Introduction
Endometriosis refers to a heterogeneous disease that
presents as endometrial tissue with growth activity (including the
glands and stroma) outside the corpus uteri and engraftment growth.
Regarding the pathomorphology, it is benign (1); however, it presents malignant
biological properties, such as infiltration and metastasis. The
incidence rate of endometriosis is 10–15% of women (2). The epidemiological data of
endometriosis reported in existing literature is incomplete since
the disease can only be definitively diagnosed with laparoscopic
and pathological diagnosis; however, many women do not receive
laparoscopic examination if disease presents no symptoms or they
are not willing to be subject to examination (3). Endometriosis can seriously affect
patient quality of life, and physical and psychological health due
to pelvic pain, infertility and high recurrence rate (4).
In recent years, researchers have detected that a
novel form of estrogen receptor in a variety of human tissues,
namely G protein-coupled receptor 30, which is also termed G
protein-coupled estrogen receptor (GPER) (5). It rapidly activates signal cascade
pathways in cells following binding to estrogen, generating second
messengers, such as Ca2+, cyclic adenosine
3′,5′-monophosphate and nitric oxide, activate a variety of protein
kinase, and regulate gene transcription. The process is termed a
‘rapid nongenomic effect’ since it only lasts for several sec or
min. GPER belongs to the G protein coupled receptor protein family
(6). It has no structural homology
with the estrogen receptor. GPER is extensively expressed in the
reproductive system, mammary glands, heart and cerebral vessels,
bone and other tissues acting with estrogen (7). GPER indirectly regulates the
biological functions of estrogen through a variety of signal
pathways, and also can has rapid effect through directly combining
with endogenous estrogen, tamoxifen and its metabolite
4-hydroxytamoxifen, environmental estrogen, specific G-1 agonists
and other ligands. At present, the researches on ER in
endometriosis have become quite developed (7). However, there are extremely few
studies regarding GPER (7). The
detection of GPER has motivated re-evaluation of the effect of
estrogen in pathogenic mechanism of endometriosis. Such studies may
be conducive to further reveal the physiological roles of estrogen,
and also provide new avenues for developing a novel generation of
drugs for the treatment of estrogen-associated diseases (8).
MicroRNAs (miRNAs) are endogenous RNA molecules (~22
nucleotides) with regulatory function discovered in eukaryotes in
recent decades. Numerous miRNAs are expressed in plants, animals
and viruses (9). miRNAs negatively
regulate gene expression by complementary base pairing with mRNA,
which leads to mRNA degradation or translation inhibition. miRNAs,
as important regulatory molecules, participate in a series of
important cellular, including viral defenses, hematopoietic
processes, organ development, cell proliferation and apoptosis, fat
metabolism and tumorigenesis (10,11).
Human leukocyte antigen-G (HLA-G) is a non-classical
major histocompatibility complex-β antigen molecule detected in
1997 (11). Under physiological
conditions, HLA-G is restrictively expressed among certain tissues
(11). Specific expression
participates in maternal-fetal immune tolerance in extravillous
trophoblasts to protect the fetus from maternal immune surveillance
and immune rejection. Recent research has reported that HLA-G can
restrain the activity of cytotoxic T cells, natural killer cells
and other effector cells (11,12).
Certain other studies have indicated that serum HLA-G levels in
patients with endometriosis is higher than in individuals without
endometriosis, suggesting that HLA-G may enhance the ability of
ectopic endometrial cells to escape from immune surveillance, which
facilitates implanting and proliferation at ectopic sites (11,13).
In the current study, the role of miRNA (miR)-148a in ovarian
endometriosis was explored and may be involved in the signaling
pathway that induces apoptosis.
Materials and methods
Human tissue samples and ethical
approval
All healthy controls (aged 52–68; n=6), patients
with endometriosis (aged 55–63; n=7) and patients with
endometriosis-associated ovarian cancer (EAOC; aged 58–69; n=7)
were recruited from the Reproductive Medicine Center and Department
of Gynecology, Affiliated Yantai Yuhuangding Hospital of Qingdao
University (Yantai, China) from June-July 2015. Endometriosis and
endometriosis-associated ovarian cancer was diagnosed and confirmed
by histological examination of laparoscopic biopsies. The research
design was approved by the ethics committee of the Affiliated
Yantai Yuhuangding Hospital of Qingdao University.
Written informed consent was obtained from each
participant prior to the study. Healthy participants were diagnosed
no evidence of ovarian pathology using laparoscopic biopsy due to a
suspicious ovarian cyst. Endometriosis and EAOC were diagnosed,
with the American Society for Reproductive Medicine stage II/III
for endometriosis (14) for
inclusion in this study. Endometrium tissue was collected from
patients with endometriosis, tumor tissue was collected from
patients with endometriosis-associated ovarian cancer and healthy
tissue was collected from healthy controls. All tissue was
collected during the laparoscopic surgical examination or
resection. Peripheral blood was centrifuged at 1,000 × g for 10 min
at 4°C and the serum was subsequently collected and stored at −80°C
until further use.
RNA isolation, reverse transcription
(RT) and quantitative polymerase chain reaction (qPCR)
Total RNA was isolated from serum or cells using
TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
cDNAs were synthesized using a Thermoscript RT kit (Thermo Fisher
Scientific, Inc.) for mRNA transcripts, at 37°C for 60 min and 85°C
for 5 min. qPCR was performed using a Roche LightCycler 480II
Real-Time PCR System (Roche Molecular Diagnostics, Pleasanton, CA,
USA) using the SensiFAST SYBR No-Rox PCR kits (Bioline Reagents
Ltd, London, UK). The 2−ΔΔCq method was used to quantify
mRNA expression (15).
Cell culture and cell
transfection
Endometriosis cell line Hs 832(C).T from a benign
ovarian cyst was purchased from Chinese Academy of Sciences,
Shanghai Cell Bank (Shanghai, China) and cultured in RPMI-1640
medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented
with 10% heat-inactivated fetal bovine serum (Sigma-Aldrich; Merck
KGaA) at 5% CO2 at 37°C. miR-148a mimics and negative
mimics were purchased from Sangon Biotech Co., Ltd. (Shanghai,
China). miR-148a mimics and negative mimics (50–100 nM) was
transfected to the cells using Oligofectamine (Invitrogen; Thermo
Fisher Scientific, Inc.). GPER inhibitor, G15 (100 nM, Cayman
Chemical Company, Ann Arbor, MI, USA) was added to Hs 832(C).T
cells transfected with miR-148a mimics after 42 h and cultured for
a further 6 h.
MTT assay of cell viability
Following transfection, cells were plated at
5×103 cells/well in 96-well plates for 0, 12, 48 and 72
h. Cell viability at each time point was measured using MTT (5
mg/ml; Sigma-Aldrich; Merck KGaA) assay for 4 h at 37°C.
Subsequently, dimethyl sulfoxide (5 mg/ml; Sigma-Aldrich; Merck
KGaA) was added to every well after 4 h and dissolved for 20 min.
Absorbance of the solution at 490 nm was read using a
spectrophotometric plate reader.
Flow cytometry analysis of
apoptosis
Cells were plated at 1×106 cells/well in
6-well plates. At 48 h after transfection, cells were stained with
5 ml Annexin-V and 5 ml propidium iodide (Nanjing KeyGen Biotech
Co., Ltd., Nanjing, China) for 30 min at 37°C in the dark.
Apoptosis was detected by flow cytometry (BD FACSCanto; BD
Biosciences, Franklin Lakes, NJ, USA).
Activity assay of caspases
Cells were plated at 1×106 cells/well in
6-well plates. At 48 h after transfection, lysates were prepared
using radioimmunoprecipitation assay (RIPA) buffer (Beyotime
Institute of Biotechnology, Haimen, China) and protein
concentration was determinate by the Coomassie blue method
(Beyotime Institute of Biotechnology). The proteins (5–10 mg) were
incubated with 2 mM Ac-DEVD-pNA (cat. no. C1116) or Ac-LEHD-pNA
(cat. no. C1158, Beyotime Institute of Biotechnology) for 1 h at
37°C in the dark. Absorbance of the solution at 405 nm was read
using a spectrophotometric plate reader.
Western blot analysis
Cells were plated at 1×106 cells/well in
6-well plates. At 48 h after transfection, lysates were prepared
using RIPA buffer and protein concentration was determinate by the
Coomassie blue method (Beyotime Institute of Biotechnology). The
proteins (30–50 mg) were separated in 8–10% SDS-PAGE gels and then
transferred to a nitrocellulose membrane (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The membrane was blocked with 5% nonfat
milk at 37°C for 1 h and then incubated with anti-Bcl-2 associated
X apoptosis regulator (Bax; cat. no. sc-6236; 1:500; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), Bcl-2 apoptosis regulator
(Bcl-2; cat. no. sc-56015; 1:500; Santa Cruz Biotechnology, Inc.),
HLA-G (cat. no. sc-53825; 1:500; Santa Cruz Biotechnology, Inc.),
GPER (cat. no. ab154069; 1:1,000; Abcam) and GAPDH (cat. no.
sc-25778; 1:5,000; Santa Cruz Biotechnology, Inc.) at 4°C
overnight. The membrane was washed with Tris-buffered saline Tween
and incubated with horseradish peroxidase conjugated anti-rabbit
antibody (1:10,000; cat. no. ab97064; Abcam, Cambridge, UK) for 1 h
at room temperature. The membrane was visualized using ECL reagents
(Beyotime Institute of Biotechnology) and analyzed using Image-Pro
Plus software (version 6.0; Media Cybernetics, Inc., Rockville, MD,
USA).
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments and was analyzed using SPSS 21.0
software (IBM Corp., Armonk, NY, USA). The Student's t-test or
one-way analysis of variance followed by Tukey's post-hoc test was
performed to compare experimental groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
Expression of miR-148a
Healthy controls, and patients with endometriosis
and EAOC were recruited in the current study. As presented in
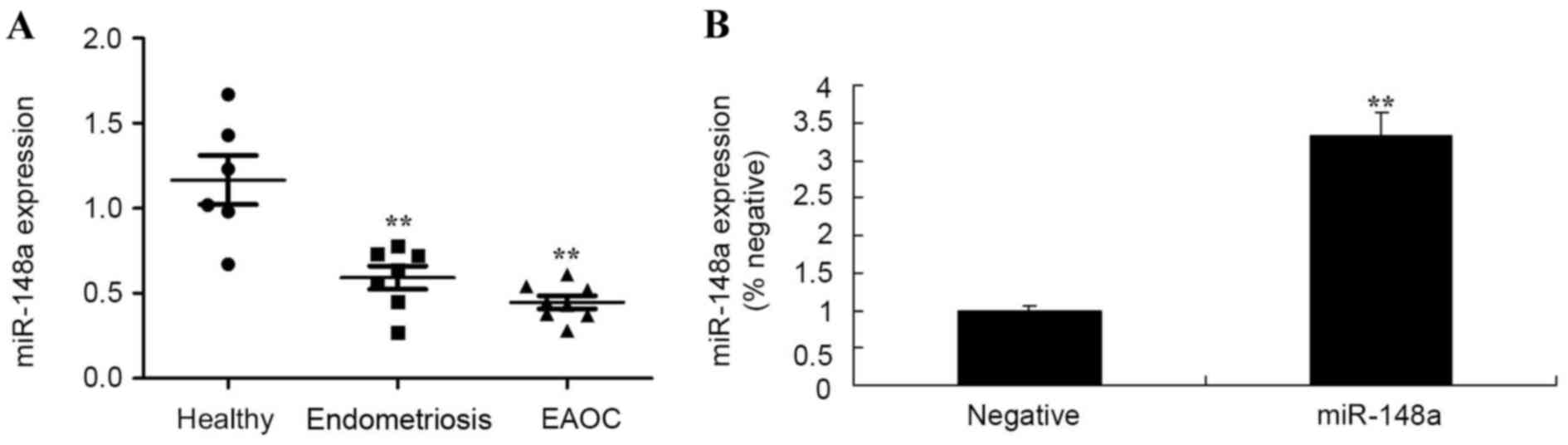
Fig. 1A, miR-148a expression of in
patients with endometriosis and EAOC were significantly lower than
that of healthy volunteers. Subsequently, miR-148a mimics were used
to increase miR-148a expression in Hs 832(C).T cells (Fig. 1B), compared with a negative control
group.
Effect of miR-148a overexpression on
cell proliferation and apoptosis of Hs 832(C).T cells
Whether overexpression of miR-148a affects cell
proliferation and apoptosis of Hs 832(C).T cells was analyzed using
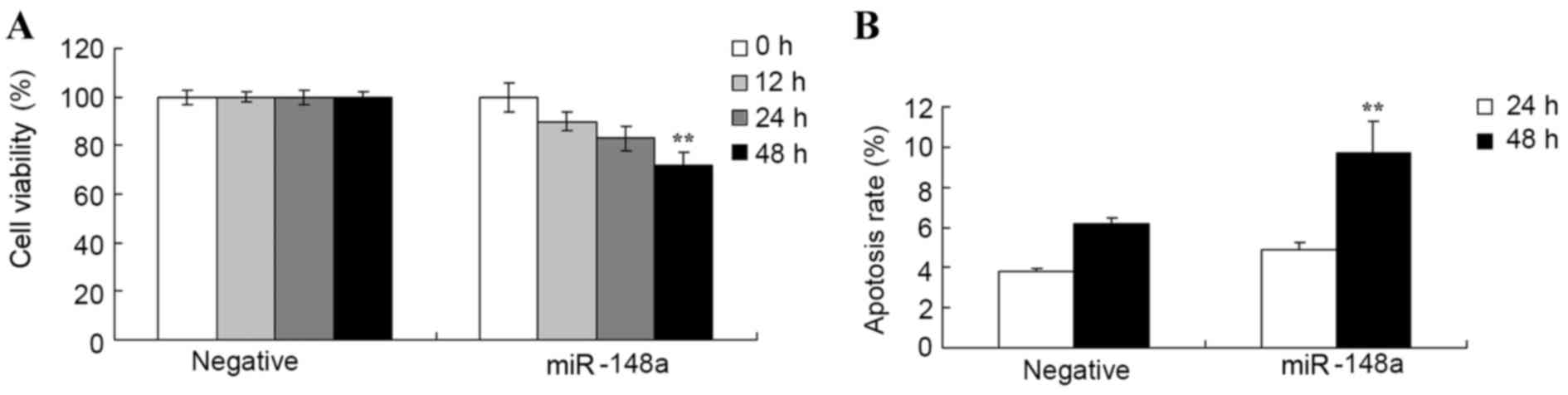
MTT and flow cytometry, respectively. Fig. 2A indicates that overexpression of
miR-148a reduced cell proliferation of Hs 832(C).T cells following
transfection at 12, 24 and 48 h. Overexpression of miR-148a
significantly reduced proliferation of Hs 832(C).T cells compared
with negative group after transfection for 48 h. Fig. 2B indicated that overexpression of
miR-148a significantly induced apoptosis of Hs 832(C).T cells after
transfection for 48 h compared with negative group.
Effect of miR-148a overexpression on
caspase-3 and caspase-9 activity, and Bax/Bcl-2 ratio in Hs
832(C).T cells
To explore the apoptotic mechanism of miR-148a in Hs
832(C).T cells, caspase-3 and caspase-9 activity, and Bax/Bcl-2
protein expression were measured using caspase-3 and caspase-9
activity kits and western blot analysis, respectively. Fig. 3A and B demonstrated that
overexpression of miR-148a significantly increased caspase-3 and
caspase-9 activity in Hs 832(C).T cells after transfection for 48 h
compared with the negative control group. Fig. 3C and D explained that
overexpression of miR-148a significantly increased the Bax/Bcl-2
ratio in Hs 832(C).T cells after transfection for 48 h compared
with the negative control group.
Effect of miR-148a overexpression on
HLA-G in Hs 832(C).T cells
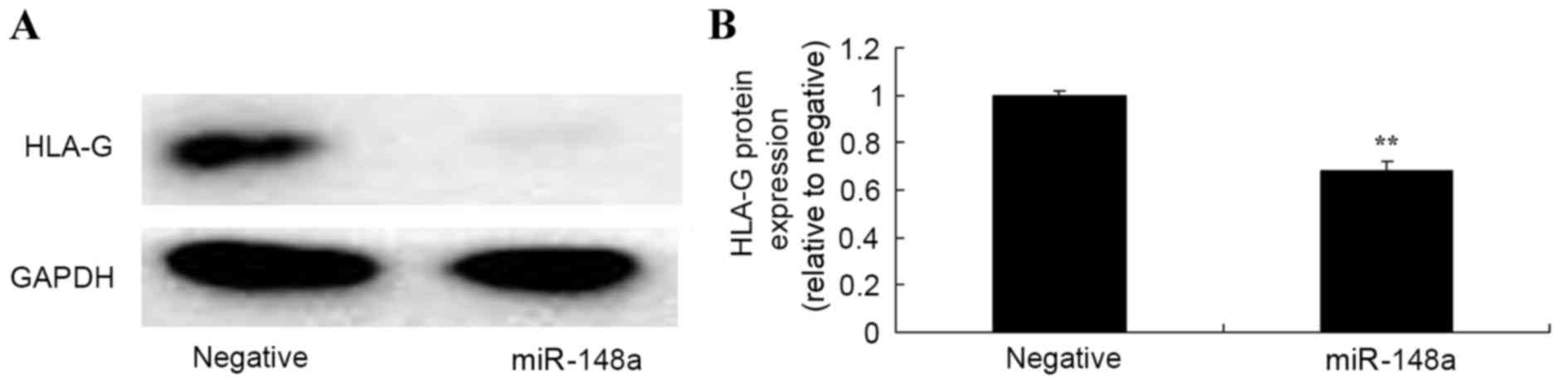
To further explore whether overexpression of
miR-148a affects HLA-G in Hs 832(C).T cells, HLA-G protein
expression was measured using western blot analysis. The results
from western blot analysis demonstrated that overexpression of
miR-148a significantly inhibited HLA-G protein expression in Hs
832(C).T cells after transfection for 48 h, compared with the
negative control group (Fig.
4).
Effect of miR-148a inhibition on
proliferation in Hs 832(C).T cells
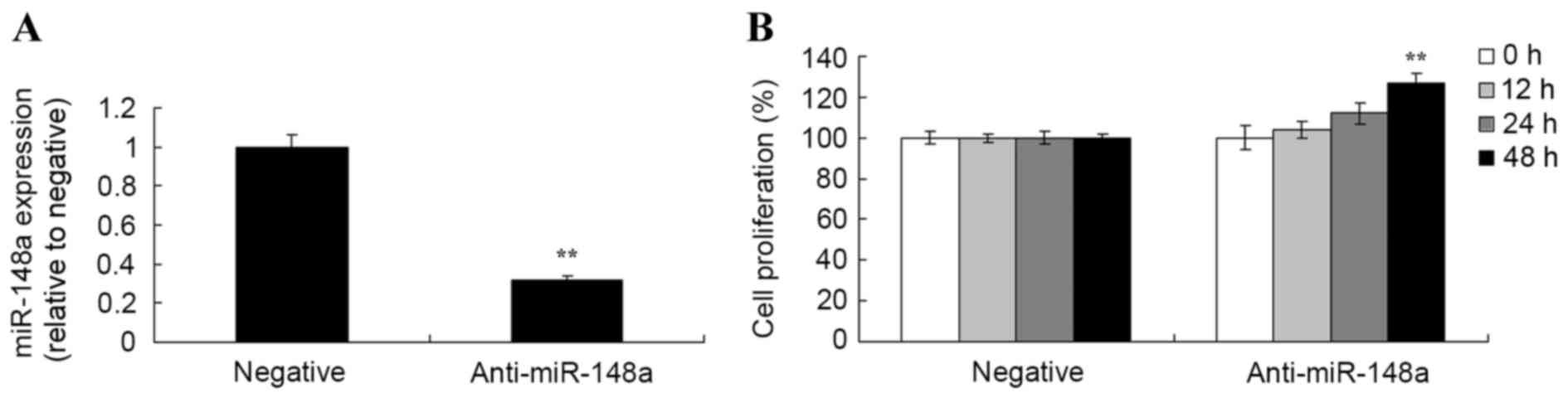
Subsequently, an MTT assay was used to analyze the
effects of miR-148a inhibition on cell viability in Hs 832(C).T
cells. As demonstrated in Fig. 5,
anti-miR-148a reduced miR-148a expression, and increased the
proliferation of Hs 832(C).T cells.
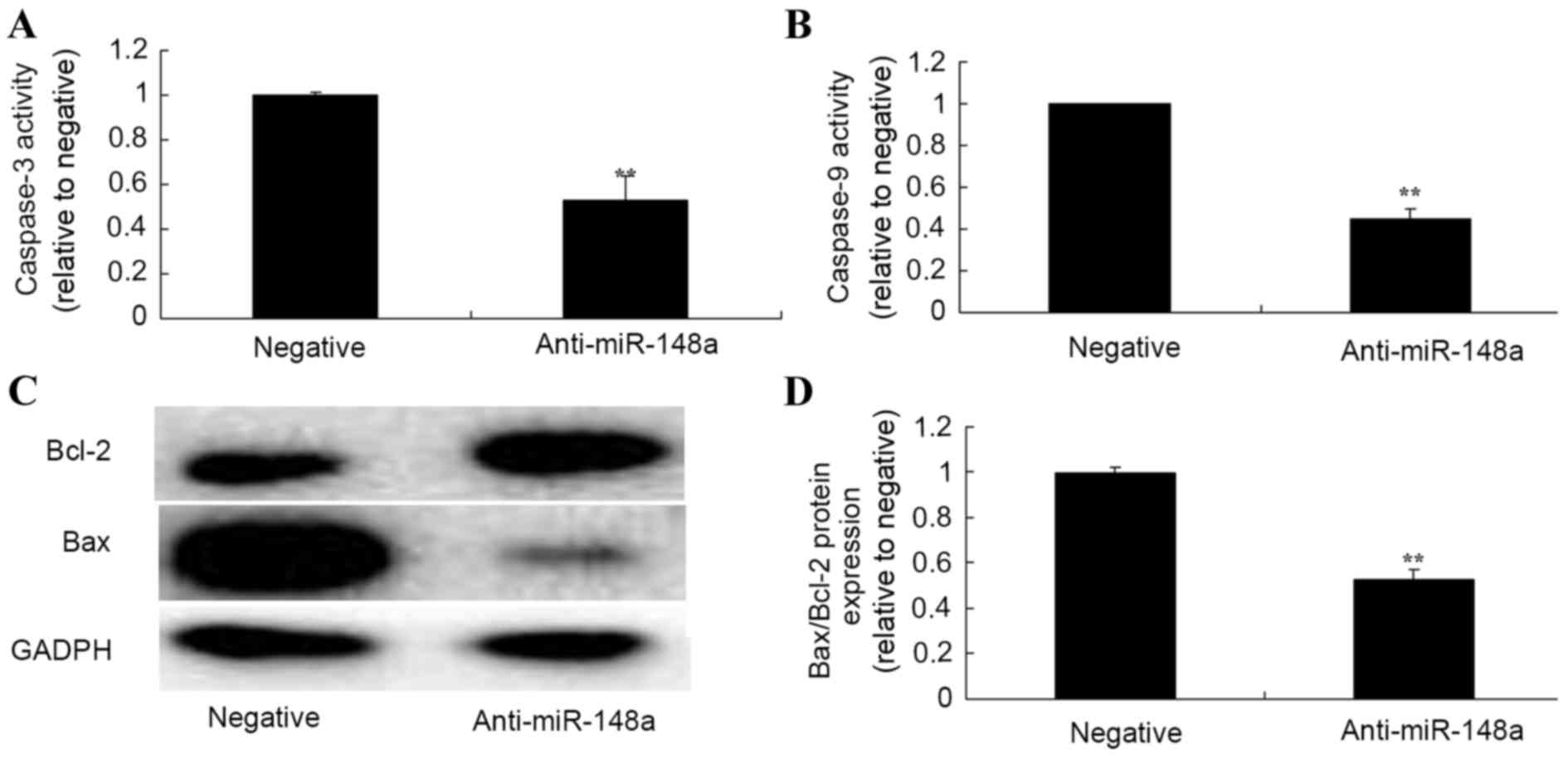
Effect of miR-148a inhibition on
caspase-3 and caspase-9 activity, and Bax/Bcl-2 ratio in Hs
832(C).T cells
To further explore the apoptotic mechanisms of
miR-148a in Hs 832(C).T cells, caspase-3 and caspase-9 activity,
and Bax/Bcl-2 protein expression were measured using caspase-3 and
caspase-9 activity kits and western blot analysis. Inhibition of
miR-148a significantly also decreased the caspase-3 and caspase-9
activities, and reduced the Bax/Bcl-2 ratio in Hs 832(C).T cells
after transfection for 48 h compared with the negative control
group (Fig. 6).
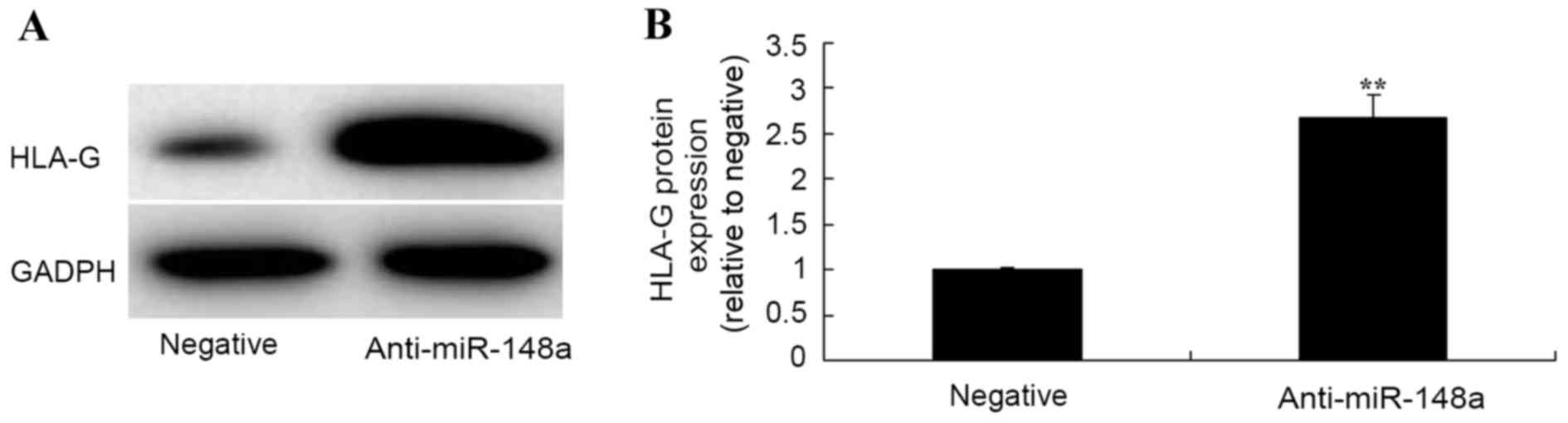
Effect of miR-148a inhibition on HLA-G
in Hs 832(C).T cells
Western blot analysis was used to measure HLA-G
protein expression Hs 832(C).T cells following miR-148a inhibition.
The results from western blot analysis demonstrated that inhibition
of miR-148a significantly increased HLA-G protein expression of Hs
832(C).T cells after transfection for 48 h compared with the
negative group (Fig. 7).
Effect of GPER inhibition on
miR-148a-induced changes to GPER and HLA-G protein expression in Hs
832(C).T cells
The role of GPER expression on the effects of
miR-148a in Hs 832(C).T cells was examined. GPER inhibitor was used
to reduce the expression of GPER protein. A GPER inhibitor, G15
(100 nM) was added to Hs 832 (C). As presented in Fig. 8, GPER inhibition significantly
suppressed GPER and HLA-G protein expression of Hs 832(C).T cell
after transfection with miR-148a, compared with transfected cells
that were not treated with G15 (Fig.
8A-C). However, the inhibition of GPER significantly increased
miR-148a expression in Hs 832(C).T cells transfected with miR-148a
for 48 h, compared with miR-148a transfection only group (Fig. 8).
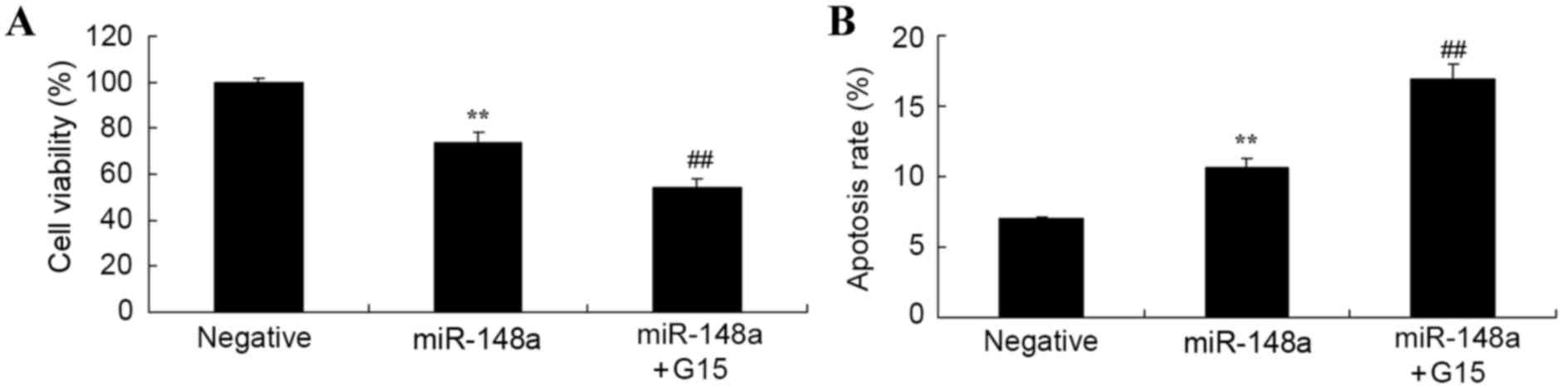
Effect of GPER inhibition on
miR-148a-induced changes to cell viability and apoptosis of Hs
832(C).T cells
It was ascertained whether the suppression of GPER
alters the effect of miR-148a on cell viability and apoptosis in Hs
832(C).T cells. Overexpression of miR-148a reduced cell viability
and induced apoptosis of Hs 832(C).T cells; these effects were
significantly intensified by the suppression of GPER using G15 in
Hs 832(C).T cells, compared with the miR-148a only group (Fig. 9).
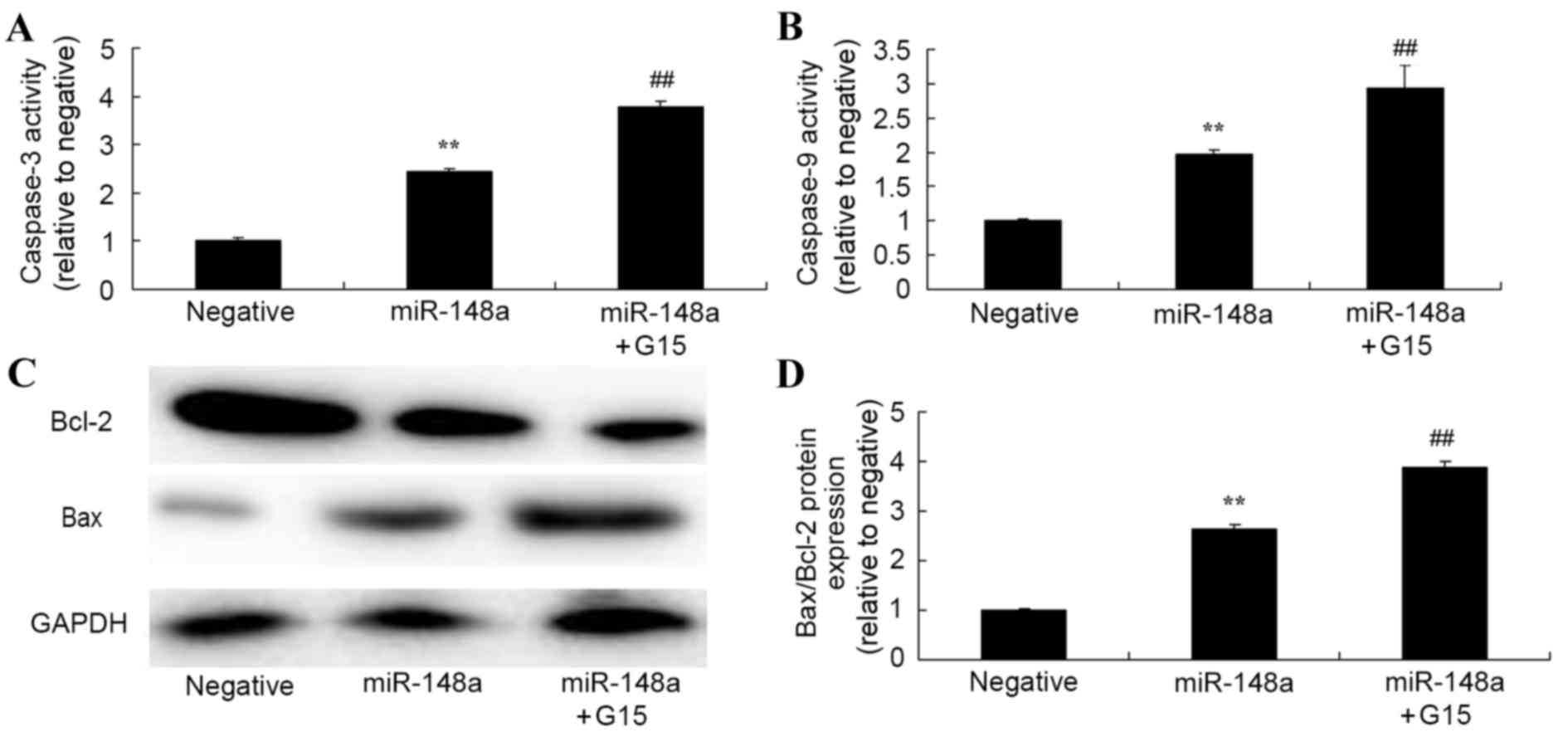
Effect of GPER inhibition on
miR-148a-induced changes to caspase-3 and caspase-9 activity, and
Bax/Bcl-2 ratio of Hs 832(C).T cells
Whether the suppression of GPER alters the effect of
miR-148a on caspase-3 and caspase-9 activity, and the Bax/Bcl-2
ratio in Hs 832(C).T cells was determined. The suppression of GPER
significantly enhanced the effect of miR-148a overexpression on
caspase-3, caspase-9 and Bax/Bcl-2 ratio in Hs 832(C).T cells
(Fig. 10).
Discussion
Endometriosis is a benign pathomorphological
feature. However, it is an estrogen-dependent disease exhibiting
malignant biological behaviors. Endometriosis is prone to occur
during the childbearing period (3). The formation of endometriosis is a
quite complex process involving numerous molecular events,
including propagation and apoptosis of endometrial cells,
migration, invasion and attaching, local hypoxia injury,
inflammatory cell aggregation, formation of new vessels and
remodeling of the cytoskeleton (16). Therefore, in-depth studies specific
to estrogen signaling pathways and functional proteins associated
with cell invasion, migration and cell apoptosis may offer novel
insight into the pathogenic mechanism of endometriosis (17). These results suggest that miR-148a
expression was lower in endometriosis tissue and patients with EAOC
compared with healthy volunteers.
The incidence rate of endometriosis is increasing
year upon year (18). However, the
pathogenic mechanism is not clear. miRNAs are endogenous RNAs with
regulatory function (19).
Existing research indicates that miRNAs are closely associated with
the development of various diseases. In addition, miRNAs have an
important role in the initiation and development of endometriosis
(9). In the current study,
overexpression of miR-148a significantly decreased cell
proliferation, promoted apoptosis, increased the Bax/Bcl-2 ratio
and caspase-3/9 activity in Hs 832(C).T cells.
Analysis of estrogen-dependent tumors, such as
breast and endometrial cancer, has verified that GPER can promote
the invasion and migration of tumor cells through the downstream
signaling pathway (5). Combining
with biological functions of GPER and the estrogen dependency
characteristics of endometriosis, it can be speculated that the
expression of GPER is associated with the formation of ectopic foci
(20). The current study reported
that overexpression of miR-148a significantly inhibited HLA-G
protein expression in Hs 832(C).T cells and the inhibition of GPER
expression significantly increased miR-148a expression in Hs
832(C).T cells. Together, the results of these studies suggest that
miR-148a may have different regulatory mechanisms of angiogenesis
in endometriosis.
HLA-G is specifically expressed in extravillous
trophoblasts to avoid sertoli cells being damaged by natural killer
immune cells, thus to induce maternal-fetal interface to generate
immune tolerance, protecting the fetus from maternal immune
surveillance and immune rejection (21). It is reported in the literature
that the pathological characteristics of endometriosis exhibit
biological behavior similar to tumor cells, which may indicate how
HLA-G protein expression in endometriotic cells promotes ectopic
endometrial cells to successfully avoid immunological surveillance
(22). In a previous study, HLA-G
expression was detected in ectopic endometrium and glandular
epithelial cells of ectopic tissue of patients with endometriosis,
but no HLA-G expression was detected in normal endometrial tissue
(23). Therefore, it is believed
that normal endometrial tissue is different from that of patients
with endometriosis, and endometrium growth on ectopia and other
areas may be facilitated through the high expression of HLA-G
protein, which promotes escape from immune surveillance (24). The results of the current study
demonstrated that overexpression of miR-148a significantly reduced
HLA-G protein expression in Hs 832(C).T cells; the inhibition of
GPER activity using G15 significantly decreased cell viability,
promoted apoptosis, increased Bax/Bcl-2 ratio and caspase-3
activity, and suppressed HLA-G protein expression in Hs 832(C).T
cells. Together, the results demonstrate that miR-148a may
negatively regulate the GPER signaling pathway in
endometriosis.
In conclusion, it was identified that
GPER/miR-148a/HLA-G signaling may be involved in mediating ovarian
endometriosis. However, further in vivo studies of
GPER/miR-148a/HLA-G are necessary in the future in order to develop
novel ovarian endometriosis treatments.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Author's contributions
YXS designed the study. SZH, JL, HCB, MMW, XRW, XH,
FHL, WZ, ALX and CFH performed the experiments. SZH and YXS
analyzed the data. YXS wrote the manuscript.
Ethics approval and consent to
participate
The research design was approved by the ethics
committee of the Affiliated Yantai Yuhuangding Hospital of Qingdao
University. Written informed consent was obtained from each
participant prior to the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen M, Huang L, Zhang W, Shi J, Lin X, Lv
Z, Zhang W, Liang R and Jiang S: MiR-23b controls TGF-β1 induced
airway smooth muscle cell proliferation via TGFβR2/p-Smad3 signals.
Mol Immunol. 70:84–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu S, Pan W, Song X, Liu Y, Shao X, Tang
Y, Liang D, He D, Wang H, Liu W, et al: The microRNA miR-23b
suppresses IL-17-associated autoimmune inflammation by targeting
TAB2, TAB3 and IKK-α. Nat Med. 18:1077–1086. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oguiza A, Recio C, Lazaro I, Mallavia B,
Blanco J, Egido J and Gomez-Guerrero C: Peptide-based inhibition of
IκB kinase/nuclear factor-κB pathway protects against
diabetes-associated nephropathy and atherosclerosis in a mouse
model of type 1 diabetes. Diabetologia. 58:1656–1667. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahmed S, Mundhe N, Borgohain M, Chowdhury
L, Kwatra M, Bolshette N, Ahmed A and Lahkar M: Diosmin modulates
the NF-kB signal transduction pathways and downregulation of
various oxidative stress markers in alloxan-induced diabetic
nephropath. Inflammation. 39:1783–1797. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu GY, Zheng GZ, Chang B, Hu QX, Lin FX,
Liu DZ, Wu CC, Du SX and Li XD: Naringin stimulates osteogenic
differentiation of rat bone marrow stromal cells via activation of
the notch signaling pathway. Stem Cells Int. 2016:71306532016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Crane CA, Han SJ, Ahn B, Oehlke J, Kivett
V, Fedoroff A, Butowski N, Chang SM, Clarke J, Berger MS, et al:
Individual patient-specific immunity against high-grade glioma
after vaccination with autologous tumor derived peptides bound to
the 96 KD chaperone protein. Clin Cancer Res. 19:205–214. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan E, Yu D, Yue B, Potthast L, Chowdhary
S, Smith P and Chamberlain M: A prospective phase II
single-institution trial of sunitinib for recurrent malignant
glioma. J Neurooncol. 110:111–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buckner JC, Shaw EG, Pugh SL, Chakravarti
A, Gilbert MR, Barger GR, Coons S, Ricci P, Bullard D, Brown PD, et
al: Radiation plus procarbazine, CCNU and vincristine in low-grade
glioma. N Engl J Med. 374:1344–1355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karremann M, Hoffmann M, Benesch M,
Kwiecien R, von Bueren AO and Kramm CM: Secondary solid
malignancies after high-grade glioma treatment in pediatric
patients. Pediatr Hematol Oncol. 32:467–473. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ducassou A, Uro-Coste E, Verrelle P,
Filleron T, Benouaich-Amiel A, Lubrano V, Sol JC, Delisle MB, Favre
G, Ken S, et al: αvβ3 Integrin and Fibroblast growth factor
receptor 1 (FGFR1): Prognostic factors in a phase I–II clinical
trial associating continuous administration of Tipifarnib with
radiotherapy for patients with newly diagnosed glioblastoma. Eur J
Cancer. 49:2161–2169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bischof J, Westhoff MA, Wagner JE,
Halatsch ME, Trentmann S, Knippschild U, Wirtz CR and Burster T:
Cancer stem cells: The potential role of autophagy, proteolysis,
and cathepsins in glioblastoma stem cells. Tumour Biol.
39:10104283176922272017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mazurowski MA, Clark K, Czarnek NM,
Shamsesfandabadi P, Peters KB and Saha A: Radiogenomics of
lower-grade glioma: Algorithmically-assessed tumor shape is
associated with tumor genomic subtypes and patient outcomes in a
multi-institutional study with the cancer genome atlas data. J
Neurooncol. 133:27–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hernández-Tiedra S, Fabriàs G, Dávila D,
Salanueva ÍJ, Casas J, Montes LR, Antón Z, García-Taboada E,
Salazar-Roa M, Lorente M, et al: Dihydroceramide accumulation
mediates cytotoxic autophagy of cancer cells via autolysosome
destabilization. Autophagy. 12:2213–2229. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Quinlan S, Kenny A, Medina M, Engel T and
Jimenez-Mateos EM: MicroRNAs in neurodegenerative diseases. Int Rev
Cell Mol Biol. 334:309–343. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ohga S, Shikata K, Yozai K, Okada S, Ogawa
D, Usui H, Wada J, Shikata Y and Makino H: Thiazolidinedione
ameliorates renal injury in experimental diabetic rats through
anti-inflammatory effects mediated by inhibition of NF-kappaB
activation. Am J Physiol Renal Physiol. 292:F1141–F1150. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang Q, Liu P, Wu X, Liu W, Shen X, Lan
T, Xu S, Peng J, Xie X and Huang H: Berberine attenuates
lipopolysaccharide-induced extracelluar matrix accumulation and
inflammation in rat mesangial cells: Involvement of NF-κB signaling
pathway. Mol Cell Endocrinol. 331:34–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li HL, Cui XL, Zhang JN and Lin S:
Chemotherapy alleviates subacute recurrent glioma-associated
refractory cerebral edema by downregulating vascular endothelial
growth factor. Med Oncol. 31:132014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bartels U, Wolff J, Gore L, Dunkel I,
Gilheeney S, Allen J, Goldman S, Yalon M, Packer RJ, Korones DN, et
al: Phase 2 study of safety and efficacy of nimotuzumab in
pediatric patients with progressive diffuse intrinsic pontine
glioma. Neuro Oncol. 16:1554–1559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo X, Zheng X and Huang H: Protective
effects of dexmedetomidine on brain function of glioma patients
undergoing craniotomy resection and its underlying mechanism. Clin
Neurol Neurosurg. 146:105–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen CM, Syu JP, Way TD, Huang LJ, Kuo SC,
Lin CT and Lin CL: BC3EE2,9B, a synthetic carbazole derivative,
upregulates autophagy and synergistically sensitizes human GBM8901
glioblastoma cells to temozolomide. Int J Mol Med. 36:1244–1252.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ding X, Ma M, Teng J, Teng RK, Zhou S, Yin
J, Fonkem E, Huang JH, Wu E and Wang X: Exposure to ALS-FTD-CSF
generates TDP-43 aggregates in glioblastoma cells through exosomes
and TNTs-like structure. Oncotarget. 6:24178–24191. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin CJ, Chen TL, Tseng YY, Wu GJ, Hsieh
MH, Lin YW and Chen RM: Honokiol induces autophagic cell death in
malignant glioma through reactive oxygen species-mediated
regulation of the p53/PI3K/Akt/mTOR signaling pathway. Toxicol Appl
Pharmacol. 304:59–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li P, Wang X, Shan Q, Wu Y and Wang Z:
MicroRNA-130b promotes cell migration and invasion by inhibiting
peroxisome proliferator-activated receptor-γ in human glioma. Oncol
Lett. 13:2615–2622. 2017. View Article : Google Scholar : PubMed/NCBI
|