Introduction
Inflammatory bowel disease (IBD), typically
referring to ulcerative colitis (UC) and Crohn's disease (CD), is a
group of chronically inflammatory disorders of gastrointestinal
tract without specific etiology (1–4).
Although these inflammatory conditions have been treated with
5-aminosalicylic acid derivatives, corticosteroids and
immunosuppressants (5), most of
these currently used treatments are inadequate due to severe side
effects such as systemic immunosuppression (6–8).
Thus, there is a need to develop novel and safer therapeutic
agents. Recently, natural product-based treatments, particularly
Traditional Chinese Medicine (TCM), have received great interest as
they have relatively few side effects and have been used as
alternative remedies for a variety of diseases including IBD
(9–12).
The pathogenic mechanisms underlying IBD development
are complex and heterogeneous, with the involvement of multiple
cellular transduction pathways including interleukin-6
(IL-6)/signal transducer and activator of transcription 3 (STAT3)
signaling. IL-6 is an important pro-inflammatory cytokine that has
been shown to play a potential role in the pathogenesis of IBD.
Elevated IL-6 levels have been detected in serum, inflammed colonic
mucosal tissues and cultivated lamina propria mononuclear cells in
IBD patients (13–16). Moreover, IL-6 level is correlated
with the inflammatory severity and therefore is considered as a
clinical IBD-relevant parameter (17–23).
Previous findings indicated that IL-6 signal transduction in IBD is
not mediated by the membrane-bound receptor for IL-6 (IL-6R), but
the soluble form of the IL-6R (sIL-6R), a process known as IL-6
trans-signaling (24–26). The IL-6/sIL-6R complex in turn
binds to a common signal transducing receptor gp130, promoting
dimerization of gp130 and then resulting in activation of the
associated Janus kinases (JAKs). Activated JAKs phosphorylate
gp130, leading to the recruitment and activation of STAT3 (27). STAT3 is an important transcription
factor that plays an essential role in cell survival and
proliferation (28,29). After activation via phosphorylation
at tyrosine 705 by JAKs, STAT3 proteins in the cytoplasm dimerize
and translocate to the nucleus where they regulate the expression
of various critical genes, eventually leading to the development of
IBD. Constitutive activation of STAT3 has been found in intestinal
T cells from IBD patients (21,30)
and commonly suggests severe disease activity (31). Therefore, suppressing the
IL-6/STAT3 pathway provides a promising therapeutic strategy in
IBD.
Pien Tze Huang (PZH) is a well-known TCM formula
that was first prescribed by a royal physician more than 450 years
ago in the Ming Dynasty. The main ingredients of PZH include
Moschus, Calculus bovis, Snake gall and Radix
notoginseng. These products together confer PZH properties of
heat-clearing and detoxification (32). Since in the TCM system the
accumulation of toxic dampness and heat is one of the major
causative factors for inflammation and inflammation-related cancer,
PZH has been used in China and Southeast Asia for centuries to
clinically treat a variety of inflammatory diseases and cancers
(33–50). However, the precise mechanism of
anti-inflammatory activity of PZH remains largely unclear. Using
dextran sulfate sodium (DSS)-induced mouse colitis model, in the
present study we evaluated the therapeutic efficacy of PZH against
UC and elucidated the possible molecular mechanisms.
Materials and methods
Materials and reagents
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), penicillin-streptomycin, Trypsin-EDTA were
obtained from Life Technologies Corporation (Grand Island, NY,
USA). A 24-well cell culture insert was purchased from BD
Biosciences (Franklin Lakes, NJ, USA). DSS (molecular weight,
40,000 kDa) was purchased from MP Biomedicals (Solon, OH, USA).
Antibodies for western blot analysis were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA). The immunoblot
detection system (ECL Plus) and bicinchoninic acid (BCA) protein
assay reagent were obtained from Pierce Biotechnology, Inc.
(Rockford, IL, USA). Mouse IL-6 or serum amyloid A (SAA) detection
ELISA kit was purchased from BioLegend, Inc. (San Diego, CA, USA)
or Immunology Consultants Laboratory, Inc. (Portland, OR, USA),
respectively. All the other chemicals, unless otherwise stated,
were obtained from Sigma Chemicals (St. Louis, MO, USA).
Preparation of PZH
PZH was obtained from and authenticated by the sole
manufacturer Zhangzhou Pien Tze Huang Pharmaceutical Co., Ltd.
(Zhangzhou, China; Chinese FDA approval no. Z35020242). The stock
solution of PZH was prepared by dissolving PZH powder in saline to
a concentration of 20 mg/ml, followed by sonication for 30 min. The
working concentrations for cell-based experiments were made by
diluting the stock solution in the cell culture medium.
Animals
Seven-week-old male BALB/c mice (weight, 20–22 g)
were purchased from Shanghai SLAC Laboratory Animal Co., Ltd.
(Shanghai, China) and were acclimatized for 1 week before the
experiment. Animals were housed individually in a room maintained
at 22°C under a 12-h day/night cycle. Food and water were given
ad libitum throughout the experiments. The animal
experiments conducted in this study were approved by the Animal
Care and Use Committee of Fujian University of Traditional Chinese
Medicine.
In vivo mouse colitis study
Mouse colitis model was established as we described
previously (51). Briefly, acute
colitis was induced by administration with 3% DSS in the drinking
water for 8 days. On the first day of model construction, the
animals were randomly divided into three groups (n=5): The normal
control group in which mice received neither DSS stimulation nor
PZH treatment; the DSS-induced model or PZH-treated group in which
mice received DSS stimulation and were given intra-gastric
administration with saline or 234 mg/kg, respectively, daily for 12
days. The progression of colitis was monitored in a blinded manner,
including measurement of body weight, evaluation of stool
consistency, and presence of rectal bleeding tested by guaiac
paper. Disease activity index (DAI) was represented as the sum of
scores for weight loss, stool consistency and rectal bleeding
(Table I).
 | Table I.Score of the disease activity
index. |
Table I.
Score of the disease activity
index.
| Score | Weight loss | Rectal
bleeding | Stool
consistency |
|---|
| 0 | 0% | Normal | Normal |
| 1 | 1–5% | – | – |
| 2 | 6–10% | Positive
hemoccult | Loose |
| 3 | 11–20% | – | – |
| 4 | >21% | Gross bleeding | Diarrhea |
Sample preparation
At the end of the experiment, the animals were
anaesthetized with Avertin. Blood was collected via right heart
ventricle puncture in lightly heparinized syringes and kept on ice.
Sera were separated by 5 min centrifugation at 5,000 × g and stored
at −80°C prior to the analysis. The colons were excised and length
was measured. One portion of each distal colon was cut and fixed in
10% formalin for histological examination. The remainder of each
distal colon was snap-frozen in liquid nitrogen and stored at −80°C
for further analysis of the tissue IL-6 level and STAT3
phosphorylation. Proteins in frozen colons were extracted using
T-PER Tissue Protein Extraction Reagent kit according to the
manufacturer's protocol. Protein concentrations were determined by
BCA protein assay kit.
Histopathological evaluation
The formalin fixed section of distal colons were
processed and stained with hematoxylin and eosin (H&E) and
evaluated under light microscopy in a blinded manner by an
experienced pathologist.
Measurement of serum SAA level or
expression of IL-6 in colon by ELISA
The level of SAA in the sera and the expression of
IL-6 in colonic tissues were measured using ELISA kits according to
the manufacturer's protocol. Absorbance was read at 450 nm using a
microplate reader (model ELX800; BioTek Instruments, Inc.,
Winooski, VT, USA). All the samples were analyzed in
triplicate.
Western blot analysis
Equivalent amounts of protein were resolved in 12%
Novex Bis-Tris gel electrophoresis (NuPAGE; Life Technologies
Corporation). Proteins were then transferred into nitrocellulose
membranes in an iBlot Western Blotting system (Invitrogen, Grand
Island, NY, USA). The membranes were blocked for 1 h at room
temperature with super SuperBlock® Blocking Buffers
(Pierce Biotechnology, Inc.). They were then incubated at 4°C
overnight with primary antibodies against p-STAT3 (rabbit,
polyclonal, 1:1,000, CST, cat. no. 9131), regular STAT3 (rabbit,
polyclonal, 1:1,000, CST, cat. no. 9132)and β-actin (rabbit,
polyclonal, 1:2,000, CST, cat. no. 4967) in blocking buffer. After
the membranes were washed, they were further incubated with
appropriate horseradish anti-rabbit IgG, HRP-linked antibody
(1:5,000, CST, cat. no. 7074) for 1 h at room temperature. The
membranes were analyzed using enhanced chemiluminescence Plus
reagents and scanned with the Storm Scanner (Amersham Pharmacia
Biotech, Inc., Piscataway, NJ, USA).
Cell culture
Human colon cancer Caco-2 cells were purchased from
the American Type Culture Collection (Rockville, MD, USA). Cells
(passages 20–40) were grown in DMEM supplemented with 20% (v/v)
FBS, 1,000 mg/l of glucose, 50 U/ml penicillin and 50 µg/ml
streptomycin in a 37°C humidified incubator with 5% CO2.
Caco-2 cells usually reached confluence 3 days after seeding, and
differentiated into enterocyte-like cells 18–20 days
post-confluence. To test the effect of PZH on IL-6-induced
phosphoralytion of STAT3, we seeded these fully differentiated
cells in 24-well biocameral inserts (area, 0.33 µm2;
pore size, 0.4 µm insert). On the day of the experiment, the medium
was removed and supplemented with 0.5% FBS medium. Differentiated
Caco-2 cells (20 days post-confluence) in 24-well biocameral
inserts were pre-incubated with 0.5 mg/ml of PZH for 2 h followed
by stimulation with 10 ng/ml of IL-6 for 30 min.
Measurement of cell viability by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Differentiated Caco-2 cells (20 days
post-confluence) in 96-well plates were treated with various
concentrations of PZH for 24 h. The cytotoxic effect of PZH on
Caco-2 cells was examined by the MTT colorimetric assay. Briefly,
MTT (100 µl) (0.5 mg/ml in PBS) was added to each well, and the
samples were incubated for an additional 4 h at 37°C. The
purple-blue MTT formazan precipitate was dissolved in 100 µl DMSO.
The absorbance was measured at 570 nm using a spectrophotometer
reader (Model ELX800; BioTek Instruments, Inc., Winooski, VT,
USA).
Statistical analysis
Data were presented as mean ± SD for the indicated
number of independently performed experiments. The data were
analyzed using the SPSS package for Windows (version 17.0; SPSS,
Inc., Chicago, IL, USA). Statistical analysis was carried out using
Student's t-test and one-way ANOVA, followed by LSD's test or
Dunnett's test. Differences with P<0.05 were considered to be
statistically significant.
Results
PZH potentially inhibits the
development of DSS-induced UC in mice
To determine whether PZH could inhibit the
development of UC, we evaluated the clinical manifestations in
experimental mice. The DAI was calculated based on the observation
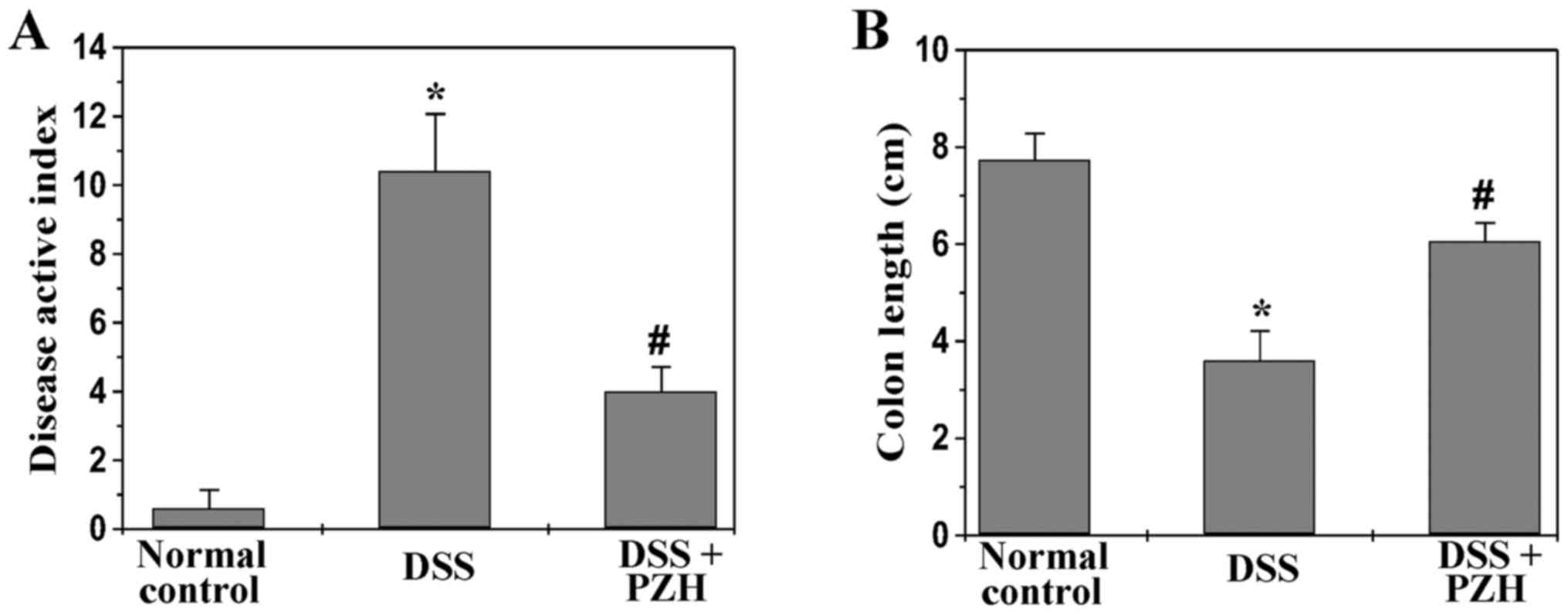
of body weight, stool consistency and rectal bleeding. As shown in
Fig. 1, upon DSS stimulation mice
exhibited apparent manifestations of colitis which was
characterized by body weight loss, diarrhea and rectal bleeding.
Administration of PZH significantly ameliorated DSS-induced colitis
symptoms. The DAI score of normal control, DSS-stimulated model or
PZH-treated group was 0.6±0.54, 10.4±1.67 or 4.0±0.71, respectively
(P<0.05; Fig. 1A). Moreover,
colons were harvested from mice in each group after sacrifice; and
length from cecum to anus was measured. We found that PZH treatment
profoundly prevented DSS-induced colon shortening. The average
colonic length in normal control, model or PZH-treated group was
7.74±0.34, 3.6±0.41 or 6.06±0.38 cm, respectively (P<0.05;
Fig. 1B). Taken together, PZH is
potent in suppressing the development of UC in vivo.
PZH alleviates colon histological
damages in mice with DSS-induced UC
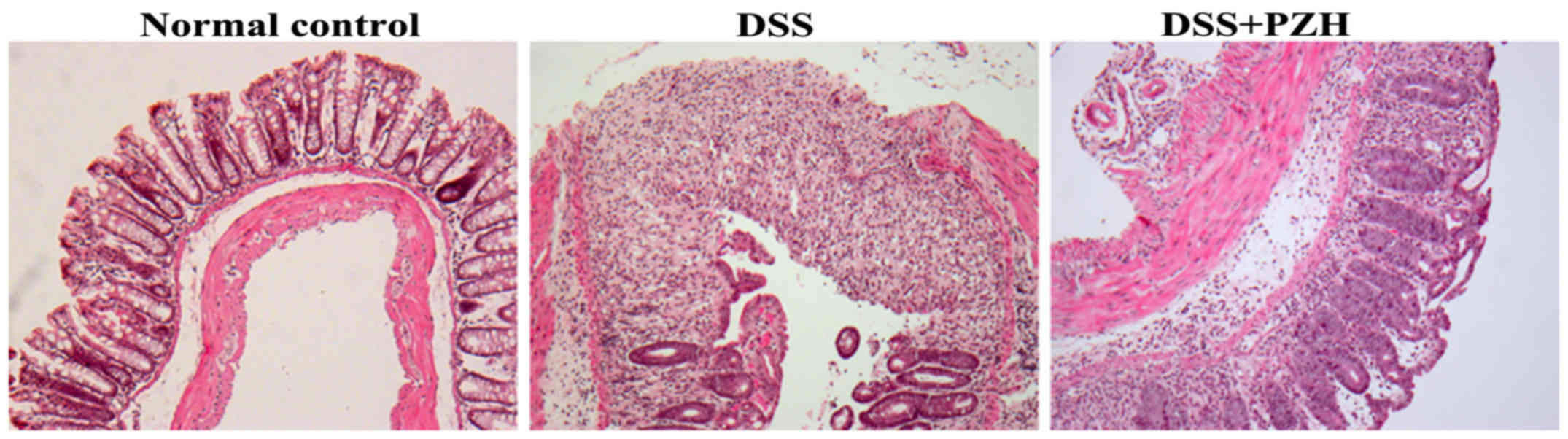
We next evaluated the histopathological changes of
colon tissues through H&E staining. As shown in Fig. 2, the normal control mice displayed
normal colonic histology with an intact epithelium, well-defined
gland lengths and no leukocyte infiltration in the mucosa, whereas
DSS induced severe colonic histological damages such as mucosal
ulceration, inflammatory cell infiltration, crypt distortion and
hyperplastic epithelium. However, PZH treatment significantly
alleviated DSS-induced histopathological changes in colonic
mucosa.
PZH reduces SAA level in mice with
DSS-induced UC
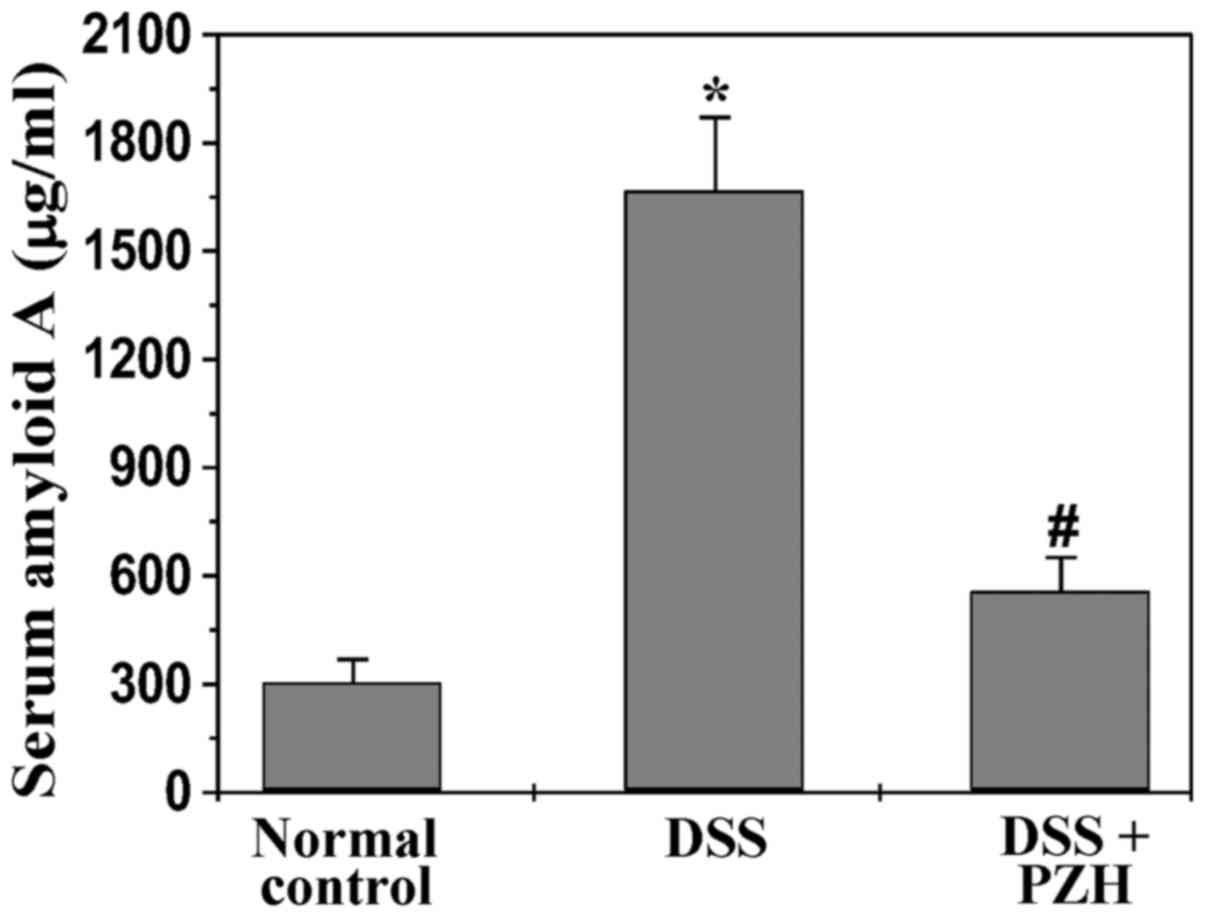
It has been shown that the level of the inflammatory
marker SAA is increased in UC patients. We therefore performed
ELISA assay to examine SAA level in experimental mice. As shown in
Fig. 3, PZH treatment
significantly inhibited DSS-induced increase of serum level of SAA
in UC mice. The SAA level in mice of normal control, model or
PZH-treated group was 304.9±62.8, 1,667.9±202.9 or 558.2±92.5
µg/ml, respectively (P<0.05).
PZH suppresses IL-6/STAT3 signaling
pathway both in mice with DSS-induced UC and in inflammatory
intestinal epithelial cells
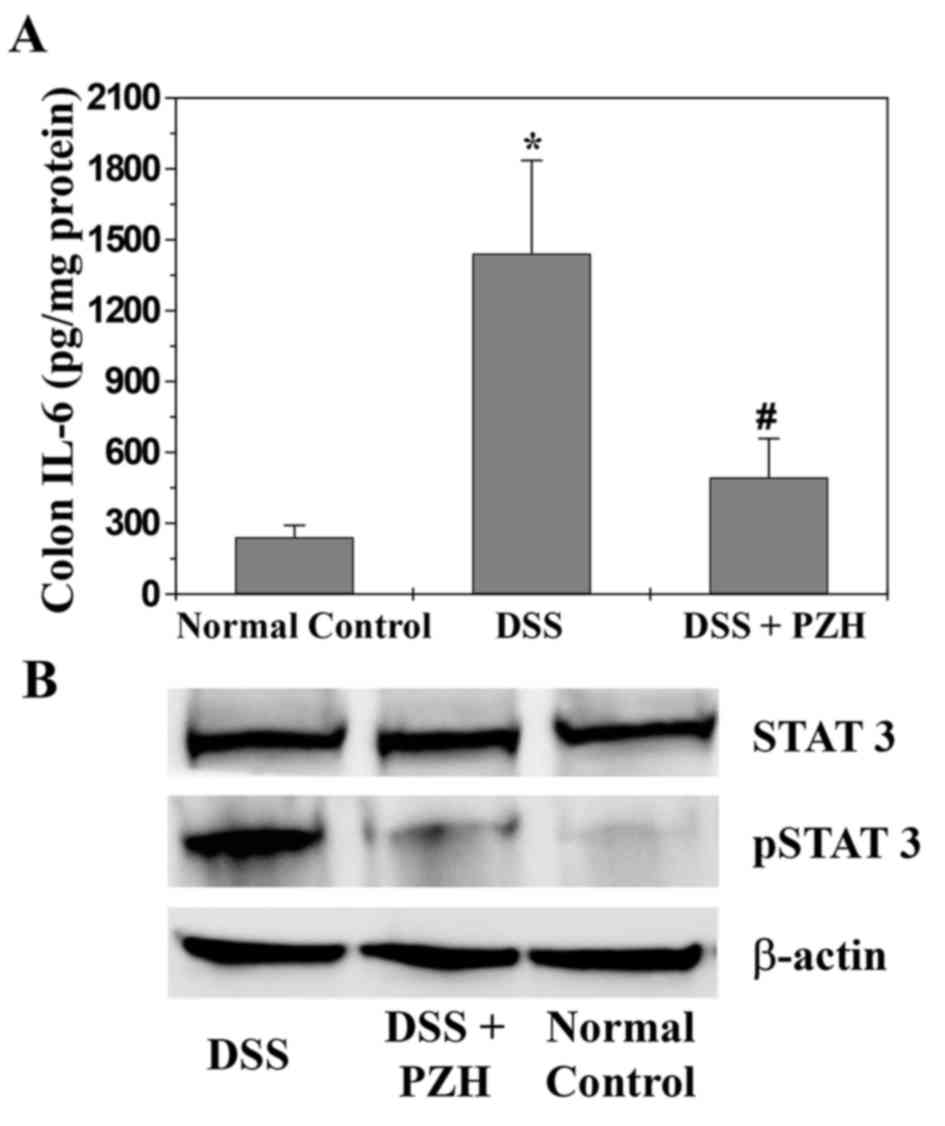
To investigate the mechanism of the
anti-inflammatory activity of PZH, we evaluated the activation of
IL-6/STAT3 pathway in colon tissues of experimental mice. The
protein expression of IL-6 in colon tissues was determined by ELISA
assay and STAT3 activation was examined by western blot analysis
using antibody that recognizes STAT3 phosphorylation at Tyr705. As
shown in Fig. 4, the expression of
IL-6 and the phosphorylation level of STAT3 in DSS-induced UC mice
was significantly increased, as compared to that in normal control
mice; which, however, was significantly inhibited by PZH
treatment.
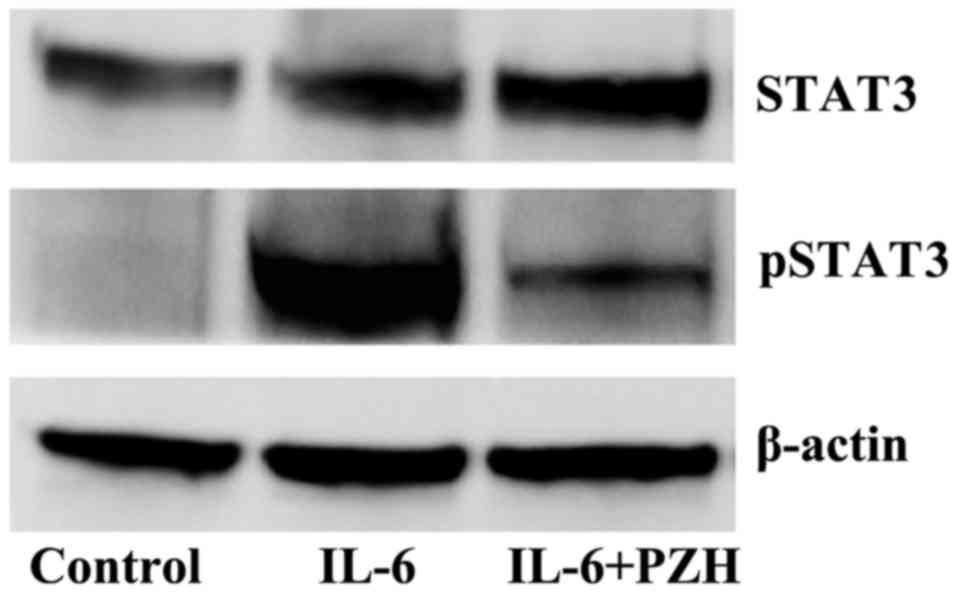
By stimulating the differentiated human colorectal
carcinoma Caco-2 cells with IL-6, we generated an inflammatory cell
model of human intestinal epithelium to examine the in vitro
effect of PZH on IL-6/STAT3 pathway. As shown in Fig. 5, IL-6 stimulation resulted in a
significant increase of pSTAT3 level in Caco-2 cells. However, PZH
treatment profoundly inhibited IL6-induced STAT3 phosphorylation.
The levels of non-phosphorylated STAT3 remained unchanged after the
treatment with IL-6 and/or PZH.
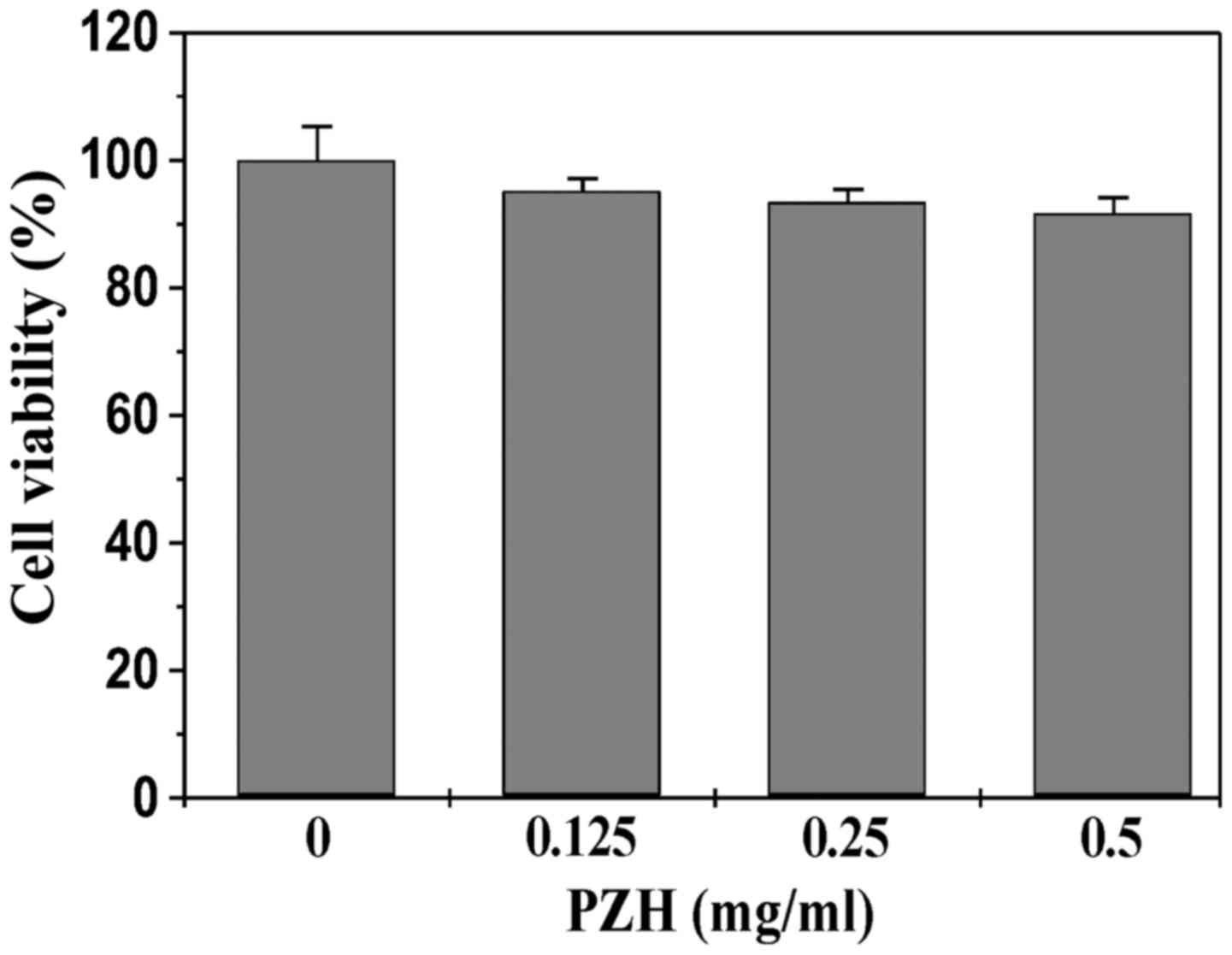
PZH did not display cytotoxicity in
intestinal epithelial cells
To exclude the possibility that the in vitro
suppressive activity of PZH on STAT3 pathway was due to cytoxicity,
we determined its effect on the viability of differentiated Caco-2
cells using MTT assay. As shown in Fig. 6, Caco-2 cell viability was not
affected by treatment with PZH, suggesting that the inhibitory
effect of PZH on IL-6-induced STAT3 activation in intestinal
epithelial cells did not result from its cytotoxic action.
Discussion
Despite recent advances in the drug treatment of
IBD, many currently used pharmacotherapies contain intrinsic side
effects such as systemic immunosuppression, which limits their
long-term use (5–8). Natural products, including TCM, have
received great interest since they have long been used to
clinically treat inflammatory diseases and are relatively
toxicity-free (9–12). PZH is a well-known TCM formula
which has been used in China and Southeast Asia for centuries to
clinically treat a variety of inflammatory diseases (33–50).
However, the precise mechanism of anti-inflammatory activity of PZH
remains largely unknown.
Using a DSS-induced mouse colitis model, in the
present study, we evaluated the therapeutic efficacy of PZH against
UC. We found that PZH significantly ameliorated DSS-induced colitis
symptoms, including body weight, stool consistency and rectal
bleeding. In addition, the administration of PZH profoundly
prevented DSS-induced colon shortening, and alleviated colonic
histopathological changes such as mucosal ulceration, infiltration
of inflammatory cells, crypt distortion and hyperplastic
epithelium. Moreover, PZH markedly inhibited the DSS-induced
increase in serum levels of SAA, one of the inflammatory biomarkers
which is commonly elevated in patients with UC. Thus, PZH is potent
in suppressing the development of UC.
The IL-6/STAT3 pathway is an important signaling
pathway that mediates inflammatory response. As a critical
pro-inflammatory cytokine, IL-6 plays essential roles in the
development of inflammatory diseases including IBD. Elevated IL-6
levels have been detected in the serum, inflammed colonic mucosal
tissues and cultivated lamina propria mononuclear cells in IBD
patients and in murine model with acute inflammation (13–16).
In addition, serum IL-6 level is positively correlated with
severity of intestinal histopathology (17–23).
Generally, IL-6 exerts its bioactivities via binding to the sIL-6R,
leading to the activation of JAKs and the downstream effectors such
as STAT3 (24–27). Activated STAT3 proteins in the
cytoplasm form homodimers and translocate to the nucleus to
regulate the expression of various critical genes mediating
antiapoptotic activities (28,29).
Constitutive activation of STAT3 has been commonly found in lamina
propria mononuclear cells from IBD patients, resulting in the
resistance against apoptosis in these cells and eventually the
progression of IBD (21,30,31).
Thus, the IL-6/STAT3 pathway has become a major target in the
treatment of IBD. Using ELISA assay we found that PZH treatment
significantly inhibited the DSS-induced expression of IL-6 in UC
mice. Moreover, the increased phosphorylation of STAT3, induced by
DSS in experimental mice or by IL-6 in the differentiated human
colorectal carcinoma cells, was profoundly suppressed by PZH.
In conclusion, in the present study we report, to
the best of our knowledge, for the first time that PZH attenuates
intestinal inflammation in murine colitis model probably through
inhibition of the IL-6/STAT3 pathway, demonstrating its potential
clinical value in the treatment of IBD.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (81673721), the International
Cooperative Project of Fujian Department of Science and Technology
(2017I0007) and the Natural Science Foundation of Fujian Province
(2018J01229).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JP and YC conceived and designed the experiments.
LL, AS, JC and XK conducted the animal experiments. LL and TJS
performed western blot and data analysis. AS and SS conducted ELISA
and analysis. LL, JP and YC wrote and revised the manuscript.
Ethics approval and consent to
participate
The animal experiments conducted in this study were
approved by the Animal Care and Use Committee of Fujian University
of Traditional Chinese Medicine.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TCM
|
Traditional Chinese Medicine
|
|
PZH
|
Pien Tze Huang
|
|
IBD
|
inflammatory bowel disease
|
|
UC
|
ulcerative colitis
|
|
IL-6
|
interleukin-6
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
References
|
1
|
Baumgart DC and Carding SR: Inflammatory
bowel disease: Cause and immunobiology. The Lancet. 369:1627–1640.
2007. View Article : Google Scholar
|
|
2
|
Baumgart DC and Sandborn WJ: Inflammatory
bowel disease: Clinical aspects and established and evolving
therapies. The Lancet. 369:1641–1657. 2007. View Article : Google Scholar
|
|
3
|
Xavier RJ and Podolsky DK: Unravelling the
pathogenesis of inflammatory bowel disease. Nature. 448:427–434.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Podolsky DK: Inflammatory bowel disease. N
Engl J Med. 347:417–429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rezaie A, Parker RD and Abdollahi M:
Oxidative stress and pathogenesis of inflammatory bowel disease: An
epiphenomenon or the cause? Dig Dis Sci. 52:2015–2021. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho EJ, Shin JS, Noh YS, Cho YW, Hong SJ,
Park JH, Lee JY, Lee JY and Lee KT: Anti-inflammatory effects of
methanol extract of Patrinia scabiosaefolia in mice with
ulcerative colitis. J Ethnopharmacol. 136:428–435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jani N and Regueiro MD: Medical therapy
for ulcerative colitis. Gastroenterol Clin N Am. 31:147–166. 2002.
View Article : Google Scholar
|
|
8
|
Lakatos PL and Lakatos L: Ulcerative
proctitis: A review of pharmacotherapy and management. Expert Opin
Pharmacother. 9:741–749. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Treasure J: Herbal medicine and cancer: An
introductory overview. Semin Oncol Nurs. 21:177–1835. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stickel F and Schuppan D: Herbal medicine
in the treatment of liver diseases. Dig Liver Dis. 39:293–304.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Langmead L and Rampton DS: Review article:
Complementary and alternative therapies for inflammatory bowel
disease. Aliment Pharmacol Ther. 23:341–349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bensoussan M, Jovenin N, Garcia B,
Vandromme L, Jolly D, Bouché O, Thiéfin G and Cadiot G:
Complementary and alternative medicine use by patients with
inflammatory bowel disease: Results from a postal survey.
Gastroenterol Clin Biol. 30:14–23. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gross V, Andus T, Cäsar I, Roth M and
Schölmerich J: Evidence for continuous stimulation of interleukin-6
production in Crohn's disease. Gastroenterol. 102:514–519. 1992.
View Article : Google Scholar
|
|
14
|
Holub M C, Mako E, Devay T, Dank M, Szalai
C, Fenyvesi A and Falus A: Increased interleukin-6 levels,
interleukin-6 receptor and gp130 expression in peripheral
lymphocytes of patients with inflammatory bowel disease. Scand J
Gastroenterol Suppl. 228:47–50. 1998.PubMed/NCBI
|
|
15
|
Isaacs KL, Sartor RB and Haskill S:
Cytokine messenger RNA profiles in inflammatory bowel disease
mucosa detected by polymerase chain reaction amplification.
Gastroenterol. 103:1587–1595. 1992. View Article : Google Scholar
|
|
16
|
Reinecker HC, Steffen M, Witthöft T,
Pflüger I, Schreiber S, MacDermott RP and Rädler A: Enhanced
secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by
isolated lamina propria mononuclear cells from patients with
ulcerative colitis and Crohn's disease. Clin Exp Immunol.
94:174–181. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reinisch W, Gasche C, Tillinger W, Wyatt
J, Lichtenberger C, Willheim M, Dejaco C, Waldhör T, Bakos S,
Vogelsang H, Gangl A and Lochs H: Clinical relevance of serum
interleukin-6 in Crohn's disease: single point measurements,
therapy monitoring, and prediction of clinical relapse. Am J
Gastroenterol. 94:2156–2164. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Belaiche J, Van Kemseke C and Louis E: Use
of the enteroscope for colo-ileoscopy: Low yield in unexplained
lower gastrointestinal bleeding. Endoscopy. 31:298–301. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Louis E, Belaiche J, van Kemseke C,
Franchimont D, deGroote D, Gueenen V and Mary JY: A high serum
concentration of interleukin-6 is predictive of relapse in
quiescent Crohn's disease. Eur J Gastroenterol Hepatol. 9:939–944.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kusugami K, Fukatsu A, Tanimoto M, Shinoda
M, Haruta J, Kuroiwa A, Ina K, Kanayama K, Ando T and Matsuura T:
Elevation of interleukin-6 in inflammatory bowel disease is
macrophage- and epithelial cell-dependent. Digestive Dis Sci.
40:949–959. 1995. View Article : Google Scholar
|
|
21
|
Atreya R, Mudter J, Finotto S, Müllberg J,
Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C, et
al: Blockade of interleukin 6 trans signaling suppresses T-cell
resistance against apoptosis in chronic intestinal inflammation:
Evidence in Crohn disease and experimental colitis in vivo. Nat
Med. 6:583–588. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Desreumaux P, Brandt E, Gambiez L, Emilie
D, Geboes K, Klein O, Ectors N, Cortot A, Capron M and Colombel JF:
Distinct cytokine patterns in early and chronic ileal lesions of
Crohn's disease. Gastroenterol. 113:118–126. 1997. View Article : Google Scholar
|
|
23
|
Brown KA, Back SJ, Ruchelli ED, Markowitz
J, Mascarenhas M, Verma R, Piccoli DA and Baldassano RN: Lamina
propria and circulating interleukin-6 in newly diagnosed pediatric
inflammatory bowel disease patients. Am J Gastroenterol.
97:2603–2608. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Becker C, Fantini MC, Schramm C, Lehr HA,
Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, et al:
TGF-beta suppresses tumor progression in colon cancer by inhibition
of IL-6 trans-signaling. Immunity. 2:491–501. 2004. View Article : Google Scholar
|
|
25
|
Becker C, Fantini MC, Wirtz S, Nikolaev A,
Lehr HA, Galle PR, Rose-John S and Neurath MF: IL-6 signaling
promotes tumor growth in colorectal cancer. Cell Cycle. 4:217–220.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dowdall JF, Winter DC, Andrews E, Laug WE,
Wang JH and Redmond HP: Soluble interleukin 6 receptor (sIL-6R)
mediates colonic tumor cell adherence to the vascular endothelium:
A mechanism for metastatic initiation? J Surg Res. 107:1–6. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Heinrich PC, Behrmann I, Haan S, Hermanns
HM, Müller-Newen G and Schaper F: Principles of interleukin
(IL)-6-type cytokine signalling and its regulation. Biochem J.
374:1–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bromberg J and Darnell JE Jr: The role of
STATs in transcriptional control and their impact on cellular
function. Oncogene. 19:2468–2473. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aggarwal BB, Kunnumakkara AB, Harikumar
KB, Gupta SR, Tharakan ST, Koca C, Dey S and Sung B: Signal
transducer and activator of transcription-3, inflammation, and
cancer: How intimate is the relationship? Ann NY Acad Sci.
1171:59–76. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lovato P, Brender C, Agnholt J, Kelsen J,
Kaltoft K, Svejgaard A, Eriksen KW, Woetmann A and Ødum N:
Constitutive STAT3 activation in intestinal T cells from patients
with Crohn's disease. J Biol Chem. 278:16777–16781. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Suzuki A, Hanada T, Mitsuyama K, Yoshida
T, Kamizono S, Hoshino T, Kubo M, Yamashita A, Okabe M, Takeda K,
et al: CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3
activation and intestinal inflammation. J Exp Med. 193:471–481.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chinese Pharmacopoeia Commission, .
Pharmacopoeia of the People's Republic of China. 1. China Medical
Science and Technology Press; Beijing: pp. 573–575. 2010
|
|
33
|
Lin JM, Wei LH, Chen YQ, Liu XX, Hong ZF,
Sferra TJ and Peng J: Pien Tze Huang-induced apoptosis in human
colon cancer HT-29 cells is associated with regulation of the Bcl-2
family and activation of caspase 3. Chin J Integr Med. 17:685–690.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhuang QC, Hong F, Shen AL, Zheng LP, Zeng
JW, Lin W, Chen YQ, Sferra TJ, Hong ZF and Peng J: Pien Tze Huang
inhibits tumor cell proliferation and promotes apoptosis via
suppressing the STAT3 pathway in colorectal cancer mouse. Int J
Oncol. 40:1569–1574. 2012.PubMed/NCBI
|
|
35
|
Shen AL, Hong F, Liu LY, Lin JM, Zhuang
QC, Hong ZF and Peng J: Effects of Pien Tze Huang on angiogenesis
in vivo and in vitro. Chin J Integr Med. 18:431–436. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shen A, Hong F, Liu L, Lin J, Wei L, Cai
Q, Hong Z and Peng J: Pien Tze Huang inhibits the proliferation of
human colon carcinoma cells by arresting G1/S cell cycle
progression. Oncol Lett. 4:767–770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shen A, Chen Y, Hong F, Lin J, Wei L, Hong
Z, Sferra TJ and Peng J: Pien Tze Huang suppresses IL-6-inducible
STAT3 activation in human colon carcinoma cells through induction
of SOCS3. Oncol Rep. 28:2125–2130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shen A, Lin J, Chen Y, Lin W, Liu L, Hong
Z, Sferra TJ and Peng J: Pien Tze Huang inhibits tumor angiogenesis
in a mouse model of colorectal cancer via suppression of multiple
cellular pathways. Oncol Rep. 3:1701–1706. 2013. View Article : Google Scholar
|
|
39
|
Lin W, Zhuang QC, Zheng LP, Cao ZY, Shen
AL, Li QY, Fu CX, Feng JY and Peng J: Pien Tze Huang inhibits liver
metastasis by targeting TGF-β signaling in an orthotopic model of
colorectal cancer. Oncol Rep. 33:1922–1028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wei L, Chen P, Chen Y, Shen A, Chen H, Lin
W, Hong Z, Sferra TJ and Peng J: Pien Tze Huang suppresses the
stem-like side population in colorectal cancer cells. Mol Med Rep.
9:261–266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen HW, Shen AL, Zhang YC, Chen YQ, Lin
JM, Lin W, Sferra TJ and Peng J: Pien Tze Huang inhibits
hypoxia-induced epithelial-mesenchymal transition in human colon
carcinoma cells through suppression of the HIF-1 pathway. Exp Ther
Med. 7:1237–1242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shen AL, Lin W, Chen YQ, Liu LL, Chen HW,
Zhuang QF, Lin JM, Sferra TJ and Peng J: Pien Tze Huang inhibits
metastasis of human colorectal carcinoma cells via modulation of
TGF-β1/ZEB/miR-200 signaling network. Int J Oncol. 46:685–690.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen H, Feng J, Zhang Y, Shen A, Chen Y,
Lin J, Lin W, Sferra TJ and Peng J: Pien Tze Huang inhibits
hypoxia-induced angiogenesis via HIF-1α/VEGF-A pathway in
colorectal cancer. Evid Based Complement Alternat Med.
2015:4542792015.PubMed/NCBI
|
|
44
|
Shen A, Chen H, Chen Y, Lin J, Lin W, Liu
L, Sferra TJ and Peng J: Pien Tze Huang overcomes multidrug
resistance and epithelial-mesenchymal transition in human
colorectal carcinoma cells via suppression of TGF-β pathway. Evid
Based Complement Alternat Med. 2014:6794362014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Qi F, Wei L, Shen A, Chen Y, Lin J, Chu J,
Cai Q, Pan J and Peng J: Pien Tze Huang inhibits the proliferation,
and induces the apoptosis and differentiation of colorectal cancer
stem cells via suppression of the Notch1 pathway. Oncol Rep.
35:511–517. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lin J, Feng J, Jin Y, Yan Z, Lai Z and
Peng J: Pien Tze Huang suppresses VEGF-C-mediated lymphangiogenesis
in colorectal cancer. Oncol Rep. 36:3568–3576. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fu C, Chu J, Shen A, Liu L, Chen H, Lin J,
Sferra TJ, Chen Y and Peng J: Pien Tze Huang alleviates
5-fluorouracil-induced intestinal mucositis in CT-26 tumor-bearing
mice. Exp Ther Med. 14:2291–2297. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen Z, Shen A, Liu L, Chen Y, Chu J, Cai
Q, Qi F, Sferra TJ and Peng J: Pien Tze Huang induces apoptosis and
inhibits proliferation of 5-fluorouracil-resistant colorectal
carcinoma via increasing miR-22 expression. Exp Ther Med.
14:3533–3540. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wan Y, Shen A, Qi F, Chu J, Cai Q, Sferra
TJ, Peng J and Chen Y: Pien Tze Huang inhibits the proliferation of
colorectal cancer cells by increasing the expression of miR-34c-5p.
Exp Ther Med. 14:3901–3907. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Qi F, Zhou S, Li L, Wei L, Shen A, Liu L,
Wang Y and Peng J: Pien Tze Huang inhibits the growth of
hepatocellular carcinoma cells by upregulating miR-16 expression.
Oncol Lett. 14:8132–8137. 2017.PubMed/NCBI
|
|
51
|
Ke X, Zhou F, Gao Y, Xie B, Hu G, Fang W,
Peng J, Chen Y and Sferra T: Qing Hua Chang Yin exerts therapeutic
effects against ulcerative colitis through the inhibition of the
TLR4/TNF-κB pathway. Int J Mol Med. 32:926–930. 2013. View Article : Google Scholar : PubMed/NCBI
|