. J Invest Dermatol. 120:1081–1086. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Wu Q, Bu M, Hu L, Du WW, Jiao C, Pan
H, Sdiri M, Wu N, Xie Y and Yang BB: Ergosterol peroxide activates
Foxo3-mediated cell death signaling by inhibiting AKT and c-Myc in
human hepatocellular carcinoma cells. Oncotarget. 7:33948–33959.
2016.PubMed/NCBI
|
|
24
|
Iida Y, Ciechanover A, Marzese DM, Hata K,
Bustos M, Ono S, Wang J, Salomon MP, Tran K, Lam S, et al:
Epigenetic regulation of KPC1 ubiquitin ligase affects the NF-κB
pathway in melanoma. Clin Cancer Res. 23:4831–4842. 2017.
View Article : Google Scholar : PubMed/NCBI
|