Introduction
Alveolar bone defect is a common clinic issue, which
may be caused by multiple factors, including inflammation, trauma,
tumor, or congenital and developmental disorders (1). It may have a serious impact on
chewing and pronunciation, and may even lead to serious facial
dysfunction and deformities (2).
Therefore, efficient alveolar bone restoration is required to
restore function and appearance. Although bone grafts are commonly
used in alveolar bone reconstruction, these methods have numerous
drawbacks, including inducing new defects, immune rejections and
potential infection (3).
Three-dimensional printed (3DP) scaffolds combined
with osteogenic cells and/or osteo-inductive molecules have emerged
as a promising alternative to grafts in bone tissue regeneration
(4,5). The fused deposition modeling (FDM)
technique, a type of 3DP technique, has been successfully used in
bone tissue engineering. Scaffolds are fabricated based on the
extrusion of molten polymer fibers in a layer-by-layer manner,
using the data collected from a computer-aided design program and
computer-aided medical imaging including computed tomography or
X-ray (6). Therefore, an FDM
scaffold is able to highly match the external morphology of the
defect and accurately restore the internal structure of the tissue,
and thus represents a powerful approach for complex tissue
engineering, particularly for cranio-maxillofacial constructs
(1).
The ideal scaffold should be biodegradable and
biocompatible. It should additionally possess a highly porous
interconnected network with excellent surface characteristics for
cell affinity and tissue regeneration (7). Selecting suitable materials for 3DP
scaffold production remains challenging. U. S. Food and Drug
Administration-approved synthetic polymers poly (ε-caprolactone)
(PCL) and poly-lactic-co-glycolic acid (PLGA) have the advantages
of being relatively low cost, with the possibility of long-term
storage and superior bioactivity, thus these polymers have received
considerable attention in bone regeneration (8). PCL is flexible and used in various
fabrication techniques; however, it has been demonstrated to have
low degradability and inferior bioactivity (9). PLGA is hydrophilic, biodegradable and
highly biocompatible; however, is more brittle (10,11).
The combined use of PCL and PLGA may overcome the shortcomings of
single use and become an excellent biocompatible composite, which
has been extensively applied in the clinic for various tissue
restoration procedures (12–18).
Previous studies have primarily focused on the biocompatibility of
the PCL/PLGA composite scaffold; however, the biodegradable
properties and surface characteristics of PCL/PLGA composite
scaffolds have rarely been reported. Furthermore, few studies have
examined the application of PCL/PLGA composite scaffold in alveolar
bone regeneration.
Human periodontal ligament stem cells (hPDLSCs)
possess immense potential in bone tissue engineering, owing to
their various sources, easy attainability (19), high rates of proliferation,
multi-lineage differentiation ability (20), and great capacity for alveolar bone
(21) and tendon regeneration
(22). In order to investigate a
suitable material for alveolar bone regeneration, scaffolds with
different ratios of PCL and PLGA were fabricated via 3DP and their
surface characteristics and degradative properties, along with the
response of hPDLSCs on these scaffolds, were subsequently
assessed.
Materials and methods
Fabrication of PCL/PLGA scaffolds
The scaffolds were fabricated using a 3DP system
(Bioplotter; EnvisionTEC GmbH, Gladbeck, Germany). PCL (molecular
weight 50,000; Polysciences, Inc., Warrington, PA, USA) and PLGA
(polylactic acid/polyglycolic acid 50:50; molecular weight
50,000–75,000; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) were
used and the ratios of PCL/PLGA were 1:9, 5:5 and 9:1. The blended
powder of PCL/PLGA was melted in a chamber at 130°C, centrifuged at
100 × g for 10 min at a temperature range of 130–150°C, and
dispensed through a 27-gauge metal needle at 135°C to create fully
interconnected structures with diameters and heights of 15 and 1
mm, respectively. The XY dispensing head speed was 100 mm/min and
the dispensing pressure was ~650 kPa.
Surface characteristics
The microscopic appearance of the scaffolds was
observed using scanning electron microscopy (SEM; LEO 1530 variable
pressure field emission SEM; Zeiss GmbH, Jena, Germany) at a
magnification of 75 and ×1,000. The surface roughness of the
scaffolds was determined using a profilometer of laser scanning
confocal microscope (LSM700; Zeiss GmbH) at a magnification of ×50
and ×200. The arithmetic average of the 3D roughness (Sa) and the
quadratic average of the 3D roughness (Sq) was measured. The
wettability of the scaffolds was evaluated by measuring the water
contact angle (CA), using a CA video-based system (OCAl5;
DataPhysics, Filderstadt, Germany) and SCA20 software (version 1.0;
DataPhysics).
Degradation characteristics
Scaffolds were cut into pieces weighing 50 mg and
were immersed separately in PBS (pH 7.4; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) at 37°C over 12 weeks. PBS was changed
every 3 weeks. At 3, 6, 9 and 12 weeks, the scaffolds were removed,
rinsed with distilled water and air-dried at room temperature for
24 h. The weight loss was measured using a sensitive scale (ME103;
Mettler-Toledo GmbH, Greifense, Switzerland). The pH value of the
PBS following removal of the scaffolds was measured using a pH
meter (SevenMulti; Mettler-Toledo). Five specimens were examined
for each scaffold at each time point and the average value was
calculated and recorded.
Cell culture and identification
The protocol was approved by the Ethics Committee of
the Hospital of Stomatology, Sun Yat-sen University (Guangzhou,
China). Informed consent from each patient was obtained. A total of
16 healthy premolars were acquired from four orthodontic patients
aged 14–18 years (two males and two females) at the Hospital of
Stomatology, Sun Yat-sen University from September 2016 to October
2016. Teeth extracted for orthodontic treatment were included in
the present study. Teeth with cavities, periodontitis, broken
sputum or not fully developed apical foramens were excluded. As
previously described (23),
hPDLSCs were isolated from the periodontal ligament of the teeth,
and cultured at 37°C with a limited dilution method in complete
culture medium, containing α modified eagle medium (MEM; Gibco;
Thermo Fisher Scientific, Inc.), 10% fetal bovine serum, 100 U/ml
penicillin and 100 µg/ml streptomycin (all Gibco; Thermo Fisher
Scientific, Inc.). The osteogenic and adipogenic differentiation
potential of the hPDLSCs was investigated by alizarin red S
staining and oil red O staining, respectively. After 1 day of
culture, hPDLSCs were cultured with osteoblast-inducing medium (0.1
µM dexamethasone, 50 µg/ml ascorbic acid and 10 mM β-glycerol
phosphate) or adipogenic medium (100 nM dexamethasone, 10 µg/ml
insulin, 0.5 mM 3-isobutyl-1-methylxanthine and 50 mM
indomethacin). After 2 weeks, adipogenic induced cells were fixed
in 4% paraformaldehyde for 15 min at room temperature and stained
with Oil Red O staining solution (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China) at room temperature, according to the
manufacturer's protocol. Briefly, cells were stained with reagent 1
for 15 min, washed with distilled water for 20 sec and subsequently
stained with reagent 2 for 5 min. Furthermore, after 4 weeks, the
osteoblast induced cells were fixed in 4% paraformaldehyde and
stained with Alizarin Red S staining solution (Sigma-Aldrich; Merck
KGaA) for 3 min at room temperature. All dishes were subsequently
dried at room temperature and observed under an optical light
microscope at ×200 magnification.
The colony-forming ability was assessed using
colony-forming assays as previously described (23). Briefly, human PDLSCs (P3) were
seeded in 10 cm diameter culture dishes at a density of
1×103 cells per dish and cultured in complete medium.
After 12 days of incubation, cells were fixed in 4%
paraformaldehyde for 15 min at room temperature and stained with
0.1% crystal violet (Sigma-Aldrich; Merck KGaA) for 15 min at room
temperature. The dishes were subsequently washed twice with PBS and
observed under an inverted microscope at ×30 magnification. An
aggregate of 50 or more cells were considered colony unit and were
included in the final statistical analysis; aggregates of fewer
cells were excluded. Cloning formation efficiency was assessed by
examining the ratio of colony units and seeding cell number.
The specific surface markers of hPDLSCs, including
integrin β-1 [cluster of differentiation (CD)29], cell surface
glycoprotein MUC18 (CD146), CD44 (antigen CD44), thy-1 membrane
glycoprotein (CD90), hematopoietic progenitor cell antigen (CD34)
and receptor-type tyrosine-protein phosphatase C (CD45) were
identified by flow cytometry. Briefly, once 90% confluence was
reached, hPDLSCs were trypsinized and incubated with all antibodies
diluted at 1:100, CD146 (cat. no. sc-53369), CD29 (cat. no.
sc-59827), CD44 (cat. no. sc-65265), CD90 (cat. no. sc-53116), CD34
(cat. no. sc-133082) and CD45 (cat. no. sc-53045) at 4°C for 16 h
in the dark, all of which were mouse IgG-flourescein isothiocyanate
(FITC) antibodies purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Cell suspensions incubated with served as
controls. Finally, cells were fixed with 4% paraformaldehyde for 15
min at room temperature, washed three times with PBS and analyzed
via fluorescence-activated cell sorting with a flow cytometer (BD
Biosciences, San Jose, CA, USA) and BD Cell Quest Pro software
(version 5.1; BD Biosciences). Cells passaged to P4 were used in
the following experiments.
Cell seeding
Prior to seeding hPDLSCs, the scaffolds were
immersed in 70% ethanol for 12 h, air-dried in a clean workstation
for 1 day and incubated with α-MEM in 24-well plates for 7 days.
Subsequently, hPDLSCs were seeded on the scaffolds at a density of
1×106 cells/well.
Cell adhesion
Following 1 and 2 days of culture, the adherent
cells were fixed in 3% paraformaldehyde for 15 min and stained with
DAPI (Beyotime Institute of Biotechnology, Shanghai, China) for 10
min at room temperature. The cell numbers on each scaffold were
counted in five random fields, under an epifluorescence microscope
(Observer.Z1; Zeiss GmbH) at ×50 magnification.
Cell proliferation
Following culturing for 1, 3, 5, and 7 days, the
cell proliferation of hPDLSCs was assessed using a Cell Counting
Kit (CCK)-8 assay (Beyotime Institute of Biotechnology). At each
time point, medium was replaced with serum-free medium containing
CCK-8 solution (0.5 mg/ml) at 37°C under 5% CO2 for 2 h.
The optical density (OD) at 450 nm was measured using a
spectrophotometer (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan).
Cell morphology
Following 3 days of incubation, the cell morphology
of the hPDLSCs was observed under SEM. The samples were fixed in
2.5% (w/w) glutaraldehyde for 4 h at 4°C, dehydrated in a 30, 50,
70, 90 and 100% ethanol series (15 min for each step), freeze-dried
and sputter coated with gold for SEM observation.
Cell adhesion morphology
Following culturing for 7 and 14 days, the
cytoskeletal morphology of the hPDLSCs was observed using confocal
laser scanning microscopy (CLSM: LSM780; Zeiss GmbH) at a
magnification of ×50 and ×200, and SEM at a magnification of ×75
and ×1,000. Samples for CLSM observation were fixed in 3%
paraformaldehyde for 10 min at room temperature, and immersed in
0.1% TritonX-100 for 10 min. The cells were subsequently stained
with 1:100 Actin-Tracker Green (Beyotime Institute of
Biotechnology) and DAPI at 37°C for 1 h. Samples for SEM
observation were prepared by the same protocol as for cell
morphology observation.
Alizarin Red S staining
Subsequent to 14 days of osteogenic induction, the
hPDLSCs on scaffolds were fixed in 3% paraformaldehyde for 10 min,
and stained with Alizarin Red S staining solution (Beyotime
Institute of Biotechnology) for 1 min, both at room temperature.
The scaffolds were subsequently washed twice with PBS until no more
color appeared and observed under an optical microscope at ×30
magnification. Finally, 2% cetylpyridinium chloride was added to
the samples to dissolve the mineralized nodules. The OD at 562 nm
was measured using a spectrophotometer.
Alkaline phosphatase (ALP)
activity
Subsequent to seeding for 1 day, the hPDLSCs were
cultured with osteoblast-inducing medium containing 0.1 µM
dexamethasone, 50 µg/ml ascorbic acid and 10 mM β-glycerol
phosphate. On day 7 and 14, protein concentration and ALP activity
were determined using a bicinchoninic acid protein assay kit
(Beijing ComWin Biotech Co., Ltd., Beijing, China) and an ALP kit
(Nanjing Jiancheng Bioengineering Institute), respectively,
according to the manufacturer's protocols. OD values were measured
at 560 and 520 nm, correspondingly, using a spectrophotometer. ALP
activity was normalized to the total protein amount.
Expression of osteogenic
biomarkers
hPDLSCs were cultured with osteoblast-inducing
medium for 7 and 14 days. Total RNA was extracted from hPDLSCs
using the RNAzol RNA extraction reagent (GeneCopoeia, Inc.,
Rockville, MD, USA; cat. no. RN190). The reverse transcription (RT)
reactions were performed using Prime Script RT Master Mix (cat. no.
RR037; Takara Bio, Inc., Otsu, Japan). The RT was performed at 37°C
for 15 min and 85°C for 5 sec. A quantitative polymerase chain
reaction (qPCR) was conducted using a SYBR PCR Master Mix kit (cat.
no. 4367218; Thermo Fisher Scientific, Inc.) and 10 µM specific
primers in 20 µl, consisting of 40 cycles at 95°C for 10 min, 95°C
for 15 sec, 60°C for 60 sec, and 72°C for 30 sec. The data were
acquired using the LightCycler480 System (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). β-actin was used to normalize the
expression level of ALP, osteocalcin (OCN) and runt-related
transcription factor 2 (RUNX2). The primers are presented in
Table I. Relative expression was
calculated using the 2−ΔΔCq method (24).
 | Table I.Primer nucleotide sequences for the
reverse transcription-quantitative polymerase chain reaction. |
Table I.
Primer nucleotide sequences for the
reverse transcription-quantitative polymerase chain reaction.
| Gene | Primer nucleotide
sequence |
|---|
| β-actin | Forward: |
5′-GCCTTCAAGGTGGTAGCCC-3′ |
|
| Reverse: |
5′-CGTTACCCGCCATGACAGTA-3′ |
| ALP | Forward: |
5′-CATGAAATACGAGATCCACCGAGAC-3′ |
|
| Reverse: |
5′-ATGCGACCACCCTCCACGAAG-3′ |
| OCN | Forward: |
5′-CCCAGGCGCTACCTGTATCAA-3′ |
|
| Reverse: |
5′-GGTCAGCCAACTCGTCACAGTC-3′ |
| RUNX2 | Forward: |
5′-TGGTTACTGTCATGGCGGGTA-3′ |
|
| Reverse: |
5′-TCTCAGATCGTTGAACCTTGCTA-3′ |
Statistical analysis
Data are presented as the mean ± standard deviation
(n=5) and were statistically analyzed using SPSS 13.0 (SPSS, Inc.,
Chicago, IL, USA). The difference among groups was analyzed by
one-way analysis of variance followed by the Student-Newman-Keuls
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
hPDLSC culture and identification
Colony-forming units of the hPDLSCs were counted and
the colony formation efficiency was 30.2% (Fig. 1A and B). The hPDLSCs displayed long
spindle morphology, and demonstrated the ability for osteogenic
(Fig. 1C) and adipogenic
differentiation (Fig. 1D). The
flow cytometric analyses demonstrated that the hPDLSCs were
positive for CD29 (adhesion molecule), CD146 (mesenchymal stem cell
marker), CD44 (receptor molecule) and CD90 (extracellular matrix
protein); however, they were negative for CD34 and CD45
(hematopoietic and angiogenic lineage markers) (Fig. 1E-J).
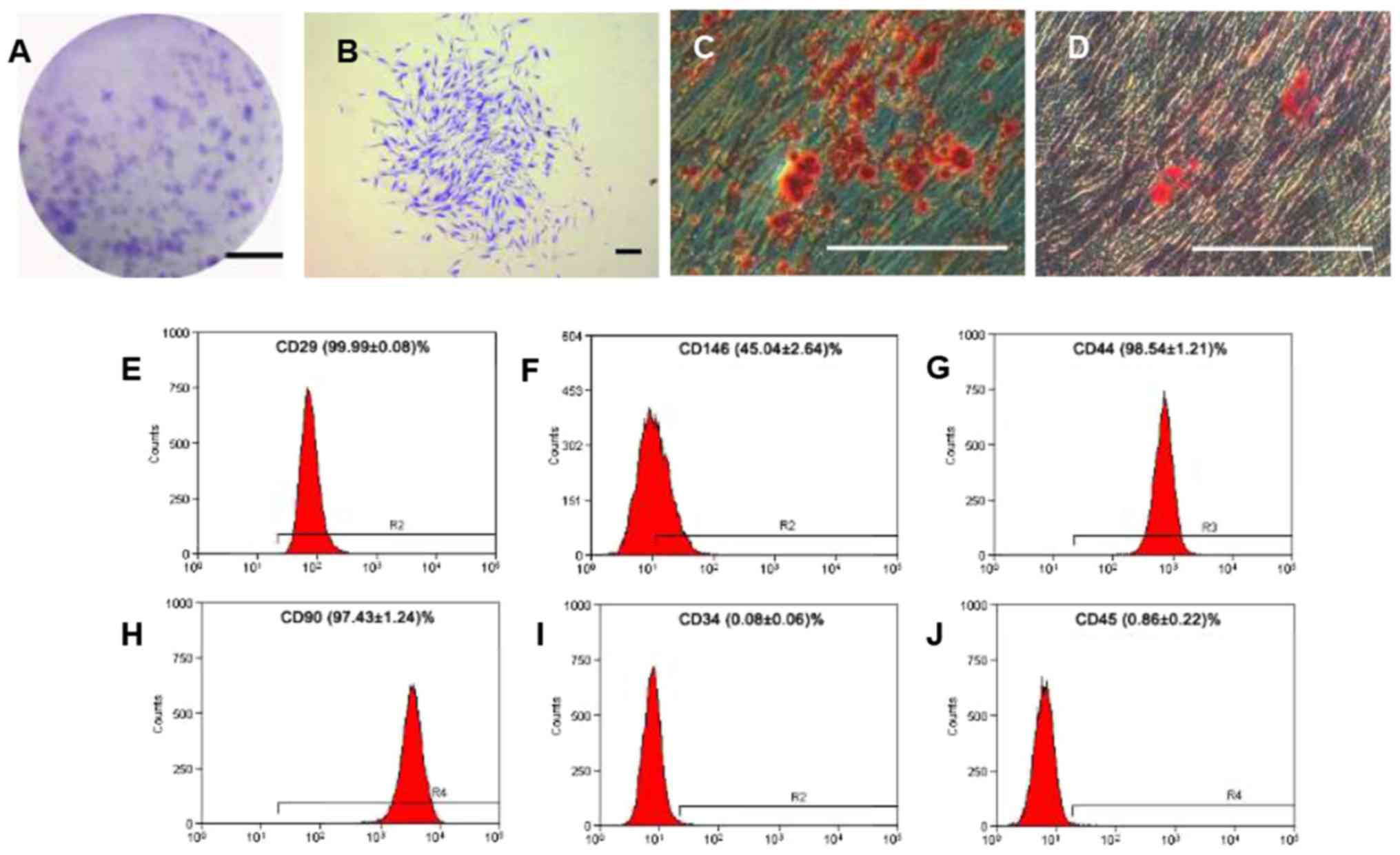 | Figure 1.Cell culture and identification of
hPDLSCs. (A) Colony forming units of hPDLSCs after cultivation for
12 days. Scale bar, 2 cm (B) Single clone of hPDLSCs. (C)
Mineralized nodules identified by alizarin red S staining following
osteogenic induction for 28 days. (D) Oil red O-positive lipid
clusters identified following adipogenic induction for 14 days.
(B-D) Scale bar, 300 µm. Flow cytometric analyses for surface
markers of hPDLSCs: (E) CD29; (F) CD146; (G) CD44; (H) CD90; (I)
CD34; and (J) CD45. hPDLSCs, human periodontal ligament stem cells;
CD, cluster of differentiation; CD29, integrin β-1; CD146, cell
surface glycoprotein, CD44, antigen CD44; CD90, thy-1 membrane
glycoprotein; CD34, hematopoietic progenitor cell antigen; CD45,
receptor-type tyrosine-protein phosphatase C. |
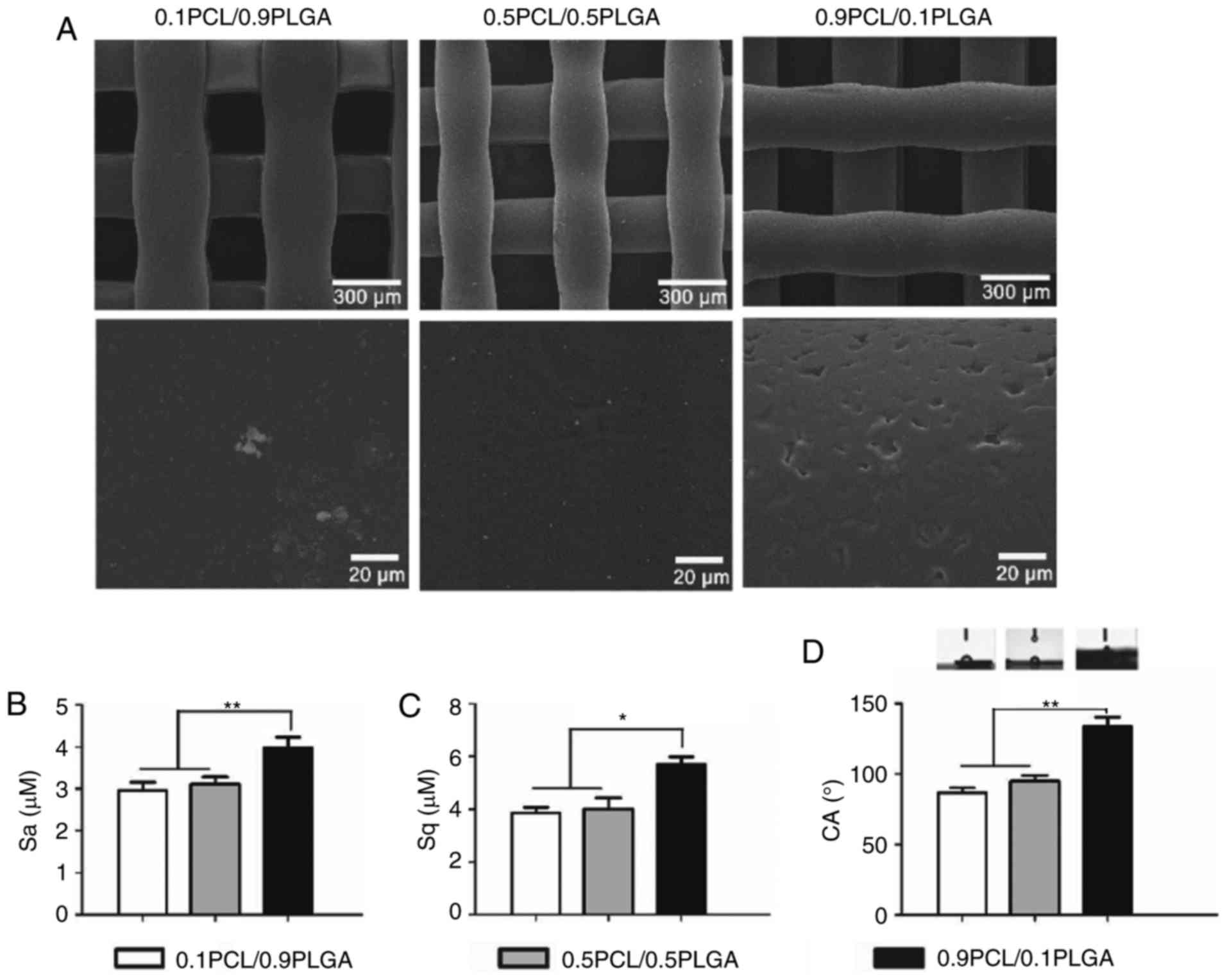
Surface characteristics (morphology,
surface roughness and wettability)
As demonstrated in Fig.
2A, the surface of the 0.9PCL/0.1PLGA scaffold was rough and
exhibited micro-sized pores. By comparison, the surfaces of the
0.1PCL/0.9PLGA and 0.5PCL/0.5PLGA scaffold were smooth.
Surface roughness is exhibited in Fig. 2B and C. The introduction of PLGA
into PCL decreased the Sa and Sq. The Sa value of 0.9PCL/0.1PLGA
was significantly higher compared with the other two groups
(P<0.01). Sq values shared the same tendency as Sa (Fig. 2C).
Water contact angle images are illustrated in
Fig. 2D. The water angle of the
composites decreased and the surface hydrophilicity increased with
the increasing proportion of PLGA. The average values of the CAs of
0.1PCL/0.9PLGA (86.60±3.60°) and 0.5PCL/0.5PLGA (94.93±4.14°) were
significantly lower compared with 0.9PCL/0.1PLGA (133.83±6.53°)
(P<0.01; Table II). There was
no significant difference observed between the two lower
groups.
 | Table II.Surface roughness and hydrophilicity
of the tested scaffolds (n=5). |
Table II.
Surface roughness and hydrophilicity
of the tested scaffolds (n=5).
| Group | Sa, µm | Sq, µm | CA, ° |
|---|
| 0.1PCL/0.9PLGA |
2.97±0.19c |
3.85±0.12c |
86.60±3.60c |
| 0.5PCL/0.5PLGA |
3.12±0.06c |
4.01±0.42c |
94.93±4.14c |
| 0.9PCL/0.1PLGA |
3.99±0.04a,b |
5.70±0.68a,b |
133.83±6.53a,b |
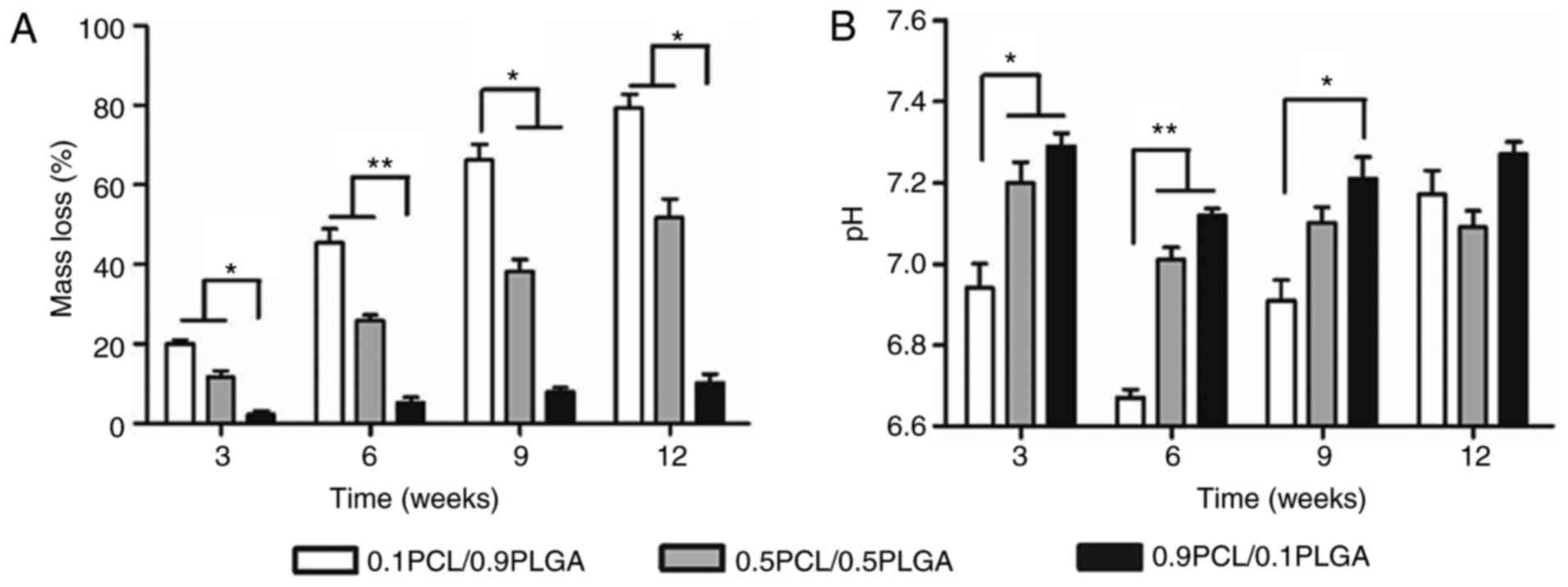
Degradation properties/alterations in
mass and pH
As demonstrated in Fig.
3A, PLGA addition may adjust the degradation rates of PCL/PLGA
scaffolds. The presence of PLGA significantly accelerated the mass
loss of the composites. Following 12 weeks, the mass loss was
79.20±4.22, 51.89±4.56 and 10.21±2.16% for the 0.1PCL/0.9PLGA,
0.5PCL/0.5PLGA and 0.9PCL/0.1PLGA scaffolds, respectively.
Overall, the pH values of the composites decreased
with the increasing ratio of PLGA at each time point (Fig. 3B). At 6 weeks of degradation, the
pH values of the three different scaffolds reached the minimum,
which was 6.67±0.02, 7.01±0.06 and 7.12±0.02 for 0.1PCL/0.9PLGA,
0.5PCL/0.5PLGA and 0.9PCL/0.1PLGA, respectively. Additionally, a
significant difference was observed in the pH values between
0.1PCL/0.9PLGA and the other two scaffolds following 3 (P<0.05)
and 6 weeks (P<0.01) of degradation, and between 0.1PCL/0.9PLGA
and 0.9PCL/0.1PLGA at 9 weeks of degradation (P<0.05).
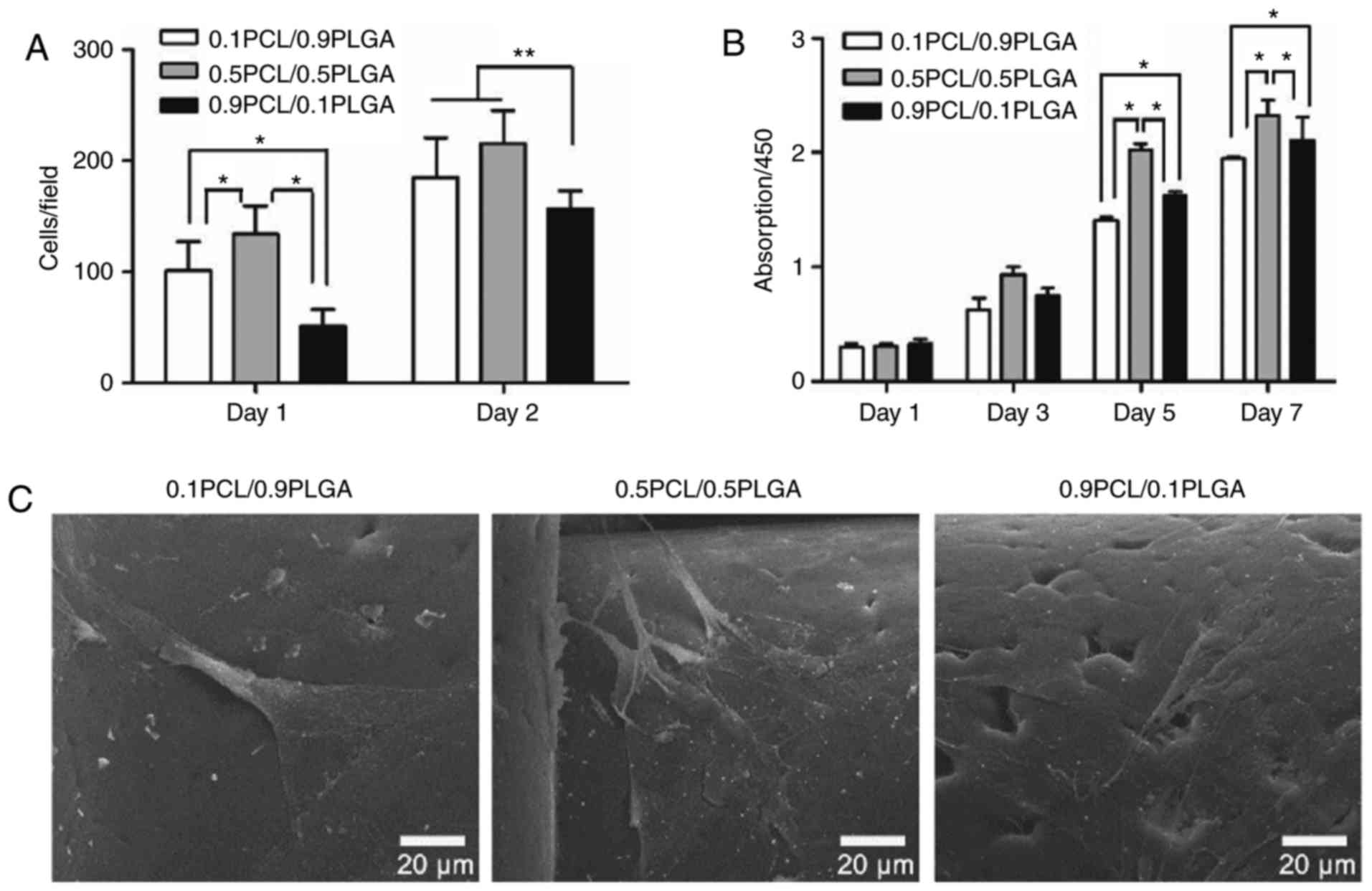
Cell adhesion
As demonstrated in Fig.
4A, the initial adherent number of hPDLSCs on 0.1PCL/0.9PLGA
and 0.5PCL/0.5PLGA was significantly higher compared with
0.9PCL/0.1PLGA on day 1 (P<0.05) and day 2 (P<0.01). No
significant difference between 0.1PCL/0.9PLGA and 0.5PCL/0.5PLGA
was revealed on day 2.
Cell proliferation
As presented in Fig.
4B, the proliferation of the hPDLSCs on different scaffolds
demonstrated a specific order at each time point with the exception
of day 1, with the ranking 0.5PCL/0.5PLGA >0.9PCL/0.1PLGA
>0.1PCL/0.9PLGA. On day 1 and day 3, no significant difference
was observed among the groups (P>0.05). On day 5 and day 7, the
proliferation level was significantly higher in 0.5PCL/0.5PLGA
(OD5d=2.03±0.05; OD7d=2.33±0.13) and
significantly lower in 0.1PCL/0.9PLGA (OD5d=1.62±0.04;
OD7d=2.11±0.20) compared with 0.9PCL/0.1PLGA
(OD5d=1.41±0.03; OD7d=1.95±0.01)
(P<0.05).
Cell morphology
The morphology of the hPDLSCs was demonstrated in
Fig. 4C following 3 days of
culture. Cells on 0.5PCL/0.5PLGA were highly branched, polygonal
shaped and exhibited clear nuclei; whereas, cells were flat on the
0.1PCL/0.9PLGA scaffold, and spindle-like with fewer branch points
on the 0.9PCL/0.1PLGA scaffold.
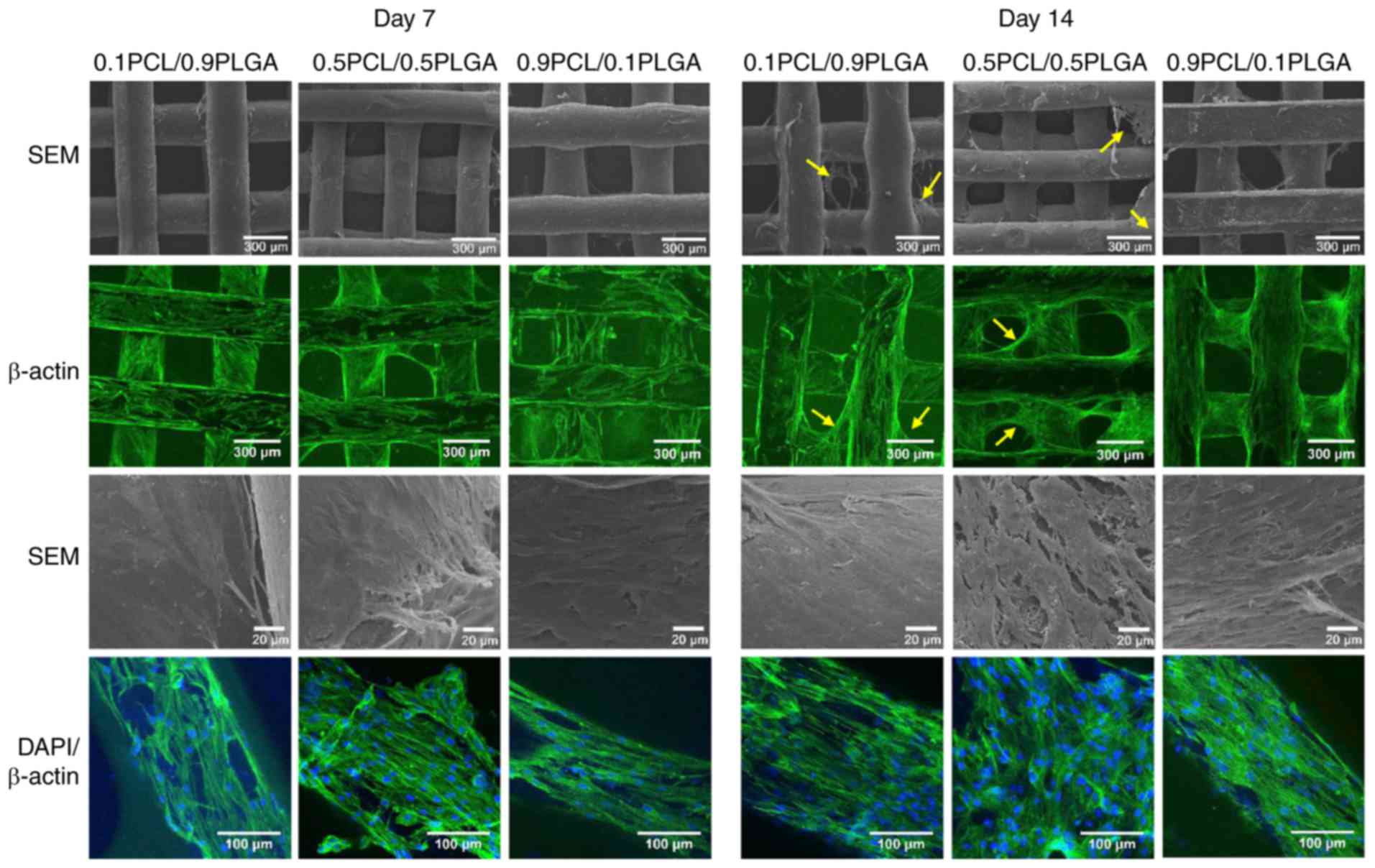
Cell adhesion morphology
The cell adhesion morphology of the hPDLSCs was
observed following 7 and 14 days of incubation on the scaffolds
(Fig. 5). SEM and CLSM images at
low magnification demonstrated that with culture time, hPDLSCs
gradually grew on the scaffold surface and formed cell layers.
Subsequent to 14 days of culture, hPDLSCs grew on the struts and
additionally over the porous structure of the 0.1PCL/0.9PLGA and
0.5PCL/0.5PLGA scaffolds (yellow arrows); whereas, hPDLSCs only
covered the struts and not the porous structure of the
0.9PCL/0.1PLGA scaffolds.
SEM images at high magnification revealed that
hPDLSCs were well adhered to the surfaces of all scaffolds. The
hPDLSCs formed denser cell layers on the 0.5PCL/0.5PLGA scaffolds
compared with on the 0.1PCL/0.9PLGA and 0.9PCL/0.1PLGA scaffolds.
CLSM images at high magnification revealed that abundant stress
fibers and actin microfilaments were observed on the 0.5PCL/0.5PLGA
scaffolds with fewer cellular junctions revealed on the
0.1PCL/0.9PLGA and 0.9PCL/0.1PLGA scaffolds.
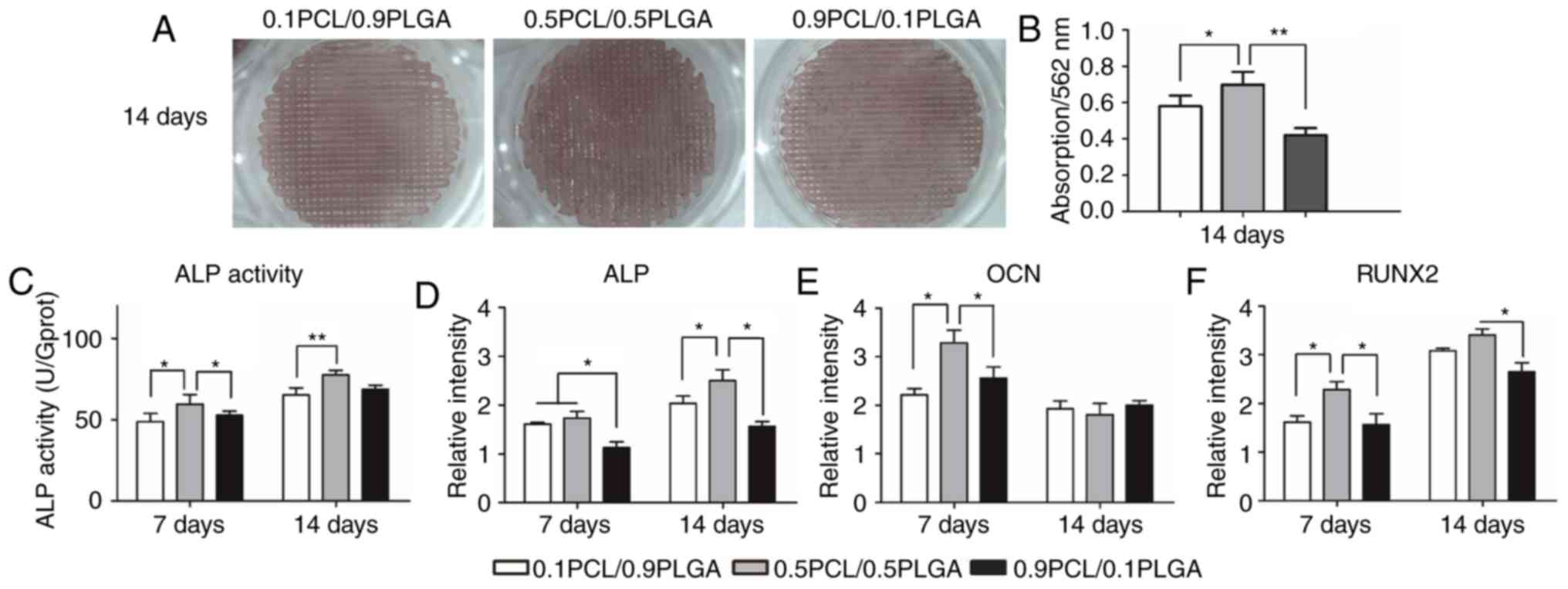
Osteogenic differentiation
Extracellular matrix mineralization of hPDLSCs on
scaffolds following 14 days of osteogenic induction is demonstrated
in Fig. 6A. Markedly dense red
color in the struts of the 0.5PCL/0.5PLGA scaffolds was observed,
with pale red color on the 0.1PCL/0.9PLGA and 0.9PCL/0.1PLGA
scaffolds. The semi-quantitative analysis (Fig. 6B) demonstrated that hPDLSCs
cultured on the 0.5PCL/0.5PLGA (OD562=0.70±0.07)
scaffolds produced a higher level of deposited calcium compared
with the 0.1PCL/0.9PLGA (OD562=0.58±0.06) and
0.9PCL/0.1PLGA (OD562=0.42±0.04) scaffolds
(P<0.05).
ALP activity was determined to assess the early
osteogenic capacity of the hPDLSCs on the scaffolds (Fig. 6C). The overall ALP activity of the
hPDLSCs on the 0.5PCL/0.5PLGA surface was significantly higher
compared with the other two groups on day 7 and day 14 (P<0.05),
apart from no significant difference compared with 0.1PCL/0.9PLGA
on day 14.
The expression of osteogenic biomarkers of hPDLSCs
on scaffolds was detected using a qPCR (Fig. 6D-F). Overall, the mRNA expression
of ALP and RUNX2 was higher on day 14 compared with on day 7, while
the expression of OCN mRNA demonstrated the opposite trend.
Following culturing for 7 days, the OCN and RUNX2 mRNA expression
of the cells grown on the 0.5PCL/0.5PLGA scaffolds was
significantly higher compared with the other two groups
(P<0.05). Additionally, the expression level of ALP mRNA was
significantly higher in the 0.1PCL/0.9PLGA and 0.5PCL/0.5PLGA
groups compared with the 0.9PCL/0.1PLGA group (P<0.05).
Subsequent to 14 days of culture, the mRNA expression of ALP and
RUNX2 were the highest, and the difference between the
0.5PCL/0.5PLGA scaffolds and the 0.9PCL/0.1PLGA scaffolds was
statistically significant (P<0.05).
Discussion
In the present study, PCL/PLGA blended scaffolds
with highly porous interconnected networks were successfully
created using the FDM technique. The topological structure of the
scaffolds directly affects cell activity and tissue regeneration
(25). Karageorgiou and Kaplan
(26) reported that a scaffold
with 300–500 µm pores optimized vascularization, and enhanced cell
adhesion and new tissue integration. Levenberg et al
(27) suggested that the size of
the struts should not exceed 400 µm in any dimension; otherwise, it
may inhibit the O2 diffusion and result in local tissue
hypoxia and necrosis. The pore diameter and the cross-section of
the beam and column of the scaffolds in the present study was 300
µm, suitable for bone regeneration.
Scaffolds should break down at a rate that is
harmonious with the rate of cell growth and tissue maturation
(28). The degradation rate of the
PCL/PLGA scaffolds was adjustable by altering the concentration of
PLGA. The 0.5PCL/0.5PLGA composite scaffold demonstrated an ideal
degradation rate; the degradation of the 0.1PCL/0.9PLGA scaffold
was too fast and the 0.9PCL/0.1PLGA scaffold was too slow. PLGA is
hydrophilic, and hence is able to accelerate the hydrolytic
degradation of hybrid PCL/PLGA (29). However, PLGA had acidic byproducts
during degradation, which may consequently inhibit cell viability
and induce tissue inflammation (30). It was revealed in the present study
that with the proportion of PLGA decreasing, the scaffolds
exhibited fewer acidic byproducts, demonstrating their potential
use in biomedical applications.
Biomaterials are required to allow cell attachment,
which is termed biocompatibility (31). Following cell attachment and
proliferation, the material should break down and be replaced by
the extracellular matrix (32,33).
A previous study indicated that hybrid PCL/PLGA may facilitate the
adhesion and proliferation of stem cells (34). The present data demonstrated that
hPDLSCs on the 0.5PCL/0.5PLGA and 0.1PCL/0.9PLGA scaffolds had a
significantly higher rate of attachment and proliferation compared
with the 0.9PCL/0.1PLGA scaffolds. Furthermore, hPDLSCs on
0.5PCL/0.5PLGA presented a uniform appearance resembling
mesenchymal stem cells and exhibited enhanced cell bridging
following 7 and 14 days of seeding. The differences may be due to
the different roughness and hydrophilicity of the scaffolds
(35).
Additionally, 0.5PCL/0.5PLGA demonstrated a superior
osteogenic capacity compared with the other two scaffolds,
confirmed with higher ALP activity and higher expression of
bone-related markers, including ALP, OCN and RUNX2, consistent with
a previous study reported by Makadia and Siegel (29), who discovered that a PCL/PLGA
composite may induce differentiation of osteoblasts. The promotion
of osteogenic capacity may be due to improved early cell attachment
and proliferation on the 0.5PCL/0.5PLGA scaffold. Cell morphology
may be another reason for osteogenesis, as stretching of cells may
affect intracellular communication and upregulate osteogenic gene
expression (36,37).
Biological behavior is associated with the surface
characteristics of the scaffolds. Generally, scaffolds must possess
adequate roughness, hydrophilicity and specific surface topography,
which are widely accepted to affect cell attachment, proliferation
and differentiation (38,39). The present data revealed that the
surface roughness of composite scaffolds increased with the
increased proportion of PCL. Thapa et al (40) discovered a rougher surface of PCL
compared with PLGA following dissolution and deposition. Other
researchers reported that different surface topographies resulted
from pores with various sizes (5–40 nm) formed on the polymer
surface during the crystallization process following deposition
(41), and the size of pores was
strongly influenced by the polymer blend ratio (42).
Another study suggested that the increased surface
roughness provides a larger surface area, and thus leads to
increased cell adhesion (43).
However, in the present study, early cell attachment and
proliferation did not alter correspondingly with the increase in
surface roughness. This result was in accordance with studies
reported by Liao et al (44) and Deng et al (45). Therefore, superior hPDLSC responses
on the PCL/PLGA scaffolds may be attained by modifying surface
roughness with different ratios of PCL/PLGA.
Surface wettability was additionally responsible for
the cell response on the material. Highly hydrophilic surfaces
facilitate the initial attachment of water molecules, promoting the
adsorption of proteins, including fibronectin (46). Furthermore, hydrophilic surfaces
demonstrate high surface energies and enhanced cell adhesion
(47,48). It was observed that the
hydrophilicity of PLGA/PCL hybrid scaffolds increased with the
higher PLGA ratio, which was positively associated with cell
attachment, viability and differentiation.
In summary, the present study demonstrated that
PCL/PLGA composite scaffolds exhibited good degradation properties,
surface characteristics and cellular activities, demonstrating
potential in alveolar bone restoration, and 0.5PCL/0.5PLGA (w/w)
possessed superior bioactivity. Further studies are required to
examine the in vivo effect in animal models.
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science
Foundation of Guangdong Province (China; grant no.
2015A030313083).
Availability of data and materials
The data sets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
LW, JZ and CP conceived and designed the
experiments. XZ, LD and CP performed the experiments. CP, ZC and DC
analyzed the data. CP drafted the manuscript. CP and LW critically
revised the manuscript.
Ethics approval and consent to
participate
The protocol was approved by the Ethics Committee of
the Hospital of Stomatology, Sun Yat-sen University (Guangzhou,
China; approval no. PKUSSIRB-201311103). Informed consent from each
patient was obtained.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Asa'ad F, Pagni G, Pilipchuk SP, Gianni
AB, Giannobile WV and Rasperini G: 3D-printed scaffolds and
biomaterials: Review of alveolar bone augmentation and periodontal
regeneration applications. Int J Dent. 2016:12398422016.PubMed/NCBI
|
|
2
|
Teven CM, Fisher S, Ameer GA, He TC and
Reid RR: Biomimetic approaches to complex craniofacial defects. Ann
Maxillofac Surg. 5:4–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sheikh Z, Hamdan N, Ikeda Y, Grynpas M,
Ganss B and Glogauer M: Natural graft tissues and synthetic
biomaterials for periodontal and alveolar bone reconstructive
applications: A review. Biomater Res. 21:92017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Obregon F, Vaquette C, Ivanovski S,
Hutmacher DW and Bertassoni LE: Three-dimensional bioprinting for
regenerative dentistry and craniofacial tissue engineering. J Dent
Res. 94 9 Suppl:143S–152S. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hung BP, Naved BA, Nyberg EL, Dias M,
Holmes CA, Elisseeff JH, Dorafshar AH and Grayson WL:
Three-dimensional printing of bone extracellular matrix for
craniofacial regeneration. ACS Biomater Sci Eng. 2:1806–1816. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu T, Li Y and Chen T: Techniques for
fabrication and construction of three-dimensional scaffolds for
tissue engineering. Int J Nanomedicine. 8:337–350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nyberg EL, Farris AL, Hung BP, Dias M,
Garcia JR, Dorafshar AH and Grayson WL: 3D-printing technologies
for craniofacial rehabilitation, reconstruction, and regeneration.
Ann Biomed Eng. 45:45–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chia HN and Wu BM: Recent advances in 3D
printing of biomaterials. J Biol Eng. 9:42015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rasperini G, Pilipchuk SP, Flanagan CL,
Park CH, Pagni G, Hollister SJ and Giannobile WV: 3D-printed
bioresorbable scaffold for periodontal repair. J Dent Res. 942 9
Supp:153S–157S. 2015. View Article : Google Scholar
|
|
10
|
Chou SF and Woodrow KA: Relationships
between mechanical properties and drug release from electrospun
fibers of PCL and PLGA blends. J Mech Behav Biomed Mater.
65:724–733. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li X, Yang C, Li L, Xiong J, Xie L, Yang
B, Yu M, Feng L, Jiang Z, Guo W and Tian W: A therapeutic strategy
for spinal cord defect: Human dental follicle cells combined with
aligned PCL/PLGA electrospun material. Biomed Res Int.
2015:1971832015.PubMed/NCBI
|
|
12
|
Jensen T, Blanchette A, Vadasz S, Dave A,
Canfarotta M, Sayej WN and Finck C: Biomimetic and synthetic
esophageal tissue engineering. Biomaterials. 57:133–141. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zong C, Wang M, Yang F, Chen G, Chen J,
Tang Z, Liu Q, Gao C, Ma L and Wang J: A novel therapy strategy for
bile duct repair using tissue engineering technique: PCL/PLGA
bilayered scaffold with hMSCs. J Tissue Eng Regen Med. 11:966–976.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Trajano VCC, Costa KJR, Lanza CRM,
Sinisterra RD and Cortes ME: Osteogenic activity of
cyclodextrin-encapsulated doxycycline in a calcium phosphate PCL
and PLGA composite. Mater Sci Eng C Mater Biol Appl. 64:370–375.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Franco RA, Nguyen TH and Lee BT:
Preparation and characterization of electrospun PCL/PLGA membranes
and chitosan/gelatin hydrogels for skin bioengineering
applications. J Mater Sci Mater Med. 22:2207–2218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baker SC, Rohman G, Southgate J and
Cameron NR: The relationship between the mechanical properties and
cell behaviour on PLGA and PCL scaffolds for bladder tissue
engineering. Biomaterials. 30:1321–1328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Subramanian A, Krishnan UM and Sethuraman
S: Fabrication, characterization and in vitro evaluation of aligned
PLGA-PCL nanofibers for neural regeneration. Ann Biomed Eng.
40:2098–2110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qian Y, Chen H, Xu Y, Yang J, Zhou X,
Zhang F and Gu N: The preosteoblast response of electrospinning
PLGA/PCL nanofibers: Effects of biomimetic architecture and
collagen I. Int J Nanomedicine. 11:4157–4171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mrozik K, Gronthos S, Shi S and Bartold
PM: A method to isolate, purify, and characterize human periodontal
ligament stem cells. Methods Mol Biol. 666:269–284. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu W and Liang M: Periodontal ligament
stem cells: Current status, concerns, and future prospects. Stem
Cells Int. 2015:9723132015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cha Y, Jeon M, Lee HS, Kim S, Kim SO, Lee
JH and Song JS: Effects of in vitro osteogenic induction on in vivo
tissue regeneration by dental pulp and periodontal ligament stem
cells. J Endod. 41:1462–1468. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moshaverinia A, Xu X, Chen C, Ansari S,
Zadeh HH, Snead ML and Shi S: Application of stem cells derived
from the periodontal ligament or gingival tissue sources for tendon
tissue regeneration. Biomaterials. 35:2642–2650. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang H, Liu S, Zhu B, Xu Q, Ding Y and
Jin Y: Composite cell sheet for periodontal regeneration: Crosstalk
between different types of MSCs in cell sheet facilitates complex
periodontal-like tissue regeneration. Stem Cell Res Ther.
7:1682016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bruzauskaite I, Bironaite D, Bagdonas E
and Bernotiene E: Scaffolds and cells for tissue regeneration:
Different scaffold pore sizes-different cell effects.
Cytotechnology. 68:355–369. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karageorgiou V and Kaplan D: Porosity of
3D biomaterial scaffolds and osteogenesis. Biomaterials.
26:5474–5491. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Levenberg S, Rouwkema J, Macdonald M,
Garfein ES, Kohane DS, Darland DC, Marini R, van Blitterswijk CA,
Mulligan RC, D'Amore PA and Langer R: Engineering vascularized
skeletal muscle tissue. Nat Biotechnol. 23:879–884. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Osorio R, Alfonso-Rodriguez CA, Osorio E,
Medina-Castillo AL, Alaminos M, Toledano-Osorio M and Toledano M:
Novel potential scaffold for periodontal tissue engineering. Clin
Oral Investig. 21:2695–2707. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Makadia HK and Siegel SJ: Poly
Lactic-co-glycolic acid (PLGA) as biodegradable controlled drug
delivery carrier. Polymers (Basel). 3:1377–1397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rhee SH and Lee SJ: Effect of acidic
degradation products of poly(lactic-co-glycolic)acid on the
apatite-forming ability of poly(lactic-co-glycolic)acid-siloxane
nanohybrid material. J Biomed Mater Res A. 83:799–805. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Prakasam M, Locs J, Salma-Ancane K, Loca
D, Largeteau A and Berzina-Cimdina L: Biodegradable materials and
metallic Implants-a review. J Funct Biomater. 8:pii: E44. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pulyala P, Singh A, Dias-Netipanyj MF,
Cogo SC, Santos LS, Soares P, Gopal V, Suganthan V, Manivasagam G
and Popat KC: In-vitro cell adhesion and proliferation of adipose
derived stem cell on hydroxyapatite composite surfaces. Mater Sci
Eng C Mater Biol Appl. 75:1305–1316. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He HY, Zhang JY, Mi X, Hu Y and Gu XY:
Rapid prototyping for tissue-engineered bone scaffold by 3D
printing and biocompatibility study. Int J Clin Exp Med.
8:11777–11785. 2015.PubMed/NCBI
|
|
34
|
Sa M and Kim JY: Effect of various
blending ratios on the cell characteristics of PCL and PLGA
scaffolds fabricated by polymer deposition system. Int J Precis Eng
Man1. 4:649–655. 2013. View Article : Google Scholar
|
|
35
|
Shahrousvand M, Sadeghi GMM, Shahrousvand
E, Ghollasi M and Salimi A: Superficial physicochemical properties
of polyurethane biomaterials as osteogenic regulators in human
mesenchymal stem cells fates. Colloids Surf B Biointerfaces.
156:292–304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Folkman J and Moscona A: Role of cell
shape in growth control. Nature. 273:345–349. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Uzer G, Pongkitwitoon S, Chan Ete M and
Judex S: Vibration induced osteogenic commitment of mesenchymal
stem cells is enhanced by cytoskeletal remodeling but not fluid
shear. J Biomech. 46:2296–2302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mathieu PS and Loboa EG: Cytoskeletal and
focal adhesion influences on mesenchymal stem cell shape,
mechanical properties, and differentiation down osteogenic,
adipogenic, and chondrogenic pathways. Tissue Eng Part B Rev.
18:436–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fu Y, Liu S, Cui SJ, Kou XX, Wang XD, Liu
XM, Sun Y, Wang GN, Liu Y and Zhou YH: Surface chemistry of
nanoscale mineralized collagen regulates periodontal ligament stem
cell fate. ACS Appl Mater Interfaces. 8:15958–15966. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Thapa A, Webster TJ and Haberstroh KM:
Polymers with nano-dimensional surface features enhance bladder
smooth muscle cell adhesion. J Biomed Mater Res A. 67:1374–1383.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Karagkiozaki V, Vavoulidis E,
Karagiannidis PG, Gioti M, Fatouros DG, Vizirianakis IS and
Logothetidis S: Development of a nanoporous and multilayer
drug-delivery platform for medical implants. Int J Nanomedicine.
7:5327–5338. 2012.PubMed/NCBI
|
|
42
|
Affrossman S, Henn G, O'Neill S, Pethrick
P and Stamm M: Surface topography and composition of deuterated
polystyrene-poly (bromostyrene) blends. Macromolecules.
29:5010–5016. 1996. View Article : Google Scholar
|
|
43
|
Baker BM, Trappmann B, Wang WY, Sakar MS,
Kim IL, Shenoy VB, Burdick JA and Chen CS: Cell-mediated fibre
recruitment drives extracellular matrix mechanosensing in
engineered fibrillar microenvironments. Nat Mater. 14:1262–1268.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liao J, Wei Q, Zou Y, Fan J, Song D, Cui
J, Zhang W, Zhu Y, Ma C, Hu X, et al: Notch signaling augments
BMP9-induced bone formation by promoting the
osteogenesis-angiogenesis coupling process in mesenchymal stem
cells (MSCs). Cell Physiol Biochem. 41:1905–1923. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Deng Y, Liu X, Xu A, Wang L, Luo Z, Zheng
Y, Deng F, Wei J, Tang Z and Wei S: Effect of surface roughness on
osteogenesis in vitro and osseointegration in vivo of carbon
fiber-reinforced polyetheretherketone-nanohydroxyapatite composite.
Int J Nanomedicine. 10:1425–1447. 2015.PubMed/NCBI
|
|
46
|
Spriano S, Chandra Sarath V, Cochis A,
Uberti F, Rimondini L, Bertone E, Vitale A, Scolaro C, Ferrari M,
Cirisano F, et al: How do wettability, zeta potential and
hydroxylation degree affect the biological response of
biomaterials? Mater Sci Eng C Mater Biol Appl. 74:542–555. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ranella A, Barberoglou M, Bakogianni S,
Fotakis C and Stratakis E: Tuning cell adhesion by controlling the
roughness and wettability of 3D micro/nano silicon structures. Acta
Biomater. 6:2711–2720. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhao G, Schwartz Z, Wieland M, Rupp F,
Geis-Gerstorfer J, Cochran DL and Boyan BD: High surface energy
enhances cell response to titanium substrate microstructure. J
Biomed Mater Res A. 74:49–58. 2005. View Article : Google Scholar : PubMed/NCBI
|