Introduction
Doxorubicin (DOX) is a broad-spectrum anthracycline
antibiotic, which can interact with topoisomerase II complex,
thereby inhibiting the progression of DNA replication (1,2);
therefore, DOX is often used to treat solid tumors and certain
types of leukemia. However, its clinical use is limited by side
effects, including vomiting, hair loss, inflammation and heart
injury (3). Among these side
effects, cardiomyopathy is the most severe and is caused by
increased generation of reactive oxygen species (ROS) (4,5).
Mitochondria serve a central role in production of intracellular
energy and are the primary target of DOX-induced toxicity (6). Once mitochondrial dysfunction occurs,
apoptosis or programmed cell death is activated. It has previously
been reported that DOX can induce apoptosis by arresting cell cycle
progression (7). DOX-induced ROS
release is mediated by signaling pathways and triggers apoptosis of
myocardial cells. Protein kinase B (AKT) is a serine/threonine
kinase, which serves a role in various biological events, including
cell survival, apoptosis and metabolism. In addition, forkhead box
protein O (FOXO) transcription factors modulate apoptosis and
resistance to oxidative stress (8). Activated AKT via phosphorylation
regulates its downstream targets via kinase activity. It has been
reported that FOXO3a is a member of the FOXO protein family, which
can be phosphorylated by this activated signal (9). Phosphorylation of FOXO3a may lead to
its nuclear export and suppression of the transcription of its
target genes. Conversely, apoptotic signals may result in
dephosphorylation of FOXO3a and therefore activate the expression
of its target genes (10–12). It has previously been revealed that
B-cell lymphoma 2 (Bcl-2)-like protein 11 (Bim) has an important
role in the underlying mechanisms associated with programmed death
and stress-mediated apoptosis, which can be regulated by FOXO3a.
Furthermore, inhibition of AKT activity has been demonstrated to
enhance the expression levels of Bim (12). Based on these findings, prevention
of apoptosis may be a potential treatment strategy for DOX-induced
cardiotoxicity.
CUE domain-containing 2 (CUEDC2) is extensively
expressed in the brain, testes and heart; it has previously been
reported that CUEDC2 promotes ubiquitination and degradation of
progesterone receptors (13).
CUEDC2 is associated with the cell cycle and inflammation (14), and it has been revealed to be
abnormally expressed in tumors, including kidney, brain, ovarian
and breast cancer (14). However,
the potential function of CUEDC2 in DOX-induced cardiotoxicity
remains to be completely elucidated. The present study focused on
determining the effects of CUEDC2 on DOX-induced cardiotoxicity and
elucidating the underlying molecular mechanism.
In the present study, downregulation of CUEDC2
alleviated DOX-induced cardiotoxicity by reducing oxidative stress
and suppressing apoptosis. Furthermore, it was demonstrated that
this attenuated cardiotoxicity may be caused by the phosphorylation
of AKT/FOXO3a and decreased expression of Bim. The results
indicated that CUEDC2 may be considered a promising target in the
prevention of DOX-induced cardiotoxicity.
Materials and methods
Cell culture and treatment groups
The H9c2 cell line (American Type Culture
Collection, Manassas, VA, USA) was grown at 37°C in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) containing 10% fetal bovine serum
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and 10%
penicillin/streptomycin in an atmosphere containing 5%
CO2. The phenotype of myocardial cells was initially
observed under a light microscope (magnification, ×40). The
negative small interfering (si) RNA scramble control (siSCR),
CUEDC2 siRNA (siCUE) and reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) primers were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). At ~80%
confluence, cells were divided into the following groups: i)
untreated cells (control); ii) cells transfected with siSCR; iii)
cells treated with 1 µM DOX (Sigma-Aldrich; Merck KGaA) at 37°C for
24 h (DOX); iv) cells transfected with siSCR and treated with 1 µM
DOX at 37°C for 24 h (DOX + siSCR); and v) cells transfected with
siCUE and treated with 1 µM DOX at 37°C for 24 h (DOX + siCUE). All
experiments were carried out independently at least three
times.
Cell transfection
According to the manufacturer's protocol, cells
(1×105) were seeded into 6-well plates and maintained in
serum-free medium overnight. Briefly, Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
was mixed with serum-free DMEM. After 5 min, the resulting regent
was divided into three centrifuge tubes, containing: i) 10 µl
Lipofectamine® 2000 alone, ii) 10 µM siScr (cat. no.
sc-37007; Santa Cruz Biotechnology, Inc.) or iii) 10 µM siCUE (cat.
no. sc-90791; Santa Cruz Biotechnology, Inc.), according to the
experimental design. The mixtures were incubated at room
temperature for 20 min. Subsequently, the liposome mixtures were
added to the cells. After a 6 h incubation at 37°C, the
transfection medium was removed and supplemented with normal
culture medium or DOX for 1 h. The cells were collected a total of
24 h post-transfection and then subjected to subsequent assays.
Cell Counting kit-8 (CCK-8) assay
The CCK-8 method was used to determine cell
viability. Briefly, cells (1×105) were seeded in 96-well
plates and treated with DOX (0.1, 0.5, 1 and 5 µM) for 24, 36 and
48 h time intervals. The collected cells were then treated with
CCK-8 solution (10 µl; cat. no. C0038; Beyotime Institute of
Biotechnology, Haimen, China) for 4 h at 37°C. The absorbance was
measured at a wavelength of 450 nm using a microplate reader
(Promega Corporation, Madison, WI, USA).
Flow cytometric analysis of ROS
Cells were stained with the oxidant-sensitive
fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate
(DCFH-DA; cat. no. 35845; Sigma-Aldrich; Merck KGaA) for 15 min and
were washed with PBS. 2′,7′-Dichlorofluorescein (DCF) was generated
from DCFH-DA in the presence of peroxide. The fluorescence of DCF
was detected using Accuri C6 flow cytometry (BD Biosciences,
Franklin Lakes, NJ, USA) with an excitation wavelength of 485 nm.
Data analysis was performed using Accuri CFlow Plus software
(version 1.0; BD Biosciences).
Mitochondrial membrane potential (MMP)
determination
The JC-1 probe can emit green fluorescence at low
membrane potentials and red fluorescence at increased potentials.
MitoProbe™ JC-1 Assay kit (cat. no. M34152; Invitrogen; Thermo
Fisher Scientific, Inc.) was used to determine MMP, according to
the manufacturer's protocol. Briefly, the cells were stained with
JC-1 for 20 min at 37°C and were then loaded onto an Accuri C6 flow
cytometer (BD Biosciences). The ratio of red/green fluorescence was
calculated and a decrease in the ratio indicated loss of MMP. Data
analysis was performed with Accuri CFlow Plus software (version
1.0; BD Biosciences).
Flow cytometric analysis of
apoptosis
The Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) kit (cat. no. V13241; Invitrogen;
Thermo Fisher Scientific, Inc.) was used to detect apoptosis.
Annexin V-FITC+/PI− indicated early apoptotic
cells, whereas late apoptotic cells were represented as Annexin
V-FITC+/PI+. According to the manufacturer's
protocol, following treatment, cells in a 24-well plate
(1×105 cells/well) were stained with Annexin V-FITC and
PI for 10 min at room temperature in the dark, after which
fluorescence intensity was analyzed using Accuri C6 flow cytometry
with Accuri CFlow Plus software version 1.0 (BD Biosciences).
Activity measurement of oxidative
indicators
Superoxide dismutase (SOD; cat. no. S0101), catalase
(CAT; cat. no. S0051) and malondialdehyde (MDA; cat no. S0131) were
spectrophotometrically determined in collected cells following
treatment using commercial assay kits, according to the
manufacturer's protocols (Beyotime Institute of Biotechnology). SOD
and CAT activity was presented as units per mg of protein, and MDA
content was presented as nmol/mg.
RNA isolation and RT-qPCR
Total RNA was isolated from the cells using
TRIzol® regent (Invitrogen; Thermo Fisher Scientific,
Inc). cDNA was synthesized from 2 µg RNA using oligo-dT primers
(New England BioLabs, Inc., Ipswich, MA, USA) and M-MLV reverse
transcriptase (Promega Corporation), according to manufacturer's
protocol. qPCR was conducted on the Mastercycler® ep
realplex system (Eppendorf, Hamburg, Germany) using SYBR Green PCR
master mix (Takara Bio, Inc., Otsu, Japan), according to the
manufacturer's protocol. The thermocycling conditions used for qPCR
were as follows: 95°C for 5 min; followed by 36 cycles of 95°C for
30 sec and 60°C for 30 sec; followed by 72°C for 7 min. The
relative expression levels of target genes were normalized to the
expression of the housekeeping gene GAPDH. The following primer
sequences were used for qPCR: Bcl-2-associated X protein (Bax),
forward 5′-TGGCCTCCTTTCCTACTTCG-3′, reverse
5′-AAAATGCCTTTCCCCGTTCC-3′; Bcl-2, forward
5′-CACACACACACATTCAGGCA-3′, reverse 5′-GGCAATTCCTGGTTCGGTTT-3′;
caspase-3, forward 5′-CTCGCGTTAACAGGAAGGTG-3′, reverse
5′-GGCAGTGGTAGCGTACAAAG-3′; cytochrome c, forward
5′-AGAGTGAGTTCCAGGACAGC-3′, reverse 5′-ACTAACGAGGCCCCTTTGAA-3′; and
GAPDH, forward 5′-GGGTCCCAGCTTAGGTTCAT-3′ and reverse
5′-CATTCTCGGCCTTGACTGTG-3′. Quantification of RT-qPCR products was
performed using the 2−ΔΔCq method (15).
Western blot analysis
Total proteins were extracted using
radioimmunoprecipitation assay (Beijing Solarbio Science &
Technology, Co., Ltd., Beijing, China) and were boiled. A BCA
protein quantification kit (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) was used to determine the protein concentration.
Subsequently, protein samples from each group (20 µg) were
separated by 10% SDS-PAGE and transferred onto PVDF membrane.
Following blocking with fat-free milk for 2 h at room temperature,
the membrane was incubated with primary antibodies overnight at
4°C. Prior to incubation with secondary antibodies at room
temperature for 1 h, the membrane was washed with Tris-buffered
saline containing 0.1% Tween. Protein bands were visualized using
BeyoECL Plus reagent (Beyotime Institute of Biotechnology). The
following antibodies were used for western blotting:
Anti-phosphorylated (p)-FOXO3a (cat. no. ab53287; 1:1,000),
anti-Bcl-2 (cat. no. ab59348; 1:700) and anti-Bax (cat. no.
ab53154; 1:1,000) from Abcam (Cambridge, UK); anti-CUEDC2 (cat. no.
12294; 1:1,000), anti-FOXO3a (cat. no. 2497; 1:1,000),
anti-cytochrome c (cat. no. 11940; 1:1,000), anti-cleaved caspase-3
(cat. no. 9664; 1:1,000), anti-p-AKT (Thr308; cat. no. 13038;
1:1,000), anti-AKT (cat. no. 4691; 1:1,000), anti-Bim (cat. no.
2819; 1:1,000) and anti-GAPDH (cat. no. 5174; 1:1,000) from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (cat. no.
sc-2004; 1:5,000) was supplied by Santa Cruz Biotechnology, Inc.
Denistometric analysis was performed using Quantity One software
(version 4.6.2; Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism (version 6.0; GraphPad Software, Inc., La Jolla, CA, USA).
The difference between groups was analyzed using one-way analysis
of variance followed by Dunnett's or Turkey's post hoc tests when
appropriate. Data are presented as the means ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cytotoxicity of DOX in H9c2 cells
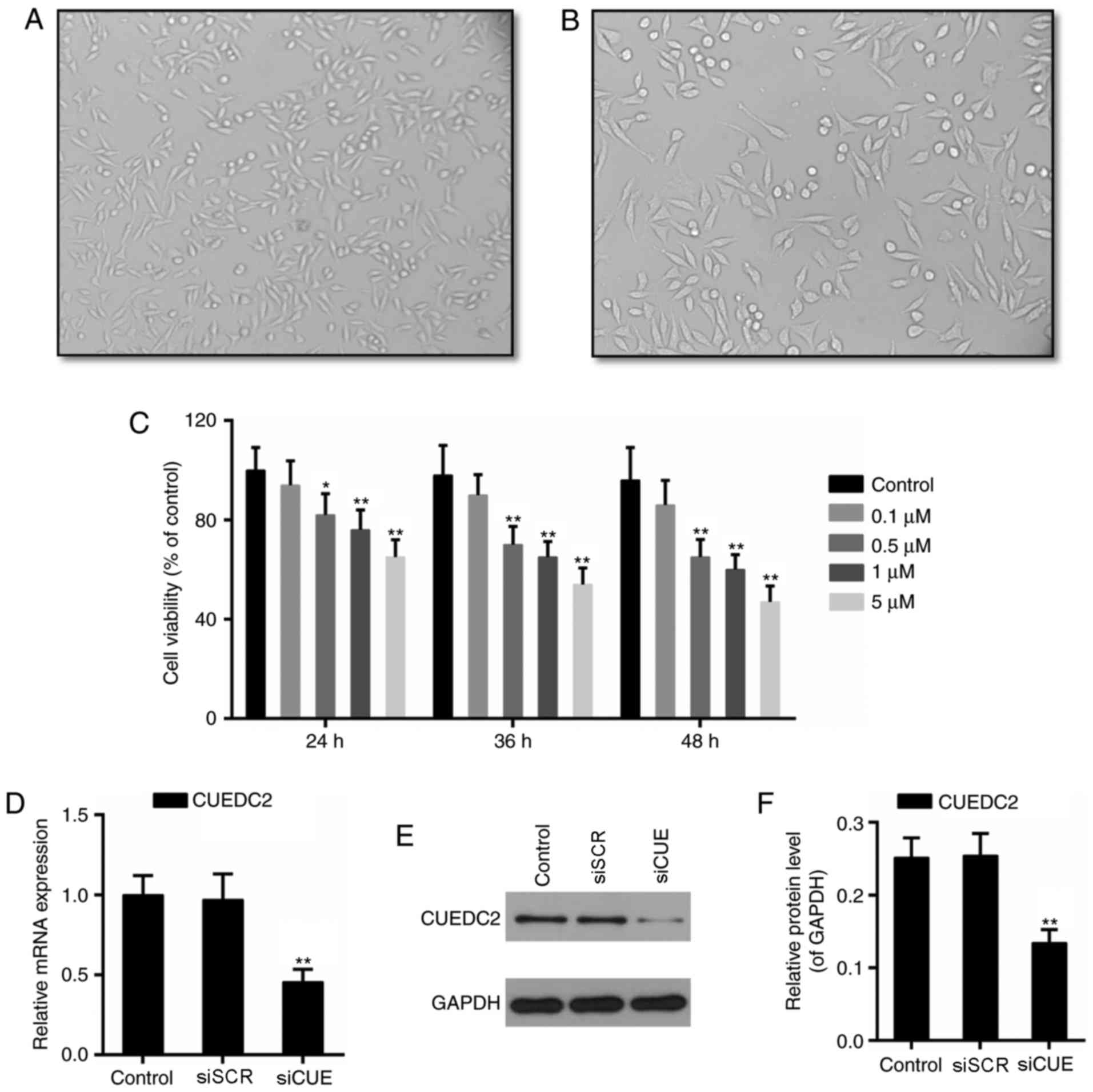
The phenotype of myocardial cells are presented in
Fig. 1A and B. Subsequently,
cytotoxicity of DOX (0.1, 0.5, 1 and 5 µM) was determined in H9c2
cells. The results of CCK-8 assay demonstrated that viability of
H9c2 cells was significantly decreased following incubation with
0.5 µM DOX for 24 h. Cytotoxicity of DOX increased in a
dose-dependent manner (Fig. 1C).
According to the results presented in Fig. 1C and a previous study (16), 1 µM DOX was selected to establish
the cardiotoxicity model in the present study.
Effects of depletion of CUEDC2 on
DOX-induced oxidative stress
CUEDC2 is extensively expressed in the heart, and it
has been reported that this protein is involved in modulation of
the oxidative capacity of cardiomyocytes (17). Therefore, the potential function of
CUEDC2 in cardiomyocytes was investigated in subsequent
experiments. Following transfection with siRNA, the mRNA and
protein expression levels of CUEDC2 were downregulated, thus
indicating that knockdown of CUEDC2 was effective (Fig. 1D-F). Formation of ROS has
previously been implicated in DOX-induced cardiotoxicity (17). Flow cytometric analysis
demonstrated that the levels of ROS were elevated in the DOX group,
whereas they were decreased in the DOX + siCUE group (Fig. 2A and B). Intracellular redox
homeostasis is dependent on the generation and removal of ROS.
Production of MDA and the activity of antioxidative enzymes,
including SOD and CAT, can be used to monitor this redox state
(18). The results of the present
study revealed that DOX markedly increased the content of MDA,
whereas depletion of CUEDC2 resulted in a significant decrease in
MDA levels. In addition, DOX-reduced SOD activity was rescued by
the depletion of CUEDC2, whereas the alterations in CAT activity
were not significantly different in the DOX + siCUE group compared
with in the DOX group (Fig.
2C).
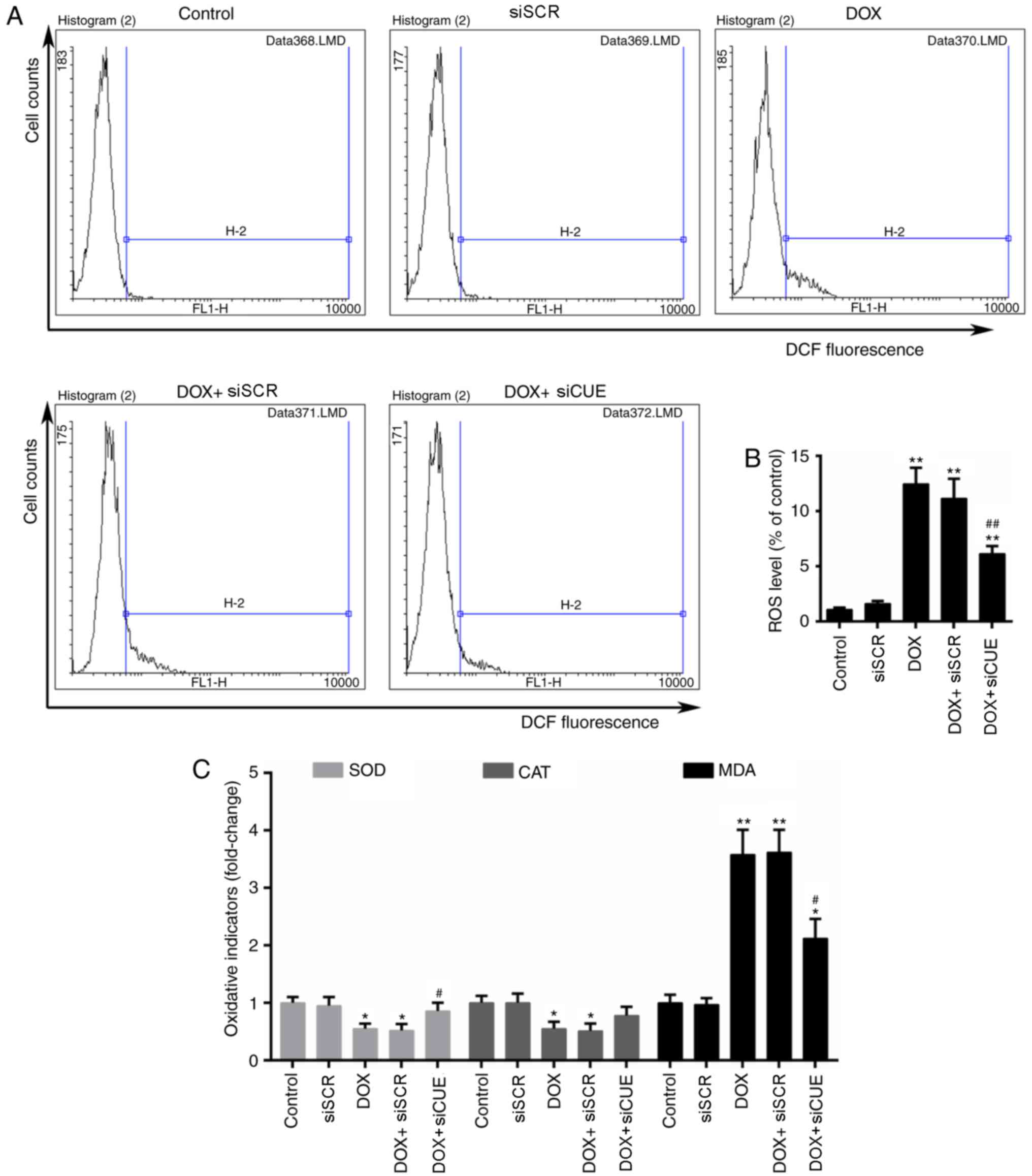 | Figure 2.Flow cytometry (A) histograms and (B)
quantitative analysis, as used to determine ROS levels. (C)
Relative activity of oxidative stress indicators MDA, SOD and CAT.
*P<0.05 and **P<0.01 vs. the control group.
#P<0.05 and ##P<0.01 vs. the DOX group.
CUEDC2, CUE domain-containing 2; CAT, catalase; DCF,
2′,7′-dichlorofluorescein; DOX, doxorubicin; MDA, malondialdehyde;
ROS, reactive oxygen species; siCUE, CUEDC2 siRNA; siRNA, small
interfering RNA; siSCR, siRNA scramble control; SOD, superoxide
dismutase. |
Effects of CUEDC2 knockdown on
MMP
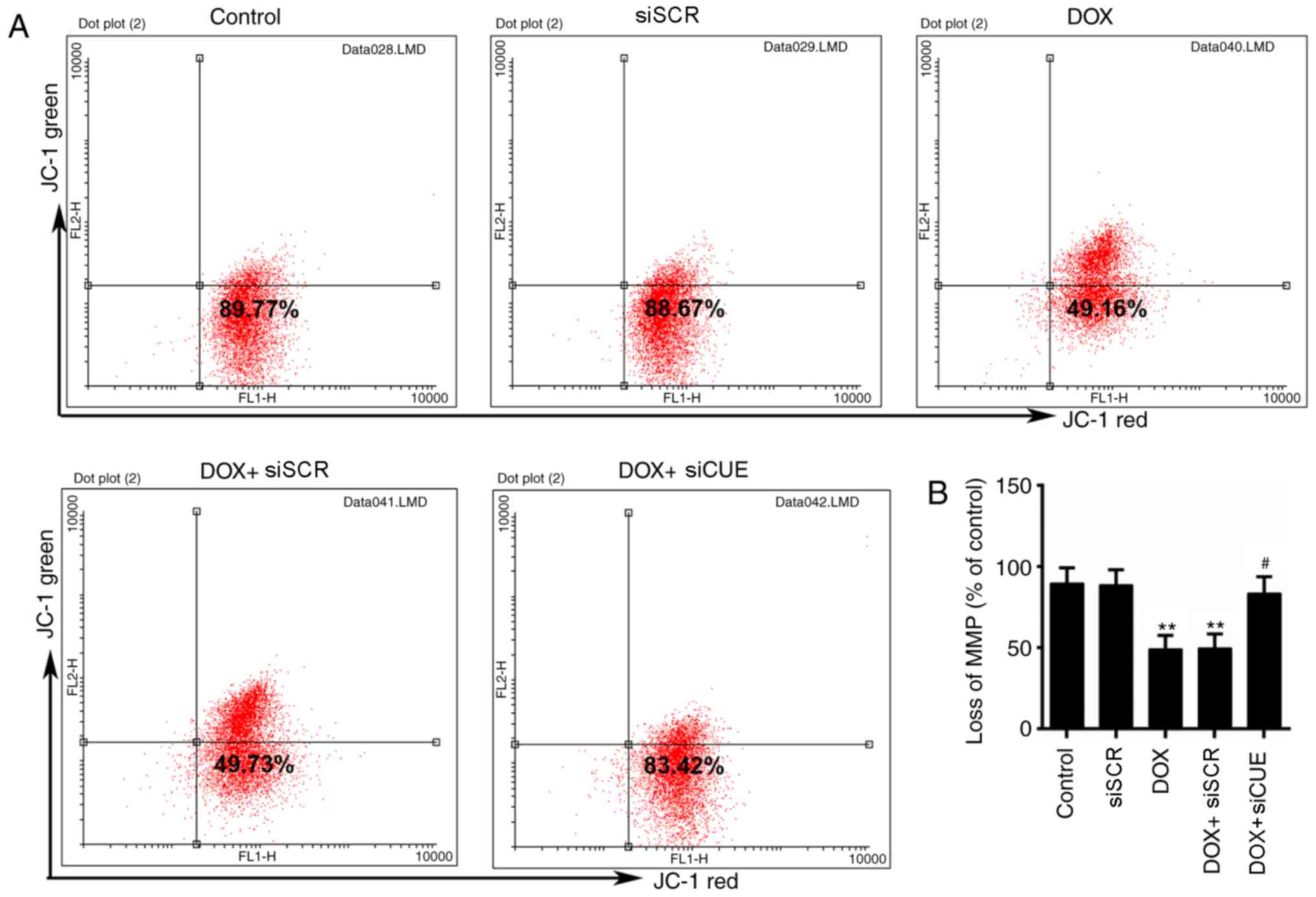
Mitochondria are the primary target organelles of
DOX-induced cardiotoxicity (19,20).
MMP is necessary for the production of ATP, which serves crucial
roles in living cells (21). The
results of flow cytometry revealed that DOX induced MMP loss;
however, MMP was recovered by the knockdown of CUEDC2 (Fig. 3A and B)
Effects of CUEDC2 knockdown on
DOX-mediated apoptosis
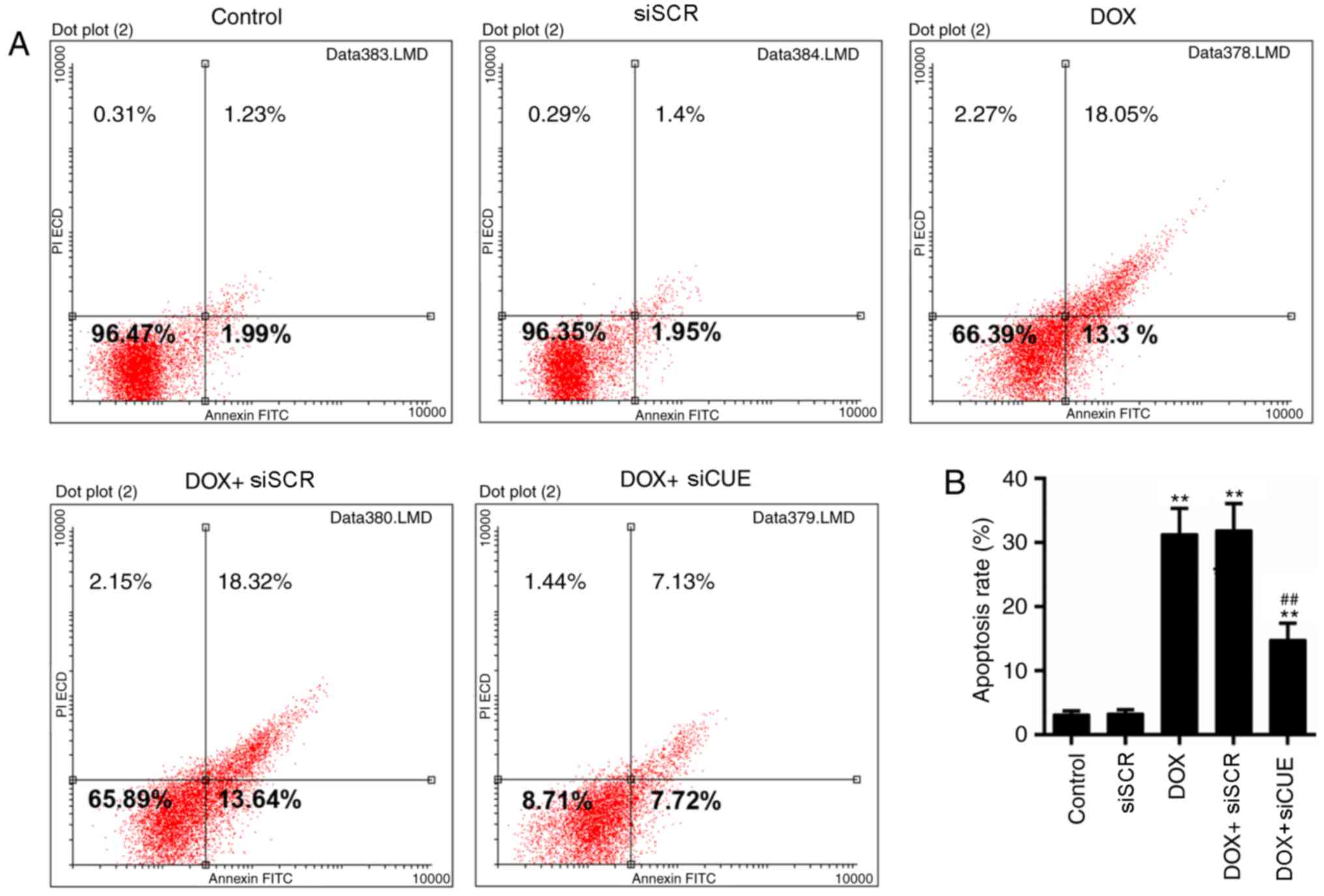
Mitochondrial dysfunction is closely associated with
apoptosis (22); therefore, the
levels of apoptosis were determined in the present study. The
results of flow cytometry demonstrated that the rate of apoptosis
was lower in the DOX + siCUE group compared with in the DOX group
(Fig. 4A and B). The expression
levels of apoptosis-associated genes were also detected in the
present study. The mRNA expression levels of Bax, caspase-3 and
cytochrome c were decreased in the DOX + siCUE group compared with
in the DOX group (Fig. 5).
Conversely, Bcl-2 expression was increased following depletion of
CUEDC2. In addition, the protein expression levels of Bax,
cleaved-caspase-3 and cytochrome c were lower in the DOX + siCUE
group compared with in the DOX group, whereas Bcl-2 expression was
elevated in the DOX + siCUE group compared with in the DOX
group.
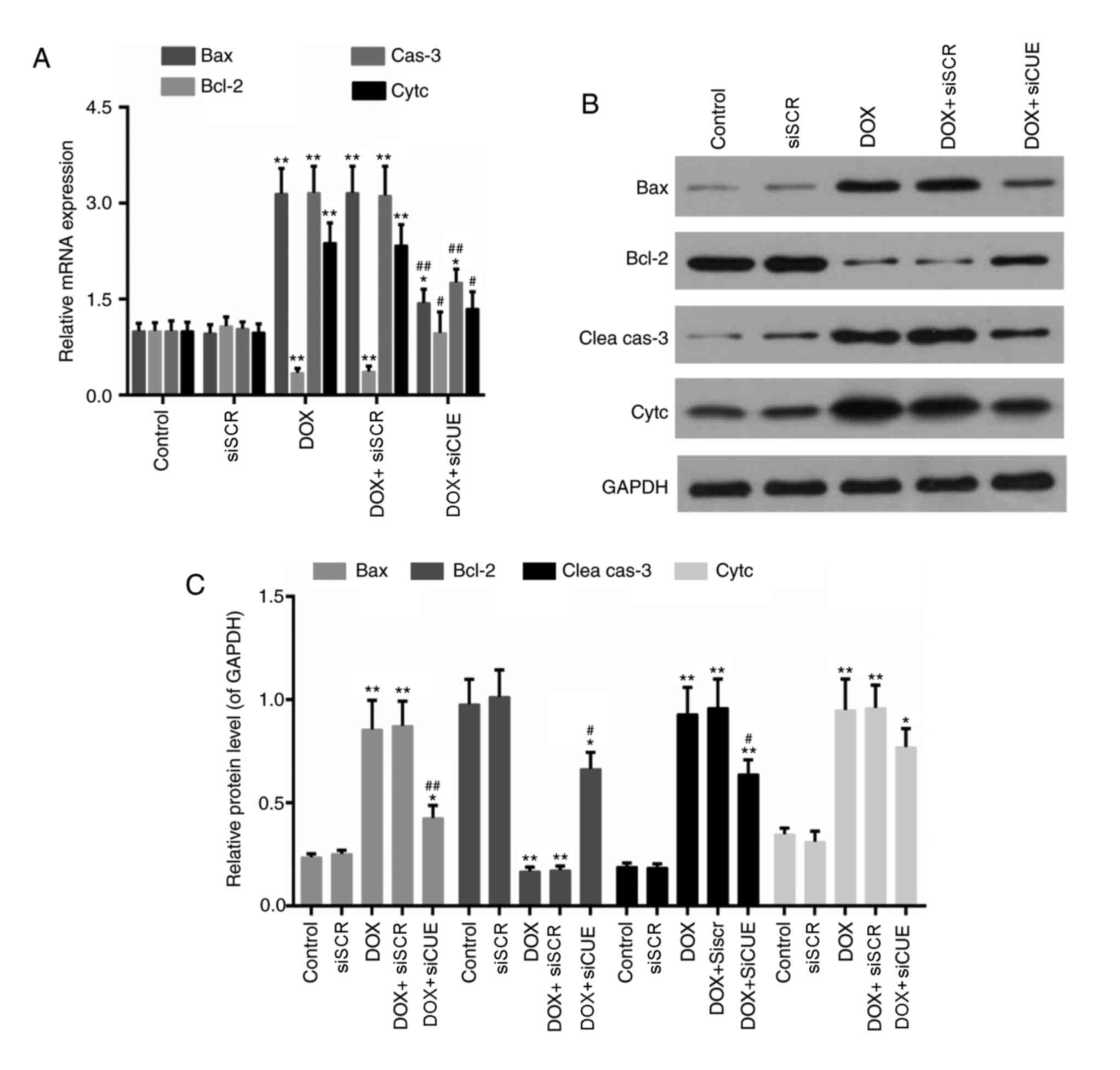 | Figure 5.(A) mRNA expression levels of Bax,
Bcl-2, cas-3 and Cytc. (B) Western blot and (C) semi-quantitative
analysis of the protein expression levels of Bax, Bcl-2, Clea cas-3
and Cytc. GAPDH was used as a loading control. *P<0.05 and
**P<0.01 vs. the control group. #P<0.05 and
##P<0.01 vs. the DOX group. Bax, Bcl-2-associated X
protein; Bcl-2, B-cell lymphoma 2; Clea cas-3, cleaved caspase-3;
Cytc, cytochrome c; DOX, doxorubicin; siCUE, CUEDC2 siRNA;
siRNA, small interfering RNA; siSCR, siRNA scramble control. |
Effects of CUEDC2 depletion on the
AKT/FOXO3a/Bim signaling pathway
FOXO3a, as a member of the FOXO family of
transcription factors, serves a role in the oxidative stress
response. It has previously been reported that AKT signaling
regulates the activity FOXO3a, which in turn modulates the
expression of Bim (23,24). Levels of phosphorylated AKT and
phosphorylated FOXO3a were significantly enhanced in the DOX +
siCUE group compared with in the DOX group (Fig. 6). Conversely, the elevated protein
expression of Bim following treatment with DOX was decreased
following depletion of CUEDC2. These findings suggested that siCUE
activated phosphorylated AKT, which subsequently phosphorylated
FOXO3a and decreased Bim transcription.
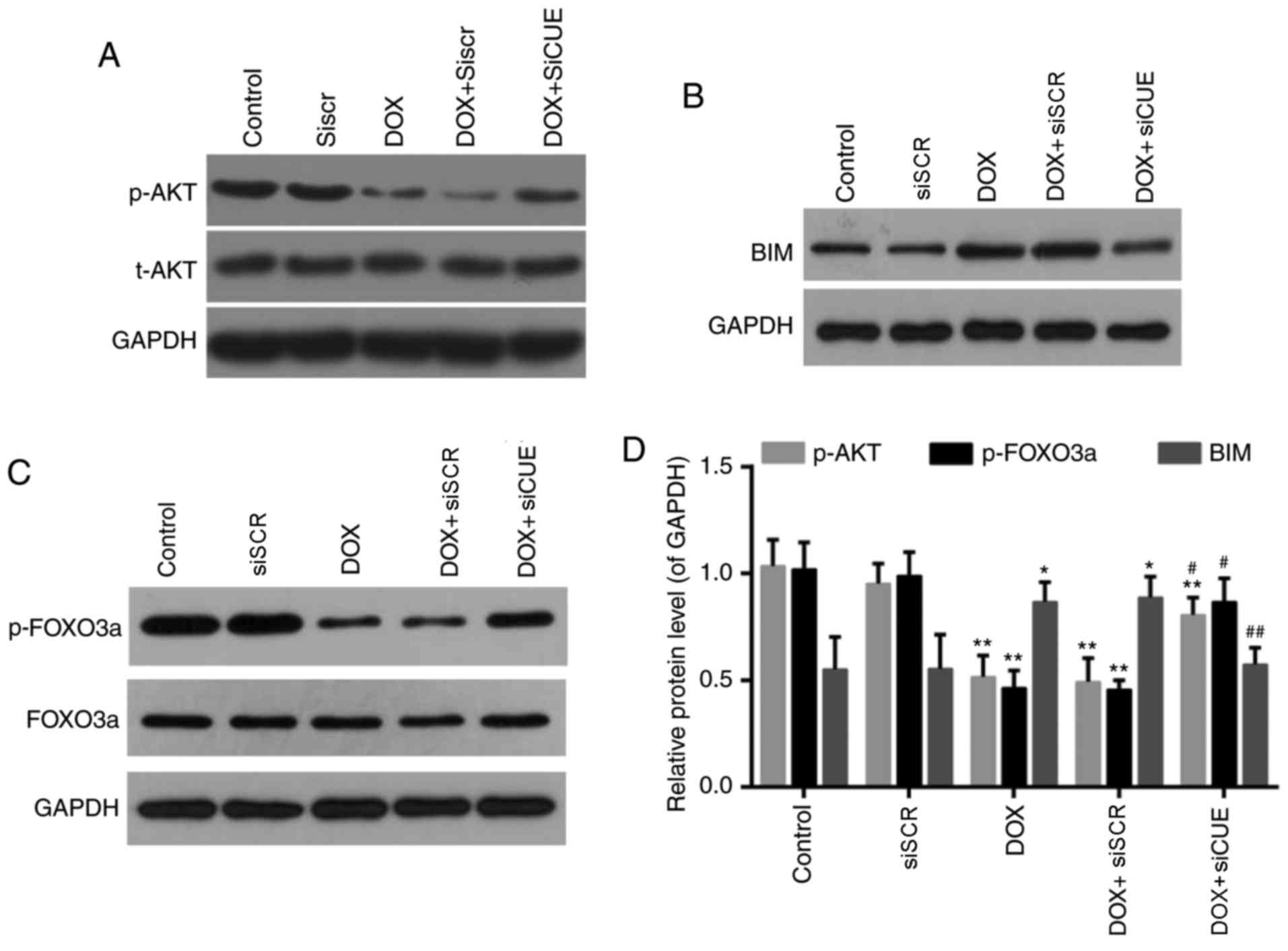 | Figure 6.Western blot analysis of (A) p-AKT
and AKT, (B) BIM, and (C) p-FOXO3a and FOXO3a. (D) Relative protein
expression levels of p-AKT, p-FOXO3a and BIM. GAPDH was used as a
loading control. *P<0.05 and **P<0.01 vs. the control group.
#P<0.05 and ##P<0.01 vs. the DOX group.
AKT, protein kinase B; BIM, B-cell lymphoma 2-like protein 11; DOX,
doxorubicin; FOXO3a, forkhead box O3a; p, phosphorylated; siCUE,
CUEDC2 siRNA; siRNA, small interfering RNA; siSCR, siRNA scramble
control. |
Discussion
Despite its high anticancer efficacy, the clinical
use of DOX is limited by its acute or cumulative cardiotoxicity
(25). CUEDC2 is considered to
serve a role in various cellular processes and is abundant in the
heart (14); however, little is
known about the potential function of CUEDC2 in DOX-induced
cardiotoxicity. Therefore, the present study aimed to investigate
its function and associated mechanisms. H9c2 cells, which were
originally derived from embryonic rat ventricular tissue, can
accurately model the response of primary cardiomyocytes (26). As opposed to non-proliferating
primary cardiomyocytes, H9c2 cells are able to proliferate
(27–29). Therefore, the present study used
H9c2 as an in vitro model for subsequent experiments.
In the present study, treatment with DOX decreased
viability of myocardial cells in a dose-dependent manner.
Production of ROS is reported as one of the mechanisms mediating
DOX-induced cardiotoxicity (25).
The results of the present study demonstrated that depletion of
CUEDC2 decreased ROS levels, which were elevated following
treatment with DOX. Furthermore, oxidative stress indicators were
modulated by depletion of CUEDC2, as demonstrated by inhibition of
the generation of MDA and increased activity of SOD and CAT;
however, the increase of CAT activity following transfection with
siCUE was not significant. The aforementioned results are
consistent with a previous study where ablation of CUEDC2 protected
cardiomyocytes against oxidative stress by facilitating stability
of the antioxidant enzyme glutathione peroxidase 1 (17). Apoptosis can be mediated by
DOX-induced oxidative stress (30). Loss of MMP has been demonstrated to
occur during the early stage of apoptosis (31). In the present study, loss of MMP
was effectively recovered by the depletion of CUEDC2, thus
indicating that silencing CUEDC2 improved mitochondrial function.
Apoptosis rate was decreased by ~50% in the CUEDC2 depletion group
compared with in the DOX group. Bcl-2 is an anti-apoptotic protein,
whereas Bax is a proapoptotic protein (32,33).
Furthermore, diverse apoptosis pathways converge on a mechanism
associated with caspase-3 (34,35),
and the release of cytochrome c from mitochondria to cytosol
can act as an intermediate to induce apoptosis (36). The results of the present study
revealed that the DOX-induced elevated expression levels of Bax,
cleaved caspase-3 and cytochrome c were decreased in the
CUEDC2 depletion group. Conversely, the decreased expression of
Bcl-2 was rescued by depletion of CUEDC2. Taken together,
downregulation of CUEDC2 may prevent DOX-induced oxidative stress
and apoptosis. FOXO transcription factors are implicated in
numerous cellular responses (8).
Among the FOXO proteins, FOXO3a has been extensively studied and
has been demonstrated to serve a role in the stress response
(37–39). It has been reported that
phosphorylated AKT, as a mediator of cellular processes, may
phosphorylate FOXO3a to inhibit its activity, thereby disrupting
transcription of its target genes, including Bim (11). As a target of FOXO3, Bim is also
associated with apoptosis caused by stress (40). To further determine the underlying
mechanisms of DOX-induced cardiotoxicity, involvement of the
AKT/FOXO3a/Bim signaling pathway was examined. The results
demonstrated that the expression levels of p-AKT and p-FOXO3a were
increased in the CUEDC2 depletion group, whereas Bim expression was
downregulated. This suggests that siCUE activated p-AKT, and
subsequently the levels of p-FOXO3a and Bim were increased and
decreased by p-AKT, respectively. However, this was not fully
determined by the present study and thus requires further
investigation. In the present study, the AKT/FOXO3a/Bim signaling
pathway exerted a positive role in the prevention of DOX-induced
cardiotoxicity, which was in agreement with the results of a
previous study (41). In addition,
NAD-dependent protein deacetylase sirtuin-1 has been reported to
exhibit synergetic effects on phosphoinositide 3-kinase/AKT that
augment the protective effects of exercise on the heart (11). Therefore, it is possible that other
signaling pathways may be involved in regulation of FOXO3a. The
present study demonstrated that downregulation of CUEDC2 was
beneficial and alleviated DOX-induced toxicity in H9c2 cells. The
results of the present study are supported by results of a previous
study where overexpression of CUEDC2 was considered a predictor of
poor prognosis of ovarian serous carcinoma (42). However, a recent study reported
that CUEDC2 knockdown could increase tumor growth in lung
adenocarcinoma (43). It has
therefore been suggested that the function of CUEDC2 may be
dependent on cell type. Therefore, it is necessary to perform
further in-depth investigations, including in vivo animal
studies, to elucidate the underlying mechanism of CUEDC2, as CUEDC2
may exhibit different functions in vitro and in vivo.
Taken together, CUEDC2 may be used as a therapeutic target for the
treatment of cardiac injury.
In conclusion, the present study demonstrated that
downregulation of CUEDC2 prevented DOX-induced cardiotoxicity by
alleviating oxidative stress, maintaining MMP and inhibiting
apoptosis. It was also demonstrated that the AKT/FOXO3a/Bim
signaling pathway serves a role in the cardioprotective mechanism
of CUEDC2 knockdown. The results of the present study indicated
that modulating the expression of CUEDC2 may be a novel therapeutic
strategy to mitigate DOX-associated cardiotoxicity in the
future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Authors' contributions
XZ and WC designed the study. JL, YC, JY and XL
performed the experiments. JL and YC performed data analysis. XZ
wrote the manuscript. XZ, JL and WC contributed to manuscript
revisions.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Capranico G, Kohn KW and Pommier Y: Local
sequence requirements for DNA cleavage by mammalian topoisomerase
II in the presence of doxorubicin. Nucleic Acids Res. 18:6611–6619.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Young RC, Ozols RF and Myers CE: The
anthracycline neoplastic drugs. N Engl J Med. 305:139–153. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thorn CF, Oshiro C, Marsh S,
Hernandez-Boussard T, McLeod H, Klein TE and Altman RB: Doxorubicin
pathways: Pharmacodynamics and adverse effects. Pharmacogenet
Genomics. 21:440–446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Orhan B: Doxorubicin cardiotoxicity:
Growing importance. J Clin Oncol. 17:2294–2296. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Samuel L: Doxorubicin-induced
cardiotoxicity. Postgrad Med J. 71:3181995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pereira GC, Pereira SP, Pereira CV, Lumini
JA, Magalhães J, Ascensão A, Santos MS, Bjork JA, Moreno AJ,
Wallace KB, et al: Doxorubicin-induced cardiac, hepatic and renal
mitochondrial toxicity in an acute versus sub-chronic treatment
model. Biochim Biophys Acta. 1797 Supple:S812010. View Article : Google Scholar
|
|
7
|
Skladanowski A and Konopa J: Adriamycin
and daunomycin induce programmed cell death (apoptosis) in tumour
cells. Biochem Pharmacol. 46:375–382. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carter ME and Brunet A: FOXO transcription
factors. Curr Biol. 17:R113–R114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li M, Chiu JF, Mossman BT and Fukagawa NK:
Down-regulation of manganese-superoxide dismutase through
phosphorylation of FOXO3a by Akt in explanted vascular smooth
muscle cells from old rats. J Biol Chem. 281:40429–40439. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Y, Zhao Y, Liao W, Yang J, Wu L,
Zheng Z, Yu Y, Zhou W, Li L, Feng J, et al: Acetylation of FoxO1
activates Bim expression to induce apoptosis in response to histone
deacetylase inhibitor depsipeptide treatment. Neoplasia.
11:313–324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin CH, Lin CC, Ting WJ, Pai PY, Kuo CH,
Ho TJ, Kuo WW, Chang CH, Huang CY and Lin WT: Resveratrol enhanced
FOXO3 phosphorylation via synergetic activation of SIRT1 and
PI3K/Akt signaling to improve the effects of exercise in elderly
rat hearts. Age (Dordr). 36:97052014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sharma G, Kar S, Palit S and Das PK:
18β-glycyrrhetinic acid induces apoptosis through modulation of
Akt/FOXO3a/Bim pathway in human breast cancer MCF-7 cells. J Cell
Physiol. 227:1923–1931. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang PJ, Zhao J, Li HY, Man JH, He K,
Zhou T, Pan X, Li AL, Gong WL, Jin BF, et al: CUE domain containing
2 regulates degradation of progesterone receptor by
ubiquitin-proteasome. Embo J. 26:1831–1842. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Man J and Zhang X: CUEDC2: An emerging key
player in inflammation and tumorigenesis. Protein Cell. 2:699–703.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kotamraju S, Konorev EA, Joseph J and
Kalyanaraman B: Doxorubicin-induced apoptosis in endothelial cells
and cardiomyocytes is ameliorated by nitrone spin traps and
ebselen. Role of reactive oxygen and nitrogen species. J Biol Chem.
275:33585–33592. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jian Z, Liang B, Pan X, Xu G, Guo SS, Li
T, Zhou T, Xiao YB and Li AL: CUEDC2 modulates cardiomyocyte
oxidative capacity by regulating GPX1 stability. EMBO Mol Med.
8:813–829. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morakinyo A, Iranloye B and Adegoke O:
Calcium antagonists modulate oxidative stress and acrosomal
reaction in rat spermatozoa. Arch Med Sci. 7:613–618. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kalivendi SV, Konorev EA, Cunningham S,
Vanamala SK, Kaji EH, Joseph J and Kalyanaraman B: Doxorubicin
activates nuclear factor of activated T-lymphocytes and Fas ligand
transcription: Role of mitochondrial reactive oxygen species and
calcium. Biochem J. 389:527–539. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Konorev EA, Kennedy MC and Kalyanaraman B:
Cell-permeable superoxide dismutase and glutathione peroxidase
mimetics afford superior protection against doxorubicin-induced
cardiotoxicity: The role of reactive oxygen and nitrogen
intermediates. Arch Biochem Biophys. 368:421–428. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gomez-Cabrera MC, Sanchis-Gomar F,
Garcia-Valles R, Pareja-Galeano H, Gambini J, Borras C and Vina J:
Mitochondria as sources and targets of damage in cellular aging.
Clin Chem Lab Med. 50:1287–1295. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takasaki A, Hanyu H, Iwamoto T, Shirato K,
Izumi R, Toyota H, Mizuguchi J, Miyazawa K and Tomoda A:
Mitochondrial depolarization and apoptosis associated with
sustained activation of c-jun-N-terminal kinasein the human
multiple myeloma cell line U266 induced by
2-aminophenoxazine-3-one. Mol Med Rep. 2:199–203. 2009.PubMed/NCBI
|
|
23
|
Luo H, Yang Y, Duan J, Wu P, Jiang Q and
Xu C: PTEN-regulated AKT/FoxO3a/Bim signaling contributes to
reactive oxygen species-mediated apoptosis in selenite-treated
colorectal cancer cells. Cell Death Dis. 4:e4812013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiong H, Wang J, Guan H, Wu J, Xu R, Wang
M, Rong X, Huang K, Huang J, Liao Q, et al: SphK1 confers
resistance to apoptosis in gastric cancer cells by downregulating
Bim via stimulating Akt/FoxO3a signaling. Oncol Rep. 32:1369–1373.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arola OJ, Saraste A, Pulkki K, Kallajoki
M, Parvinen M and Voipio-Pulkki LM: Acute doxorubicin
cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res.
60:1789–1792. 2000.PubMed/NCBI
|
|
26
|
Kimes BW and Brandt BL: Properties of a
clonal muscle cell line from rat heart. Exp Cell Res. 98:367–381.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hescheler J, Meyer R, Plant S, Krautwurst
D, Rosenthal W and Schultz G: Morphological, biochemical, and
electrophysiological characterization of a clonal cell (H9c2) line
from rat heart. Circ Res. 69:1476–1486. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sipido KR and Marban E: L-type calcium
channels, potassium channels, and novel nonspecific cation channels
in a clonal muscle cell line derived from embryonic rat ventricle.
Circ Res. 69:1487–1499. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Watkins SJ, Borthwick GM and Arthur HM:
The H9C2 cell line and primary neonatal cardiomyocyte cells show
similar hypertrophic responses in vitro. In vitro Cell Dev Biol
Anim. 47:125–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arstall MA, Sawyer DB, Fukazawa R and
Kelly RA: Cytokine-mediated apoptosis in cardiac myocytes: The role
of inducible nitric oxide synthase induction and peroxynitrite
generation. Circ Res. 85:829–840. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (deltapsi(m)) in apoptosis; an
update. Apoptosis. 8:115–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sharifi AM, Mousavi SH and Jorjani M:
Effect of chronic lead exposure on pro-apoptotic Bax and
anti-apoptotic Bcl-2 protein expression in rat hippocampus in vivo.
Cell Mol Neurobiol. 30:769–774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhai D, Jin C, Huang Z, Satterthwait AC
and Reed JC: Differential regulation of Bax and Bak by
anti-apoptotic Bcl-2 family proteins Bcl-B and Mcl-1. J Biol Chem.
283:9580–9586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Salvesen GS: Caspases: Opening the boxes
and interpreting the arrows. Cell Death Differ. 9:3–5. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ghavami S, Hashemi M, Ande SR, Yeganeh B,
Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ and
Los M: Apoptosis and cancer: Mutations within caspase genes. J Med
Genet. 46:497–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang J, Liu X, Bhalla K, Kim CN, Ibrado
AM, Cai J, Peng TI, Jones DP and Wang X: Prevention of apoptosis by
Bcl-2: Release of cytochrome c from mitochondria blocked. Science.
275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lam EW, Francis RE and Petkovic M: FOXO
transcription factors: Key regulators of cell fate. Biochem Soc
Trans. 34:722–726. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sunters A, de Mattos Fernández S, Stahl M,
Brosens JJ, Zoumpoulidou G, Saunders CA, Coffer PJ, Medema RH,
Coombes RC and Lam EW: FoxO3a transcriptional regulation of Bim
controls apoptosis in paclitaxel-treated breast cancer cell lines.
J Biol Chem. 278:49795–49805. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sunters A, Madureira PA, Pomeranz KM,
Aubert M, Brosens JJ, Cook SJ, Burgering BM, Coombes RC and Lam EW:
Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer
cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res.
66:212–220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Concannon CG, Tuffy LP, Weisová P, Bonner
HP, Davila D, Bonner C, Devocelle MC, Strasser A, Ward MW and Prehn
JH: AMP kinase-mediated activation of the BH3-only protein Bim
couples energy depletion to stress-induced apoptosis. J Cell Biol.
189:83–94. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Samuel SM, Thirunavukkarasu M, Penumathsa
SV, Paul D and Maulik N: Akt/FOXO3a/SIRT1-mediated cardioprotection
by n-tyrosol against ischemic stress in rat in vivo model of
myocardial infarction: Switching gears toward survival and
longevity. J Agric Food Chem. 56:9692–9698. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang A, Guo C, Sun Y, Lu L, Wang Y, Wang
Q, Zhang Y, Zhang H, Wang L, Gu Y, et al: Overexpression of CUEDC2
predicts poor prognosis in ovarian serous carcinomas. J Cancer.
6:542–547. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sun L, Bai L, Lin G, Wang R, Liu Y, Cai J,
Guo Y, Zhu Z and Xie C: CUEDC2 down-regulation is associated with
tumor growth and poor prognosis in lung adenocarcinoma. Oncotarget.
6:20685–20696. 2015. View Article : Google Scholar : PubMed/NCBI
|