Introduction
Breast cancer is a major health issue affecting
women worldwide, with ~1.4 million novel diagnoses and >450,000
mortalities occurring annually (1). Overexpression of human epidermal
growth factor receptor 2 (HER2) has been reported in ~20% of
patients with breast cancer and is associated with aggressive
clinical behavior and poor prognosis (2). HER2-targeting agents, including the
anti-HER2 monoclonal antibody trastuzumab, have been demonstrated
to improve the overall survival rate of patients with HER2-positive
breast cancer (3). However,
resistance to trastuzumab may eventually develop and, in certain
cases, relapse may occur following adjuvant therapy.
Lapatinib is a small-molecule tyrosine kinase
inhibitor that suppresses the expression of epidermal growth factor
receptor (EGFR) and HER2, as well as the activity of the downstream
phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/Akt pathway,
which have important roles in cell proliferation and survival
(4). Administration of lapatinib
in combination with the oral fluoropyrimidine capecitabine has been
previously demonstrated to be an effective treatment option for
trastuzumab-resistant HER2-positive metastatic breast cancer
(5). This combination treatment
was approved by the United States Food and Drug Administration and
European Medicines Agency based on evidence from a phase III study
that demonstrated an increased time to progression (TTP) in
patients treated with lapatinib in combination with capecitabine,
as compared with capecitabine alone (6). Capecitabine was designed to
preferentially generate 5-fluorouracil (5-FU) in tumor tissue.
Correlations have been previously demonstrated between decreased
thymidylate synthase (TS) expression and a higher response rate to
5-FU-based chemotherapy (7,8). In
gastric cancer, lapatinib induces the downregulation of E2F
transcription factor 1 (E2F1), which enhances the transcription of
the TS gene (9). Therefore, we
hypothesized that lapatinib may downregulate TS expression in
HER2-positive breast cancer cells via downregulation of E2F1
expression.
The efficacy of combination therapy involving
lapatinib and other cytotoxic agents in HER2-positive breast cancer
remains undetermined. E2F1 regulates the expression of
ribonucleotide reductase catalytic M1 subunit (RRM1), an important
determinant of gemcitabine resistance (10), and DNA topoisomerase II-α (TOP2A),
a molecular target of anthracyclines (11). Therefore, we further hypothesized
that lapatinib may exhibit a synergistic antitumor effect in
combination with not only capecitabine, but also gemcitabine, and
it may also exhibit an antagonistic effect when used in combination
with anthracyclines.
In the present study, the molecular mechanisms
associated with the synergistic antitumor effects observed
following treatment with lapatinib and capecitabine were
investigated, as well as the efficacy of interactions between
lapatinib and other cytotoxic agents for the treatment of patients
with breast cancer exhibiting overexpression of cellular HER2.
Materials and methods
Cell lines and cell culture
The SKBR3 HER2-overexpressing human breast cancer
cell line and the T47D human breast cancer cell line with moderate
expression of HER2 (12,13) were obtained from the American Type
Culture Collection (Manassas, VA, USA). SKBR3 cells were cultured
in Dulbecco's modified Eagle's medium supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific Inc., Waltham,
MA, USA) and 5% 0.1 mM penicillin-streptomycin. T47D cells were
cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific Inc.)
supplemented with 10% FBS and 5% 0.1 mM penicillin-streptomycin.
All cell lines were incubated at 37°C in 95% humidified atmosphere
containing 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
SKBR3 and T47D cells (3×105 cells/well)
were seeded in 6-well plates and were serum-starved at 37°C for 24
h. Cells were treated with epidermal growth factor (EGF; 100 ng/ml;
Gibco; Thermo Fisher Scientific Inc.) at 37°C for 30 min and
subsequently treated with dimethyl sulfoxide (DMSO; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) vehicle control, LY294002 (10 µM;
Sigma-Aldrich; Merck KGaA), wortmannin (200 nM; Sigma-Aldrich;
Merck KGaA) or lapatinib (1 µM; Selleck Chemicals, Houston, TX,
USA) at 37°C for 48 h. Total RNA was extracted from the cells using
an RNeasy Plus Mini kit (Qiagen, Inc., Valencia, CA, USA) and
first-strand cDNA was synthesized using the PrimeScript RT reagent
kit (Takara Bio, Inc., Otsu, Japan) to investigate the expression
levels of genes of interest. The protocol for cDNA synthesis was
37°C for 15 min, followed by 85°C for 5 sec. qPCR analysis was
performed using Power SYBR Green Master Mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.) on a 7300 Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The following
program was run: Predenaturing for 10 min at 95°C, amplification
for 40 cycles (15 sec of denaturation at 95°C, 1 min of
annealing/extension at 60°C). GAPDH was used as the internal
control and the 2−∆∆Cq method (14) was used to determine protein
expression levels. The sequences of all primers used in the present
study were as follows: TOP2A, 5′-ACCAGCACATCAAAGGAAGC-3′ (forward)
and 5′-AATCCTCAGGAAGCCCAAGT-3′ (reverse); TS,
5′-GCCTCGGTGTGCCTTTCA-3′ (forward) and 5′-CCCGTGATGTGCGCAAT-3′
(reverse); RRM1, 5′-ACTAAGCACCCTGACTATGCTATCC-3′ (forward) and
5′-CTTCCATCACATCACTGAACACTTT-3′ (reverse); E2F1,
5′-CAAGAAGTCCAAGAACCACATCC-3′ (forward) and
5′-AGATATTCATCAGGTGGTCCAGC-3′ (reverse); and GAPDH,
ATCATCCCTGCCTCTACTGG-3′ (forward) and 5′-TTTCTAGACGGCAGGTCAGGT-3′
(reverse). The experiment was performed three times.
Western blot analysis
SKBR3 and T47D cells were serum-starved at 37°C for
24 h prior to treatment with EGF (100 ng/ml) at 37°C for 30 min.
Cells were subsequently washed and treated with DMSO vehicle
control, LY294002 (40 µM), wortmannin (500 nM) or lapatinib (1 µM)
at 37°C for 30 min, 24 or 48 h. Cells were lysed using a ReadyPrep
Protein Extraction kit (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) supplemented with a protease inhibitor cocktail (Roche
Diagnostics, Basel, Switzerland) and phosphatase inhibitor cocktail
(Roche Diagnostics), according to the manufacturer's protocol.
Protein samples were quantified by the Bradford method and total
protein (60 µg per lane) was fractionated on 4–15%, 10-well comb
Mini-PROTEAN TGX gels (Bio-Rad Laboratories, Inc.) and
electrophoretically transferred onto TransBlot Turbo Mini
polyvinylidene fluoride membranes (Bio-Rad Laboratories, Inc.).
Membranes were subsequently blocked with Amersham ECL Blocking
Agent (GE Healthcare Life Sciences, Little Chalfont, UK) in
Tris-buffered saline and 0.1% Tween for 30–60 min at room
temperature. Following this, membranes were exposed to the
following primary antibodies overnight at 4°C: Anti-Akt (mouse;
1:800; cat. no. 2920S; Cell Signaling Technology, Inc., Danvers,
MA, USA), anti-phosphorylated-Akt (p-Akt; rabbit; 1:800; cat. no.
4060S; Cell Signaling Technology, Inc.), anti-E2F1 (mouse; 1:1,000;
cat. no. sc-251; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-lamin (mouse; 1:800; cat. no. ab8980; Abcam, Cambridge, UK)
and anti-GAPDH (mouse; 1:2,000; cat. no. sc-47724; Santa Cruz
Biotechnology, Inc.). Membranes were subsequently exposed to the
horseradish peroxidase (HRP)-conjugated anti-rabbit (1:2,000; cat.
no. NA934V; GE Healthcare Life Sciences) and anti-mouse (1:1,000;
cat. no. NA931V; GE Healthcare Life Sciences) secondary antibodies
at room temperature for 1 h. Proteins were visualized using
Luminata Forte Western HRP substrate (EMD Millipore, Billerica, MA,
USA).
MTT cell proliferation assay
An MTT assay was performed to investigate cell
proliferation. SKBR3 and T47D cells were plated in 96-well plates
at a density of 1×104 cells/well and cultured at 37°C
for 24 h. Following this, cells were treated with various
concentrations of lapatinib, 5-FU (Sigma-Aldrich; Merck KGaA),
gemcitabine (Sigma-Aldrich; Merck KGaA) and epirubicin
(Sigma-Aldrich; Merck KGaA) at 37°C for 5 days. The concentrations
of each drug was as follows: 5-FU and lapatinib for T47D were 0.1,
0.2, 1, 2, 10 and 20 µM, and 0.05, 0.1, 0.5, 1, 5 and 10 µM,
respectively; 5-FU and lapatinib for SKBR3 were 0.5, 1, 5, 10, 50
and 100 µM, and 0.25, 0.5, 2.5, 5, 25 and 50 µM, respectively;
gemcitabine and lapatinib for T47D were 0.01, 0.1, 0.5, 1, 5 and 10
µM, and 0.005, 0.05, 0.25, 0.5, 2.5 and 5 µM, respectively;
gemcitabine and lapatinib for SKBR3 were 0.05, 0.1, 1, 5, 10 and
100 µM, and 0.025, 0.05, 0.5, 2.5, 5 and 50 µM, respectively;
epirubicin and lapatinib for T47D were 0.01, 0.1, 0.5, 1, 5 and 10
µM; epirubicin and lapatinib for SKBR3 were 0.005, 0.025, 0.1, 1,
10 and 100 µM. MTT (10 µl) was subsequently added to each well and
the cells were incubated at 37°C for a further 3 h. Following
incubation, DMSO (>99%; 130 µl) was added to each well and the
absorbance at 540 nm was determined using a SUNRISE Rainbow RC-R
(Tecan Group, Ltd., Mannedorf, Switzerland). The antitumor effect
of the different combinations of lapatinib with various cytotoxic
drugs (5-FU, gemcitabine and epirubicin) was analyzed according to
a previously described method (15). Interactions between two drugs
(lapatinib and 5-FU, lapatinib and gemcitabine, and lapatinib and
epirubicin) were estimated via the combination index (CI) using
CalcuSyn software (version 2; Biosoft, Cambridge, UK). CI <1, CI
=1 and CI >1 scores revealed synergistic, additive and
antagonistic effects, respectively.
Statistical analysis
Statistical analysis was performed using SPSS
statistical software (version 19; IBM Corp., Armonk, NY, USA).
RT-qPCR was performed in triplicate and the mRNA expression (mean ±
standard deviation) was determined using one-way analysis of
variance followed by Games Howell post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
E2F1, TS, TOP2A and RRM1 expression
levels are attenuated following treatment with lapatinib and PI3K
inhibitors
To investigate the molecular mechanisms associated
with interactions between lapatinib and capecitabine, the effect of
treatment with lapatinib and PI3K inhibitors on E2F1 and TS
expression in HER2-positive breast cancer cells was investigated.
The results of RT-qPCR demonstrated that treatment with lapatinib
and PI3K inhibitors decreased the mRNA expression of E2F1 and TS in
T47D and SKBR3 cells at 48 h post-treatment (Fig. 1A-C). Decreased TS expression has
been reported to be associated with a higher response rate to
5-FU-based chemotherapy (7,8), and
the E2F1 transcription factor promotes TS expression (16). Therefore, these results indicate
that downregulation of E2F1 expression by lapatinib may induce TS
downregulation, which subsequently enhances the effect of
capecitabine. Furthermore, treatment with lapatinib and PI3K
inhibitors also downregulated the expression of TOP2A (Fig. 1B and C), a molecular target of
anthracyclines, in both cell lines. In addition, treatment with
lapatinib and PI3K inhibitors suppressed RRM1 expression, an
important determinant of gemcitabine resistance, in T47D cells
(Fig. 1B). In SKBR3 cells, RRM1
downregulation was demonstrated following treatment with wortmannin
and lapatinib; however, RRM1 expression was enhanced in SKBR3 cells
following treatment with LY294002 (Fig. 1C), indicating different responses
to different PI3K inhibitors.
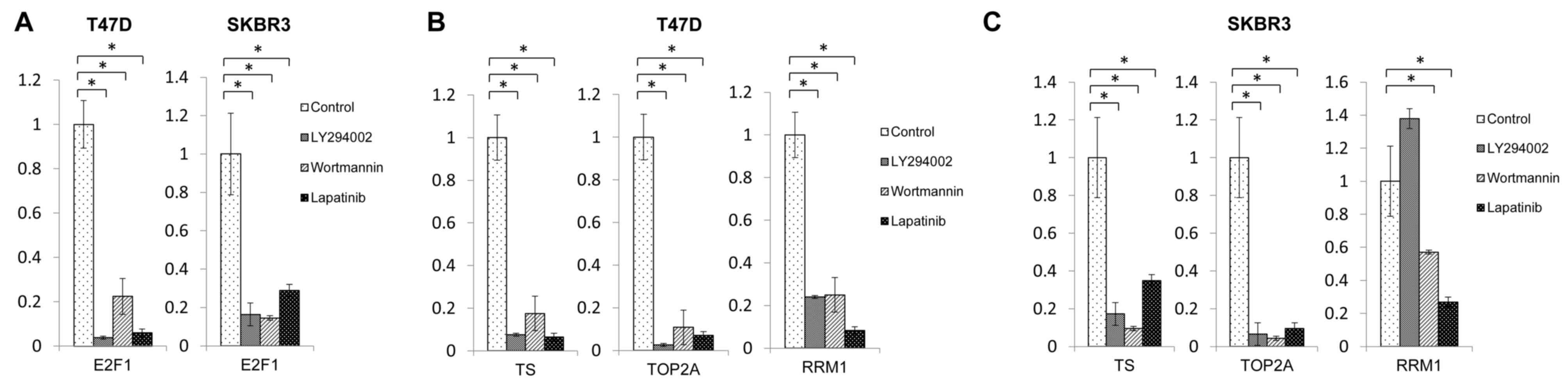 | Figure 1.Expression levels of E2F1, TS, TOP2A
and RRM1 mRNAs following treatment with lapatinib and
phosphatidylinositol-4,5-bisphosphate 3-kinase inhibitors. (A) E2F1
expression levels following treatment with lapatinib, LY294002 and
wortmannin in T47D and SKBR3 cells. TS, TOP2A and RRM1 expression
levels following treatment with lapatinib, LY294002 and wortmannin
in (B) T47D cells and (C) SKBR3 cells. *P<0.05, as indicated.
E2F1, E2F transcription factor 1; TS, thymidylate synthase; TOP2A,
DNA topoisomerase II-α; RRM1, ribonucleotide reductase M1
subunit. |
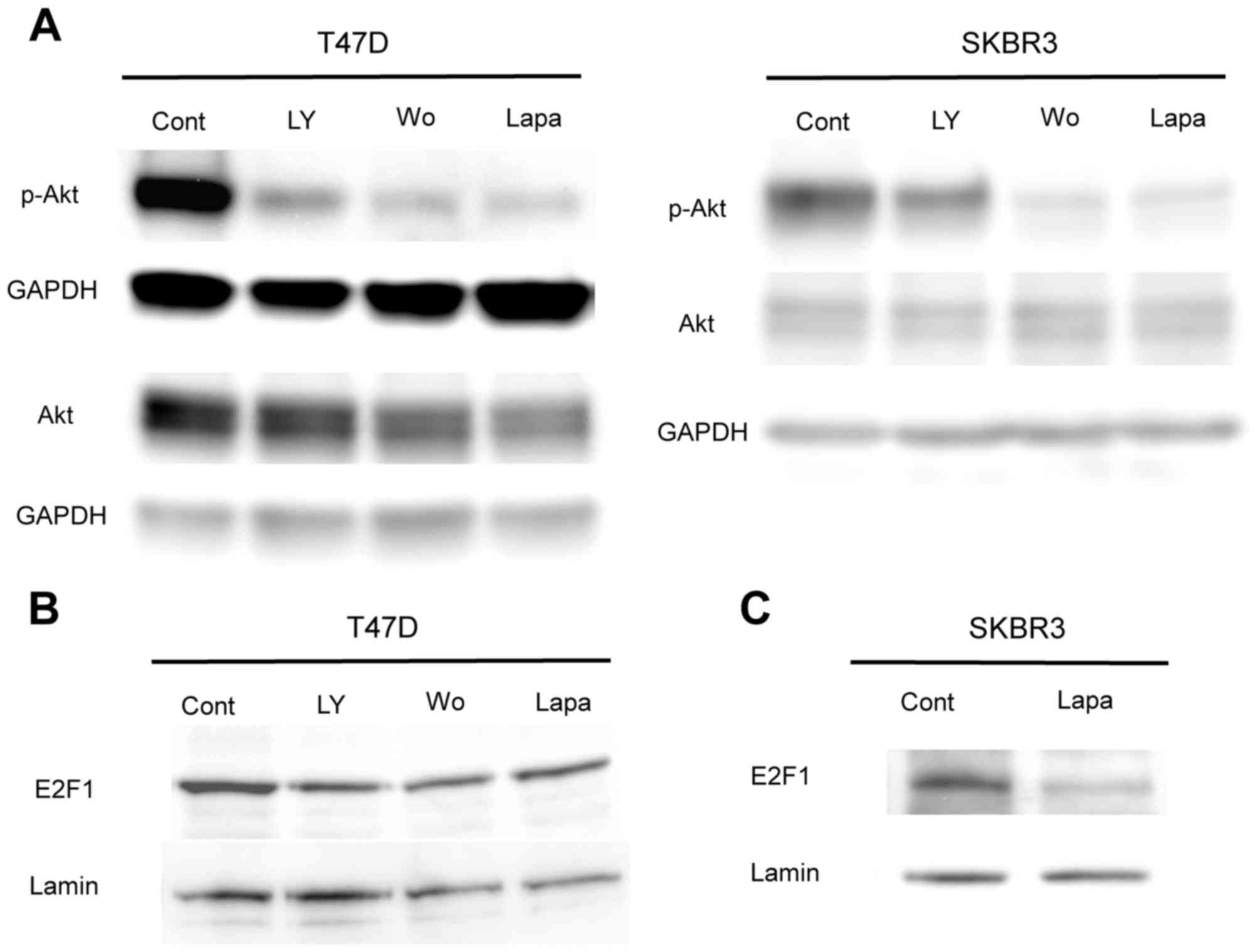
Treatment with lapatinib and PI3K
inhibitors downregulates E2F1 protein expression via the PI3K/Akt
pathway
To investigate the effects of lapatinib treatment on
the PI3K/Akt signaling pathway, levels of p-Akt and E2F1 were
investigated using western blot analysis. Following treatment with
PI3K inhibitors and lapatinib for 30 min, levels of p-Akt were
revealed to be inhibited in both T47D and SKBR3 cells (Fig. 2A). Western blot analysis also
revealed that E2F1 protein expression was downregulated following
treatment with PI3K inhibitors and lapatinib for 24 h in T47D cells
(Fig. 2B). In SKBR3 cells, it was
revealed that E2F1 protein levels were suppressed by treatment with
lapatinib for 48 h (Fig. 2C);
however, these levels were unaffected by treatment with PI3K
inhibitors or lapatinib for 24 h (data not shown). Following 48 h
of treatment with PI3K inhibitors, it was not possible to determine
the expression levels of E2F1 in SKBR3 cells, as the majority of
the cells were died unexpectedly (Fig.
2B). These results indicate that E2F1 downregulation following
treatment with lapatinib may be a result of inhibition of the
PI3K/Akt signaling pathway.
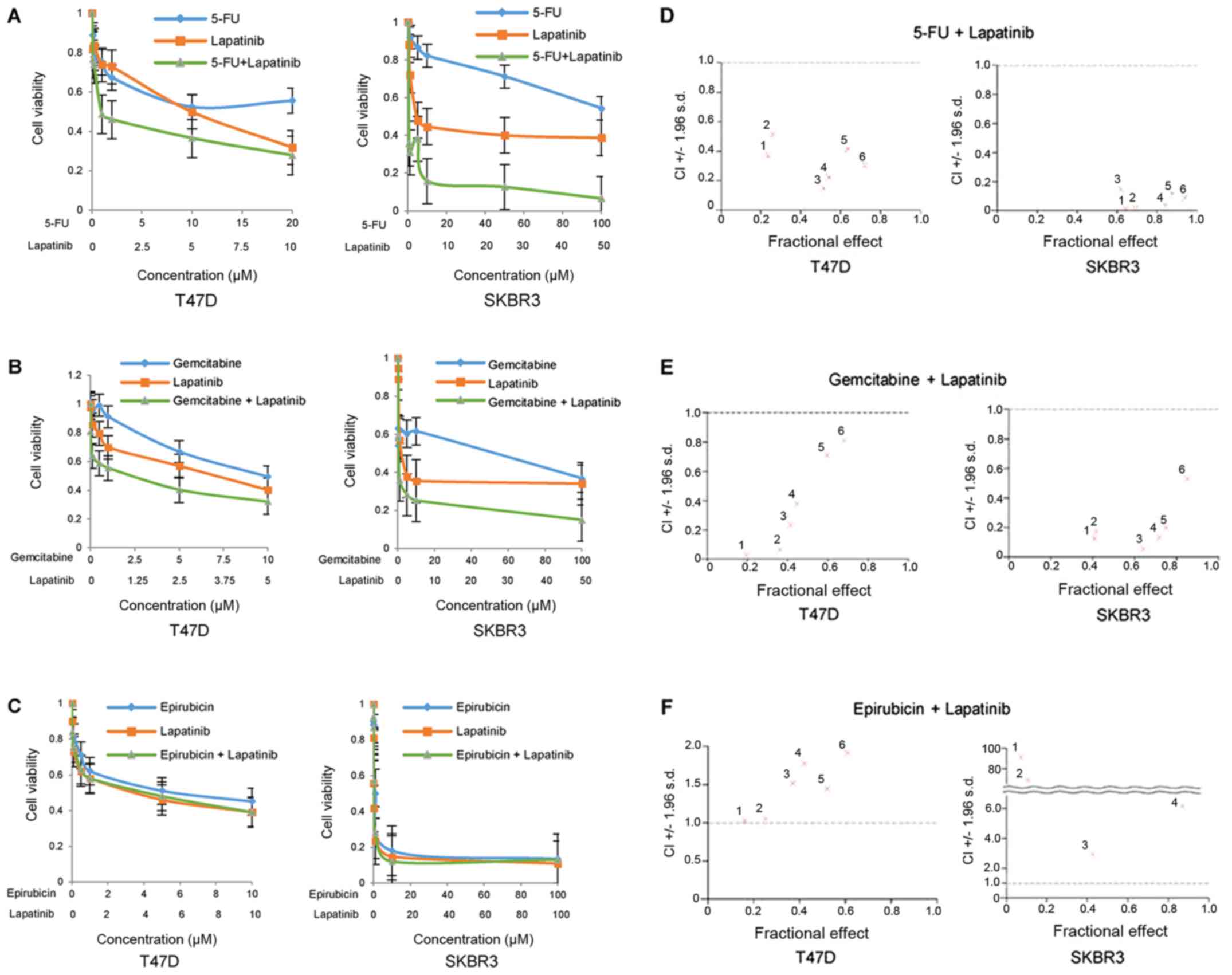
Antitumor effect of combination
therapy using lapatinib and cytotoxic agents
An MTT assay was performed to investigate the cell
proliferative effects of combination therapy involving lapatinib
and various cytotoxic agents (5-FU, gemcitabine and epirubicin) on
HER2-positive breast cancer cells. Capecitabine is designed to
preferentially generate 5-FU in tumor tissue. Therefore, 5-FU was
used instead of capecitabine for the MTT assay. The results of the
MTT assay revealed that cell viability was markedly decreased in
T47D and SKBR3 cells following treatment with 5-FU or lapatinib
alone at various concentrations, and the inhibitory effect was
enhanced when 5-FU was administered in combination with lapatinib
in a dose-dependent manner (Fig.
3A). Similar inhibitory effects were also observed in both cell
lines following treatment with gemcitabine and lapatinib (Fig. 3B), whereas combinatory treatment
with epirubicin (an anthracycline drug) and lapatinib did not
markedly suppress cell proliferation compared with that observed
following treatment with epirubicin or lapatinib alone (Fig. 3C). In addition, the interaction
between two drugs based on CI values was investigated. A
synergistic antitumor effect (CI <1.0) on cell proliferation was
demonstrated when T47D and SKBR3 cells were treated with a
combination of 5-FU and lapatinib at each concentration (Fig. 3D). A similar synergistic effect
involving combined treatment of gemcitabine and lapatinib was also
observed in both cell lines (Fig.
3E). By contrast, the combination of epirubicin and lapatinib
demonstrated antagonistic effects (CI >1.0) at each
concentration (Fig. 3F). These
results indicate that combination treatment with lapatinib and
either 5-FU or gemcitabine exhibited synergistic antitumor effects,
whereas lapatinib treatment in combination with epirubicin
exhibited antagonistic antitumor effects in HER2-positive breast
cancer cells.
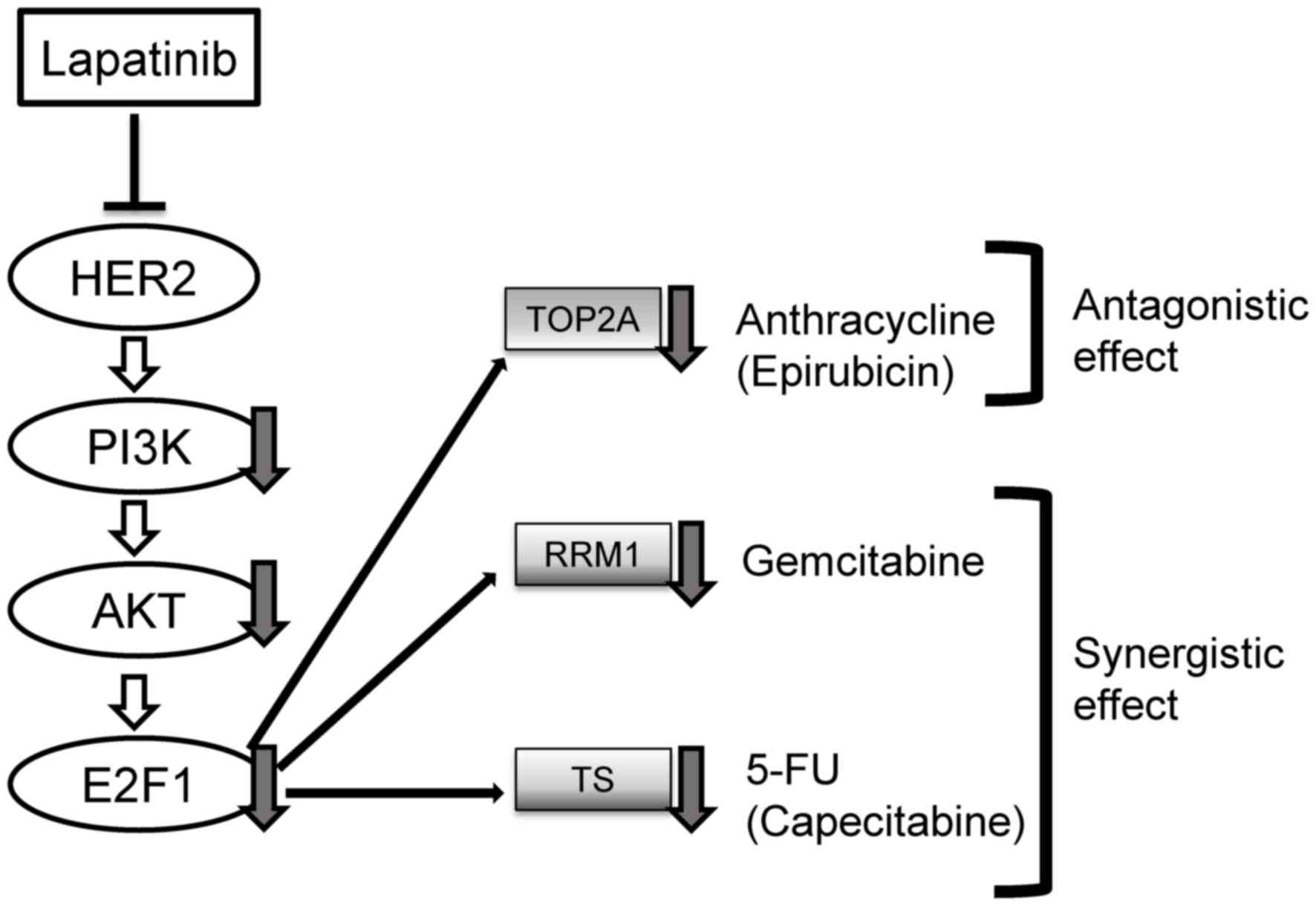
Overall, the results of the present study indicate
that downregulation of TS, RRM1 and TOP2A in HER2-positive breast
cancer cells following lapatinib treatment is attributable to E2F1
downregulation, which may be a result of inhibition of the PI3K/Akt
pathway, a process which is summarized in Fig. 4.
Discussion
The results of the present study demonstrated that
combinatory treatment with lapatinib and 5-FU, a capecitabine
metabolite, induced a synergistic antitumor effect on HER2-positive
breast cancer cells. In addition, RT-qPCR and western blot analyses
revealed that treatment of cells with lapatinib or PI3K inhibitors
downregulated E2F1 and TS expression. These results indicated that
inhibition of the PI3K/Akt signaling pathway may contribute to the
downregulation of E2F1 and TS expression by lapatinib. These
results are consistent with those reported by Tanizaki et al
(9), who demonstrated that in
gastric cancer cells exhibiting overexpression of HER2, treatment
with lapatinib downregulated TS expression via the PI3K/Akt
signaling pathway, which may be a result of downregulated E2F1
expression.
Lapatinib is a dual EGFR and HER2 tyrosine kinase
inhibitor approved by the United States Food and Drug
Administration for the treatment of patients with HER2-positive
metastatic or locally advanced breast cancer. In a phase III trial
of combined treatment with lapatinib and capecitabine vs. treatment
with capecitabine alone in patients with HER2-positive advanced
breast cancer (6), the combinatory
treatment significantly improved TTP compared with capecitabine
treatment alone (6.2 vs. 4.3 months; hazard ratio, 0.57;
P<0.001). Despite the development of novel and effective
molecular-targeted therapies, such as pertuzumab (17) and trastuzumab emtansine (18), lapatinib continues to represent an
important treatment option for patients with trastuzumab-resistant
HER2-positive metastatic breast cancer. Therefore, the results of
the present study concerning the molecular mechanism associated
with the synergistic antitumor effects of combination treatment
with lapatinib and capecitabine provide important insight into
effective options for treatment of HER2-positive breast cancer.
The efficacy of interactions between lapatinib and
other cytotoxic agents, such as gemcitabine and epirubicin, was
also investigated with regards to HER2-positive breast cancer
treatment. The results revealed that treatment with lapatinib or
PI3K inhibitors markedly downregulated the expression of RRM1, an
important determinant of gemcitabine resistance, as well as TOP2A,
a molecular target of anthracyclines such as epirubicin. Activation
of the PI3K/Akt signaling pathway has been associated with
upregulated E2F1 expression (19,20).
E2F1 is involved in the regulation of RRM1 (10) and TOP2A (11) expression. Therefore, the results of
the present study indicate that lapatinib may synergistically
interact with gemcitabine, resulting in a combinatorial
antagonistic effect with epirubicin and leading to the
downregulation of E2F1 and RRM1, or the downregulation of TOP2A,
via the PI3K/Akt pathway.
In a phase III trial, combination therapy involving
lapatinib and capecitabine frequently resulted in adverse side
effects, such as diarrhea, hand-foot syndrome, and nausea, which
were reported in 60, 49, and 44% of the patients, respectively
(6). By contrast, gemcitabine
administered to patients with metastatic breast cancer in previous
phase II trials rarely exhibited symptomatic toxicities, such as
gastrointestinal toxicity and hand-foot syndrome (21). Therefore, combination treatment
with lapatinib and gemcitabine may represent a more effective
treatment option for patients with HER2-positive metastatic breast
cancer. The results of the present study regarding epirubicin
treatment, a member of the anthracycline class of drugs, indicate
that it was ineffective in combination with lapatinib. Furthermore,
the combination of anti-HER2 therapy, including lapatinib, with
anthracycline has not been recommended in clinical practice, as it
has been revealed to be associated with high incidences of cardiac
toxicity (22).
In conclusion, the present study investigated the
molecular mechanisms associated with the combined treatment of
lapatinib and capecitabine in HER2-positive breast cancer cells,
and determined the potential efficacy of interactions between
lapatinib and gemcitabine, as well as the effect of lapatinib and
epirubicin. The results of the present study lead to the suggestion
that a clinical trial of combination chemotherapy using lapatinib
and gemcitabine for the treatment of breast cancer exhibiting an
overexpression of HER2 may be promising.
Acknowledgements
The authors would like to thank Mr. H. Okazaki, Mr.
K. Miyao and Dr Y. Nakazawa (Keio University School of Medicine,
Tokyo, Japan) for their technical support.
Funding
The current study was supported by the Ministry of
Education, Culture, Sports, Science and Technology (Tokyo, Japan)
Grant-in-Aid for Young Scientists (grant no. JP25861163).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TH, MT, HJ and YK conceived and designed the
experiments. AM and TH performed the experiments. AM and TH
analyzed the data. AM wrote the manuscript. AM, TH, MT, HJ and YK
discussed the results and revised the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Medina PJ and Goodin S: Lapatinib: A dual
inhibitor of human epidermal growth factor receptor tyrosine
kinases. Clin Ther. 30:1426–1447. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blackwell KL, Kaplan EH, Franco SX, Marcom
PK, Maleski JE, Sorensen MJ and Berger MS: A phase II, open-label,
multicenter study of GW572016 in patients with
trastuzumab-refractory metastatic breast cancer. Proc Ann Meeting
Am Soc Clin Oncol. 23:1962004.
|
|
6
|
Geyer CE, Forster J, Lindquist D, Chan S,
Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A,
Kaufman B, et al: Lapatinib plus capecitabine for HER2-positive
advanced breast cancer. N Engl J Med. 355:2733–2743. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Johnston PG, Drake JC, Trepel J and
Allegra CJ: Immunological quantitation of thymidylate synthase
using the monoclonal antibody TS 106 in 5-fluorouracil-sensitive
and -resistant human cancer cell lines. Cancer Res. 52:4306–4312.
1992.PubMed/NCBI
|
|
8
|
Johnston PG, Fisher ER, Rockette HE,
Fisher B, Wolmark N, Drake JC, Chabner BA and Allegra CJ: The role
of thymidylate synthase expression in prognosis and outcome of
adjuvant chemotherapy in patients with rectal cancer. J Clin Oncol.
12:2640–2647. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanizaki J, Okamoto I, Takezawa K,
Tsukioka S, Uchida J, Kiniwa M, Fukuoka M and Nakagawa K:
Synergistic antitumor effect of S-1 and HER2-targeting agents in
gastric cancer with HER2 amplification. Mol Cancer Ther.
9:1198–1207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yasui K, Okamoto H, Arii S and Inazawa J:
Association of over-expressed TFDP1 with progression of
hepatocellular carcinomas. J Hum Genet. 48:609–613. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakajima T, Yasui K, Zen K, Inagaki Y,
Fujii H, Minami M, Tanaka S, Taniwaki M, Itoh Y, Arii S, et al:
Activation of B-Myb by E2F1 in hepatocellular carcinoma. Hepatol
Res. 38:886–895. 2008.PubMed/NCBI
|
|
12
|
Gschwantler-Kaulich D, Grunt TW, Muhr D,
Wagner R, Kölbl H and Singer CF: HER Specific TKIs exert their
antineoplastic effects on breast cancer cell lines through the
involvement of STAT5 and JNK. PLoS One. 11:e01463112016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ludyga N, Anastasov N,
Gonzalez-Vasconcellos I, Ram M, Höfler H and Aubele M: Impact of
protein tyrosine kinase 6 (PTK6) on human epidermal growth factor
receptor (HER) signalling in breast cancer. Mol Biosyst.
7:1603–1612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: The combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
DeGregori J, Kowalik T and Nevins JR:
Cellular targets for activation by the E2F1 transcription factor
include DNA synthesis- and G1/S-regulatory genes. Mol Cell Biol.
15:4215–4224. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baselga J, Cortés J, Kim SB, Im SA, Hegg
R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, et al:
Pertuzumab plus trastuzumab plus docetaxel for metastatic breast
cancer. N Engl J Med. 366:109–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hurvitz SA, Dirix L, Kocsis J, Bianchi GV,
Lu J, Vinholes J, Guardino E, Song C, Tong B, Ng V, et al: Phase II
randomized study of trastuzumab emtansine versus trastuzumab plus
docetaxel in patients with human epidermal growth factor receptor
2-positive metastatic breast cancer. J Clin Oncol. 31:1157–1163.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hallstrom TC and Nevins JR: Specificity in
the activation and control of transcription factor E2F-dependent
apoptosis. Proc Natl Acad Sci USA. 100:10848–10853. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu K, Paik JC, Wang B, Lin FT and Lin WC:
Regulation of TopBP1 oligomerization by Akt/PKB for cell survival.
EMBO J. 25:4795–4807. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blackstein M, Vogel CL, Ambinder R, Cowan
J, Iglesias J and Melemed A: Gemcitabine as first-line therapy in
patients with metastatic breast cancer: A phase II trial. Oncology.
62:2–8. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Curigliano G, Cardinale D, Suter T,
Plataniotis G, de Azambuja E, Sandri MT, Criscitiello C, Goldhirsch
A, Cipolla C and Roila F: ESMO Guidelines Working Group:
Cardiovascular toxicity induced by chemotherapy, targeted agents
and radiotherapy: ESMO clinical practice guidelines. Ann Oncol. 23
Suppl 7:vii155–vii166. 2012. View Article : Google Scholar : PubMed/NCBI
|