Introduction
Bone remodeling comprises bone-forming osteoblast
and bone-resorbing osteoclast processes (1,2). The
normal balance between resorption and formation is essential for
fracture healing and skeletal development (3). There are four stages of bone
formation: Proliferation, differentiation, maturation and
mineralization. These stages guide the osteoblasts to gradually
differentiate into osteocytes and facilitate osteogenesis (4); therefore, high bioactivity and
biocompatibility, osteoconduction and biodegradability are
necessary characteristics in an alternative material that may
facilitate bone regeneration (5).
In the past three decades, various bone substitutes with high
biocompatibility and good osteoconduction have been reported and
have been applied to repair bone defects (6–9);
however, insufficient bioactivity of these materials has restricted
the repair process (8). Therefore,
high biocompatibility, biodegradability and osteogenic potential
are considered desirable for bone tissue engineering.
Nacre (mother of pearl) is a naturally formed
composite material constituting inorganic calcium carbonate plates
and a complex organic matrix. A previous study demonstrated that
nacre exhibits excellent biocompatibility, biodegradability and
osteogenic properties, and may be used as a potential biomaterial
in tissue engineering (7).
Numerous studies have reported that nacre functions to promote
osteoblast proliferation and differentiation (10,11).
Lamghari et al (12)
observed the osteogenic potential of nacre in vitro and
in vivo. Asvanund et al (13) reported that pearls may promote the
osteogenic differentiation of human bone cells by increasing
alkaline phosphatase (ALP) and osteocalcin expression in
vitro. Furthermore, it is possible that nacre contains
signaling molecules capable of stimulating the osteogenic pathway
in mammalian cells. The organic matrix of nacre has also been
suggested to contain biological molecules capable of activating
osteoblast activity (14,15). The water-soluble matrix (WSM) of
nacre promotes antioxidative and osteogenic differentiation
activities in MC3T3-E1 cells (14); however, the purification and
classification of WSM is complex, and one of the main goals in the
research of nacre is to determine its mechanism for promoting bone
differentiation. Therefore, it is important to investigate the most
effective factors of extracted pearl powder and the mechanism by
which it effectively improves bone formation.
The present study aimed to investigate the role of
water-soluble nano-pearl powder (WSNNP) on osteoblast
differentiation and its underlying mechanisms. The results
indicated that WSNNP stimulated osteoblast differentiation and
enhanced cell mineralization by activating the extracellular
signal-regulated kinase (ERK)-autophagy signaling pathway. The
present study provides evidence of a promising biological material
for bone-grafting procedures.
Materials and methods
Scanning electron microscopy (SEM) and
transmission electron microscopy (TEM)
The nano-pearl powder (NNP) samples were sent to the
Southern Medical University (Shenzhen, China) to verify the
presence of NNP by SEM (S-3000; Hitachi, Ltd., Tokyo, Japan) and
TEM (JEM-2100; JEOL, Ltd., Tokyo, Japan).
Cell culture
MC3T3-E1 cells can differentiate into osteoblasts
and induce mineralization, and were purchased from the American
Type Culture Collection (Manassas, VA, USA). The cells were
cultured in phenol-red-free a-Minimum Essential medium (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (Thermo Fisher Scientific, Inc.), 5 mM
β-glycerophosphate and 25 mg/ml ascorbic acid at 37°C in an
atmosphere containing 5% CO2.
Cell treatment
NNP (100 mg; Jingrun Pearl Biological, Inc., Hainan,
China) was dissolved in 100 ml PBS, magnetically stirred for 24 h
at 4°C and centrifuged at 30,000 × g at 4°C for 20 min. The
supernatant was freeze-dried at −80°C to obtain the WSNNP in
powdered form. The freeze-dried powder was then dissolved in PBS
and a bicinchoninic acid (BCA) protein assay was performed.
Subsequently treatment for 48 h with 0, 10, 25 and 50 µg protein/ml
WSNNP was used for further study (14). An autophagy inhibitor
3-methyladenine (3-MA), and a mitogen-activated protein kinase
kinase (MEK) signaling inhibitor, U0126, were obtained from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). WSNNP protein (25
µg/ml), and either 5 mmol 3-MA or 15 µM U0126, were added to the
cells and incubated for 48 h at 37°C prior to further analysis.
Immunofluorescence assay
For the immunofluorescence assay, 4% formaldehyde
was used to fix the cells (1×105 cells/ml) onto
coverslips for 30 min at 4°C, followed by the addition of PBS
containing 0.5% Triton-X-100 to the coverslips for 20 min to
increase permeability. The cells were then blocked in 5% non-fat
milk in Tris-buffered saline with Tween-20 (TBST; 0.05% Tween-20)
for 60 min at room temperature and incubated with anti-Beclin1
antibody (ab62472; 1:100; Abcam, Cambridge, UK) at 4°C overnight.
The cells were then exposed to Cy3 goat anti-rabbit immunoglobulin
G (sc-2004; 1:200; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
at 37°C for 1 h. The coverslips were stained with DAPI (sc-3598;
1:1,000; Santa Cruz Biotechnology, Inc.) for 2 min and mounted on
slides using anti-fade mounting medium. Immunofluorescence images
were captured using the Nikon ECLIPSE 80i microscope (Nikon
Corporation, Tokyo, Japan).
MTT assay
The MTT assay was performed every 24 h to measure
cell viability. After the indicated treatment, 3×103
cells were seeded into each well of 96-well plates. Briefly, 4 µl
MTT (Sigma-Aldrich; Merck KGaA) was added to the cells, which were
incubated for 4 h at 37°C. Subsequently, 150 µl dimethyl sulfoxide
(Sigma-Aldrich; Merck KGaA) was used to dissolve the formazan
crystals and the absorbance was measured at 570 nm using a
microplate reader.
Western blotting
RIPA lysis buffer (P002A, Auragene, Changsha, China)
was added to the indicated cells to extract the proteins. The
protein concentration was then measured using a BCA Protein Assay
kit (Auragene, Changsha, China); 50 µg protein was separated by 12%
SDS-PAGE and blotted onto 0.22-µm nitrocellulose membranes. The
membranes were blocked with 5% milk-TBS for 2 h at room temperature
and were incubated with the following primary antibodies overnight
at 4°C: Mouse monoclonal anti-collagen I (ab138492; 1:1,000;
Abcam), mouse monoclonal anti-secreted phosphoprotein 1 (SPP1;
AM4093; 1:1,000; Abzoom Biolabs, Inc., Dallas, TX, USA), rabbit
polyclonal anti-runt-related transcription factor 2 (RUNX2; ab3931;
1:500; Abcam), rabbit polyclonal anti-microtubule-associated light
chain 3 (LC3)II/I (ab51520; 1:500; Abcam), rabbit polyclonal
anti-Beclin1 (ab62472; 1:1,000; Abcam) and rabbit polyclonal
anti-autophagy-related 7 (ATG7; 10088-2-AP; 1:1,000; ProteinTech
Group, Inc., Chicago, IL, USA), rabbit polyclonal anti-ERK (YM0244;
1:1,000; ImmunoWay Biotechnology Co., Plano, TX, USA), rabbit
polyclonal anti-phosphorylated (p)-ERK (YP0497; 1:1,000; ImmunoWay
Biotechnology Co.), rabbit polyclonal anti-MEK (YM0435; 1:1,000;
ImmunoWay Biotechnology Co.), rabbit polyclonal anti-p-MEK (YP0169;
1:500; ImmunoWay Biotechnology Co.), rabbit polyclonal
anti-mammalian target of rapamycin (mTOR; YT2913; 1:1,000;
ImmunoWay Biotechnology Co.), rabbit polyclonal anti-p-mTOR
(YP0176: 1:200; ImmunoWay Biotechnology Co.), rabbit polyclonal
anti-p70 S6 kinase (p70S6K; ab9366; 1:1,000; Abcam), rabbit
polyclonal anti-p-p70S6K (ab126818; 1:200; Abcam) and mouse
monoclonal anti-β-actin (ab8226; 1:2,000; Abcam). The membranes
were then washed with TBST and incubated with the appropriate
horseradish peroxidase-conjugated secondary antibodies (goat
anti-rabbit; sc-2004; 1:2,000 and goat anti-mouse; sc-2039 1:2,000;
Santa Cruz Biotechnology, Inc.) for 1 h at 37°C. Finally, the
membranes were exposed to enhanced chemiluminescence (P020WB;
Auragene, Changsha, China) for visualization and the optical
density of the objective protein levels was analyzed by
densitometry using Image-Pro Plus version 6.0 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
ALP activity
Triton X-100 (1%) was used to lyse the cells for 30
min at 4°C and centrifuged at 8,000 × g for 5 min at 4°C. The
protein was extracted from supernatant and the ALP Assay kit
(ab83369; Abcam) was used to detect ALP activity, according to the
manufacturer's protocol.
ALP staining
MC3T3-E1 cells (1×105 cells/ml) were
treated with 25 µg/ml WSNNP for 3 days at 37°C. The ALP Staining
kit (D001-2; Nanjing Jiancheng Bioengineering Institute, Nanjing,
China) was then used to detect the extent of ALP staining,
according to the manufacturer's protocol and the cells examined
under a light microscope (AE41; Motic, Xiamen, China).
Alizarin red S staining
MC3T3-E1 cells (1×105 cells/ml) were
treated with WSNNP 25 µg/ml for 14 days at 37°C. Subsequently, the
Alizarin Red S staining kit (G1450; Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) was used to detect the
intensity of Alizarin Red S staining according to the manufacturer’
protocol and the cells examined under a light microscope (AE41;
Motic).
Statistical analysis
GraphPad Prism 5 (GraphPad Software, Inc., La Jolla,
CA, USA) was used for statistical analyses, and the data are
presented as the means ± standard deviation. One-way analysis of
variance and the Bonferroni post hoc test were used to analyze data
from more than two groups. An unpaired two-tailed Student's t-test
was used to analyze data from two groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
WSNNP enhances the differentiation and
maturation of cultured MC3T3-E1 cells
SEM and TEM were used to confirm the presence of
WSNNP prepared from the NNP (Fig. 1A
and B). Subsequently, the effects of WSNNP on the viability of
MC3T3-E1 cells were determined using the MTT assay. The present
study revealed that 10, 25 and 50 µg/ml WSNNP stimulated MC3T3-E1
cell viability, and of these concentrations, 50 µg/ml WSNNP had the
maximum effect (Fig. 1C). To
investigate the effects of WSNNP on osteoblast differentiation,
western blotting and ALP activity assays were performed. The
results indicated that increases in the expression levels of
collagen I, SPP1and RUNX2 may have promoted osteogenic
differentiation. Collagen I is known to stimulate cell migration,
proliferation and osteogenic differentiation of rat bone-marrow
stromal cells (15). SPP1, also
known as osteopontin, is a marker of the later period of osteoblast
mineralization, whereas RUNX2 acts as the key regulator of bone
formation by regulating osteoblast differentiation (16). In the present study, WSNNP
treatment significantly enhanced the protein expression levels of
collagen I, SPP1 and RUNX2 in a dose-dependent manner (Fig. 1D and E). ALP activity served as an
initial indicator of osteoblast differentiation; WSNNP treatment
also increased ALP activity and Alizarin Red S staining in a
time-and dose-dependent manner (Fig.
1F-H). These results suggested that WSNNP may promote
osteoblast proliferation and differentiation.
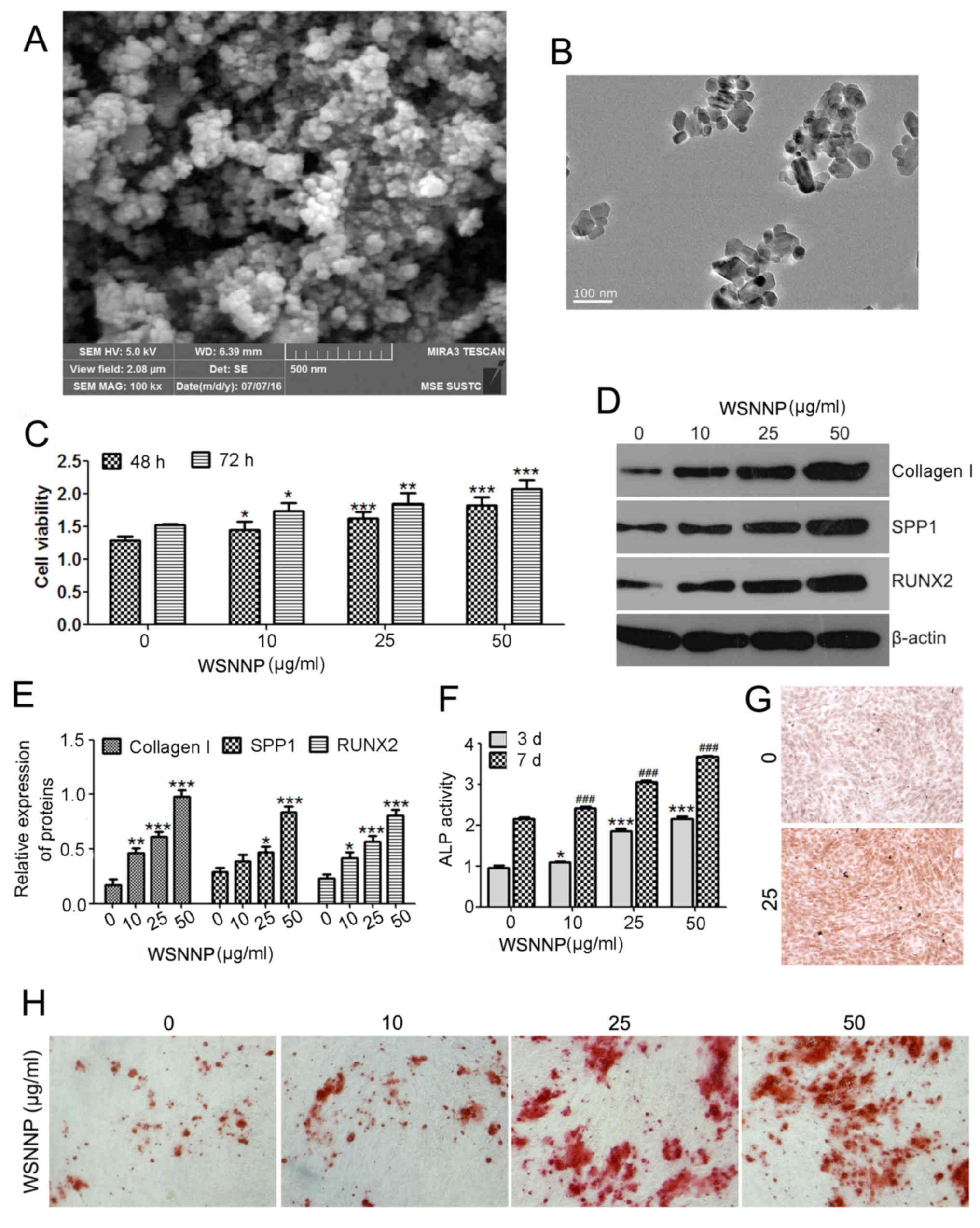 | Figure 1.WSNNP stimulates MC3T3-E1 cell
viability and differentiation. NNP observed by (A) scanning
electron microscopy and (B) transmission electron microscopy. (C)
MTT assay was performed to measure cell viability following WSNNP
treatment (0, 10, 25 and 50 µg/ml) for 48 and 72 h. (D and E)
Expression levels of collagen I, SPP1 and RUNX2 were determined by
western blotting following WSNNP treatment (0, 10, 25 and 50 µg/ml)
for 48 h. (F) ELISA assay was used to detect the ALP expression
following treatment with WSNNP (0, 10, 25 and 50 µg/ml) for 3 and 7
days. (G) ALP staining of cells following WSNNP treatment (0 and 25
µg/ml) for 3 days (magnification, ×200). (H) Alizarin red S
staining of cells treated with WSNNP (0, 10, 25 and 50 µg/ml) for
14 days (magnification, ×200). Data are presented as the means ±
standard deviation, n=3. *P<0.001, **P<0.01, ***P<0.05 and
###P<0.001 vs. non-WSNNP-treated cells on day 7. ALP,
alkaline phosphatase; RUNX2, runt-related transcription factor 2;
SPP1, secreted phosphoprotein1; WSNNP, water-soluble nano-pearl
powder. |
WSNNP contributes to MC3T3-E1 cell
differentiation by enhancing autophagy
To understand the potential mechanism by which WSNNP
regulates osteoblast differentiation, and considering the close
association between autophagy and bone development, the present
study investigated whether WSNNP may regulate autophagy. MC3T3-E1
cells were treated with various concentrations of WSNNP (10, 25 and
50 µg/ml); immunofluorescence and western blotting were conducted
to measure the expression levels of autophagy markers. The results
of the present study revealed that WSNNP enhanced Beclin1
fluorescence (Fig. 2A). Increasing
concentrations of WSNNP also upregulated the expression levels of
LC3II/I, Beclin1 and ATG7, which are key molecules of autophagy
signaling, with the exception of 50 µg/ml WSNNP on the expression
of LC3II/I (Fig. 2B and C). These
findings indicated that WSNNP may stimulate autophagy.
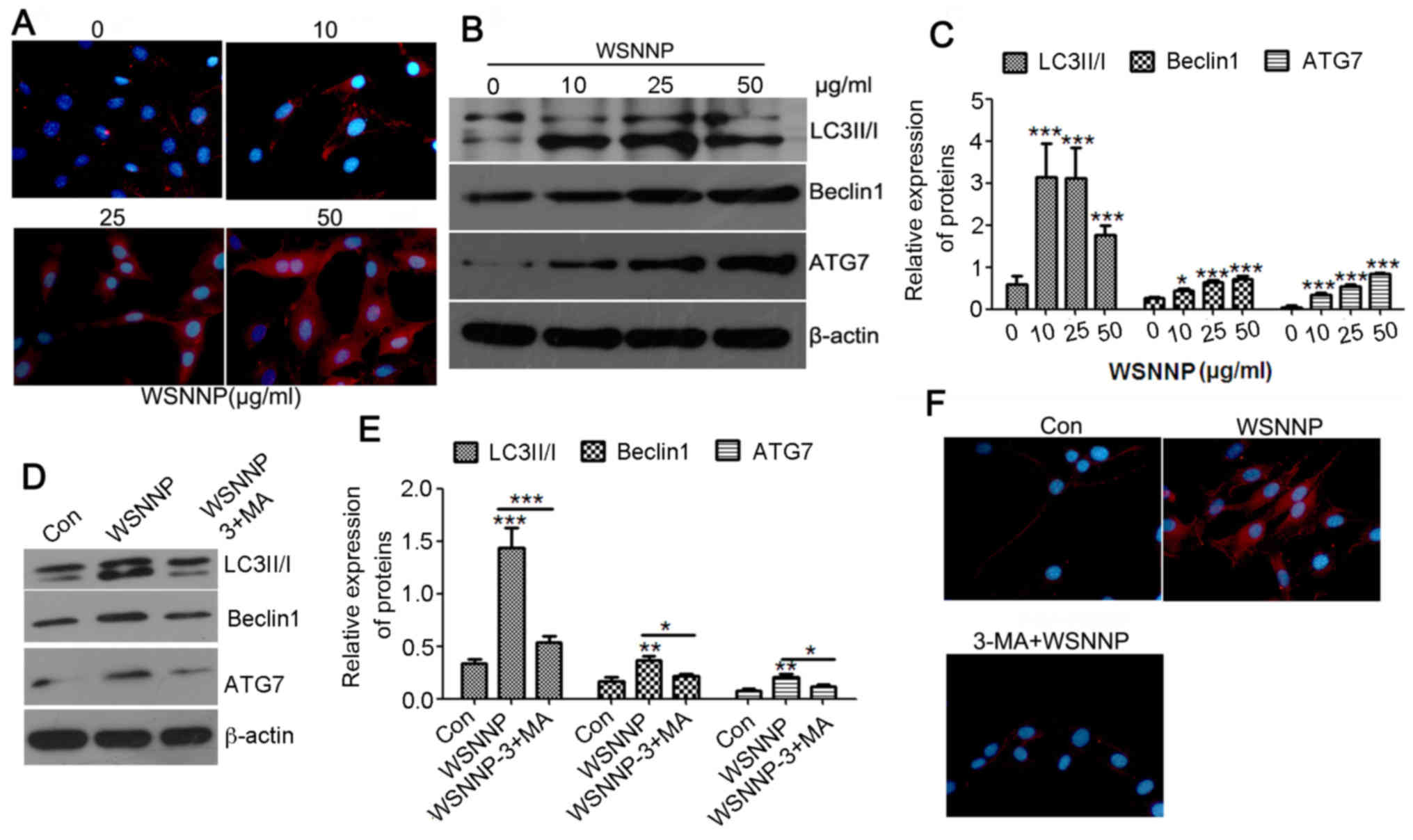 | Figure 2.WSNNP stimulates autophagy in MC3T3-E1
cells, which is reversed by 3-MA. (A) Expression of Beclin1 (red)
following WSNNP treatment (0, 10, 25 and 50 µg/ml) for 48 h in
MC3T3-E1 cells was analyzed by an immunofluorescence assay. Blue
staining indicates nuclei. Magnification, ×400. (B and C) Protein
expression levels of LC3II/I, Beclin1 and ATG7 were detected by
western blotting following WSNNP treatment (0, 10, 25 and 50 µg/ml)
for 48 h. (D and E) LC3II/I, Beclin1 and ATG7 expression levels
were detected by western blotting following treatment with 25 µg/ml
WSNNP, with or without 5 mmol 3-MA, for 48 h. (F)
Immunofluorescence was performed to measure the expression of
Beclin1 (red) following treatment with 25 µg/ml WSNNP, with or
without 5 mmol 3-MA, for 48 h in MC3T3-E1 cells. Blue staining
indicates nuclei. Magnification, ×400. Data are presented as the
means ± standard deviation, n=3. *P<0.001, **P<0.01 and
***P<0.05 vs. non-WSNNP-treated cells at 48 h. 3-MA,
3-methyladenine; ATG7, autophagy-related 7; Con, control; LC3,
microtubule-associated light chain 3; WSNNP, water-soluble
nano-pearl powder. |
The present study also investigated whether
autophagy was required for WSNNP-mediated osteoblast
differentiation using 25 µg/ml WSNNP. Briefly, 3-MA was used as an
autophagy inhibitor; autophagy and osteoblast differentiation of
MC3T3-E1 cells were evaluated using western blotting,
immunofluorescence and ALP activity assays in the presence of WSNNP
treatment with or without 3-MA. Western blotting results
demonstrated that the expression levels of LC3II/I, Beclin1 and
ATG7 were significantly inhibited by 3-MA compared with in cells
treated with WSNNP alone (Fig. 2D and
E). Immunofluorescence assays revealed that the expression
levels of Beclin1 were enhanced in the WSNNP-treated group and
reduced in the 3-MA and WSNNP cotreatment group (Fig. 2F). The results of the present study
indicated that 3-MA significantly inhibited WSNNP-induced
autophagy. Notably, cotreatment with WSNNP and 3-MA also
significantly downregulated the protein expression levels of
collagen I, SPP1and RUNX2 compared with in cells treated with WSNNP
alone (Fig. 3A and B). ALP
activity was also significantly inhibited by 3-MA compared with in
the WSNNP group, but was enhanced by WSNNP alone (Fig. 3C). These results suggested that
WSNNP may contribute to MC3T3-E1 cell differentiation by enhancing
autophagy.
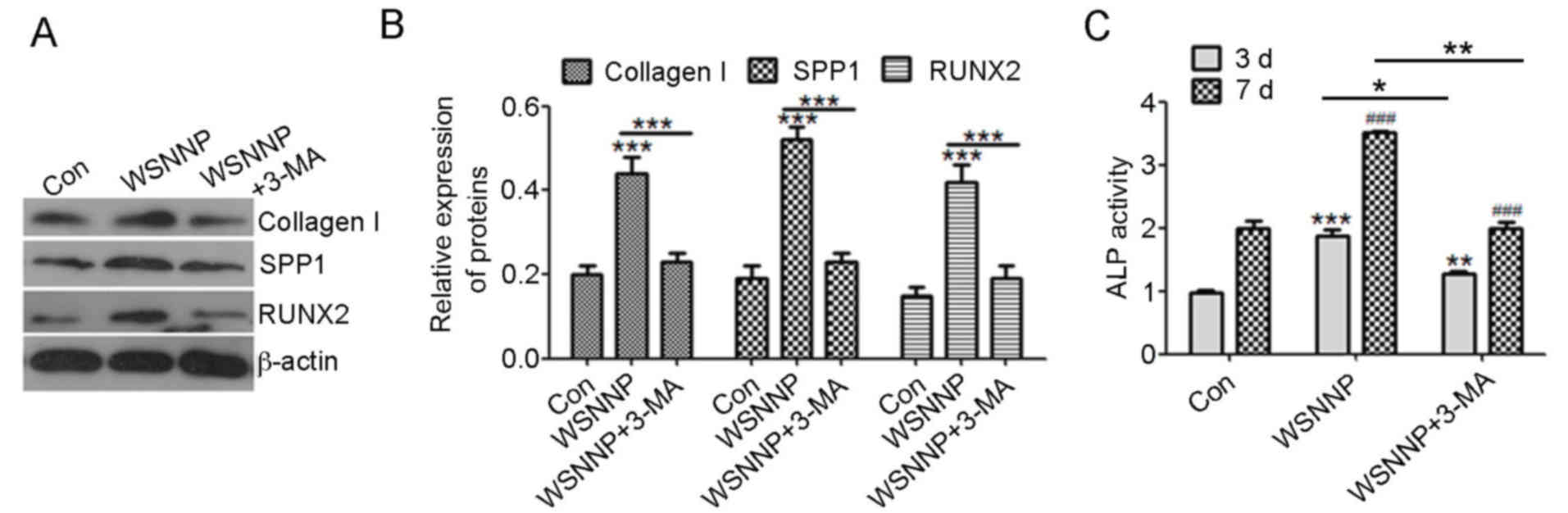 | Figure 3.3-MA reverses WSNNP-mediated
differentiation of MC3T3-E1 cells. (A and B) Collagen I, SPP1 and
RUNX2 expression levels were measured by western blotting following
treatment with 25 µg/ml WSNNP, in the presence or absence of 5 mmol
3-MA, for 48 h. (C) ELISA was used to detect ALP activity following
treatment with 25 µg/ml WSNNP, with or without 5 mmol 3-MA, for 3
and 7 days. Data are presented as the means ± standard deviation,
n=3. ***P<0.001, **P<0.01 and *P<0.05.
###P<0.001 vs. non-WSNNP-treated cells on day 7.
3-MA, 3-methyladenine; ALP, alkaline phosphatase; Con, control;
RUNX2, runt-related transcription factor 2; SPP1, secreted
phosphoprotein1; WSNNP, water-soluble nano-pearl powder. |
WSNNP stimulates MC3T3-E1 cell
autophagy via the MEK/ERK signaling pathway
The present study also investigated whether WSNNP
may stimulate autophagy via the MEK/ERK signaling pathway. ERK/MEK
is a known activator of autophagy (17,18).
The present study demonstrated that WSNNP significantly upregulated
the phosphorylation levels of ERK and MEK, and inhibited the
phosphorylation of mTOR and p70S6K compared with in the control
group (Fig. 4A and B). These
results suggested that WSNNP may stimulate autophagy via the
MEK/ERK signaling pathway. To confirm this, the MEK signaling
inhibitor, U0126, was employed to inhibit ERK signaling. Autophagy
levels were evaluated using western blotting and
immunofluorescence, and the potential of osteoblast differentiation
was analyzed via western blotting and ALP activity assays. The
results of the present study revealed that U0126 significantly
diminished WSNNP-mediated autophagy, which was determined by the
decrease in LC3II/I, Beclin1 and ATG7 expression levels (Fig. 4C and D). In addition, inhibition of
ERK signaling by U0126 significantly attenuated the expression of
collagen I, SPP1 and RUNX2, and ALP activity, which were
upregulated by WSNNP (Fig. 4E and
F). These results revealed that WSNNP may promote MC3T3-E1 cell
differentiation by enhancing autophagy via the MEK/ERK/mTOR
signaling pathway.
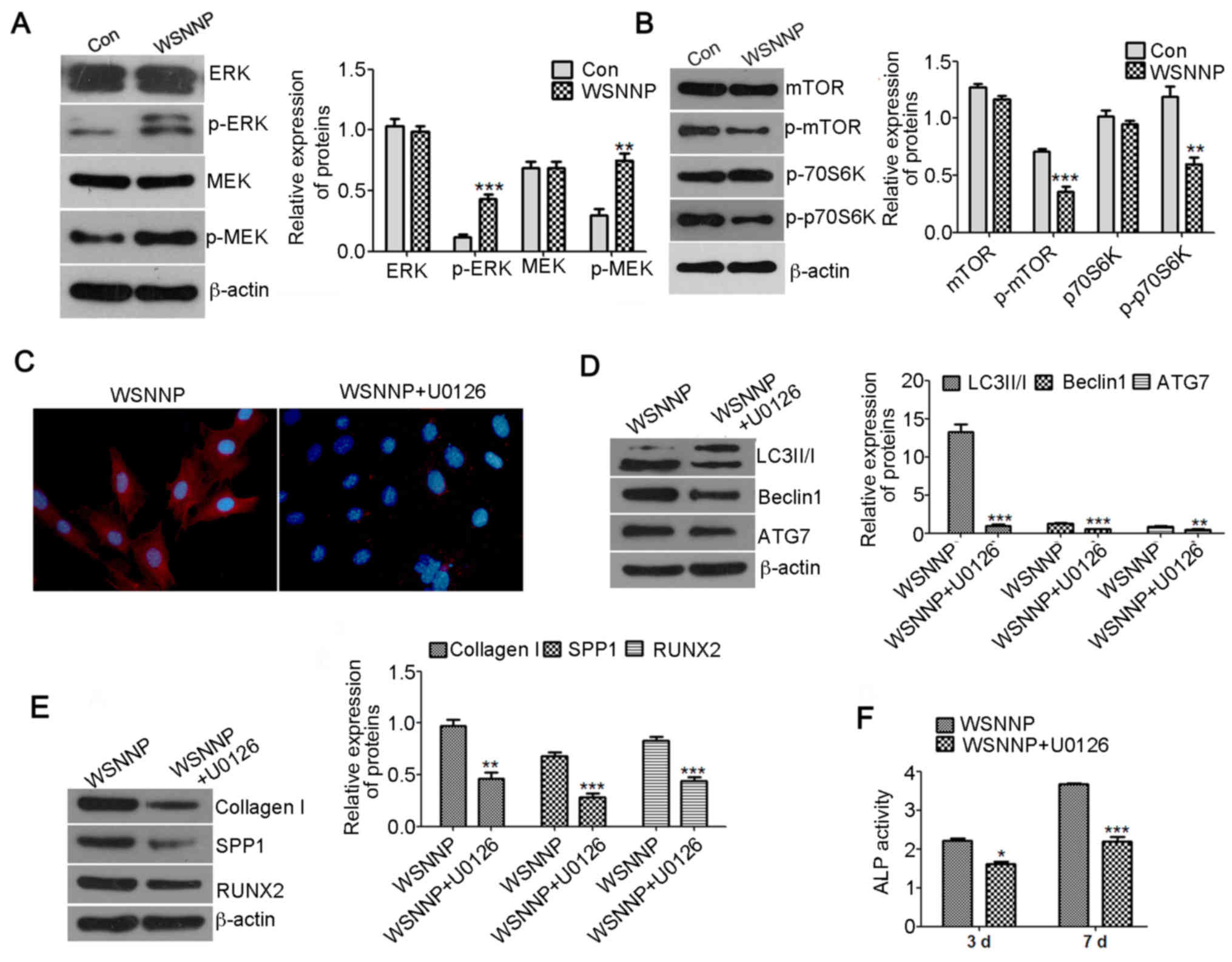 | Figure 4.U0126 reverses WSNNP-mediated
autophagy and differentiation. (A) Expression levels of ERK, p-ERK,
MEK and p-MEK were detected by western blotting following treatment
with 25 µg/ml WSNNP for 48 h. β-actin was used as a loading
control. (B) mTOR, p-mTOR, p70S6K and p-p70S6K levels were measured
by western blotting following treatment with 25 µg/ml WSNNP for 48
h. β-actin was used as a loading control. (C) Expression levels of
Beclin1 (red) were assessed by immunofluorescence following
treatment of MC3T3-E1 cells with 25 µg/ml WSNNP, in the presence or
absence of 15 µM U0126, for 48 h. Blue staining indicates nuclei.
Magnification, ×400. (D) LC3II/I, Beclin1 and ATG7 expression
levels were assessed by western blotting following treatment with
25 µg/ml WSNNP, in the presence or absence of 15 µM U0126, for 48
h. (E) Expression levels of Collagen I, SPP1 and RUNX2 were
detected by western blotting following treatment with 25 µg/ml
WSNNP, in the presence or absence of 15 µM, U0126 for 48 h. (F) ALP
activity was assessed by ELISA assay following treatment with 25
µg/ml WSNNP, in the presence or absence of 15 µM U0126, for 3 and 7
days. Data are presented as the means ± standard deviation, n=3.
***P<0.001, **P<0.01, and *P<0.05 vs. non-U0126-treated
group. ALP, alkaline phosphatase; Con, control; ERK, extracellular
signal-regulated kinase; LC3, microtubule-associated light chain 3;
MEK, mitogen-activated protein kinase kinase; mTOR, mammalian
target of rapamycin; p, phosphorylated; p70S6K, p70 S6 kinase;
RUNX2, runt-related transcription factor 2; SPP1, secreted
phosphoprotein1; WSNNP, water-soluble nano-pearl powder. |
Discussion
In the present study, WSNNP promoted osteoblast
differentiation in a time- and dose-dependent manner. Further
investigation revealed that WSNNP contributed to osteoblast
differentiation by enhancing autophagy via the MEK/ERK signaling
pathway. To the best of our knowledge, the present study is the
first to report that overactivated autophagy may be a potential
mechanism underlying the effects of WSNNP on osteoblast
differentiation.
Nacre has been demonstrated to promote osteoblast
differentiation and bone formation in vitro and in
vivo (19,20). Lamghari et al (12) demonstrated that 12 weeks following
the use of nacre powder for treating loss of vertebral bone in
sheep, newly matured bone trabeculae occupied the experimental
cavity, which indicated bone formation. The water-soluble extract
of nacre also resulted in ALP enhancement in bone marrow cells
(21). A series of studies
revealed that peptides (14–57 Da) of nacre may serve a role in
oxidative activity and as proteinase inhibitors (22–25).
However, Duplat et al (26)
suggested that the small molecules of water soluble nacre may
decrease bone resorption by inhibiting osteoclast cathepsin K.
Conversely, nacre molecules exposed to MC3T3-E1 cells were revealed
to significantly upregulate the mRNA expression levels of
collagenI, RUNX2 and osteopontin (27), indicating the potential use for
in vivo bone repair. Laothumthut et al (28) demonstrated that WSM promotes the
proliferation of human dental pulp cells. The results of these
studies are consistent with those of the present study, in which
the effects of WSNNP on the differentiation of MC3T3-E1 cells were
investigated. More protein may be extracted from WSNNP than
micro-pearl powder and significantly increase cell differentiation
compared with the water solution of micro-pearl powder (15,607 vs.
985 µg/ml, respectively) (data not shown). Additionally, the
present study demonstrated that WSNNP may stimulate MC3T3-E1 cell
differentiation and enhance the protein expression levels of
collagen I, SPP1 and RUNX2, and ALP activity; however, the
mechanism by which WSNNP stimulates MC3T3-E1 cell differentiation
requires further investigation.
Autophagy is a mechanism by which cells are
protected and regulated to allow the removal of excess organelles,
in order to maintain stability of the cell environment. Numerous
factors, including inflammation, immune response, medication and
external stimuli, may lead to cell autophagy (29–31).
It has previously been reported that autophagy may serve a latent
role in the pathogenesis of osteogenesis (32), and activation of autophagy may
inhibit cadmium-induced osteoblast apoptosis (33). Nollet et al (34) revealed that autophagy is activated
during bone mineralization and that the inhibition of autophagy by
interfering with relative gene expression associated with autophagy
in mice may reduce bone mineralization. In addition, autophagy
serves an important role in osteoblast differentiation.
Insulin-like growth factor-1 and insulin-like growth factor-binding
protein 2 stimulate 5′adenosine monophosphate-activated protein
kinase and activate autophagy, which are important factors for
osteoblast differentiation (35).
Notably, fibroblast growth factor (FGF)18, via FGF receptor 4 and
c-Jun N-terminal kinase-dependent activation of autophagy, promotes
bone growth (36). The results of
the present study demonstrated that WSNNP treatment enhanced
autophagy. However, co-treatment of MC3T3-E1 cells with WSNNP and
3-MA downregulated the protein expression levels of collagen I,
SPP1 and RUNX2, and ALP activity, compared with WSNNP treatment
alone. The present study revealed that WSNNP stimulated osteoblast
differentiation by enhancing autophagy and that autophagy may serve
an important role in MC3T3-E1 cell differentiation.
The ERK/MEK signaling pathway is known to be
involved in autophagy; one study reported the presence of the
autophagy marker Beclin1 and the conversion of LC3-I to LC3-II,
activated by ERK-dependent autophagic activity (37). Liu et al (38) also demonstrated that autophagy may
be evoked by lithium chloride to promote spinal cord injury through
the ERK-dependent pathway. Wang et al (39) demonstrated that the MEK inhibitor
U0126 attenuates ischemia/reperfusion-induced apoptosis and
autophagy in the myocardium via the ERK/MEK/early growth response
protein 1 pathway. The present study demonstrated that WSNNP may
activate the phosphorylation of ERK and MEK, and that U0126 may
reverse WSNNP-induced MC3T3-E1 cell autophagy and differentiation.
Therefore, WSNNP may promote osteoblast differentiation by
activating ERK-associated autophagy. However, only the
WSNNP-affected signaling pathway associated with osteoblast
differentiation was investigated in the present study. There may be
crosstalk between WSNNP and MEK; however, further investigation is
required.
In conclusion, in the present study, a novel
mechanism by which WSNPP promotes osteoblast differentiation by
regulating autophagy via the MEK/ERK signaling pathway was
demonstrated. These findings may provide insight for optimization
of biological materials employed for bone implants.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key Research
and Development Projects of Hainan Province (grant no. ZDYF2016018)
and Natural Science Foundation of Hainan Province (grant nos.
814378 and 20168313).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
Study design by YC and PX, statistical analysis by
WZ, data interpretation by HF and manuscript writing and revision
by PX.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maruotti N, Corrado A and Cantatore FP:
Osteoblast role in osteoarthritis pathogenesis. J Cell Physiol.
232:2957–2963. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Terrier Saint-Pastou C and Gasque P: Bone
responses in health and infectious diseases: A focus on
osteoblasts. J Infect. 75:281–292. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dallas SL, Prideaux M and Bonewald LF: The
osteocyte: An endocrine cell … and more. Endocr Rev. 34:658–690.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Insua A, Monje A, Wang HL and Miron RJ:
Basis of bone metabolism around dental implants during
osseointegration and peri-implant bone loss. J Biomed Mater Res A.
105:2075–2089. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jazayeri HE, Tahriri M, Razavi M, Khoshroo
K, Fahimipour F, Dashtimoghadam E, Almeida L and Tayebi L: A
current overview of materials and strategies for potential use in
maxillofacial tissue regeneration. Mater Sci Eng C Mater Biol Appl.
70:913–929. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Uzeda MJ, de Brito Resende RF, Sartoretto
SC, Alves ATNN, Granjeiro JM and Calasans-Maia MD: Randomized
clinical trial for the biological evaluation of two nanostructured
biphasic calcium phosphate biomaterials as a bone substitute. Clin
Implant Dent Relat Res. 19:802–811. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Flausse A, Henrionnet C, Dossot M, Dumas
D, Hupont S, Pinzano A, Mainard D, Galois L, Magdalou J, Lopez E,
et al: Osteogenic differentiation of human bone marrow mesenchymal
stem cells in hydrogel containing nacre powder. J Biomed Mater Res
A. 101:3211–3218. 2013.PubMed/NCBI
|
|
8
|
Pilliar RM, Filiaggi MJ, Wells JD, Grynpas
MD and Kandel RA: Porous calcium polyphosphate scaffolds for bone
substitute applications-in vitro characterization. Biomaterials.
22:963–972. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lopez E, Le Faou A, Borzeix S and Berland
S: Stimulation of rat cutaneous fibroblasts and their synthetic
activity by implants of powdered nacre (mother of pearl). Tissue
Cell. 32:95–101. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shen Y, Zhu J, Zhang H and Zhao F: In
vitro osteogenetic activity of pearl. Biomaterials. 27:281–287.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mouries LP, Almeida MJ, Milet C, Berland S
and Lopez E: Bioactivity of nacre water-soluble organic matrix from
the bivalve mollusk Pinctada maxima in three mammalian cell types:
Fibroblasts, bone marrow stromal cells and osteoblasts. Comp
Biochem Physiol B Biochem Mol Biol. 132:217–229. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lamghari M, Almeida MJ, Berland S, Huet H,
Laurent A, Milet C and Lopez E: Stimulation of bone marrow cells
and bone formation by nacre: In vivo and in vitro studies. Bone. 25
2 Suppl:91S–94S. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Asvanund P, Chunhabundit P and
Suddhasthira T: Potential induction of bone regeneration by nacre:
An in vitro study. Implant Dent. 20:32–39. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chaturvedi R, Singha PK and Dey S: Water
soluble bioactives of nacre mediate antioxidant activity and
osteoblast differentiation. PLoS One. 8:e845842013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hesse E, Hefferan TE, Tarara JE, Haasper
C, Meller R, Krettek C, Lu L and Yaszemski MJ: Collagen type I
hydrogel allows migration, proliferation, and osteogenic
differentiation of rat bone marrow stromal cells. J Biomed Mater
Res A. 94:442–449. 2010.PubMed/NCBI
|
|
16
|
Leboy PS: Regulating bone growth and
development with bone morphogenetic proteins. Ann N Y Acad Sci.
1068:14–18. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Whiteman MW, Lian H, Wang G, Singh
A, Huang D and Denmark T: A non-canonical MEK/ERK signaling pathway
regulates autophagy via regulating Beclin 1. J Biol Chem.
284:21412–21424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cagnol S and Chambard JC: ERK and cell
death: Mechanisms of ERK-induced cell death-apoptosis, autophagy
and senescence. FEBS J. 277:2–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X, Liu S, Xie L, Zhang R and Wang Z:
Pinctada fucata mantle gene 3 (PFMG3) promotes differentiation in
mouse osteoblasts (MC3T3-E1). Comp Biochem Physiol B Biochem Mol
Biol. 158:173–180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y, Huang Q and Feng Q: 3D scaffold of
PLLA/pearl and PLLA/nacre powder for bone regeneration. Biomed
Mater. 8:650012013. View Article : Google Scholar
|
|
21
|
Almeida MJ, Milet C, Peduzzi J, Pereira L,
Haigle J, Barthelemy M and Lopez E: Effect of water-soluble matrix
fraction extracted from the nacre of Pinctada maxima on the
alkaline phosphatase activity of cultured fibroblasts. J Exp Zool.
288:327–334. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bedouet L, Marie A, Dubost L, Péduzzi J,
Duplat D, Berland S, Puisségur M, Boulzaguet H, Rousseau M, Milet C
and Lopez E: Proteomics analysis of the nacre soluble and insoluble
proteins from the oyster Pinctada margaritifera. Mar Biotechnol
(NY). 9:638–649. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bédouet L, Rusconi F, Rousseau M, Duplat
D, Marie A, Dubost L, Le Ny K, Berland S, Péduzzi J and Lopez E:
Identification of low molecular weight molecules as new components
of the nacre organic matrix. Comp Biochem Physiol B Biochem Mol
Biol. 144:532–543. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bedouet L, Duplat D, Marie A, Dubost L,
Berland S, Rousseau M, Milet C and Lopez E: Heterogeneity of
proteinase inhibitors in the water-soluble organic matrix from the
oyster nacre. Mar Biotechnol (NY). 9:437–449. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oliveira DV, Silva TS, Cordeiro OD, Cavaco
SI and Simes DC: Identification of proteins with potential
osteogenic activity present in the water-soluble matrix proteins
from Crassostrea gigas nacre using a proteomic approach.
ScientificWorldJournal. 2012:7659092012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duplat D, Gallet M, Berland S, Marie A,
Dubost L, Rousseau M, Kamel S, Milet C, Brazier M, Lopez E and
Bédouet L: The effect of molecules in mother-of-pearl on the
decrease in bone resorption through the inhibition of osteoclast
cathepsin K. Biomaterials. 28:4769–4778. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rousseau M, Boulzaguet H, Biagianti J,
Duplat D, Milet C, Lopez E and Bédouet L: Low molecular weight
molecules of oyster nacre induce mineralization of the MC3T3-E1
cells. J Biomed Mater Res A. 85:487–497. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Laothumthut T, Jantarat J, Paemanee A,
Roytrakul S and Chunhabundit P: Shotgun proteomics analysis of
proliferating STRO-1-positive human dental pulp cell after exposure
to nacreous water-soluble matrix. Clin Oral Investig. 19:261–270.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
De Nunzio C, Giglio S, Stoppacciaro A,
Gacci M, Cirombella R, Luciani E, Tubaro A and Vecchione A:
Autophagy deactivation is associated with severe prostatic
inflammation in patients with lower urinary tract symptoms and
benign prostatic hyperplasia. Oncotarget. 8:50904–50910. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takashi O and Matsu A: Autophagy. Arerugi.
66:1018–1019. 2017.(In Japanese). PubMed/NCBI
|
|
31
|
Shetty R, Sharma A, Pahuja N, Chevour P,
Padmajan N, Dhamodaran K, Jayadev C, M M A, Nuijts R, Ghosh A and
Nallathambi J: Oxidative stress induces dysregulated autophagy in
corneal epithelium of keratoconus patients. PLoS One.
12:e1846282017. View Article : Google Scholar
|
|
32
|
Piemontese M, Onal M, Xiong J, Han L,
Thostenson JD, Almeida M and O'Brien CA: Low bone mass and changes
in the osteocyte network in mice lacking autophagy in the
osteoblast lineage. Sci Rep. 6:242622016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu W, Dai N, Wang Y, Xu C, Zhao H, Xia P,
Gu J, Liu X, Bian J, Yuan Y, et al: Role of autophagy in
cadmium-induced apoptosis of primary rat osteoblasts. Sci Rep.
6:204042016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nollet M, Santucci-Darmanin S, Breuil V,
Al-Sahlanee R, Cros C, Topi M, Momier D, Samson M, Pagnotta S,
Cailleteau L, et al: Autophagy in osteoblasts is involved in
mineralization and bone homeostasis. Autophagy. 10:1965–1977. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xi G, Rosen CJ and Clemmons DR: IGF-I and
IGFBP-2 stimulate AMPK activation and autophagy, which are required
for osteoblast differentiation. Endocrinology. 157:268–281. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cinque L, Forrester A, Bartolomeo R,
Svelto M, Venditti R, Montefusco S, Polishchuk E, Nusco E, Rossi A,
Medina DL, et al: FGF signalling regulates bone growth through
autophagy. Nature. 528:272–275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheng Y, Qiu F, Tashiro S, Onodera S and
Ikejima T: ERK and JNK mediate TNFalpha-induced p53 activation in
apoptotic and autophagic L929 cell death. Biochem Biophys Res
Commun. 376:483–488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu P, Zhang Z, Wang Q, Guo R and Mei W:
Lithium chloride facilitates autophagy following spinal cord injury
via ERK-dependent pathway. Neurotox Res. 32:535–543. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang A, Zhang H, Liang Z, Xu K, Qiu W,
Tian Y, Guo H, Jia J, Xing E, Chen R, et al: U0126 attenuates
ischemia/reperfusion-induced apoptosis and autophagy in myocardium
through MEK/ERK/EGR-1 pathway. Eur J Pharmacol. 788:280–285. 2016.
View Article : Google Scholar : PubMed/NCBI
|


















