Introduction
Diabetic nephropathy (DN) is among the most common
microvascular complications of type 1 and type 2 diabetes mellitus,
and is the major cause of end-stage renal disease globally
(1,2). Previous studies have reported that
podocyte damage, and reduced nephrin and podocin expression, are
apparent even during the early stages of DN pathogenesis, and may
be involved in the acceleration of DN development (3,4). The
generation of reactive oxygen species (ROS), cell apoptosis and
immune-mediated inflammatory responses in the kidneys are involved
in the development and progression of DN. Glucose-induced increases
in ROS generation have been reported to promote the apoptosis of
podocytes (5), and mesangial
(6) and tubular cells (7), thus promoting the progression of DN.
In addition, increasing evidence indicates an important role for
proinflammatory cytokines, including tumor necrosis factor (TNF)-α,
interleukin (IL)-1β and IL-6, in DN (8,9). The
apoptosis of renal glomerular cells, particularly podocytes, has
been reported in human and experimental DN (5,10);
however, to the best of our knowledge, whether podocyte apoptosis
may initiate or contribute to the progression of DN is yet to be
elucidated. Furthermore, the involvement of podocytes in
inflammatory responses in the kidneys is yet to be
investigated.
Glucagon-like peptide-1 (GLP-1) is an incretin
hormone that is secreted by intestinal L-cells, which are
enteroendocrine cells present throughout the gastrointestinal
tract, from the duodenum to the rectum. GLP-1 has been previously
associated with various biological functions (11), including reducing β-cell apoptosis
and food intake, decreasing the rate of gastric emptying and
secretion, and regulating blood glucose levels; thus, GLP-1 has
been successfully used in the treatment of patients with type 2
diabetes mellitus (12). Notably,
GLP-1 has been reported to exhibit a direct cytoprotective effect
against oxidative stress in the aorta of diabetic mice (13). GLP-1 acts directly on glomerular
mesangial cells through the GLP-1 receptor (GLP-1R), where it
exerts anti-inflammatory effects against advanced glycation
end-products (AGEs) by suppressing the expression of the receptor
for AGEs (14). GLP-1 has also
been reported to protect cardiac microvessels against oxidative
stress and apoptosis, and suppress the development of microvascular
barrier dysfunction, in diabetes by inhibiting Rho through a cyclic
AMP/protein kinase A-mediated signaling pathway (15). Notably, evidence indicates that
long-term treatment with the GLP-1R agonistexendin-4 may alleviate
DN in rat and mouse models (16,17).
Sirtuins (SIRTs) are proteins that are members of
the silent information regulation-2 family and possess nicotinamide
adenine dinucleotide (NAD)-dependent deacetylase activity. Sirtuin
(SIRT)1 is involved in the deacetylation of histones and numerous
transcriptional regulators, thus regulating diverse biological
processes (18,19). It has been reported that SIRT1
expression was increased in an acute kidney injury model (20), while it was reduced in the kidneys
of streptozotocin-induced diabetic mice (21). In addition, SIRT1 has been
associated with the pathogenesis of DN, including the development
of age-associated renal lesions, renal hypoxia, renal interstitial
fibrosis and tubular cell apoptosis. Therefore, SIRT1 activation
may have therapeutic potential in DN due to its antifibrotic,
anti-inflammatory, antiapoptotic and blood pressure-controlling
effects (22). These findings
indicate that SIRT1 may act as a factor for conferring
susceptibility to DN.
In the present study, treatment of podocytes with
high glucose (HG) was demonstrated to induce the generation of ROS,
promote podocyte apoptosis and enhance the secretion of
proinflammatory cytokines, thus indicating that podocyte apoptosis
or depletion may represent a novel early mechanism implicated in
the pathogenesis of DN. SIRT1 may act as a key mediator of podocyte
apoptosis in vitro and therefore function as a connection
between ROS, podocyte apoptosis and DN.
Materials and methods
Cell culture and treatments
MPC-5 mouse podocytes were obtained from BeNa
Culture Collection (Kunshan, China) and were cultured in RPMI-1640
medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Invitrogen; Thermo
Fisher Scientific, Inc.), 100X penicillin-streptomycin solution
(100 U/ml penicillin and 100 mg/l streptomycin) and 10 U/ml
interferon (IFN)-γ (ProSpec-Tany TechnoGene Ltd., East Brunswick,
NJ, USA). Cells were maintained in a humidified atmosphere at 33°C
with 5% CO2. Following proliferation to 80% confluence,
podocytes were cultured in the aforementioned medium without 10
U/ml IFN-γ and were incubated in a humidified atmosphere at 37°C
with 5% CO2 for 10–14 days.
To establish the diabetic injury model, mouse
podocytes (1×105 cells/well) were exposed to normal
glucose (5.5 mM) or HG (15, 30 and 50 mM) for 48 h. Cells
maintained in normal glucose (5.5 mM) were used as the control
group. In addition to HG induction, mouse podocytes were
simultaneously treated with GLP-1 (10, 100 and 500 nM;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) or 10 µM resveratrol
(RSV; Shanghai Aladdin Bio-Chem Technology Co., Ltd., Shanghai,
China) for 48 h.
ROS detection
A 2′,7′-dichlorodihydrofluorescein diacetate
(DCFH-DA) fluorescent probe combined with flow cytometric analysis
was used to detect alterations in the ROS levels generated in
podocytes 48 h after the various treatments, as described
previously (23). Briefly,
podocytes were resuspended in PBS and the density was adjusted to
1×105 cells/well. The podocytes were subsequently
incubated with 10 µM DCFH-DA (Beyotime Institute of Biotechnology,
Shanghai, China) for 20 min in the dark at 37°C and subjected to
flow cytometric analysis (BD Biosciences, Franklin Lakes, NJ, USA)
using a BD Accuri C6 flow cytometer and BD Cell Quest software
version 1.0.264.21 (BD Biosciences).
Cell apoptosis assay
Cell apoptosis was analyzed 48 h after the various
treatments using flow cytometry and an Annexin V-FITC Apoptosis
Detection kit (C1062; Beyotime Institute of Biotechnology).
Briefly, mouse podocytes were plated in 6-well plates at a density
of 1×105 cells/well and incubated with 195 µl Annexin
V-fluorescein isothiocyanate (FITC) and 5 µl propidium iodide (PI)
for 15 min in the dark at 4°C. Analysis of cell apoptosis was
subsequently performed using a flow cytometer. The early apoptotic
cells are presented in the lower right quadrant of the
fluorescence-activated cell sorting (FACS) histograms, and the late
apoptotic cells, which were stained with FITC and PI, emitted
red-green fluorescence and are presented in the upper right
quadrant of the FACS histograms. FACS was performed on an LSRII
flow cytometer (BD Biosciences), and data were analyzed using
FlowJo software (version 9.0.2; FlowJo LLC, Ashland, OR, USA).
ELISA
TNF-α, IL-6 and IL-1β levels present in the culture
supernatants of mouse podocytes 48 h after the various treatments
were determined using Mouse Interleukin 1β (IL-1β) ELISA kit
(PI301; Beyotime Institute of Biotechnology), Mouse Interleukin 6
(IL-6) ELISA kit (KMC0061; Thermo Fisher Scientific, Inc.), Mouse
Tumor necrosis factor α (TNF-α) ELISA kit (PT512; Beyotime
Institute of Biotechnology), respectively, according to the
manufacturer's protocol.
Caspase activity assays
The activity of caspase-3 and caspase-9 was analyzed
using Caspase-3 and Caspase-9 Colorimetric Assay kits (Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China), according to the
manufacturer's protocol. Briefly, mouse podocytes (1×105
cells/well) 48 h after the various treatments were collected,
resuspended in 50 µl chilled cell lysis buffer (Nanjing KeyGen
Biotech Co., Ltd.) and incubated on ice for 10 min. Following
centrifugation for 1 min at 400 × g at 4°C, the supernatants were
transferred to a fresh tube and the protein concentration was
assessed using a bicinchoninic acid protein assay kit (PICPI23223;
Thermo Fisher Scientific, Inc.). 100 µg protein was diluted in 50
ml cell lysis buffer for each assay. The absorbance of each sample
was measured at 405 nm using a Multiskan EX microplate reader
(Thermo Fisher Scientific, Inc.).
SIRT1 knockdown in mouse
podocytes
The pLKO.1 lentiviral vector, psPAX2 packaging
plasmid and pMD2G envelope plasmid were obtained from Addgene, Inc.
(Cambridge, MA, USA). The SIRT1-targeting short hairpin (sh)RNA
(position 516–534: GCG GAT AGG TCC ATA TAC T; forward: CCG GGC GGA
TAG GTC CAT ATA CTT TCT CGA GGA ATA CCT CAT CTT TCC TCT TTT TTT C;
reverse: AAT TGA AAA AAA GAG GAA AGA TGA GGT ATT CCT CGA GAA AGT
ATA TGG ACC TAT CCG CGG CCT) or a scramble shRNA sequence (AGA GCT
ATC GGC ATC ATG T; forward: CCG GAG AGC TAT CGG CAT CAT GTT TCT CGA
GGA ATA CCT CAT CTT TCC TCT TTT TTT C; reverse: AAT TGA AAA AAA GAG
GAA AGA TGA GGT ATT CCT CGA GAA ACA TGA TGC CGA TAG CTC TGG CCT;
Sangon Biotech Co., Ltd., Shanghai, China) was cloned into the
pLKO.1 lentiviral vector using Agel I and Ecol I restriction
enzymes. The pLKO.1 lentiviral vector with scramble shRNA was used
as the negative control (NC). 293T cells (American Type Culture
Collection, Manassas, VA, USA) at the density of 1×105
cells/well cultured in DMEM (Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Invitrogen; Thermo Fisher
Scientific, Inc.) were seeded in 60-mm culture dishes 37°C and,
after 24 h, they were co-transfected with 1 µg pLKO.1-SIRT1-shRNA
or pLKO.1-NC-shRNA, and 0.1 µg psPAX2 and 0.9 µg pMD2G, using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) as the transfection reagent, according to the
manufacturer's protocol. The recombinant lentiviral particles were
collected 48 h post-transfection and were used to infect mouse
podocytes. Mouse podocytes (1×105 cells/well) cultured
in RPMI-1640 medium 10% fetal bovine serum were infected with 2 µl
lentivirus at a multiplicity of infection of 20 in the presence of
8 µg/ml polybrene (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
for 4–6 h at 37°C. Sequencing was performed by Shanghai Majorbio
Pharmaceutical Technology Co., Ltd. (Shanghai, China) to confirm
the recombinant virus.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from podocytes 48 h after
the various treatments using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Total RNA was reverse-transcribed into cDNA using an AMV reverse
transcriptase kit (Fermentas; Thermo Fisher Scientific, Inc.) for
60 min at 37°C, 5 min at 85°C and 5 min at 4°C. qPCR was performed
on cDNA using SYBR-Green 10X Supermix (Takara Biotechnology Co.,
Ltd., Dalian, China) on an ABI 7500 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The PCR cycling
conditions were as follows: 95°C for 10 min, followed by 40 cycles
at 95°C for 15 sec and 60°C for 45 sec, and a final extension step
of 95°C for 15 sec, 60°C for 1 min, 95°C for 15 sec and 60°C for 15
sec. The sequences of the primers that were used in the present
study were: SIRT1 forward, 5′-TGACGCTGTGGCAGATTG-3′ and reverse,
5′-CAAGGCGAGCATAGATACCG-3′; nephrin forward,
5′-GGACCCACACTACTACTC-3′ and reverse, 5′-CTCTCCACCTCGTCATAC-3′;
podocin forward, 5′-TTGTTTCCTGGCTCCTTC-3′ and reverse,
5′-TGCCTTGGGACTACTTTC-3′; caspase-3 forward,
5′-CTGACTGGAAAGCCGAAAC-3′ and reverse, 5′-GCAAAGGGACTGGATGAAC-3′;
caspase-9 forward, 5′-GTGAAGAACGACCTGACTG-3′ and reverse,
5′-GCATCCATCTGTCCCATAG-3′; and GAPDH forward,
5′-ATCACTGCCACCCAGAAG-3′ and reverse, 5′-TCCACGACGGACACATTG-3′.
GAPDH was used the internal control for normalization. Relative
gene expression was calculated using the 2−ΔΔCq method
(24). The experiment was repeated
three times.
Western blot analysis
Total proteins were isolated from mouse podocytes 48
h after the various treatments in radioimmunoprecipitation assay
buffer (Beyotime Institute of Biotechnology) containing 0.01%
protease and phosphatase inhibitor (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). The protein concentration was assessed using a
bicinchoninic acid protein assay kit (Thermo Fisher Scientific,
Inc.). Proteins (20–30 µg) were separated by 12% SDS-PAGE and
transferred onto polyvinylidene difluoride membranes (Roche
Diagnostics GmbH, Mannheim, Germany). Following blocking with 5%
skimmed milk at 4°C overnight, the membranes were incubated at 4°C
overnight with the following primary antibodies: Anti-SIRT1
(ab28170; 1:1,000), anti-nephrin (ab58968; 1:500), anti-podocin
(ab181143; 1:2,000), anti-caspase-3 (ab44976; 1:500),
anti-caspase-9 (ab2013; 1:1,000) and anti-GAPDH (5174; 1:2,000).
All primary antibodies were purchased from Abcam (Cambridge, MA,
USA). The membranes were subsequently incubated with horseradish
peroxidase-conjugated secondary antibodies (A0208, A0181, A0216;
1:1,000; Beyotime Institute of Biotechnology) for 1 h at 37°C.
Protein bands were visualized using Immobilon Western
Chemiluminescent HRP Substrate (EMD Millipore, Billerica, MA, USA)
and blots were semi-quantified by densitometry using Quantity One
software version 4.5.2 (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. Statistical analysis was
performed using SPSS software version 19.0 (IBM Corp., Armonk, NY,
USA). One-way analysis of variance followed by Tukey's post-hoc
test was performed to statistically analyze the differences among
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
GLP-1 attenuates oxidative stress
induced by HG in mouse podocytes
It is established that ROS are implicated in DN
(25). To investigate whether
GLP-1 may attenuate HG-induced oxidative stress in mouse podocytes,
the present study assessed ROS production by mouse podocytes in
vitro. As presented in Fig.
1A, HG (15, 30 and 50 mM) treatment enhanced ROS production in
mouse podocytes following 48 h incubation compared with cells
maintained in normal glucose conditions. Notably, GLP-1 (10, 100
and 500 nM) was revealed to suppress ROS production in a
dose-dependent manner in 30 mM HG-stimulated mouse podocytes
(Fig. 1B).
GLP-1 suppresses HG-induced apoptosis
in mouse podocytes
To determine the effect of GLP-1 on HG-induced
apoptosis in mouse podocytes, flow cytometry was performed.
Treatment with HG (15, 30 and 50 mM) induced a significant
dose-dependent increase in apoptotic cells (Fig. 2A), compared with cells maintained
in normal glucose conditions. Compared with podocytes cultured in
30 mM HG, the GLP-1 (10, 100 and 500 nM)-treated groups exhibited a
significant decrease in cell apoptosis (30.6±0.26% in 10 nM GLP-1
group, 23.9±0.23% in 100 nM GLP-1 group and 15.8±0.56% in 500 nM
GLP-1 group vs. 36.8±0.06% in HG group; P<0.01; Fig. 2B).
GLP-1 attenuates HG-induced
downregulation of SIRT1 expression in mouse podocytes
To investigate whether GLP-1 may counteract
HG-induced alterations in SIRT1 expression in mouse podocytes,
RT-qPCR and western blot analysis were performed. The results
demonstrated that 30 mM glucose induced a significant decrease in
the mRNA and protein expression of SIRT1, compared with cells
maintained under normal glucose conditions (Fig. 3A and B). However, compared with
podocytes cultured in HG, GLP-1 (10, 100 and 500 nM)-treated cells
exhibited increases in the mRNA and protein expression levels of
SIRT1 (Fig. 3A and B).
GLP-1 attenuates HG-induced depletion
of podocyte markers in mouse podocytes
To determine whether HG treatment may induce
podocyte depletion, the expression of podocyte-specific markers,
including nephrin and podocin, was assessed using RT-qPCR and
western blot analysis. As presented in Fig. 4A-C, the mRNA and protein expression
of nephrin and podocin was significantly suppressed following
treatment of mouse podocytes with 30 mM glucose, compared with
podocytes cultured in normal glucose, thus indicating that exposure
to HG may induce podocyte depletion. However, compared with
podocytes cultured in HG, cells treated with 500 nM GLP-1 exhibited
a significant increase in the mRNA and protein expression of
nephrin and podocin (Fig. 4A-C).
Notably, treatment with GLP-1 was revealed to mimic the protective
effects of 10 µM RSV administration in HG-induced mouse podocytes
(Fig. 4A-C). Consistent with the
aforementioned results for SIRT1 expression, 500 nM GLP-1
significantly attenuated the HG-induced decrease in SIRT1
expression in mouse podocytes (Fig.
4A-C).
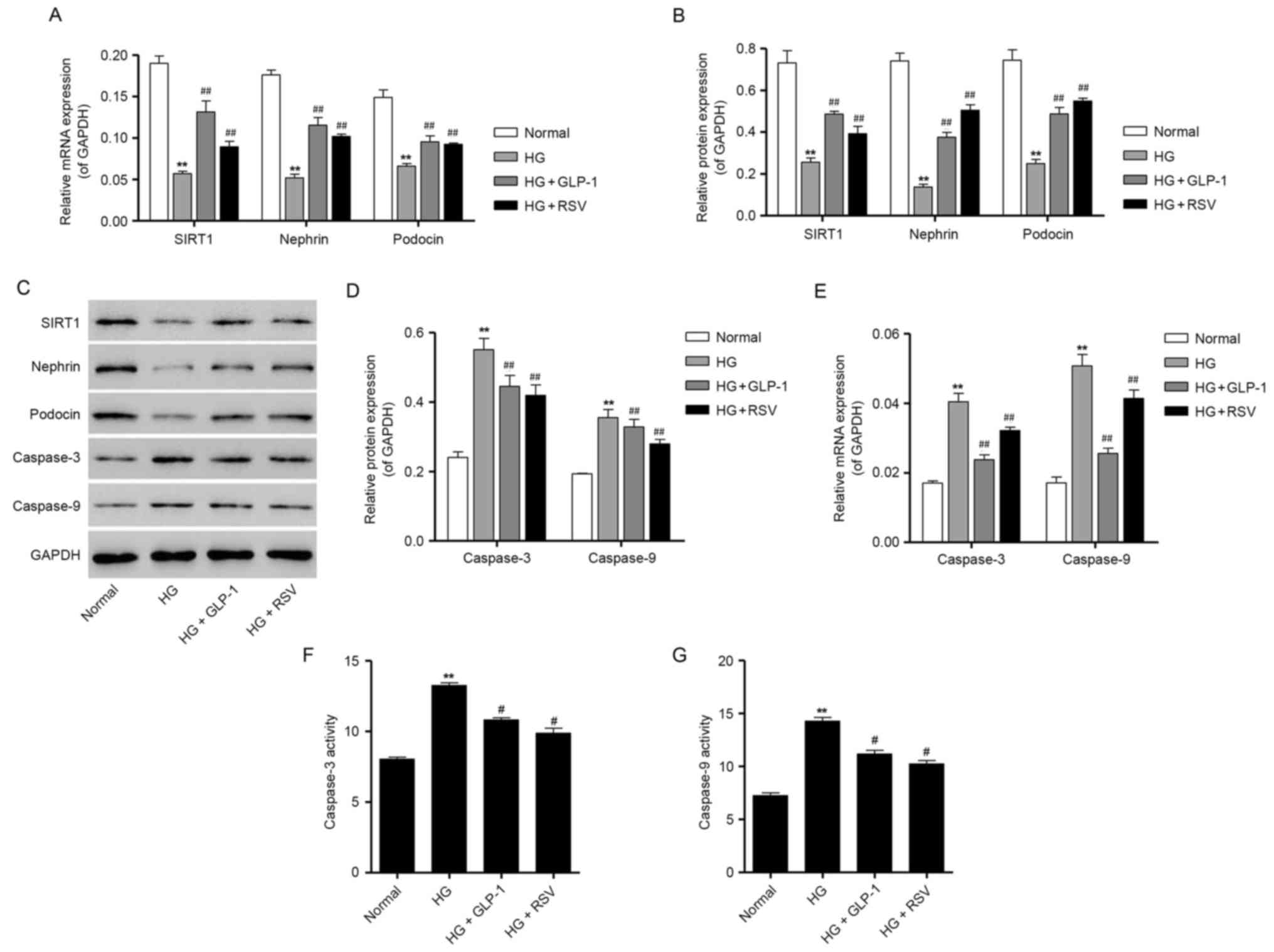 | Figure 4.Effects of GLP-1 on the expression of
podocyte-specific markers and apoptosis-associated proteins in
HG-treated mouse podocytes. (A) Mouse podocytes were treated with
30 mM HG in the absence or presence of 500 nM GLP-1 or 10 µM RSV,
and the mRNA expression of SIRT1, nephrin and podocin was
determined by RT-qPCR. (B) Protein expression levels of SIRT1,
nephrin and podocin were quantified by densitometric analysis
following treatment with 30 mM HG in the absence or presence of 500
nM GLP-1 or 10 µM RSV. (C) Representative western blot bands for
SIRT1, nephrin, podocin, caspase-3 and caspase-9 are presented.
GAPDH was employed as the loading control. (D) Protein expression
levels of caspase-3 and caspase-9 were quantified by densitometric
analysis. (E) RT-qPCR results for caspase-3 and caspase-9 mRNA
expression following treatment with 30 mM HG in the absence or
presence of 500 nM GLP-1 or 10 µM RSV. The activity of (F)
caspase-3 and (G) caspase-9 in HG-treated mouse podocytes was also
measured using colorimetric biochemical assays. Data are presented
as the mean ± standard deviation. **P<0.01 vs. normal group;
##P<0.01 vs. HG group. GLP, glucagon-like peptide;
HG, high glucose; RSV, resveratrol; SIRT, sirtuin; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
GLP-1 attenuates HG-induced
upregulation of caspase expression and activity in mouse
podocytes
RT-qPCR and western blot analysis were performed to
assess the mRNA and protein expression of caspase-3 and caspase-9
in podocytes. The results demonstrated that 30 mM glucose
significantly enhanced the mRNA and protein expression of caspase-3
and caspase-9, compared with cells maintained under normal glucose
(Fig. 4C-E). However, GLP-1
treatment was demonstrated to significantly suppress the mRNA and
protein expression of caspase-3 and caspase-9, which was induced by
HG in mouse podocytes (Fig. 4C-E).
Furthermore, the results of caspase activity assays demonstrated
that, compared with cells treated with normal levels of glucose, 30
mM glucose significantly increased the activity ofcaspase-3 and
caspase-9 (Fig. 4F and G).
Consistent with mRNA and protein results, compared with podocytes
cultured in HG, 500 nM GLP-1-treated cells exhibited a significant
decrease in the activity of caspase-3 and caspase-9, which was
similar to the protective effects produced by RSV administration in
HG-cultured mouse podocytes (Fig. 4F
and G). Taken together, these results indicate that GLP-1 may
exert an antiapoptotic effect in mouse podocytes cultured in HG
conditions.
GLP-1 suppresses HG-induced secretion
of proinflammatory cytokines in mouse podocytes
To determine the effect of GLP-1 on HG-induced
inflammatory responses in mouse podocytes, ELISA assays were
performed to detect the levels of proinflammatory cytokines
secreted by podocytes. The results demonstrated that treatment with
30 mM glucose induced a significant increase in the levels of
TNF-α, IL-1β and IL-6 secreted by mouse podocytes (Fig. 5). Compared with HG-cultured
podocytes, cells treated with 500 nM GLP-1 exhibited a significant
decrease in the secretion of TNF-α, IL-1β and IL-6; the effects of
GLP-1 were similar to the protective effects induced by RSV
administration in HG-stimulated mouse podocytes (Fig. 5).
GLP-1 reduces SIRT1 shRNA-induced
upregulation of caspase-3 and caspase-9 expression in mouse
podocytes
To investigate the roles of SIRT1 in the protective
effects of GLP-1 in mouse podocytes, SIRT1 expression was silenced
in mouse podocytes following infection with a pLKO.1-SIRT1-shRNA
recombinant lentiviral vector. The results demonstrated that the
mRNA and protein expression of SIRT1 in mouse podocytes following
transduction with the pLKO.1-SIRT1-shRNA vector was significantly
decreased by 81.1±0.03 and 60.3±0.03%, respectively (Fig. 6A-C), thus confirming successful
knockdown of SIRT1 following transduction. In addition, SIRT1-shRNA
transduction was demonstrated to enhance the protein and mRNA
expression of caspase-3 and caspase-9, compared with the cells
maintained in normal glucose and the NC shRNA group, which was
similar to the effect of HG exposure in mouse podocytes (Fig. 6D-F). Notably, western blotting and
RT-qPCR results demonstrated that treatment of podocytes with 500
nM GLP-1 significantly suppressed the expression of caspase-3 and
caspase-9, and upregulated the expression of SIRT1, in
pLKO.1-NC-shRNA and pLKO.1-SIRT1-shRNA lentiviral infected mouse
podocytes (Fig. 6D-F). These
results indicate that GLP-1 may inhibit the HG-induced apoptosis of
mouse podocytes through the activation of SIRT1 in
vitro.
 | Figure 6.Effects of GLP-1 on caspase-3 and
caspase-9 expression in mouse podocytes following SIRT1 knockdown.
(A) SIRT1 mRNA expression in mouse podocytes transduced with
pLKO.1-SIRT1-shRNA lentiviral vectors was determined by RT-qPCR.
(B) Protein levels of SIRT1 in mouse podocytes transduced with
pLKO.1-SIRT1-shRNA lentiviral vectors were determined by western
blot analysis and quantified by densitometric software. (C)
Representative western blot bands for SIRT1 protein expression in
control cells and mouse podocytes transduced with NC- or
SIRT1-shRNA. (D) Western blot analysis was performed to measure the
protein expression of SIRT1, caspase-3 and caspase-9 following
transduction of mouse podocytes with NC- or SIRT1-shRNA lentiviral
vectors with or without 500 nM GLP-1. (E) Densitometric analysis
was performed to quantify the protein expression in different
groups. (F) RT-qPCR was performed to measure the mRNA expression of
SIRT1, caspase-3 and caspase-9 in NC- or SIRT1-shRNA-transduced
mouse podocytes with or without treatment with 500 nM GLP-1. Data
are presented as the mean ± standard deviation. For parts A and B,
**P<0.01 vs. NC group; for parts E and F, **P<0.01 vs. normal
group, ##P<0.01 vs. NC group and
ΔΔP<0.01 vs. SIRT1-shRNA group. GLP, glucagon-like
peptide; SIRT, sirtuin; shRNA, short hairpin RNA; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; Control,
untransduced cells; NC, negative control; HG, high glucose; NC,
NC-shRNA-transduced cells. |
Discussion
In the present study, the protective effects of
GLP-1 against cellular damage were investigated in HG-treated mouse
podocytes in vitro, in order to determine the potential
roles of GLP-1 in the pathogenesis of DN. The results of the
current study demonstrated that knockdown of SIRT1 expression
enhanced cell apoptosis in mouse podocytes, while treatment with
GLP-1 counteracted these effects. A previous study reported that
SIRT1 exerted its cytoprotective effects through a number of
mechanisms, including via antiapoptotic, antioxidative and
anti-inflammatory functions, and its involvement in the regulation
of mitochondrial biogenesis and autophagy (26).
One of the major biomarkers for the prediction of DN
progression is a reduced density of podocytes (27); however, the molecular pathways and
pathological mechanisms that are associated with podocyte depletion
in DN are yet to be fully elucidated. Nephrin and podocin are
podocyte-specific protein markers that are critical for the
establishment of the podocyte slit diaphragm structure and for the
maintenance of the intact filtration barrier (28). In the present study, the expression
of nephrin and podocin was detected in mouse podocytes, and the
results revealed that HG exposure significantly suppressed nephrin
and podocin expression; notably, GLP-1 treatment was demonstrated
to enhance the expression of nephrin and podocin in HG-treated
cells.
Based on in vitro evidence presented in the
current study, it may be hypothesized that the exposure of
podocytes to HG leads to cytotoxic effects, as indicated by the
increased ROS production and enhanced cell apoptosis following HG
treatment. These results are consistent with previous reports that
demonstrated that glucose caused impairments in the mitochondrial
membrane potential and increased ROS generation in podocytes
(29), and the HG-induced ROS
increase initiated podocyte apoptosis and podocyte depletion in
vitro and in vivo (27), which was alleviated following GLP-1
treatment. In cardiac microvascular endothelial cells, the
protective effects of GLP-1 have been proposed to be mediated
through the inhibition of Rho/Rho-associated protein kinase
activation, which may subsequently reduce HG-induced oxidative
stress and apoptosis (15).
Members of the caspase family, particularly caspase-3 and
caspase-9, have been reported to serve important roles in the
HG-induced apoptosis of proximal tubular epithelial cells (30). In addition, high levels of glucose
promoted ROS generation through NAD phosphate oxidase and
mitochondrial mechanisms, which in turn activated caspase-3 in
podocytes in vitro (27).
In the present study, treatment of podocytes with HG significantly
increased the mRNA and protein expression levels of capase-3 and
caspase-9 in podocytes, compared with in normal glucose-treated
cells. GLP-1 treatment was revealed to counteract the effects of HG
in podocytes, as indicated by the significant downregulation of the
mRNA and protein expression of caspase-3 and caspase-9. In
accordance with the present study, GLP-1 has been reported to
suppress caspase-3 activation in pancreatic β cells (31) and reverse the increase in apoptotic
cells induced by chronic hyperglycemia in the kidneys of Zucker
diabetic fatty rats (1).
Inflammatory responses are an important factor
contributing to renal injury in HG-induced DN (32). In the present study, HG treatment
was revealed to induce cytotoxic and oxidative stress responses, as
well as inflammatory responses, in mouse podocytes. The results of
the current study demonstrated that the levels of TNF-α, IL-1β and
IL-6 secreted by mouse podocytes were increased in response to HG,
as indicated using ELISA. These results are consistent with those
of a previous study, which reported increased TNF-α, IL-1β and IL-6
levels in the kidneys of diabetic rats (1). Notably, in the present study, GLP-1
treatment significantly suppressed the secretion of TNF-α, IL-1β
and IL-6, which was consistent with a previous report that
demonstrated that the GLP-1 agonist exendin-4 reduced the
infiltration of macrophages and the secretion of inflammatory
cytokines, including TNF-β, IL-6 and IL-1β, by macrophages
(33).
The present study demonstrated that SIRT1 expression
was downregulated in HG-treated mouse podocytes, while it was
upregulated following GLP-1 treatment in a dose-dependent manner.
RSV has been reported to exert protective effects against numerous
diseases, including diabetes, neurodegenerative disorders,
cognitive disorders, cancer, kidney diseases and cardiovascular
diseases, through the activation of SIRT1 (18,34).
In the present study, RSV was used as a positive control to compare
the effect of GLP-1 on SIRT1 expression. The results of the present
study demonstrated that RSV inhibited the HG-induced increase in
the ROS generation and apoptosis of mouse podocytes (data not
shown). SIRT1 activation by RSV has been reported to suppress the
urinary excretion of albumin and reduce the apoptosis of proximal
tubule epithelial cells in vitro and in vivo
(35). Furthermore, in the present
study, knockdown of SIRT1 expression resulted in the upregulation
of capase-3 and caspase-9 expression in mouse podocytes, thus
indicating that SIRT1 knockdown may promote the progression of DN.
Notably, treatment with GLP-1 was revealed to inhibit the SIRT1
knockdown-induced increase in capase-3 and caspase-9 expression,
which indicates that GLP-1 may protect mouse podocytes against
HG-induced damage and apoptosis through the activation of SIRT1
in vitro.
In conclusion, to the best of our knowledge, the
present study demonstrated for the first time that GLP-1 may exert
protective effects against HG-induced damage in mouse podocytes, as
indicated by the suppression of HG-induced ROS production,
apoptosis and inflammatory responses, through the activation of
SIRT1 in vitro. The results of the current study may provide
novel insights into the roles of SIRT1 in DN, and indicate that the
modulation of SIRT1 expression may have potential as a novel
therapeutic strategy to alleviate podocyte injury induced by HG in
patients with diabetes.
Acknowledgements
The present study was supported by the Youth
Scientific Research Funds of Changhai Hospital in 2014 (grant no.
CH 201508).
References
|
1
|
Marques C, Mega C, Goncalves A,
Rodrigues-Santos P, Teixeira-Lemos E, Teixeira F, Fontes-Ribeiro C,
Reis F and Fernandes R: Sitagliptin prevents inflammation and
apoptotic cell death in the kidney of type 2 diabetic animals.
Mediators Inflamm. 2014:5387372014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Panduru NM, Saraheimo M, Forsblom C, Thorn
LM, Gordin D, Wadén J, Tolonen N, Bierhaus A, Humpert PM and Groop
PH: FinnDiane Study Group: Urinary adiponectin is an independent
predictor of progression to end-stage renal disease in patients
with type 1 diabetes and diabetic nephropathy. Diabetes Care.
38:883–890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu Y, Dong J, Yuan L, Liang C, Ren K,
Zhang W, Fang F and Shen J: Nephrin and podocin loss is prevented
by mycophenolate mofetil in early experimental diabetic
nephropathy. Cytokine. 44:85–91. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jim B, Ghanta M, Qipo A, Fan Y, Chuang PY,
Cohen HW, Abadi M, Thomas DB and He JC: Dysregulated nephrin in
diabetic nephropathy of type 2 diabetes: A cross sectional study.
PLoS One. 7:e360412012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu BC, Song X, Lu XY, Li DT, Eaton DC,
Shen BZ, Li XQ and Ma HP: High glucose induces podocyte apoptosis
by stimulating TRPC6 via elevation of reactive oxygen species.
Biochim Biophys Acta. 1833:1434–1442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shah A, Xia L, Goldberg H, Lee KW, Quaggin
SE and Fantus IG: Thioredoxin-interacting protein mediates high
glucose-induced reactive oxygen species generation by mitochondria
and the NADPH oxidase, Nox4, in mesangial cells. J Biol Chem.
288:6835–6848. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brezniceanu ML, Liu F, Wei CC, Chénier I,
Godin N, Zhang SL, Filep JG, Ingelfinger JR and Chan JS:
Attenuation of interstitial fibrosis and tubular apoptosis in db/db
transgenic mice overexpressing catalase in renal proximal tubular
cells. Diabetes. 57:451–459. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Purwata TE: High TNF-alpha plasma levels
and macrophages iNOS and TNF-alpha expression as risk factors for
painful diabetic neuropathy. J Pain Res. 4:169–175. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu L, Shen P, Bi Y, Chen J, Xiao Z, Zhang
X and Wang Z: Danshen injection ameliorates STZ-induced diabetic
nephropathy in association with suppression of oxidative stress,
pro-inflammatory factors and fibrosis. Int Immunopharmacol.
38:385–394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bălăşescu E, Cioplea M, Brinzea A, Nedelcu
R, Zurac S and Ion DA: Immunohistochemical aspects of cell death in
diabetic nephropathy. Rom J Intern Med. 54:54–62. 2016.PubMed/NCBI
|
|
11
|
Drucker DJ: The biology of incretin
hormones. Cell Metab. 3:153–165. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta A, Jelinek HF and Al-Aubaidy H:
Glucagon like peptide-1 and its receptor agonists: Their roles in
management of Type 2 diabetes mellitus. Diabetes Metab Syndr.
11:225–230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen M, Sun D, Li W, Liu B, Wang S, Zhang
Z and Cao F: The synergistic effect of valsartan and LAF237
((S)-1-((3-hydroxy-1-adamantyl)ammo)acetyl-2-cyanopyrrolidine) on
vascular oxidative stress and inflammation in type 2 diabetic mice.
Exp Diabetes Res. 2012:1461942012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishibashi Y, Nishino Y, Matsui T, Takeuchi
M and Yamagishi S: Glucagon-like peptide-1 suppresses advanced
glycation end product-induced monocyte chemoattractant protein-1
expression in mesangial cells by reducing advanced glycation end
product receptor level. Metabolism. 60:1271–1277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang D, Luo P, Wang Y, Li W, Wang C, Sun
D, Zhang R, Su T, Ma X, Zeng C, et al: Glucagon-like peptide-1
protects against cardiac microvascular injury in diabetes via a
cAMP/PKA/Rho-dependent mechanism. Diabetes. 62:1697–1708. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kodera R, Shikata K, Kataoka HU, Takatsuka
T, Miyamoto S, Sasaki M, Kajitani N, Nishishita S, Sarai K, Hirota
D, et al: Glucagon-like peptide-1 receptor agonist ameliorates
renal injury through its anti-inflammatory action without lowering
blood glucose level in a rat model of type 1 diabetes.
Diabetologia. 54:965–978. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park CW, Kim HW, Ko SH, Lim JH, Ryu GR,
Chung HW, Han SW, Shin SJ, Bang BK, Breyer MD and Chang YS:
Long-term treatment of glucagon-like peptide-1 analog exendin-4
ameliorates diabetic nephropathy through improving metabolic
anomalies in db/db mice. J Am Soc Nephrol. 18:1227–1238. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kitada M, Kume S, Takeda-Watanabe A,
Kanasaki K and Koya D: Sirtuins and renal diseases: Relationship
with aging and diabetic nephropathy. Clinical sci (Lond).
124:153–164. 2013. View Article : Google Scholar
|
|
19
|
Chalkiadaki A and Guarente L: Sirtuins
mediate mammalian metabolic responses to nutrient availability. Nat
Rev Endocrinol. 8:287–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He W, Wang Y, Zhang MZ, You L, Davis LS,
Fan H, Yang HC, Fogo AB, Zent R, Harris RC, et al: Sirt1 activation
protects the mouse renal medulla from oxidative injury. J Clin
Invest. 120:1056–1068. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kume S, Uzu T, Horiike K, Chin-Kanasaki M,
Isshiki K, Araki S, Sugimoto T, Haneda M, Kashiwagi A and Koya D:
Calorie restriction enhances cell adaptation to hypoxia through
Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J
Clin Invest. 120:1043–1055. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kume S, Kitada M, Kanasaki K, Maegawa H
and Koya D: Anti-aging molecule, Sirt1: A novel therapeutic target
for diabetic nephropathy. Arch Pharm Res. 36:230–236. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi JX, Wang QJ, Li H and Huang Q:
Silencing of USP22 suppresses high glucose-induced apoptosis, ROS
production and inflammation in podocytes. Mol Biosyst.
12:1445–1456. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishikawa T, Brownlee M and Araki E:
Mitochondrial reactive oxygen species in the pathogenesis of early
diabetic nephropathy. J Diabetes Investig. 6:137–139. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yacoub R, Lee K and He JC: The Role of
SIRT1 in diabetic kidney disease. Front Endocrinol (Lausanne).
5:1662014.PubMed/NCBI
|
|
27
|
Susztak K, Raff AC, Schiffer M and
Bottinger EP: Glucose-induced reactive oxygen species cause
apoptosis of podocytes and podocyte depletion at the onset of
diabetic nephropathy. Diabetes. 55:225–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saleem MA, Ni L, Witherden I, Tryggvason
K, Ruotsalainen V, Mundel P and Mathieson PW: Co-localization of
nephrin, podocin, and the actin cytoskeleton: Evidence for a role
in podocyte foot process formation. Am J Pathol. 161:1459–1466.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bock F, Shahzad K, Wang H, Stoyanov S,
Wolter J, Dong W, Pelicci PG, Kashif M, Ranjan S, Schmidt S, et al:
Activated protein C ameliorates diabetic nephropathy by
epigenetically inhibiting the redox enzyme p66Shc. Proc Natl Acad
Sci USA. 110:648–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Allen DA, Harwood S, Varagunam M, Raftery
MJ and Yaqoob MM: High glucose-induced oxidative stress causes
apoptosis in proximal tubular epithelial cells and is mediated by
multiple caspases. FASEB J. 17:908–910. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tews D, Lehr S, Hartwig S, Osmers A,
Paslack W and Eckel J: Anti-apoptotic action of exendin-4 in INS-1
beta cells: comparative protein pattern analysis of isolated
mitochondria. Horm Metab Res. 41:294–301. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lim AK and Tesch GH: Inflammation in
diabetic nephropathy. Mediators Inflamm. 2012:1461542012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo C, Huang T, Chen A, Chen X, Wang L,
Shen F and Gu X: Glucagon-like peptide 1 improves insulin
resistance in vitro through anti-inflammation of macrophages. Braz
J Med Biol Res. 49:e58262016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kitada M, Kume S, Kanasaki K,
Takeda-Watanabe A and Koya D: Sirtuins as possible drug targets in
type 2 diabetes. Curr Drug Targets. 14:622–636. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang XL, Wu LY, Zhao L, Sun LN, Liu HY,
Liu G and Guan GJ: SIRT1 activator ameliorates the renal tubular
injury induced by hyperglycemia in vivo and in vitro via inhibiting
apoptosis. Biomed Pharmacother. 83:41–50. 2016. View Article : Google Scholar : PubMed/NCBI
|




















