Introduction
Thyroid cancer is the most common type of tumor of
the endocrine system with a rising morbidity rate of >5%
annually (1,2). Papillary thyroid carcinoma is the
predominant subtype, accounting for ~70% of all thyroid cancer
cases and the overall chance of survival is favorable (3). However, patients with poorly
differentiated thyroid cancer suffer a poorer prognosis (4). Therefore, urgent investigation into
novel agents and treatments for this malignant disease is required.
There have been extensive studies on the molecular alterations in
thyroid carcinoma and the biomarkers associated with tumor
progression, including telomerase reverse transcriptase,
transcription factor SOX2 (SOX2) and zinc finger protein SNAI1
(5–7). Recently comprehensive genetic
characterization has been reported, and genome mutations and
abnormalities have been demonstrated (8).
MicroRNA (miRs) are comprised of small endogenous
noncoding RNAs of 20–30 nucleotides in length (9). miRs may regulate target gene
expression by complementary binding to the 3′ untranslated region
(3′-UTR) of mRNAs, leading to repression of translation and
inhibition of protein activity. Recently, studies demonstrated that
miRs were aberrantly expressed in tumors, including thyroid cancer,
and functioned in pro-oncogenic or tumor suppression roles
(10). A number of miRs, including
miR-187, let-7, miR-146 and miR-222, have been consistently
identified to be deregulated in thyroid cancer (11,12).
These results indicate that investigating the association between
miRs and thyroid cancer is critical for an improved understanding
of tumorigenesis.
miR-205 is reported to be deregulated in several
types of tumors, including breast, prostate and lung cancer
(13–15). However, its role in thyroid
carcinoma remains unknown. In the present study, it was
demonstrated that miR-205 was critical in regulating the
proliferation, migration and invasion of thyroid carcinoma cells.
Additionally, miR-205 was downregulated in thyroid carcinoma
tissues and the same observation was demonstrated in other studies.
Further study of the mechanism of action revealed that miR-205
inhibited thyroid carcinoma tumorigenesis by targeting YAP1
expression.
Materials and methods
Human tissue specimens
A total of 132 paired thyroid carcinoma and
non-tumor tissues were obtained from patients who underwent surgery
at the Department of Thyroid Surgery at the Shanxi Provincial
People's Hospital (Taiyuan, China) between January 2005 and June
2010. The clinicopathological features are demonstrated in Table I. All tissues samples were
immediately frozen in liquid nitrogen and stored at −80°C prior to
use. The present study was approved by the Ethics Committee of
Shanxi Provincial People's Hospital. Written informed consent was
obtained from all patients.
 | Table I.Clinicopathological features of 132
patients with thyroid cancer. |
Table I.
Clinicopathological features of 132
patients with thyroid cancer.
| Clinicopathological
features | Value |
|---|
| Median age, years
(range) | 49 (22–78) |
| Gender, n |
|
|
Male | 45 |
|
Female | 87 |
| Age, n |
|
| >35
years-old | 41 |
| ≤35
years-old | 91 |
| T classification,
n |
|
|
T1-T2 | 109 |
|
T3-T4 | 23 |
| N classification,
n |
|
| N0 | 52 |
|
N1-N2 | 80 |
| Stage, n |
|
|
I–II | 89 |
|
III–IV | 43 |
| Histological type,
n |
|
|
Papillary thyroid
carcinoma | 96 |
|
Undifferentiated thyroid
carcinoma | 36 |
Cell culture and RNA oligonucleotide
transfection
The human thyroid cancer cell lines 8505-C (cat. no.
SCSP-540), BCPAP (cat. no. SCSP-543) and BHT-101 (cat. no.
SCSP-544) were obtained from the Cell Bank at the Chinese Academy
of Sciences (Shanghai, China), and TPC-1 was from the American Type
Culture Collection (Manassas, VA, USA). Human embryonic kidney 293T
(HEK293T) cells (ATCC cat. no. CRL-11268) cells were cultured in
our laboratory. Cells were maintained in RPMI1640 medium
supplemented with 10% fetal bovine serum (both from Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), penicillin (100 U/ml)
and streptomycin (100 µg/ml). Cells were cultured at 37°C in a
humidified atmosphere containing 5% CO2. For
overexpression or knockdown of miR-205, cells were seeded into
6-well plates and transfected with an miR-205 mimic (5-UCC UUC AUU
CCA CCG GAG UCU G-3), normal control (NC) or miR-205 antagonist
(anti-miR-205; 5′-CAGACUCCGGUGGAAUGAA′-3′) at 5 µM concentration
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). cells were used 48 h following transfection. For
inhibiting endogenous YAP1 expression, small interfering RNA
targeting YAP1 (CCGUUUCCCAGACUACCUU) was purchased from Chang Jing
Bio-Tech, Ltd. (Changsha, China) and was transfected at 5 µM
concentration to the cells using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) and the cells cultured
for the next 48 h.
RNA extraction and reverse
transcriptase-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells or human tissues
by TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
first-strand cDNA was synthesized using the PrimeScript™
RT reagents kit (Takara Biotechnology Co., Ltd., Dalian, China).
qPCR was performed using SYBR-Green method as described previously
(16). YAP1 was normalized to the
expression of GAPDH and miR-205 was normalized to the small nuclear
(sn)RNA U6. The gene expression was quantified using the
2−ΔΔCq method (17).
Primers used were as followed: YAP1 forward,
5′-TAGCCCTGCGTAGCCAGTTA-3′ and reverse,
5′-TCATGCTTAGTCCACTGTCTGT-3′; snRNA U6 forward, CTCGCTTCGGCAGCACA
and reverse, AACGCTTCACGAATTTGCGT and GAPDH forward,
5′-ACAACTTTGGTATCGTGGAAGG-3′ and reverse,
5′-GCCATCACGCCACAGTTTC-3′.
Western blotting
Cells were lysed using the radioimmunoprecipitation
assay buffer (Thermo Fisher Scientific, Inc.) containing a protease
inhibitor and phosphatase inhibitor cocktail (Roche Diagnostics,
Indianapolis, IN, USA). Total protein concentration was measured by
the bicinchoninic acid assay method. Equal amounts of protein (60
µg) were separated by 10% SDS-PAGE and then transferred to
nitrocellulose membranes. Following blocking with bovine serum
albumin (Wuhan Boster Biological Technology, Ltd., Wuhan, China)
for 1 h at room temperature, the membranes were incubated with
specific primary antibodies at 4°C overnight. Then the membranes
were incubated with the secondary antibody (HRP conjugated mouse
anti-rabbit antibody; sc-2357; 1:5,000; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) at room temperature for 1 h and visualized
by enhanced chemiluminescence method (Pierce; Thermo Fisher
Scientific, Inc.). GAPDH was used as internal control. The primary
antibodies used were as followed: Anti-YAP1 (cat. no. ab52771;
1:1,000), anti-Translin-associated zinc finger protein 1 (TAZ; cat
no. ab110239; 1:1,000) and anti-Bcl2-like protein 1 (Bcl-xl; cat.
no. ab32370; 1:1,000; all Abcam, Cambridge, MA, USA) and anti-GAPDH
(cat. no. sc-365062; 1:2,000; Santa Cruz Biotechnology, Inc.).
Predicting the miRNA target
For exploring the miRNAs targeting YAP1, Targetscan
(http://www.targetscan.org/vert_71/)
was used. By typing the gene symbol, the database could give the
miRNAs which could potentially bind to the target.
Dual-luciferase reporter assay
Luciferase reporter vectors of wild-type (WT) and
mutant (MUT) YAP1 3′-UTR were synthesized. The sequence region was
cloned into the pMIR-REPORT miR vector (Ambion; Thermo Fisher
Scientific, Inc.) and the open reading frame of firefly luciferase.
For the Dual-luciferase assay, 293T cells were seeded in 6-well
plate (1×105 cells/well) and transfected with the above
vectors and co-transfected with miR-205 or control using
Lipofectamine RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.).
Following 24 h culturing, cells were harvested and the firefly and
Renilla luciferase activities were detected by Dual-Glo
luciferase assay kit (Promega Corporation, Madison, WI, USA),
according to the manufacturer's protocol.
Cell proliferation and colony
formation assay
Cell proliferation was measured using the Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). Cells were seeded in 96-well plates at a
concentration of 2,000 cells/well. The proliferation rates were
recorded at 24, 48, 72 and 96 h at 450 nm following the
manufacturer's protocol. For the colony formation assay, 1,000
cells were seeded in 6-wells plates and cultured for 2 weeks. Then
cells were fixed with 4% paraformaldehyde and stained with crystal
violet at room temperature for 30 min. Colony numbers were
calculated under a phase contrast microscope (Leica Microsystems
GmbH, Wetzlar, Germany).
Wound healing assay
Cells were cultured in a 6-well plate to form a
monolayer. The wounds were scratched using a sterile 200 µl
tip/well and cells were cultured in low serum media (1% fetal
bovine serum; FBS). The wound distance was measured at the times of
0 and 72 h. Representative images were taken by phase contrast
microphotography.
Matrigel invasion assay
A total of 105 cells in serum-free medium
were seeded into the upper chamber of a Transwell insert (8-µm pore
size) coated with Matrigel® (BD Biosciences, Franklin
Lakes, NJ, USA). Then media containing 15% FBS were added to the
lower chamber and following 48 h of culture, the cells remaining on
the upper membrane were removed and cells that invaded to the lower
membrane were fixed in 4% paraformaldehyde at room temperature for
10 min and stained with 0.1% crystal violet at room temperature for
10 min and imaged. The cell numbers were calculated under an
upright metallurgical microscope (Leica Microsystems GmbH) at 100×
magnification.
Statistical analysis
In vitro results were presented as the mean ±
standard error of the mean from at least three independent
experiments. Mann-Whitney-U test, Spearman rank correlation
coefficient test, Student's t test, analysis of variance and
multiple comparison between the groups using the S-N-K method were
performed using SPSS software (version 21.0; IBM Corp., Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-205 is repressed in human thyroid
cancer tissues and cell lines
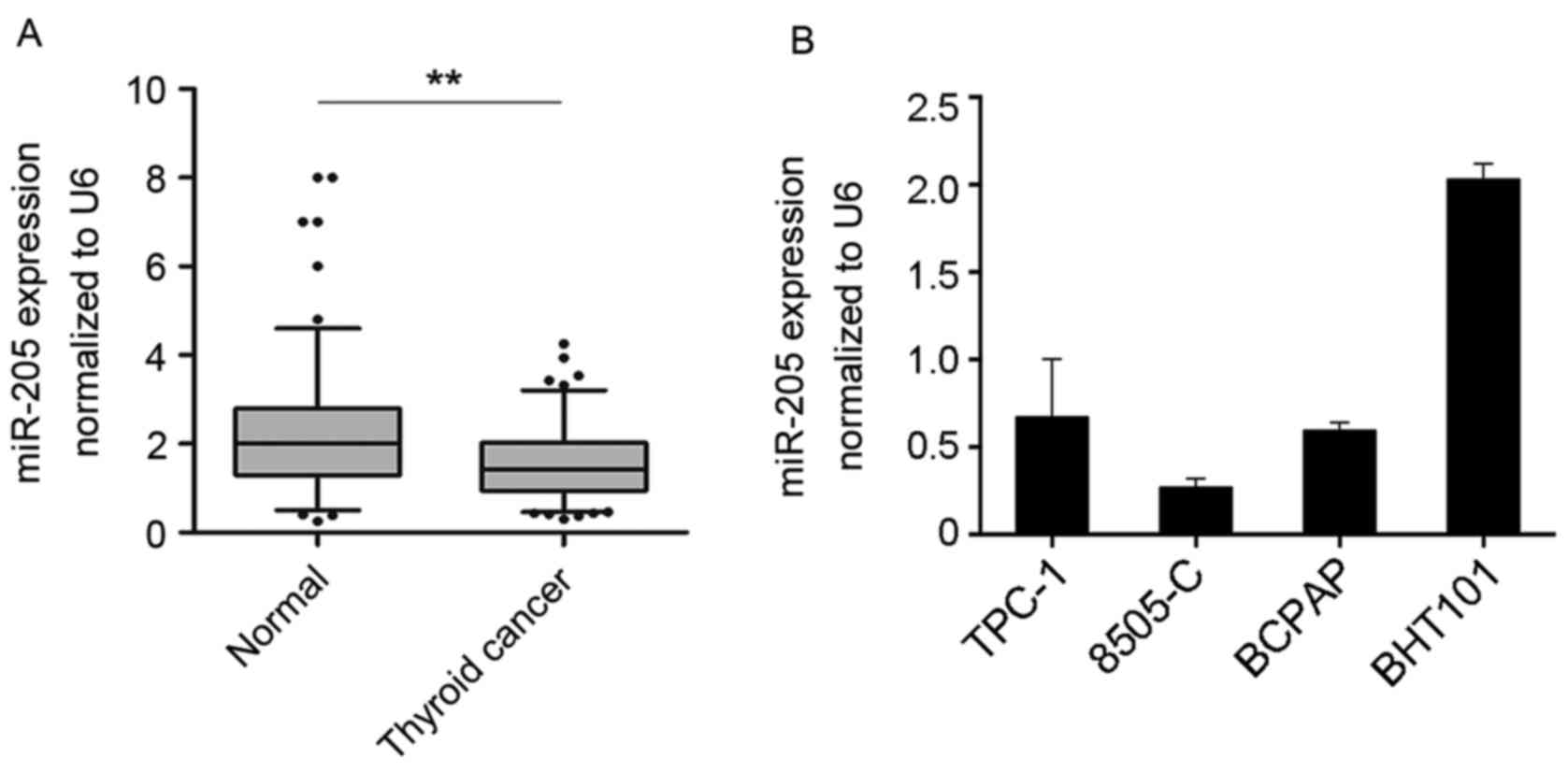
To investigate the expression of miR-205 in human
thyroid cancer tissues, the expression profile of miR-205 in 132
paired thyroid cancer and non-tumor tissues was detected by
RT-qPCR. The data demonstrated that the expression levels of
miR-205 were significantly decreased in tumor tissues compared with
non-tumor tissues (P<0.01; Fig.
1A). In addition, a panel of thyroid cancer cell lines was
tested, including TPC-1, 8505-C, BCPAP and BHT-101. The results
indicated that miR-205 expression was relatively high in BHT101
cells (Fig. 1B). These data
suggested that miR-205 may be involved in thyroid cancer
development.
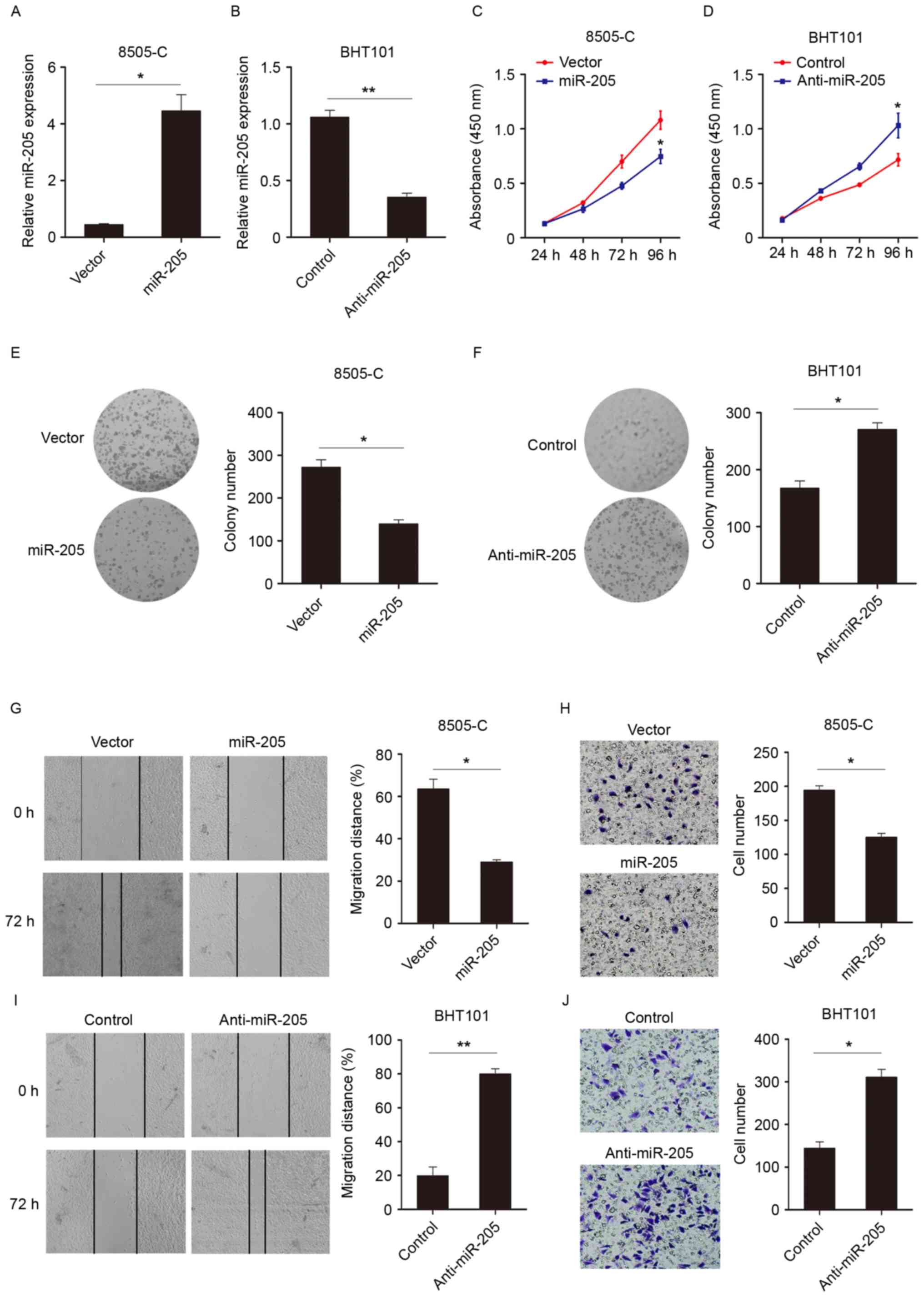
miR-205 inhibits proliferation and
invasion of thyroid cancer cells
To investigate the biological functions of miR-205
in thyroid cancer, the expression of miR-205 was modulated using
exogenous transfecting miR mimics (Fig. 2A) or inhibitors (Fig. 2B). The effect of miR-205 on cell
growth using CCK-8 assays was then examined. As exhibited in
Fig. 2C, the miR-205
overexpression group demonstrated significantly decreased
proliferation compared with the control group at 96 h. Similarly,
when miR-205 expression was knocked down, cell growth was increased
(Fig. 2D). Also, the colony
formation assay confirmed the observations (Fig. 2E and F) that miR-205 expression
suppressed cell viability. Wound healing and Transwell assays were
performed to evaluate the impact of miR-205 on the migratory and
invasive capability of thyroid cancer cells. As illustrated in
Fig. 2G and H, compared with the
control group, overexpression of miR-205 decreased migratory and
invasive. However, knocking down miR-205 expression promoted cell
migration and invasion (Fig. 2I and
J). These data suggested that miR-205 could suppress cell
proliferation, migration and invasion.
YAP1 is a direct target of
miR-205
The potential targets of miR-205 were further
investigated by predicting the binding site using TargetScan and
combined with the protein expression in GEO database (18). As demonstrated in Fig. 3A, miR-205 could bind to the 3′-UTR
of YAP1. Therefore, luciferase reporter vectors containing WT and
MU 3′-UTR were constructed and transfected into 293T cells together
with miR-205 or NC. Following 48 h culture, the luciferase activity
was significantly reduced in cells transfected with YAP1 WT 3′-UTR
and miR-205, rather than with MU 3′-UTR (Fig. 3B). Then the expression of YAP1 was
determined by RT-qPCR and western blotting. As demonstrated in
Fig. 3C and D, the expression of
YAP1 mRNA and protein was decreased in miR-205 mimic-transfected
cells, as well as TAZ and Bcl-xl, which are considered to be
downstream genes of YAP1 (19,20).
Inhibition of miR-205 could restore YAP1 expression and activate
the serine/threonine-protein kinase 4 (hippo) signaling pathway.
The YAP1 level in thyroid cancer tissue was measured and the
results demonstrated that YAP1 was significantly upregulated in
tumor tissues compared with the non-tumor tissues (Fig. 3E). As YAP1 was a potential target
of miR-205, the correlation between YAP1 and miR-205 was also
examined in thyroid cancer tissues. An inverse correlation between
the expression of YAP1 and miR-205 (r=−0.21; P=0.01; Fig. 3F) was demonstrated. These data
revealed that YAP1 was a potential target of miR-205 in thyroid
cancer.
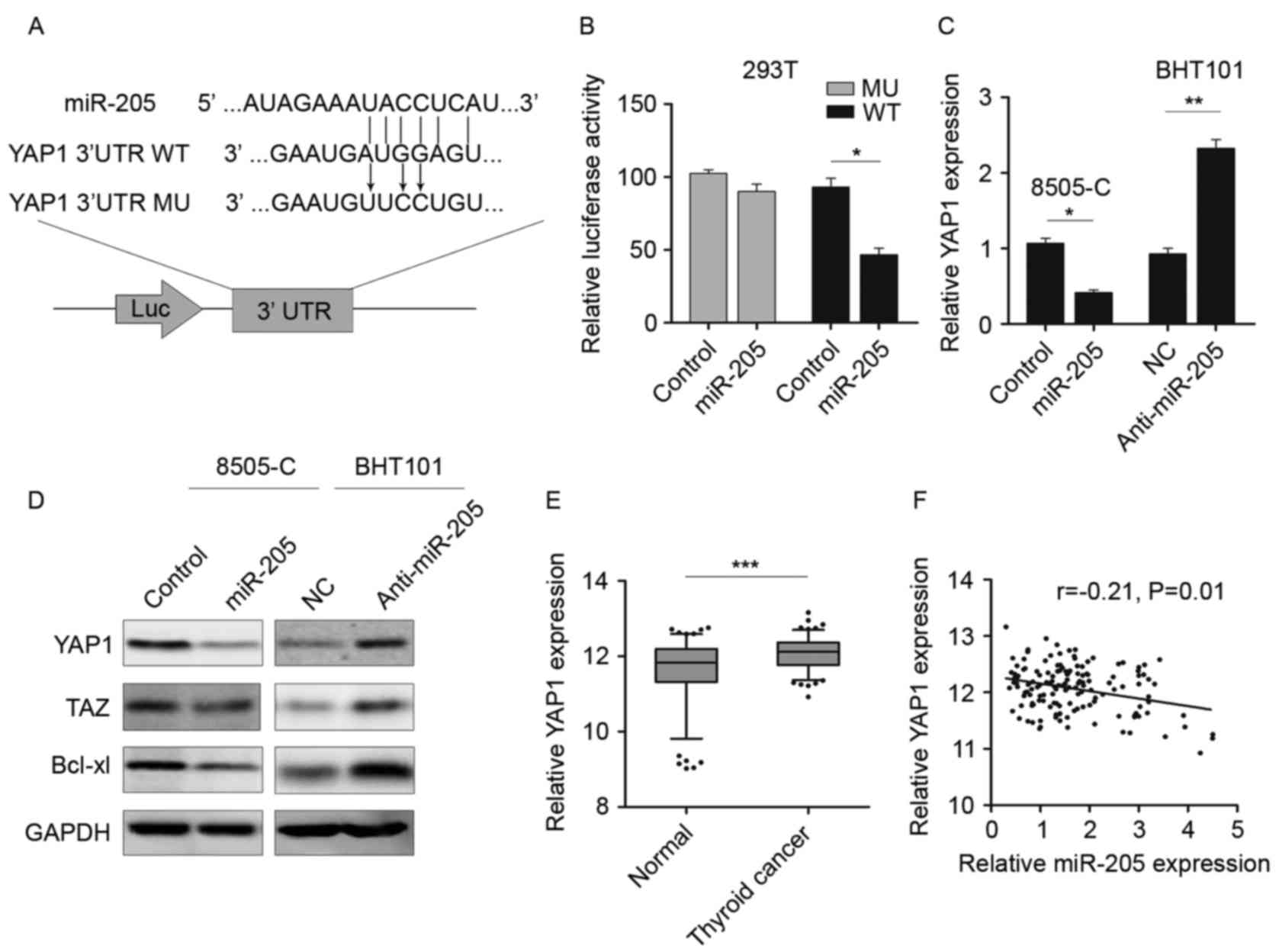 | Figure 3.YAP1 is a direct target of miR-205.
(A) Schematic of the binding sites of miR-205 in the 3′UTR of YAP1.
(B) Luciferase reporter assay in 293T cells cotransfected with
miR-205 and luciferase vector. (C) RT-qPCR was performed to detect
the YAP1 expression following miR-205 overexpression or knockdown.
(D) Western blotting was performed to detect the YAP1, TAZ and
Bcl-xl expression following miR-205 overexpression or knockdown.
(E) RT-qPCR was performed to detect YAP1 expression in thyroid
cancer tissues. The data were presented graphically using a box and
whiskers plot. The box represents the upper and lower quartiles and
the median; the whiskers indicate the 5–95% data points. The
external points represent the data out of the 5–95% range.
Mann-Whitney-U test was used to compare the expression of YAP1 in
two groups. (F) Spearman's rank correlation coefficient analysis
was performed to measure the correlation between the YAP1 and
miR-205 in thyroid cancer tissues. *P<0.05, **P<0.01,
***P<0.001. YAP1, Yes associated protein 1; Taz,
anti-Translin-associated zinc finger protein 1; miR, microRNA;
Bcl-xl, Bcl2-like protein 1; NC, normal control; RT-qPCR, reverse
transcriptase-quantitative polymerase chain reaction; UTR,
untranslated region; Luc, luciferase. |
YAP1 is involved in the inhibitory
effects of miR-205
To further investigate the role of YAP1 in miR-205
mediated tumor suppression, RNA interference was used to knockdown
YAP1 expression. As demonstrated in Fig. 4A, knockdown of miR-205 increased
YAP1 and activated hippo signaling pathway to induce TAZ
expression. When YAP1 was subsequently knocked down, the expression
of TAZ was reduced. This result indicated that miR-205 suppressed
the hippo signaling pathway by targeting YAP1. Further experiments
demonstrated that knocking down YAP1 reversed the
anti-miR-205-dependent effect on cell growth and invasion in
thyroid cancer cells (Fig. 4B-D).
These data revealed that downregulation of YAP1 could attenuate the
protumorigenic effects of miR-205 inhibitors, suggesting that YAP1
is involved in miR-205-mediated tumor suppression.
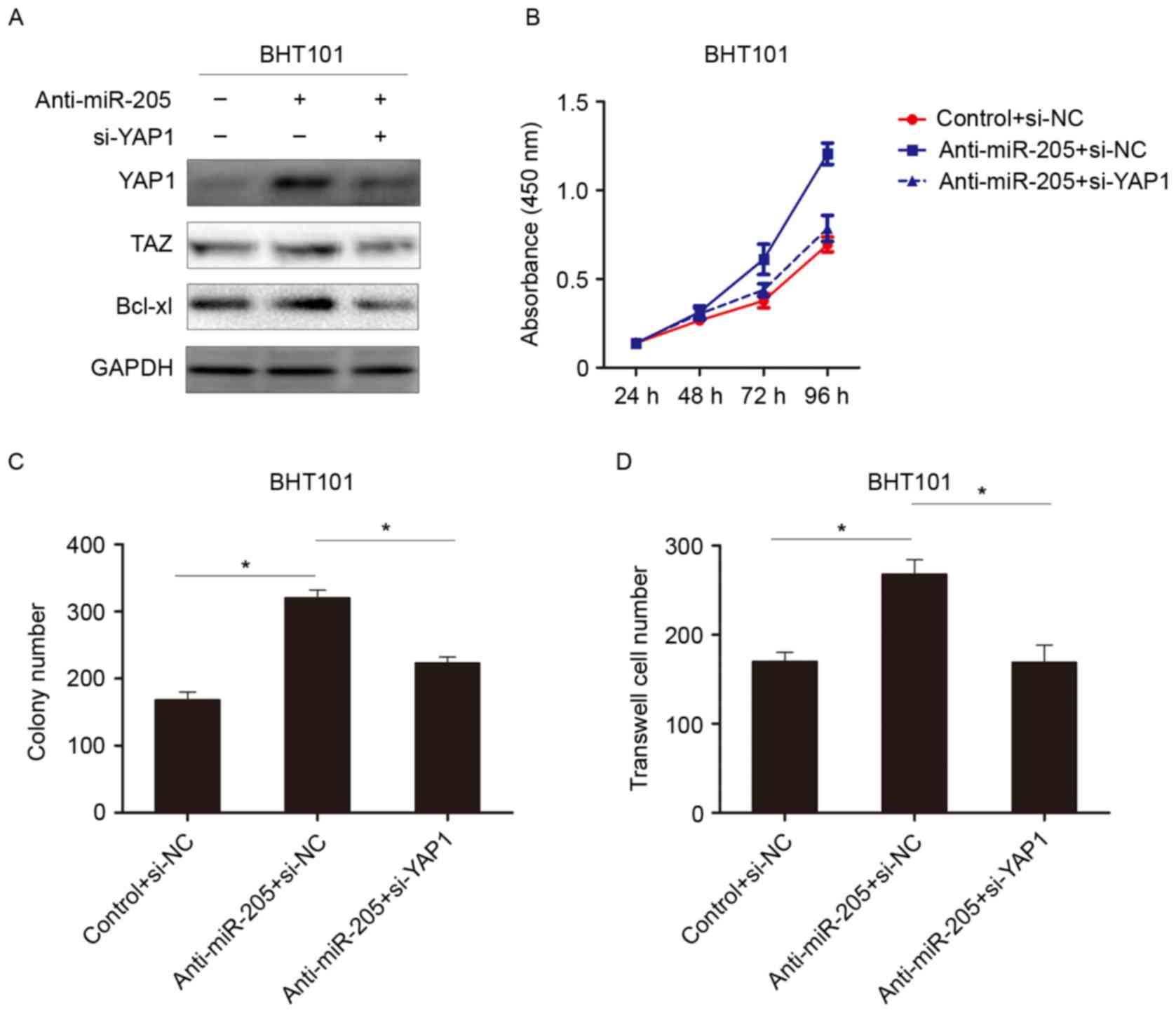 | Figure 4.YAP1 is involved in suppressing the
function of miR-205. (A) Western blot analysis was performed to
detect the indicated protein expression when cells were treated
with an miR-205 inhibitor, or YAP1-siRNA and an miR-205 inhibitor.
(B) Analysis of the absorbance at 450 nm of cells transfected with
anti-miR-205, anti-miR-205 and siYAP1, or NC. (C) Colony assay and
(D) invasion assay following treatment with anti-miR-205,
anti-miR-205 and siYAP1, or NC. *P<0.05. si, small interfering;
YAP1, Yes associated protein 1; Taz, anti-Translin-associated zinc
finger protein 1; miR, microRNA; Bcl-xl, Bcl2-like protein 1; NC,
normal control. |
Discussion
Previous studies have indicated that the deregulated
miRs are associated with tumor initiation and development. miR-205
has been identified to be downregulated miR in several types of
cancers, including breast, bladder cancer and head and neck
squamous cell carcinoma. miR-205 targets phosphatidylinositol
3,4,5-trisphosphate 3-phosphatase and dual-specificity protein
phosphatase PTEN, and inhibits renal cell carcinoma progression
(21). However, miR-205 was
demonstrated to target tumor protein p53-inducible nuclear protein
1, resulting in the tumorigenesis of nasopharyngeal carcinoma.
These results indicated that the function of miR-205 was tumor
dependent. miR-205 could be an accurate marker for squamous lung
cancer (22). Low expression of
miR-205 could be a prognostic factor for head and neck squamous
cell carcinoma (23). However, the
expression and roles of miR-205 in thyroid carcinoma remain
unclear.
In the present study, the expression of miR-205 was
observed to decrease in thyroid tumor samples compared with the
non-tumor tissues, suggesting that miR-205 may be a potential tumor
suppressor in thyroid cancer. In terms of the mechanism of action,
miR-205 could be epigenetically regulated by hypermethylation of
its promoter (24). The mechanism
of downregulation of miR-205 in thyroid cancer requires further
investigation. miR-205 expression in thyroid cancer cells was
examined and it was demonstrated that miR-205 was highly expressed
in BHT101 cells but exhibited low expression in 8505-C cells. Based
on the miR-205 expression level identified, miR-205 was increased
or inhibited to assess the biological function in the present
study. It was demonstrated that miR-205 suppressed proliferation,
migration and invasion of thyroid cancer cells, which may explain
miR-205 downregulation in cancer tissues. Furthermore, YAP1 was
identified as a direct target of miR-205. To investigate the role
of YAP1 further, a luciferase reporter assay was performed, and the
results indicated that miR-205 could bind to the 3′-UTR of YAP1 and
decrease the YAP1 mRNA level. In addition, it was demonstrated that
the levels of miR-205 and YAP1 were inversely correlated in thyroid
cancer samples.
While miR-205 inhibited cell migration and invasion,
it has been demonstrated to regulate epithelial to mesenchymal
transition by targeting zinc finger E-box-binding homeobox 1 and
Smad-interacting protein 1, together with the miR-200 family
(25). Also, protein kinase Cε,
receptor tyrosine-protein kinase erbB-3, vascular endothelial
growth factor A and Bcl-2-like protein 2 were reported to be the
targets of miR-205 in prostate and breast cancer (26–29).
These data imply the complex regulatory networks that miRs regulate
have multiple targets and a target could be regulated by various
miRs. The previous studies indicated that miR-205 level exhibited
prognostic value in non-small cell lung cancer, and endometrial and
breast cancer (22,30,31).
Furthermore, Zhang et al (24) demonstrated that hepatitis B virus X
protein induced hypermethylation of the miR-205 promoter and
suppressed its expression. Also, it is reported that miR-205 could
be regulated by long non-coding RNA HOTAIR in bladder cancer cells
(32). The regulation of miR-205
in thyroid cancer requires further investigation.
YAP1 is located on chromosome 11q13, coding a
downstream nuclear effector of the hippo signaling pathway. As
consequence, YAP1 could regulate octamer binding protein 4 activity
and SOX2 expression, facilitating a self-renewing ability (33). Also YAP1 could confer resistance to
chemotherapy in esophageal cancer (34). YAP1 frequently functions as an
oncogene in numerous types of cancer, including hepatocellular
carcinoma, breast cancer and oral squamous cell carcinoma. However,
its role in thyroid cancer remained unclear. Hudson et al
(35) has reported the inhibition
of YAP1 may be important for medullary thyroid carcinoma
development. However Garcia-Rendueles et al (36) demonstrated that YAP1 dependent
transactivation of RAS promoted poorly differentiated thyroid
cancer progression. YAP1-transcriptional enhancer factor TEF-1
could be activated by the hippo signaling pathway and enhance
transcription of target genes. In the present study, YAP1
expression was also detected and the downstream genes of hippo
signaling pathways, including TAZ and Bcl-xl. The results
demonstrated that miR-205 could regulate hippo signaling pathway by
targeting YAP1 and knockdown of YAP1 abrogated the
pro-tumorigenesis roles of anti-miR-205, which meant YAP1 could
promote tumor cell growth and invasion in the present study.
Previous studies have demonstrated that several miRs could regulate
YAP1, including miR-132 and miR-16-1 (37,38),
indicating the key role of the miR-regulatory network in tumor
initiation and progression.
In conclusion, it was demonstrated that miR-205 was
downregulated in thyroid cancer tissues. Through knockdown and
overexpression experiments, it was revealed that miR-205 inhibited
tumor cell proliferation and invasion. In addition, miR-205
expression was inversely correlated with YAP1 in thyroid cancer
samples. miR-205 suppressed the hippo signaling pathway through
targeting YAP1, and inhibited thyroid cancer cell growth and
invasion. The results of the present study suggested that
overexpression of miR-205 could be a potential therapeutic target
for thyroid cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Health and Family Planning Commission of Shanxi Province (grant no.
2015014).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DL contributed significantly to the design of the
study and writing and analyzing the data and the manuscript. QW and
NL helped with collecting and arranging the patients information.
SZ helped to perform the statistical analysis. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Shanxi Provincial People's Hospital. Written informed consent was
obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ulisse S, Baldini E, Sorrenti S, Barollo
S, Prinzi N, Catania A, Nesca A, Gnessi L, Pelizzo MR, Mian C, et
al: In papillary thyroid carcinoma BRAFV600E is associated with
increased expression of the urokinase plasminogen activator and its
cognate receptor, but not with disease-free interval. Clin
Endocrinol. 77:780–786. 2012. View Article : Google Scholar
|
|
4
|
Benvenga S and Koch CA: Molecular pathways
associated with aggressiveness of papillary thyroid cancer. Curr
Genomics. 15:162–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu X, Bishop J, Shan Y, Pai S, Liu D,
Murugan AK, Sun H, El-Naggar AK and Xing M: Highly prevalent
TERT promoter mutations in aggressive thyroid cancers.
Endocr Relat Cancer. 20:603–610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carina V, Zito G, Pizzolanti G, Richiusa
P, Criscimanna A, Rodolico V, Tomasello L, Pitrone M, Arancio W and
Giordano C: Multiple pluripotent stem cell markers in human
anaplastic thyroid cancer: The putative upstream role of SOX2.
Thyroid. 23:829–837. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yasui K, Shimamura M, Mitsutake N and
Nagayama Y: SNAIL induces epithelial-to-mesenchymal transition and
cancer stem cell-like properties in aldehyde
dehydroghenase-negative thyroid cancer cells. Thyroid. 23:989–996.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Landa I, Ibrahimpasic T, Boucai L, Sinha
R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP,
Xu B, et al: Genomic and transcriptomic hallmarks of poorly
differentiated and anaplastic thyroid cancers. J Clin Invest.
126:1052–1066. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dettmer MS, Perren A, Moch H, Komminoth P,
Nikiforov YE and Nikiforova MN: MicroRNA profile of poorly
differentiated thyroid carcinomas: New diagnostic and prognostic
insights. J Mol Endocrinol. 52:181–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pallante P, Battista S, Pierantoni GM and
Fusco A: Deregulation of microRNA expression in thyroid neoplasias.
Nat Rev Endocrinol. 10:88–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Damanakis AI, Eckhardt S, Wunderlich A,
Roth S, Wissniowski TT, Bartsch DK and Di Fazio P: MicroRNAs let7
expression in thyroid cancer: Correlation with their deputed
targets HMGA2 and SLC5A5. J Cancer Res Clin Oncol. 142:1213–1220.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Piovan C, Palmieri D, Di Leva G, Braccioli
L, Casalini P, Nuovo G, Tortoreto M, Sasso M, Plantamura I, Triulzi
T, et al: Oncosuppressive role of p53-induced miR-205 in triple
negative breast cancer. Mol Oncol. 6:458–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boll K, Reiche K, Kasack K, Mörbt N,
Kretzschmar AK, Tomm JM, Verhaegh G, Schalken J, von Bergen M, Horn
F, et al: MiR-130a, miR-203 and miR-205 jointly repress key
oncogenic pathways and are downregulated in prostate carcinoma.
Oncogene. 32:277–285. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang M, Zhang P, Hu G, Xiao Z, Xu F,
Zhong T, Huang F, Kuang H and Zhang W: Relative expressions of
miR-205-5p, miR-205-3p, and miR-21 in tissues and serum of
non-small cell lung cancer patients. Mol Cell Biochem. 383:67–75.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Zhao X, Wang D, He W, Zhang S, Cao
W, Huang Y, Wang L, Zhou S and Luo K: Up-regulated expression of
phospholipase C, beta1 is associated with tumor cell proliferation
and poor prognosis in hepatocellular carcinoma. Onco Targets Ther.
9:1697–1706. 2016.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
ELife. 4:2015. View Article : Google Scholar
|
|
19
|
Pan D: The hippo signaling pathway in
development and cancer. Dev Cell. 19:491–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rosenbluh J, Nijhawan D, Cox AG, Li X,
Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC, et
al: β-Catenin-driven cancers require a YAP1 transcriptional complex
for survival and tumorigenesis. Cell. 151:1457–1473. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang H, Chen B, Duan B, Zheng J and Wu X:
miR205 suppresses cell proliferation, invasion, and metastasis via
regulation of the PTEN/AKT pathway in renal cell carcinoma. Mol Med
Rep. 14:3343–3349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lebanony D, Benjamin H, Gilad S, Ezagouri
M, Dov A, Ashkenazi K, Gefen N, Izraeli S, Rechavi G, Pass H, et
al: Diagnostic assay based on hsa-miR-205 expression distinguishes
squamous from nonsquamous non-small-cell lung carcinoma. J Clin
Oncol. 27:2030–2037. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Childs G, Fazzari M, Kung G, Kawachi N,
Brandwein-Gensler M, McLemore M, Chen Q, Burk RD, Smith RV,
Prystowsky MB, et al: Low-level expression of microRNAs let-7d and
miR-205 are prognostic markers of head and neck squamous cell
carcinoma. Am J Pathol. 174:736–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang T, Zhang J, Cui M, Liu F, You X, Du
Y, Gao Y, Zhang S, Lu Z, Ye L and Zhang X: Hepatitis B virus X
protein inhibits tumor suppressor miR-205 through inducing
hypermethylation of miR-205 promoter to enhance carcinogenesis.
Neoplasia. 15:1282–1291. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gandellini P, Folini M, Longoni N, Pennati
M, Binda M, Colecchia M, Salvioni R, Supino R, Moretti R, Limonta
P, et al: miR-205 Exerts tumor-suppressive functions in human
prostate through down-regulation of protein kinase Cepsilon. Cancer
Res. 69:2287–2295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bhatnagar N, Li X, Padi SK, Zhang Q, Tang
MS and Guo B: Downregulation of miR-205 and miR-31 confers
resistance to chemotherapy-induced apoptosis in prostate cancer
cells. Cell Death Dis. 1:e1052010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu Y, Qiu Y, Yague E, Ji W, Liu J and
Zhang J: miRNA-205 targets VEGFA and FGF2 and regulates resistance
to chemotherapeutics in breast cancer. Cell Death Dis. 7:e22912016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yue X, Wang P, Xu J, Zhu Y, Sun G, Pang Q
and Tao R: MicroRNA-205 functions as a tumor suppressor in human
glioblastoma cells by targeting VEGF-A. Oncol Rep. 27:1200–1206.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karaayvaz M, Zhang C, Liang S, Shroyer KR
and Ju J: Prognostic significance of miR-205 in endometrial cancer.
PLoS One. 7:e351582012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Quesne JL, Jones J, Warren J, Dawson SJ,
Ali HR, Bardwell H, Blows F, Pharoah P and Caldas C: Biological and
prognostic associations of miR-205 and let-7b in breast cancer
revealed by in situ hybridization analysis of micro-RNA expression
in arrays of archival tumour tissue. J Pathol. 227:306–314. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun X, Du P, Yuan W, Du Z, Yu M, Yu X and
Hu T: Long non-coding RNA HOTAIR regulates cyclin J via inhibition
of microRNA-205 expression in bladder cancer. Cell Death Dis.
6:e19072015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bora-Singhal N, Nguyen J, Schaal C,
Perumal D, Singh S, Coppola D and Chellappan S: YAP1 regulates OCT4
activity and SOX2 expression to facilitate self-renewal and
vascular mimicry of stem-like cells. Stem Cells. 33:1705–1718.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Song S, Honjo S, Jin J, Chang SS, Scott
AW, Chen Q, Kalhor N, Correa AM, Hofstetter WL, Albarracin CT, et
al: The Hippo coactivator YAP1 mediates EGFR overexpression and
confers chemoresistance in esophageal cancer. Clin Cancer Res.
21:2580–2590. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hudson J, Duncavage E, Tamburrino A,
Salerno P, Xi L, Raffeld M, Moley J and Chernock RD: Overexpression
of miR-10a and miR-375 and downregulation of YAP1 in medullary
thyroid carcinoma. Exp Mol Pathol. 95:62–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Garcia-Rendueles ME, Ricarte-Filho JC,
Untch BR, Landa I, Knauf JA, Voza F, Smith VE, Ganly I, Taylor BS,
Persaud Y, et al: NF2 loss promotes oncogenic RAS-induced thyroid
cancers via YAP-dependent transactivation of RAS proteins and
sensitizes them to MEK inhibition. Cancer Discov. 5:1178–1193.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kang W, Tong JH, Lung RW, Dong Y, Zhao J,
Liang Q, Zhang L, Pan Y, Yang W, Pang JC, et al: Targeting of YAP1
by microRNA-15a and microRNA-16-1 exerts tumor suppressor function
in gastric adenocarcinoma. Mol Cancer. 14:522015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lei CJ, Li L, Gao X, Zhang J, Pan QY, Long
HC, Chen CZ, Ren DF and Zheng G: Hsa-miR-132 inhibits proliferation
of hepatic carcinoma cells by targeting YAP. Cell Biochem Funct.
33:326–333. 2015. View
Article : Google Scholar : PubMed/NCBI
|