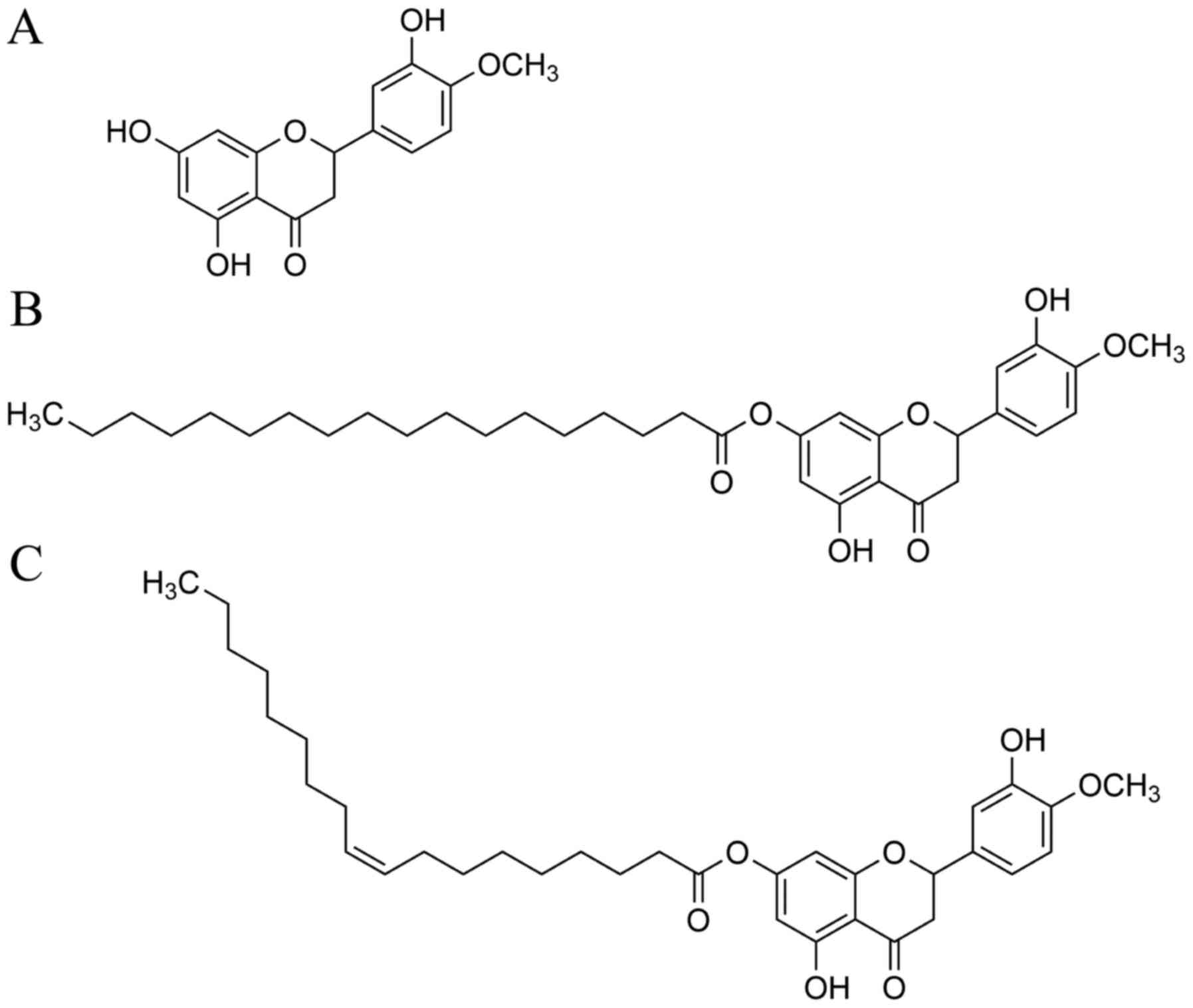
Introduction
Cataracts refers to a clouding of the lens of the
eye, which affect vision and remain a major cause of blindness in
the world (1,2). According to the World Health
Organization (WHO), cataracts account for approximately 51% of
global blindness. Therefore, cataracts are a critical public health
and social problem worldwide. At present, it is impossible to
entirely prevent cataract formation, and cataract surgery remains
the most common method of treatment. In order to decrease the
burden of surgery in older adults, it is of great interest to
establish alternative therapies to delay or prevent the development
of cataracts.
Lenses that are chronically exposed to ultraviolet
(UV) radiations generate reactive oxygen species (ROS) and
oxidative modifications (3) and
have high levels of reduced glutathione (GSH) and ascorbic acid
(AsA) to maintain a constant redox state, protecting against
oxidative stress and preserving lens transparency. Consequently,
the levels of these compounds in the lens are frequently used as
markers of cataract formation or development in both human and
animal models (4–7). The lens also uses chaperone activity
to maintain its transparency. α-Crystallin, which constitutes up to
30% of total water-soluble proteins in the lens, acts as a
molecular chaperone. Molecular chaperone activity plays an
important role in in vivo due to the longevity and
negligible turnover of lens proteins. Furthermore, it is well-known
that lens proteins in cataracts have weaker chaperone activity than
those in the non-disease state. Therefore, we measured the effect
of hesperetin and hesperetin derivatives on the changes in
chaperone activity in lenses with cataracts.
We have previously reported that hesperetin, one of
the natural flavonoids in orange rinds, could delay cataract onset
as assessed by observing cataract grade and measuring lens GSH and
AsA levels. We also showed that treatment with hesperetin prevented
down-regulation of chaperone activity in the lens (8,9). In
the current study, we assessed the therapeutic ability of
hesperetin derivatives to produce strong-acting anti-cataract
activity using an Se-induced cataract model, a well-established
rodent model used for screening potential anti-cataract
molecules.
In this study, we determined whether anti-cataract
properties of these derivatives could be altered by linking fatty
acids. Either hesperetin or hesperetin derivatives were
administered to rats with Se-induced cataracts in order to assess
the anti-cataract effect of these compounds.
Materials and methods
Materials
Sodium selenite (Na2SeO3: Se),
hesperetin, isoflurane, GSH, dithionitrobenzene (DTNB), AsA,
metaphosphoric acid, and 2-vinylpyridine were purchased from Wako
Pure Chemical Industries, Ltd. (Osaka, Japan). Stearic acid and
oleic acid were purchased from Tokyo Chemical Industry Co., Ltd.
(Tokyo, Japan). Trichloroacetic acid was purchased from Nacalai
Tesque Inc. (Kyoto, Japan). 2,6-Dichlorophenol-indophenol (DCPIP)
was purchased from Merck KGaA, (Darmstadt, Germany). Sprague Dawley
(SD) rats were obtained from Sankyo Labo Service Corporation
(Tokyo, Japan).
From hesperetin (Hes, Fig. 1A), we synthesized hesperetin
stearic acid ester (Hes-S, Fig.
1B) and oleic acid ester (Hes-O, Fig. 1C) according to a previously
reported procedure (10), and
purified materials were used for the anti-cataract experiments.
Animals
SD rats were housed in temperature-controlled (25°C
± 5°C) and light-controlled rooms (12 h cycle of light and dark).
Animals were fed balanced rat chow (CE-2; Clea Japan, Inc., Tokyo,
Japan) and provided water ad libitum. Keio University Animal
Research Committee (Tokyo, Japan) approved all of the animal
procedures that were performed in the present study
[11014-(4)]. Rats were euthanized
with isoflurane (5%, inhalation). Blood samples were immediately
collected form the vena cava; as much blood as possible was
obtained from each rat for subsequent measurements. All of the
animals in this work were treated according to the National
Institutes of Health (NIH) guide for the care and use of laboratory
animals.
Se-induced cataracts and hesperetin
treatment
A total of 168 female rats that were 13 days old
were randomized into 12 groups: Group 1, Control group (G1); Group
2, Treatment with hesperetin (G2); Group 3, Treatment with stearic
acid (G3); Group 4, Treatment with oleic acid (G4); Group 5,
Treatment with Hes-S (G5); Group 6, Treatment with Hes-O (G6);
Group 7, Treatment with Se (G7); Group 8, Treatment with hesperetin
and Se (G8); Group 9, Treatment with stearic acid and Se (G9);
Group 10, Treatment with oleic acid and Se (G10); Group 11,
Treatment with Hes-S and Se (G11); and Group 12, Treatment with
Hes-O and Se (G12). (Table I; n=6
or 8 per group in each experiment).
 | Table I.Experimental groups used in the
present study. |
Table I.
Experimental groups used in the
present study.
| Group | Challenge | Test compound | Administration
route |
|---|
| Group 1 | PBS | Vehicle | S.C. |
| Group 2 | PBS | Hes | S.C. |
| Group 3 | PBS | Stealic acid | S.C. |
| Group 4 | PBS | Oleic acid | S.C. |
| Group 5 | PBS | Hes-S | S.C. |
| Group 6 | PBS | Hes-O | S.C. |
| Group 7 | Se | Vehicle | S.C. |
| Group 8 | Se | Hes | S.C. |
| Group 9 | Se | Stealic acid | S.C. |
| Group 10 | Se | Oleic acid | S.C. |
| Group 11 | Se | Hes-S | S.C. |
| Group 12 | Se | Hes-O | S.C. |
Either test compound or vehicle was administered to
each group of rats as described in Table I. Rats in groups G1-G6 were
subcutaneously injected with phosphate-buffered saline (PBS) and
those in G7-G12 were subcutaneously injected with Se at 20 µmol/kg
body weight. PBS or Se was injected into 13-day-old rats (day 0) 4
h after administration of test compound. Hes, stearic acid, oleic
acid, Hes-S, or Hes-O was dissolved in 7% ethanol and 93% olive oil
solution and administered on Days 0, 1 and 2 subcutaneously at 10
nmol/kg body weight per day. The doses of hesperetin and its
derivatives were decided according to our previous reports
(8,9). On day 6, when the rats were 19 days
old, following euthanization, enucleated eyes were analyzed for
levels of GSH and AsA, and lens chaperone activities were
determined. Plasma samples were separated by centrifugation of
whole blood with heparin; plasma was stored at −80°C before
analysis.
Cataract classification
Cataract classifications were defined as previously
described (11). Briefly, cataract
stage 1 was defined as <5% opacity in the lens, stage 2 was
defined as 5–20% opacity, stage 3 was defined as 20–40% opacity,
stage 4 was defined as 40–60% opacity, stage 5 was defined as
60–80% opacity, and stage 6 was defined as >80% opacity.
Following lens observation, rats were euthanized by isoflurane
inhalation and lenses were recovered for further analyses.
Measurement of GSH
The level of lens GSH was determined according to a
method previously described by Sedlak & Lindsay, with minor
modifications (12). Briefly,
lenses were homogenized in 0.1 M sodium phosphate buffer (pH 8.0)
and centrifuged. The water-soluble fraction was deproteinized using
trichloroacetic acid and centrifuged to remove the proteins. The
supernatant was diluted with sodium phosphate buffer according to
the wet lens weight (1 mg lens weight/ml). The sample was divided
into two tubes: one tube contained 10 mM 2-vinylpyridine to
sequester GSH for measuring oxidative GSH, and the other tube
contained the same volume of sodium phosphate buffer to measure the
total GSH content. Both tubes were incubated for 1 h at room
temperature in a fume hood. After incubation, the excess
2-vinylpyridine was neutralized with triethanolamine. DTNB was then
added to both tubes, and the mixture was incubated for 30 min at
room temperature. Absorbance at 412 nm was then measured in an
Infinite M200PRO microplate reader (Tecan Ltd., Männedorf,
Switzerland). The levels of lens GSH were calculated by subtracting
total GSH concentration from two times the concentration of
oxidative GSH.
Measurement of AsA
Levels of AsA were determined using DCPIP as
described previously (6). Lenses
were homogenized in 0.1 M PBS (pH 7.4) and de-proteinized by using
metaphosphoric acid. The lens homogenate was centrifuged to remove
the proteins. The supernatant was titrated with DCPIP. Absorbance
at 540 nm was measured in a microplate reader, Infinite M1000
(Tecan Ltd.).
Chaperone activity measurement
Chaperone activity was measured according to methods
described previously, with minor modifications (13). Briefly, water-soluble lens proteins
were mixed with aldehyde dehydrogenase (ALDH) in 50 mM sodium
phosphate buffer containing 100 mM NaCl (pH 7.0). ALDH aggregation
was induced with 1,10-phenanthroline at 42°C. Protein aggregation
was monitored by measurement of light scattering at 360 nm using an
Infinite M200PRO microplate reader (Tecan Ltd.).
Statistical analysis
All data are reported as means ± standard error.
Statistical analysis of data was performed using one-way analysis
of variance with a post-hoc Tukey's multiple comparison test. SPSS
version 24 software (IBM Corp., Armonk, NY, USA) was used for
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cataract classification
Thirteen-day-old SD rats were randomized into two
groups and injected with either PBS (control groups: G1-G6) or Se
(cataract groups: G7-G12), and each group was divided into five
subgroups to further examine the effects of Hes, Hes-S, and Hes-O
(Table I). Hes-S and Hes-O were
synthesized as previously reported (10), and administered 4 h prior to
injecting the rats with either PBS or Se, and then once daily for
two days (total of three days). Six days after the PBS or Se
injection, cataract classifications were determined as previously
described (11). Fig. 2A-F show nuclear cataracts in rats
from groups 7 to 12, respectively (Fig. 2A-F). More than 80% of lenses from
G7 (Se-treatment only) had mature grade 6 nuclear cataracts, with
grade 5 cataracts present in the lenses of the remaining rats in
that group (Fig. 2G). All lenses
from control groups were transparent, and all had grade 1 cataracts
(data not shown). The lenses of rats in G8, G11, and G12 (Se-Hes,
Se-Hes-S, or Se-Hes-O co-treatment, respectively) lacked central
opacity and/or had lower-grade cataracts compared to those of rats
in groups G7, G9, or G10 (Se treatment only, Se-stearic acid, or
Se-oleic acid co-treatment, respectively). In G8, 8, 8, 33, 33, and
17% of rat lenses had cataract grades 5, 4, 3, 2, and 1,
respectively. In contrast, 8, 8, 42, 25, and 17% of rat lenses from
G11 had cataract grades 5, 4, 3, 2, and 1, and 17, 58, 17%, and 8
lenses in G12 had cataract grades 4, 3, 2, and 1, respectively
(Fig. 2G). These data suggest that
treatment of the lens with hesperetin or hesperetin-derived
compounds can delay Se-induced onset of cataracts.
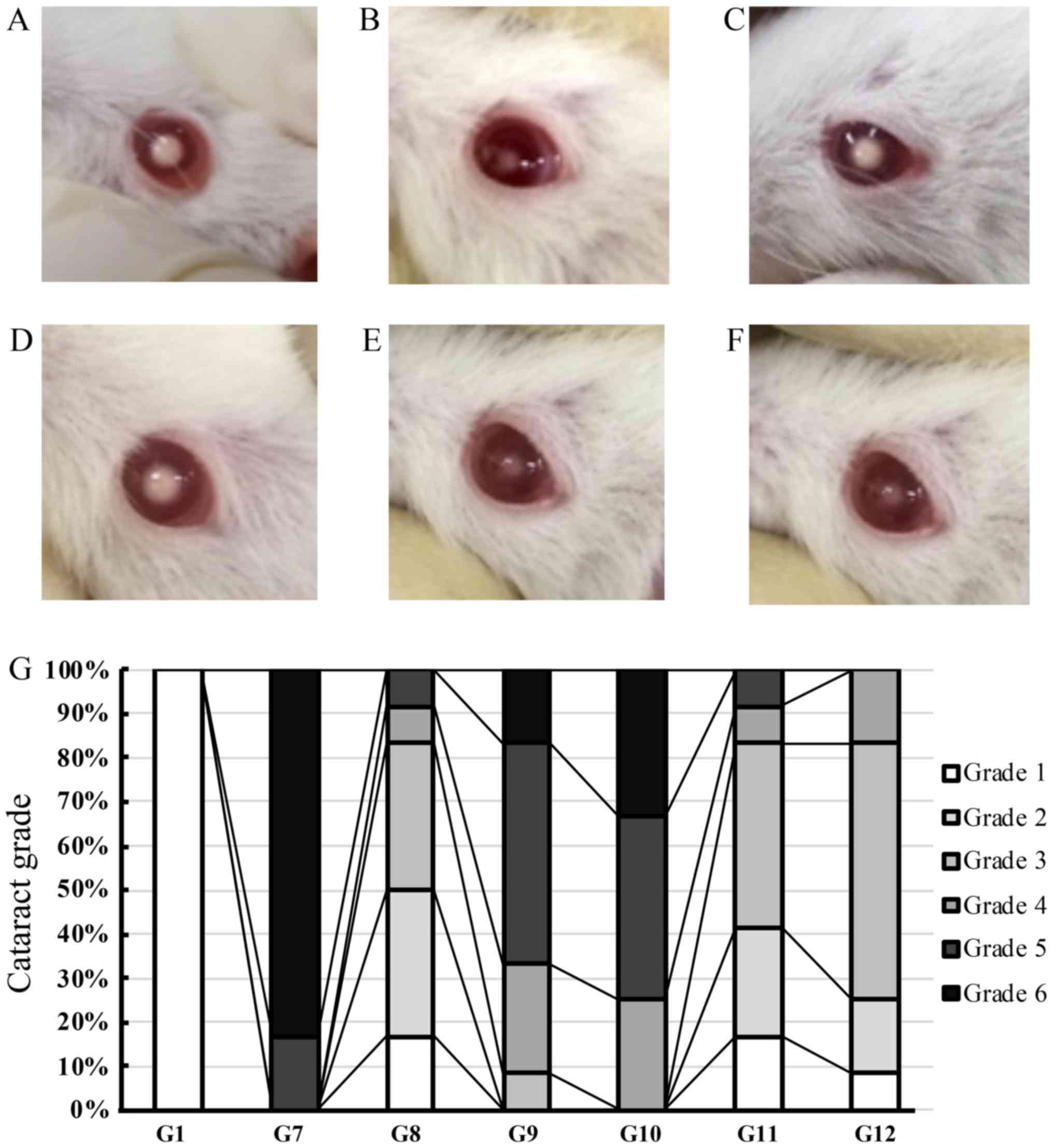 | Figure 2.Anti-cataract effects of hesperetin
and hesperetin derivatives. (A-F) Lenses from rats with
selenite-induced cataracts: Lenses from a rat from (A) the
Se-treated group (group 7: Cataract grade 6), (B) Se-Hes treatment
group (group 8: Cataract grade 2), (C) Se-oleic acid treatment
group (group 9: Cataract grade 5), (D) Se-stearic acid treatment
group (group 10: Cataract grade 6), (E) Se-Hes-S treatment group
(group 11: Cataract grade 2) or (F) Se-Hes-O treatment group (group
12: Cataract grade 2). (G) Cataract grade was determined for lenses
from rats in the groups of rats that were treated with either
hesperetin or hesperetin derivatives (n=6 or 8 per group). G1,
control group; G7-12, groups 7–12; Se, selenite; Hes, hesperetin;
Hes-S, hesperetin stearic acid ester; Hes-O, hesperetin oleic acid
ester. |
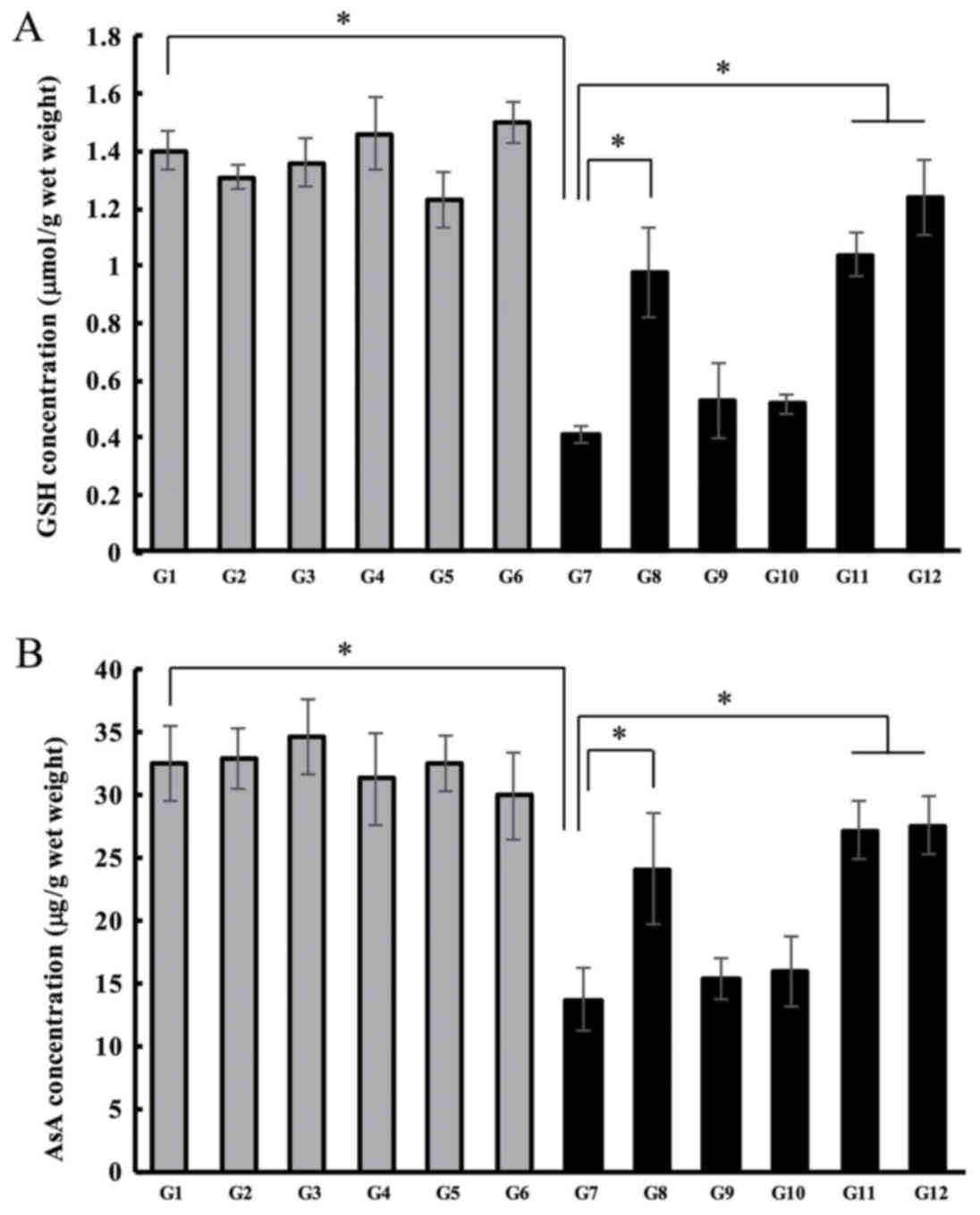
GSH and AsA levels in the lens and
plasma of Se-treated rats
The GSH and AsA levels in the lens and plasma were
determined to evaluate the effects of hesperetin derivatives on the
levels of antioxidant compounds in the lens. In the control groups
(G1-G6), GSH levels in the lens showed no change either with or
without treatment with hesperetin-derived compounds. In the G7
lenses, GSH levels were significantly decreased compared to the
levels observed in control G1 lenses (1.40 µmol/wet weight vs. 0.41
µmol/wet weight) (Fig. 3A).
Hesperetin treatment of rats with Se-induced cataracts rats
prevented a reduction in GSH levels. The concentration of GSH in
the lenses of rats in G8 lens was 0.98 µmol/wet weight.
Interestingly, the lens GSH levels in G11 or G12 rats were higher
than those in G8. GSH concentrations in the lenses of rats in G11
were 1.04 µmol/wet weight and in G12 were 1.2 µmol/wet weight
(Fig. 3A). Next, we measured the
AsA concentrations in the lenses of rats with selenite-induced
cataracts. In the control groups, AsA levels did not change
regardless of treatment (G1-6). In the lenses of rats with
Se-induced cataracts (G7), AsA levels were significantly lower than
those in the lenses of rats in the control group. The
concentrations were 13.70 µg/lens wet weight in G7, and 32.53
µg/wet weight in G1 (Fig. 3B).
Co-treatment of lenses with Hes and Se (G8) prevented the reduction
in AsA levels (24.12 µg/wet weight). AsA concentrations were higher
in G11 and G12, those observed in in G7 rat lenses. AsA levels in
G11 were 27.20 µg/wet weight and 27.55 µg/wet weight in G12.
Subsequently, we quantified the levels of plasma
antioxidant compounds in the lenses. We did not observe any changes
in the levels of GSH or AsA concentrations in the plasma following
treatment with Hes-S or Hes-O, regardless of whether the lenses had
cataracts or not (were transparent) (Fig. 4A and B). These results suggest that
Hes-S and Hes-O prevent the reduction of antioxidants exclusively
in the cataract lens, with no effect on systemic antioxidant
levels.
Lens chaperone activity in cataract
rats
The lens possesses chaperoning ability that helps to
prevent protein aggregation and cataract formation. Cataract lenses
are known to have weaker chaperone activity than normal,
transparent lenses. Therefore, we measured the effect of both
hesperetin and hesperetin-derived compounds on chaperone activity
in cataract lenses. Chaperone activity was evaluated by the
measurement of the time course of aldehyde dehydrogenase (ALDH)
light scattering using 1,10-phenanthroline at 360 nm (Fig. 5A). The sample in ALDH alone did not
show any light scattering (Fig.
5A, curve 9) and a mixture of ALDH and 1,10-phenanthroline in
the absence of lens proteins demonstrated the largest amount of
light scattering (Fig. 5A, curve
1). Lens proteins from mature cataracts in G7 were not able to
suppress light scattering (Fig.
5A, curve 7). However, the lens proteins from Se-induced
cataracts in rats treated with either Hes, Hes-S, or Hes-O showed
reduced ALDH light scattering (Fig.
5A, curve 5, 11, or 12, respectively). A mixture of ALDH,
1,10-phenanthroline, and lens proteins from transparent, normal
lenses from G1 rats showed the lowest amount of ALDH light
scattering (Fig. 5A, curve 8).
 | Figure 5.Effect of hesperetin and hesperetin
derivatives on lens chaperone activity. (A) The time course of
light scattering of ALDH at 360 nm. Curve 1 represents the light
scattering of the ALDH and 1,10-phenanthroline mixture without lens
proteins. Curves 2–8 represent the light scattering of a mixture of
ALDH and lens proteins from each group (from G7, G10, G9, G8, G11,
G12 and G1, respectively). Curve 10 is that of ALDH alone. (B)
Relative chaperone activity of cataract lens proteins with
antioxidants was calculated using ALDH light scattering at 180 min
following the addition of 1,10-phenanthroline. The change of light
scattering of ALDH in the absence of water-soluble lens proteins
was defined as 100%. Bars represent the mean ± standard error (n=6
or 8 per group). *P<0.05, as indicated. G, group; ALDH, aldehyde
dehydrogenase. |
Light scattering in the absence of lens proteins was
defined as 100% scattering. Suppression of ALDH light scattering
was represented as relative ΔA360, as indicated in the bar graph in
Fig. 5B. The lens proteins from G1
suppressed ALDH light scattering, and those from G7 did not affect
ALDH light scattering (Fig. 5B).
The lens proteins from G8 protected against an increase in ALDH
light scatter. However, light scattering suppression ability in G11
or G12 lens proteins was significantly stronger than that of G8
lens proteins. Light scattering reflected ALDH protein aggregation
and the suppression of this aggregation was assumed to be dependent
upon chaperone activity.
Discussion
As age-related cataract progression in humans is
very slow and therapeutics must be applied for a long period of
time to effectively ameliorate or prevent the development of
cataracts, it is vital to study the long-term safety of
pharmacological therapies for cataracts. Furthermore, identifying
and developing effective anti-cataract agents in the human diet
that can be consumed daily would provide significant health
economic benefits.
In this study, we used an Se-induced cataract model
that is well-characterized for screening potential anti-cataract
agents. The selenite-induced cataract animal model can be
efficiently induced by the administration of sodium selenite to rat
pups younger than 16 days old. Cataracts appear within 3–5 days
following sodium selenite administration. We previously reported
that a 3 day course of administration of antioxidant compounds can
ameliorate selenite-induced cataracts (11,13).
Manikandan et al (14)
previously reported that curcumin injected 24 h before the
administration of selenite could prevent the onset of cataract.
Furthermore, Aydemir et al (15) reported that the administration of
ebselen for 4 days inhibited oxidative stress in the lens and
prevented selenite-induced cataract development in rats.
Hesperetin, a natural flavonoid isolated from orange
rinds, has a flavanone backbone structure and is known to have
strong antioxidant activity (16).
As humans are unable to synthesize hesperetin, it is usually
acquired from oranges and other orange-colored fruits. Hesperetin
exhibits antioxidant activity by regulating the expression of
antioxidant enzymes such as catalase, GSH peroxides, and GSH
reductase (17,18). Consumption of antioxidant compounds
and maintaining a constant redox state in the lens are currently
the recommended methods for preserving lens transparency, as
cataracts are mainly caused by oxidative stress. We synthesized two
hesperetin derivatives, Hes-S and Hes-O, that have a fatty acid
linked to the 7-hydroxy position of hesperetin, to evaluate the
effect of hesperetin derivatives on cataract onset by measuring
cataract development, antioxidant levels, and lens chaperone
activity.
In this report, we show that fatty acid-linked
hesperetins have greater anti-cataract effects compared to that of
the original chemical compound, hesperetin, especially for lens
chaperone activity. We hypothesize that hesperetin fatty acid ester
compounds may improve hesperetin pharmacokinetics due to the
following effects: i) Improvement of uptake from subcutaneous areas
into the bloodstream and systemically; ii) greater permeability
across the blood-aqueous barrier; iii) hydrolysis of Hes-S or Hes-O
into hesperetin by esterase(s) localized in the lens; and iv)
trapping of hesperetin within the lens due to poor water
solubility. Firstly, cellular uptake of Hes-S or Hes-O is thought
to be enhanced by improving the lipophilicity of hesperetin which
was achieved by linking it to either stearic acid or oleic acid. It
is well known that drugs are generally absorbed by passive
diffusion into the systemic circulation and that improving the
lipophilicity of the drug can increase absorption rate.
Furthermore, the absorption of lipophilic compounds is thought to
be mediated primarily by membrane diffusion, whereas hydrophilic
compounds appear to be absorbed via passive diffusion through
intercellular junction pores (19,20).
In this current study, we were unable to detect any
differences in cataract classification or levels of antioxidant
compounds in the between the hesperetin treatment and hesperetin
derivative treatment groups. Indeed, neither hesperetin nor its
derived compounds could be detected in the serum or lens by HPLC 4
h after injection (data not shown). However, we did observe
significant differences in the retention of chaperone activity, we
hypothesized that tiny amounts of hesperetin or its derivatives
could reach the lens. It will be necessary to use a detector and/or
HPLC system with a greater sensitivity to detect the presence of
these compounds, and further studies will be needed to decipher how
hesperetin and its derivatives reach the lens and/or interact with
α-crystallin at a molecular level to maintain lens chaperone
activity.
The results of the current study indicate that
hesperetin and hesperetin-derived compounds may delay or prevent
cataract onset by preserving chaperone activity in lens proteins,
and fatty acid-linked hesperetins had greater chaperone activity
than that of the original compound (hesperetin). We previously
reported that hesperetin treatment could prevent chaperone activity
decreasing in the lens by preventing oxidative modification of
α-crystallin and retaining water solubility (9). Generally, α-crystallin purified from
the lens is used to measure chaperone activity. However, the water
solubility of this protein and its concentration in the
water-soluble fraction was changed after cataract onset. Therefore,
we used measurements of total water-soluble protein to determine
the lens chaperone activity.
Hes-O-treated rats displayed lower cataract grades,
higher lens GSH levels, and stronger chaperone activity than
Hes-S-treated rats. Although the carbon number of stearic acid is
the same as that of oleic acid, but oleic acid has a
cis-double bond in its structure. This double bond structure
may affect the membrane permeability and pharmacokinetics of the
hesperetin esters. Interestingly, cataract stages and markers of
cataract development were improved in the animals treated with
fatty acid (groups G9 and G10). We hypothesize that this
improvement may be due to enhanced lens membrane stability. Further
in-depth investigation is required to assess the pharmacokinetics
of these compounds. In addition, further studies are needed to
determine how Hes-S and Hes-O interact with lens proteins and
prevent cataract onset at the molecular level, and how they affect
the molecular disposition of antioxidants in vivo.
In addition to the anti-cataract effect, hesperetin
has several general health benefits, such as anti-inflammatory
properties, antihypertensive effects, and the improvement of very
low-density lipoprotein (VLDL) metabolic abnormalities (21–23).
We speculate that these health benefits may be more pronounced in
Hes-S or His-O compared to that in the original compound
(hesperetin).
As age-related cataract progression in humans is
very slow and treatments must be applied for long periods of time
to delay or prevent the development of cataracts, it is vital to
study the long-term safety of pharmacological therapies. As
anti-cataract pharmaceutical therapies require long-term treatment,
identifying affordable anti-cataract compounds that can be found
ubiquitously in the human diet and that have no adverse effects is
of paramount importance.
Acknowledgements
MP would like to thank the Graduate School of SIGMA
Clermont, France, for the opportunity to be a visiting research
student (2017) under an agreement between SIGMA Clermont and Keio
University.
Funding
The present study was supported by grants from the
Japan Society for the Promotion of Science KAKENHI (grant no.
16K18957), and from the Ministry of Education, Culture, Sports,
Science, and Technology (MEXT)-supported program for Strategic
Research Foundation at Private Universities (grant no.
S1101003).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
YN and HT conceived and designed the present study.
YN, MFT, TS and HT designed the methods. YN, MP, KF and NN
performed the laboratory experiments. YN, NN and HT analyzed and
interpreted the data. YN was major contributor in the writing of
the manuscript.
Ethics approval and consent to
participate
Keio University Animal Research Committee (Tokyo,
Japan) approved all of the animal procedures that were performed in
the present study [11014-(4)].
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GSH
|
glutathione
|
|
AsA
|
ascorbic acid
|
|
DTNB
|
dithionitrobenzene
|
|
DCPIP
|
2,6-dichlorophenol-indophenol
|
|
ALDH
|
aldehyde dehydrogenase
|
|
SD
|
Sprague Dawley
|
|
Se
|
selenite
|
References
|
1
|
Khairallah M, Kahloun R, Bourne R, Limburg
H, Flaxman SR, Jonas JB, Keeffe J, Leasher J, Naidoo K, Pesudovs K,
et al: Number of people blind or visually impaired by cataract
worldwide and in world regions, 1990 to 2010. Invest Ophthalmol Vis
Sci. 56:6762–6769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu YC, Wilkins M, Kim T, Malyugin B and
Mehta JS: Cataract. Lancet. 390:600–612. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bergamini CM, Gambetti S, Dondi A and
Cervellati C: Oxygen, reactive oxygen species and tissue damage.
Curr Pharm Des. 10:1611–1626. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pau H, Graf P and Sies H: Glutathione
levels in human lens: Regional distribution in different forms of
cataract. Exp Eye Res. 50:17–20. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamei A: Glutathione levels of the human
crystalline lens in aging and its antioxidant effect against the
oxidation of lens proteins. Biol Pharm Bull. 16:870–875. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakazawa Y, Oka M, Bando M, Inoue T and
Takehana M: The role of ascorbic acid transporter in the lens of
streptozotocin-induced diabetic rat. Biomed Prev Nutr. 1:43–48.
2011. View Article : Google Scholar
|
|
7
|
Grey AC, Demarais NJ, West BJ and
Donaldson PJ: A quantitative map of glutathione in the aging human
lens. Int J Mass Spectrom. (In press).
|
|
8
|
Nakazawa Y, Oka M, Bando M and Takehana M:
Hesperetin prevents selenite-induced cataract in rats. Mol Vis.
21:804–810. 2015.PubMed/NCBI
|
|
9
|
Nakazawa Y, Oka M, Tamura H and Takehana
M: Effect of hesperetin on chaperone activity in selenite-induced
cataract. Open Med (Wars). 11:183–189. 2016.PubMed/NCBI
|
|
10
|
Jeong TS, Kim EE, Lee CH, Oh JH, Moon SS,
Lee WS, Oh GT, Lee S and Bok SH: Hypocholesterolemic activity of
hesperetin derivatives. Bioorg Med Chem Lett. 13:2663–2665. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ishimori N, Oguchi J, Nakazawa Y, Kobata
K, Funakoshi-Tago M and Tamura H: Roasting enhances the
anti-cataract effect of coffee beans: Ameliorating Selenite-induced
cataracts in rats. Curr Eye Res. 42:864–870. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sedlak J and Lindsay RH: Estimation of
total, protein-bound and nonprotein sulfhydryl groups in tissue
with Ellman's reagent. Anal Biochem. 25:192–205. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakazawa Y, Nagai N, Ishimori N, Oguchi J
and Tamura H: Administration of antioxidant compounds affects the
lens chaperone activity and prevents the onset of cataracts. Biomed
Pharmacother. 95:137–143. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Manikandan R, Thiagarajan R, Beulaja S,
Sudhandiran G and Arumugam M: Effect of curcumin on
selenite-induced cataractogenesis in Wistar rat pups. Curr Eye Res.
35:122–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aydemir O, Güler M, Kaya MK, Deniz N and
Üstündağ B: Protective effects of ebselen on
sodium-selenite-induced experimental cataract in rats. J Cataract
Refract Surg. 38:2160–2166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hwang SL and Yen GC: Effect of hesperetin
against oxidative stress via ER- and TrkA-mediated actions in PC12
cells. J Agric Food Chem. 59:5779–5785. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
St-Pierre J, Drori S, Uldry M, Silvaggi
JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, et al:
Suppression of reactive oxygen species and neurodegeneration by the
PGC-1 transcriptional coactivators. Cell. 127:397–408. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu X, Kambe F, Cao X, Kozaki Y, Kaji T,
Ishii T and Seo H: 3beta-Hydroxysteroid-delta24 reductase is a
hydrogen peroxide scavenger, protecting cells from oxidative
stress-induced apoptosis. Endocrinology. 149:3267–3273. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Effros RM and Mason GR: Measurements of
pulmonary epithelial permeability in vivo. Am Rev Respir Dis.
127:S59–S65. 1983.PubMed/NCBI
|
|
20
|
Schneeberger EE: Airway and alveolar
epithelia cell junctionsThe lung: Scientific foundations. Crystal
RG, West JB, Barnes PJ, Cherniack NS and Weibel ER: Raven Press;
New York: pp. 205–214. 1991
|
|
21
|
Ohtsuki K, Abe A, Mitsuzuwi H, Kondo M,
Uemura K, Iwasaki Y and Kondo Y: Effects of long-term
administration of hesperidin and glucosyl hesperidin to
spontaneously hypertensive rats. J Nutr Sci Vitaminol (Tokyo).
48:420–422. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miwa Y, Mitsuzumi H, Sunayama T, Yamada M,
Okada K, Kubota M, Chaen H, Mishima Y and Kibata M: Glucosyl
hesperidin lowers serum triglyceride level in hypertriglyceridemic
subjects through the improvement of very low-density lipoprotein
metabolic abnormality. J Nutr Sci Vitaminol (Tokyo). 51:460–470.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Parhiz H, Roohbakhsh A, Soltani F, Rezaee
R and Iranshahi M: Antioxidant and anti-inflammatory properties of
the citrus flavonoids hesperidin and hesperetin: An updated review
of their molecular mechanisms and experimental models. Phytother
Res. 29:323–331. 2015. View
Article : Google Scholar : PubMed/NCBI
|