Introduction
A link between age-related hearing loss and
cognitive decline has been supported by numerous studies (1–4). In
recent years, such studies have demonstrated that hearing loss may
serve a causal role in cognitive decline (1–4). In
addition, it has been reported that central auditory dysfunction
significantly increases the risk of dementia (5). The rates of cognitive decline and the
risk for incident cognitive impairment are linearly associated with
the severity of an individual's baseline hearing loss, which
indicates that hearing loss is independently associated with
accelerated cognitive decline and incident cognitive impairment in
community-dwelling older adults (2,6).
Although the mechanisms underlying this association
are unclear, a reliable relationship between age-related hearing
loss and cognitive decline is considered to exist. If hearing loss
can lead to cognitive decline, the use of hearing aids or other
rehabilitative strategies much earlier in the course of hearing
loss may be valuable in the prevention of cognitive decline or
Alzheimer's disease, which has important healthcare and public
policy implications (7,8). Therefore, it is necessary to
determine the effects of age-related hearing loss on cognitive
decline and to explore the mechanisms underlying the association
between them.
Age-related hearing loss, also referred to as
presbycusis, is associated with the deterioration of central and
peripheral auditory systems (9–12).
Age-related dysfunction and degeneration of the auditory cortex in
age-related hearing loss have been detected in humans and in
numerous animal models (13–16).
It is well known that the hippocampus is associated with memory
formation and recognition, and participates in auditory information
processing (17,18). Direct and indirect connections
between the auditory pathway and the hippocampus exist, and
auditory system activity induces hippocampal plasticity (12,19).
A direct connection between the CA1 hippocampal region and the
auditory association cortex, and even to the primary auditory
cortex, has been detected in rats (19). Therefore, auditory
cortex-hippocampus interactions may be involved in the mechanisms
underlying the association between age-related hearing loss and
cognitive decline.
C57BL/6J mice are widely used as a model of
presbycusis, since they exhibit progressive hearing loss (20–22).
C57BL/6J mice, but not CBA/CaJ mice, clearly exhibit decreased
performance in the Morris water maze test and degeneration of
synapses within the hippocampus, which demonstrate that the
age-related hearing loss is accompanied by the degeneration of
synapses in the hippocampal CA3 region (23). Conversely, CBA/CaJ mice retain the
majority of their hearing sensitivity up to 18 months of age,
further supporting the association between age-related hearing loss
and cognitive decline (24). The
present study aimed to determine the association between
age-related hearing loss and cognitive decline in C57BL/6J mice,
and to explore the mechanisms underlying this association.
Materials and methods
Animals
The present study was approved by the Ethics
Committee of Shanghai University of Traditional Chinese Medicine
(Shanghai, China). Male C57BL/6J mice at the following ages were
used in the present study: Young mice (25±2 g), 3 months old; adult
mice (31±2 g), 6 months old; and middle-aged mice (40±3 g), 15
months old (n=10 mice/group). All of the mice were obtained from
Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). The
animals were maintained under a 12-h light/dark cycle at 20–26°C
and 40–70% humidity, and were allowed free access to normal food
and water until experimentation.
Auditory brainstem response (ABR)
test
Each mouse was anesthetized via intraperitoneal
injection with sodium pentobarbital (100 mg/kg). ABRs were recorded
using a TDT-III system with BioSig32 software (both Tucker-Davis
Technologies, Alachua, FL, USA). Tone burst stimuli were generated
at the following frequencies: 8, 16, 24 and 32 kHz. The average
response to 1,000 repetitive stimuli was obtained by reducing the
intensity at 5 dB sound pressure level (SPL) intervals from 90 dB
SPL. The ABR threshold was defined as the lowest dB level at which
a response could be visually detected.
Morris water maze
The water maze used in the present study consisted
of a circular pool (diameter, 120 cm; height, 30 cm), which
included a hidden platform, and a video camera-based computer
tracking system (DigBehv-MM water maze; Shanghai Jiliang Software
Technology Co., Ltd., Shanghai, China). The water temperature in
the circular pool was maintained at 22±1°C. The platform was
submerged 1 cm below the water level. During the 4-day hidden
platform test, the time spent taken to find the hidden platform
(latency) was recorded. If the mouse could not find the platform,
it was placed on the platform for 30 sec. On the fifth day, the
platform was removed and the probe trial was performed. The
percentage of time spent in the previous platform quadrant, also
known as the target quadrant, was recorded.
Hematoxylin and eosin staining
Following anesthetization via intraperitoneal
injection with sodium pentobarbital (120 mg/kg), animals were
immediately sacrificed by cervical dislocation. The brain tissues
were removed quickly on ice and fixed in 4% paraformaldehyde
overnight at 4°C. Following dehydration in a graded series of
alcohol, tissues were embedded in paraffin and sliced into 4 µM
sections. The sections were stained with hematoxylin for 7 min and
eosin for 2 min. Subsequently, the sections were observed and
images were captured under a microscope (BX53; Olympus Corporation,
Tokyo, Japan) after mounting. The location of the hippocampus and
auditory cortex was identified in accordance with the mouse brain
stereotaxic coordinates described by Paxinos and Franklin (25).
Transmission electron microscopy
(TEM)
The hippocampus and auditory cortex tissues were
perfused and fixed with 2.5% glutaraldehyde overnight at 4°C.
Following post-fixation in 1% osmium tetroxide for 2 h at room
temperature, the tissues were dehydrated in an ascending graded
ethanol series at 4°C and acetone series at room temperature.
Subsequently, tissues were immersed in acetone/Epoxy 618 mixture
for 2 h and Epoxy 618 for 2 h, and were finally embedded in Epoxy
618 for 12 h at 37°C and for 8 h at 60°C. Serial ultrathin sections
(50 nm) were collected on copper grids and stained with lead
citrate. The ultrastructure of the stained sections was examined
under a transmission electron microscope (Tecnai G2 Spirit; FEI;
Thermo Fisher Scientific, Inc., Hillsboro, OR, USA).
Western blot analysis
The hippocampus and auditory cortex tissues were
lysed in radioimmunoprecipitation acid lysis buffer containing
protease inhibitor (Shanghai Weiao Biotechnology Co., Ltd.,
Shanghai, China). Protein concentrations were determined using a
Bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology, Haimen, China). Proteins (40 µg) were then subjected
to electrophoresis on 12% polyacrylamide gels and were transferred
to polyvinylidene difluoride membranes by electrotransfer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The membranes were blocked
with 5% non-fat milk in Tris-buffered saline containing 0.5%
Tween-20 for 1 h at room temperature, and were then incubated
overnight with matrix metalloproteinase-9 (MMP-9; cat. no. AF909;
1:800; R&D systems, Minneapolis, MN, USA) and GAPDH (cat. no.
2118S; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA)
primary antibodies at 4°C. Subsequently, the membranes were washed
and incubated with the following corresponding secondary
antibodies: HRP conjugated rabbit anti-goat IgG (cat. no. ab6741;
1:2,000; Abcam, Cambridge, UK) and HRP conjugated goat anti-rabbit
IgG (cat. no. AP132P; 1:2,000; Merck KGaA, Darmstadt, Germany) for
2 h at room temperature. Finally, the protein bands were visualized
with Enhanced Chemiluminescence Plus Western blotting detection
reagents (GE Healthcare, Chicago, IL, USA). The relative levels of
the target protein compared with GAPDH were determined via
densitometric analysis using ImageJ software (version 2.1.4.7;
National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical analysis was conducted using GraphPad Prism
5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Multiple
comparisons were made by one-way analysis of variance and Turkey's
post hoc multiple comparison test. P<0.05 was considered to
indicate a statistically significant difference. Pearson
correlation analysis was performed to investigate the association
between hearing threshold with the escape latency and percentage of
time in the target quadrant.
Results
Age-related hearing loss
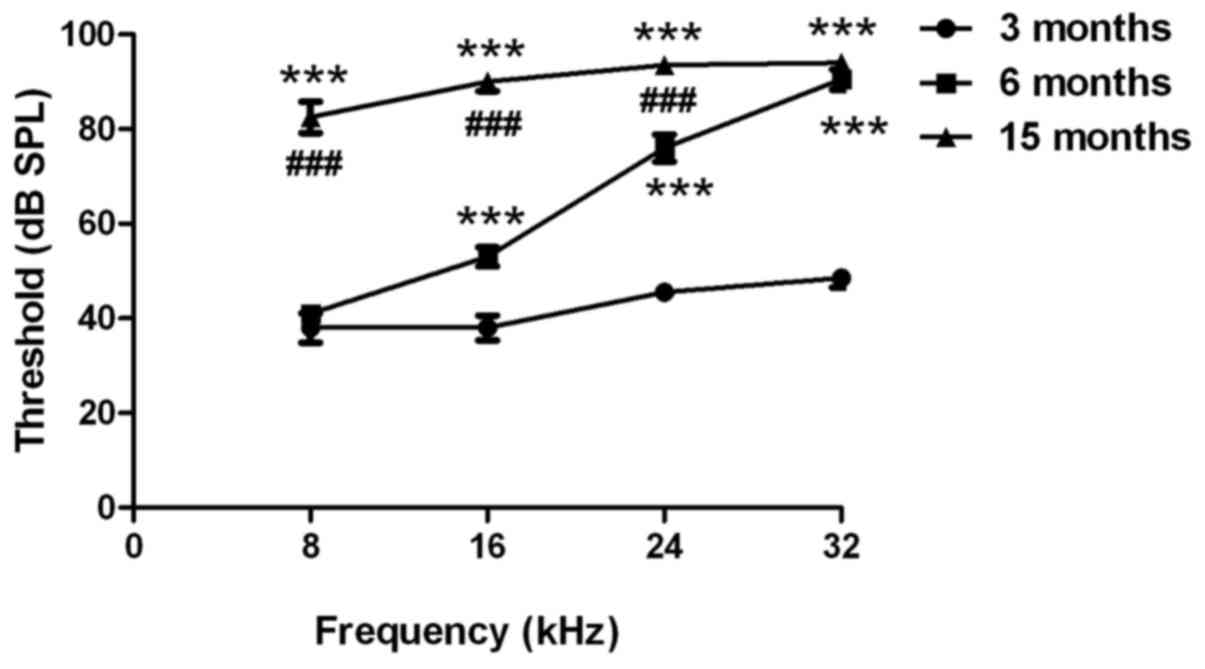
An ABR test was used to evaluate the hearing
function of C57BL/6J mice. As presented in Fig. 1, the hearing thresholds at 16, 24
and 32 kHz were significantly higher in 6-month-old mice compared
with in 3-month-old mice (P<0.001). The hearing thresholds at 8,
16, 24 and 32 kHz were significantly higher in the middle-aged
group (age, 15 months) compared with in the 3-month-old mice
(P<0.001). In addition, the hearing thresholds at 8, 16 and 24
kHz were significantly higher in the 15-month-old mice compared
with in the 6-month-old mice (P<0.001). These findings suggested
that the C57BL/6J mice exhibited age-related hearing loss.
Age-related spatial learning and
memory decline
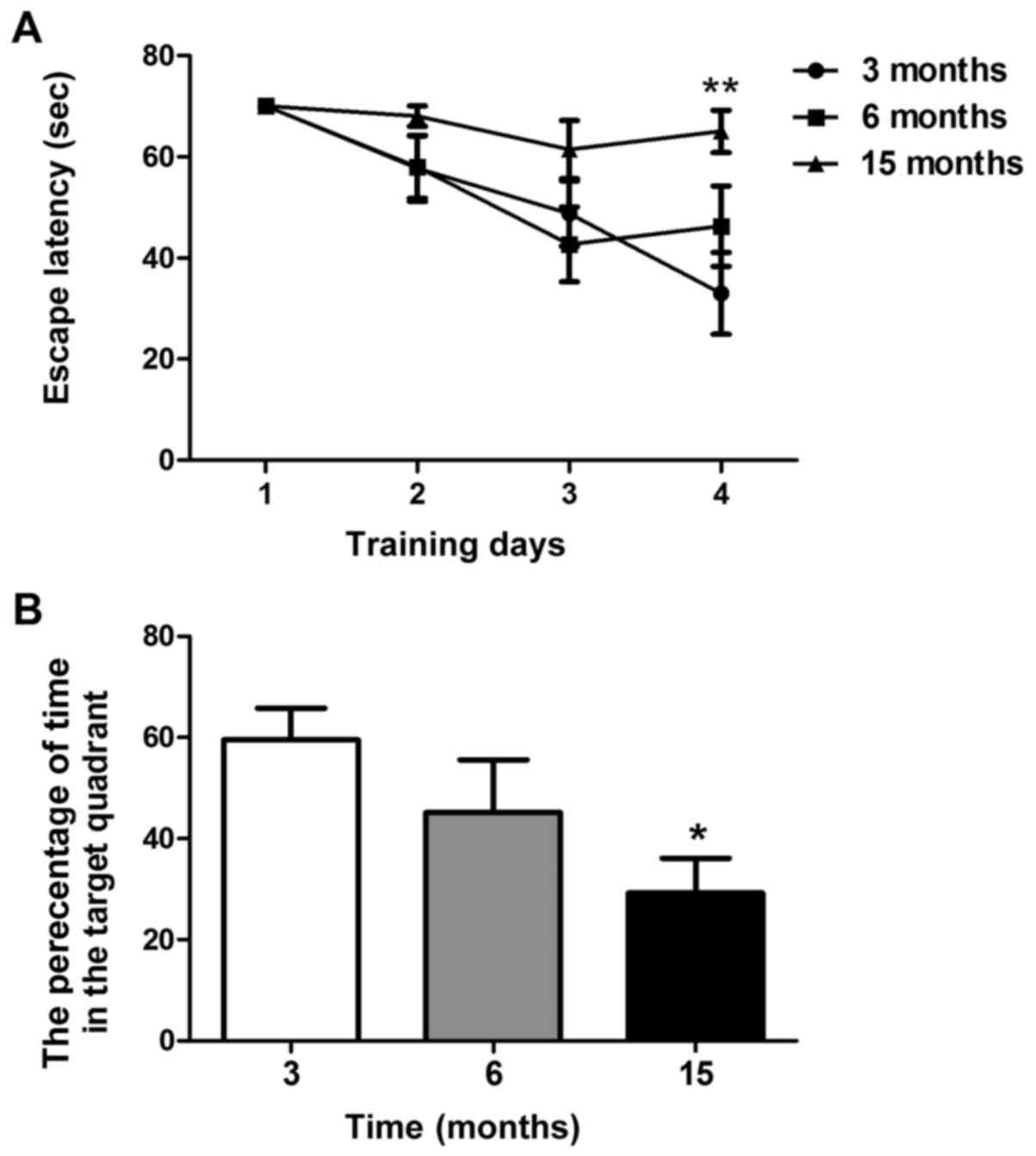
A Morris water maze test was used to examine spatial
learning and memory in C57BL/6J mice. As shown in Fig. 2A, the time taken to find the
submerged platform decreased every day in mice aged 3 and 6 months;
however, this phenomenon was not observed in 15-month-old mice. On
the fourth training day, the escape latency of 15-month-old mice
was significantly higher than that of 3-month-old mice (P<0.01).
However, there was no significant difference between the
6-month-old and 3-month-old mice (Fig.
2A). As shown in Fig. 2B,
15-month-old mice spent less time in the target quadrant compared
with the 3-month-old mice in the probe test on day 5 (P<0.05).
No significant differences were revealed between the 6-month-old
and 3-month-old mice. These findings suggested that 15-month-old
mice, but not 6-month-old mice, may exhibit significant spatial
learning and memory decline.
Correlation between age-related
hearing loss and cognitive decline
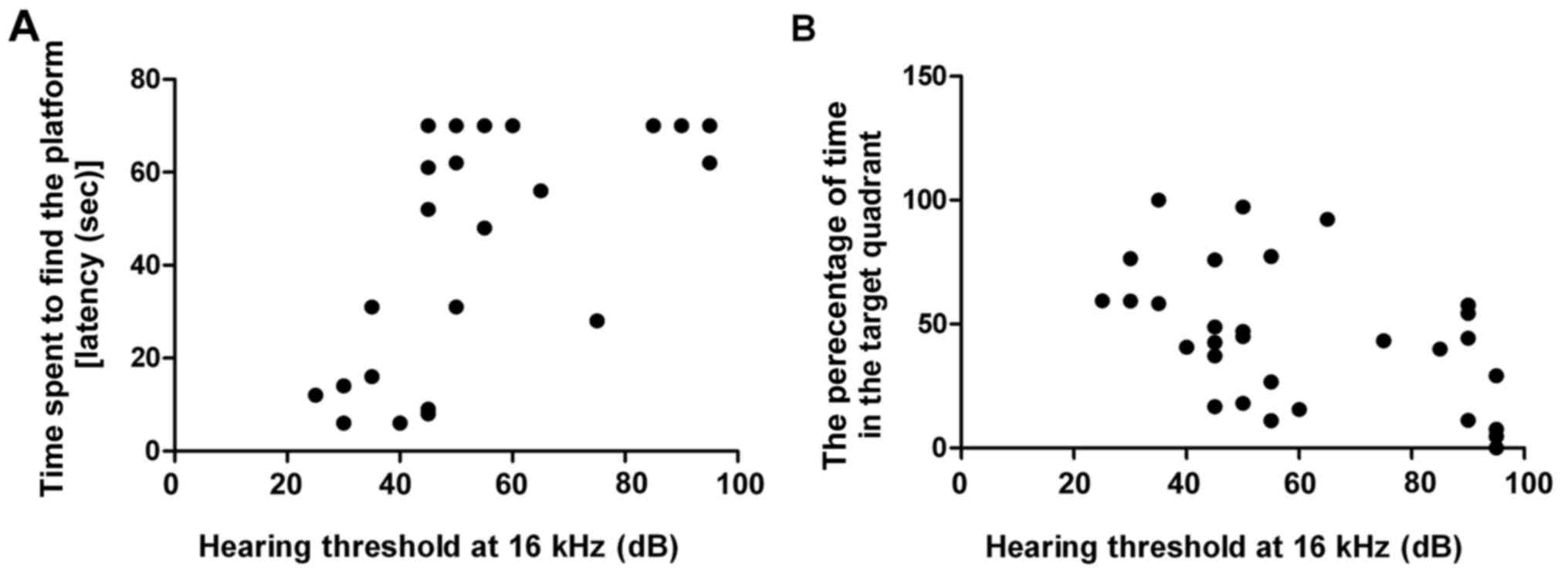
Pearson correlation analysis was used to determine
the correlation between age-related hearing loss and cognitive
decline. Results demonstrated that escape latency was positively
correlated with hearing threshold at 16 kHz (R=0.691, P<0.001;
Fig. 3B) and percentage of time in
the target quadrant was negatively correlated with hearing
threshold at 16 kHz (R=−0.469, P<0.01; Fig. 3B). These findings indicated that a
positive correlation exists between age-related hearing loss and
cognitive decline.
Pathological alterations in the
auditory cortex and hippocampus
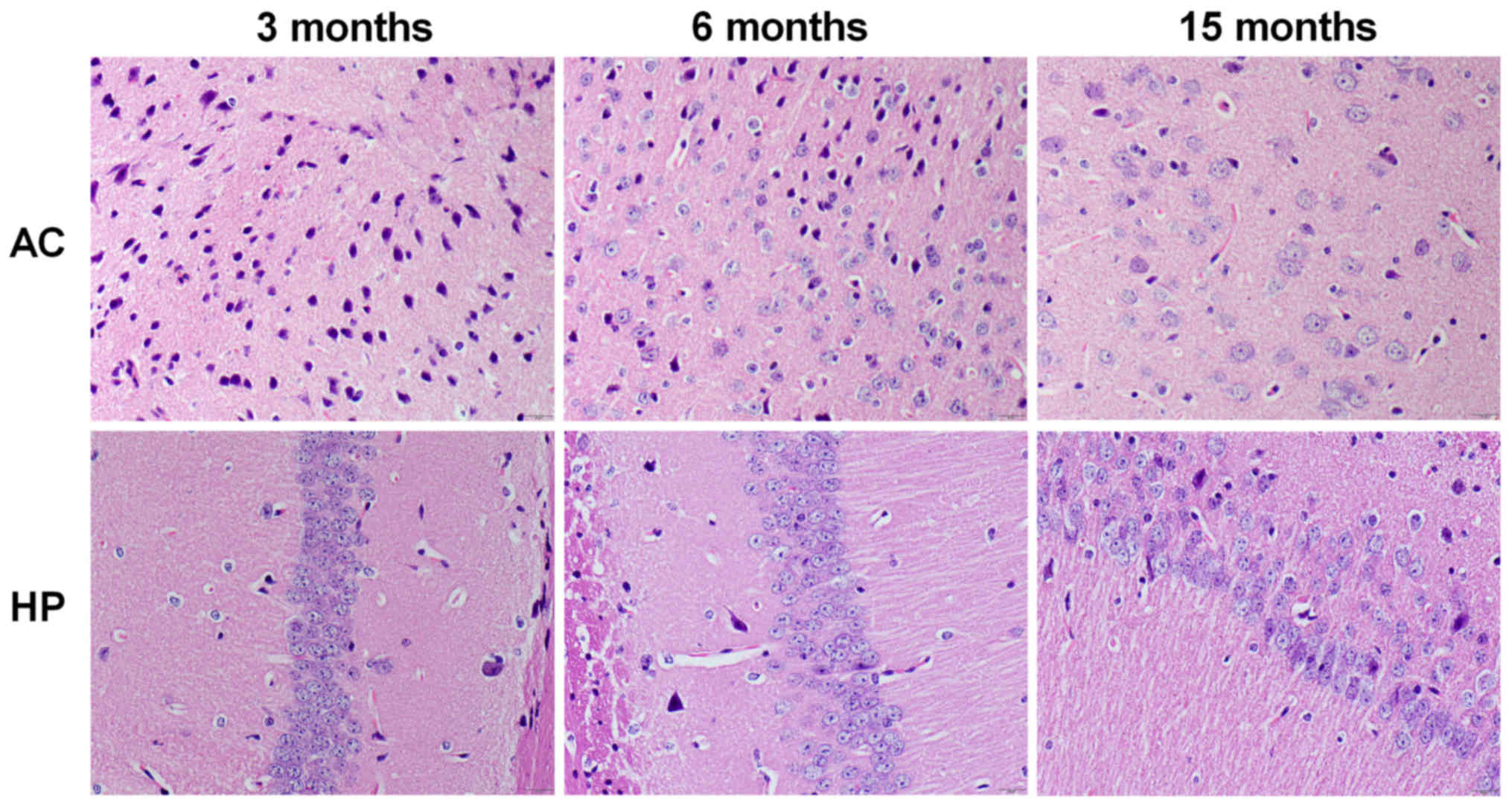
As presented in Fig.
4, the number of neurons in the auditory cortex and hippocampal
CA1 region was markedly decreased in 15-month-old mice compared
with in 3-month-old mice, but not in 6-month-old mice. Furthermore,
pyramidal neurons in the hippocampal CA1 region exhibited a
disorganized arrangement in the 15-month-old mice; however, no
marked alterations were detected in 6-month-old mice.
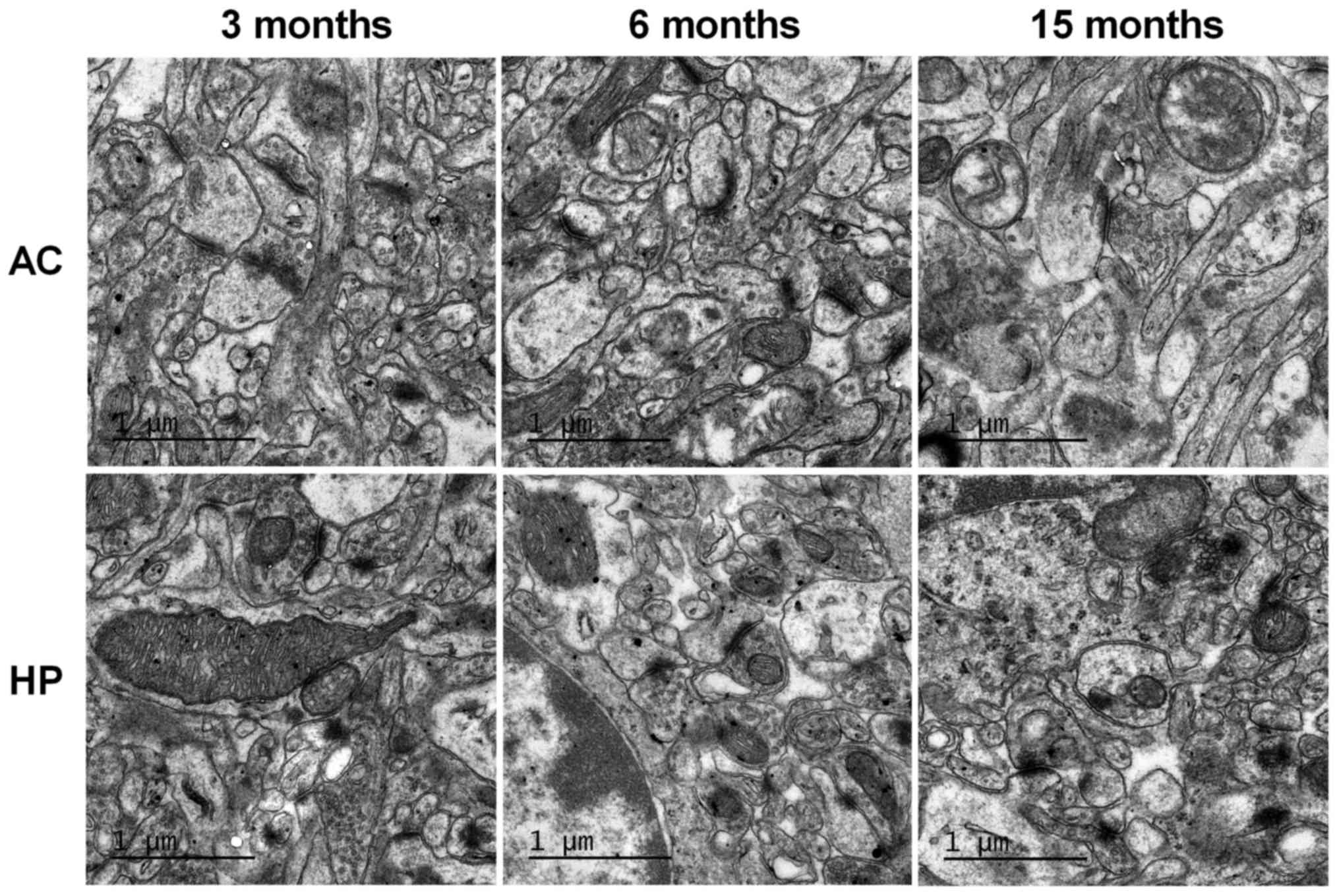
Ultrastructural alterations in the
auditory cortex and hippocampus
TEM was used to observe synaptic ultrastructure in
the auditory cortex and hippocampus. As shown in Fig. 5, there was no marked difference
between the 6-month-old and 3-month-old mice. However, synaptic
number and synaptic vesicle density were markedly decreased in the
15-month-old mice compared with in the 3-month-old mice.
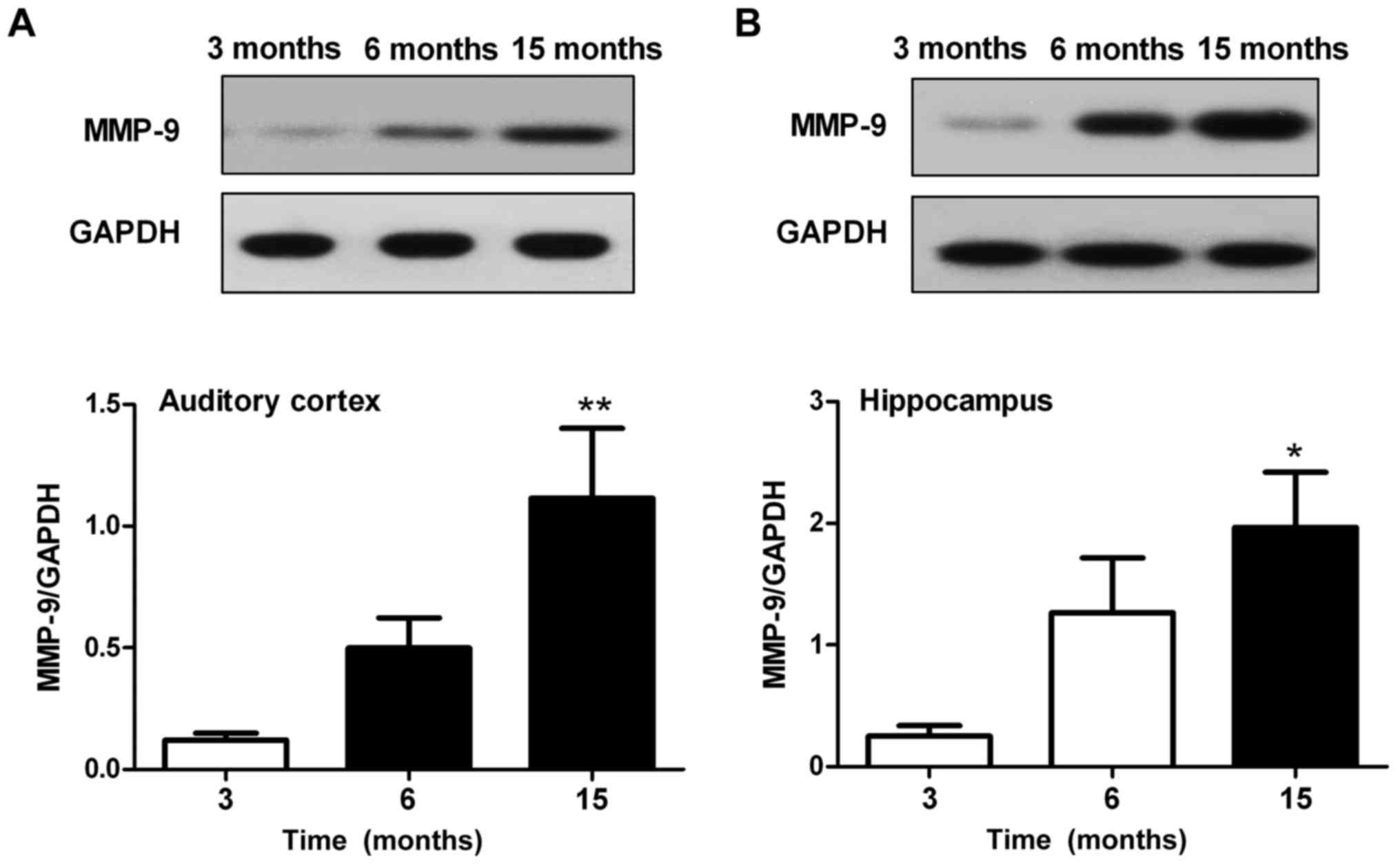
Alterations in the protein expression
levels of MMP-9 in the auditory cortex and hippocampus
Western blotting was used to assess the protein
expression levels of MMP-9 in the auditory cortex and hippocampus.
Results revealed that MMP-9 expression in the auditory cortex
(Fig. 6A) and hippocampus
(Fig. 6B) was significantly
enhanced in the 15-month-old mice compared with in the 3-month-old
mice (P<0.05). There were no significant differences in MMP-9
expression between the 6-month-old and 3-month-old mice in the
auditory cortex and hippocampus; however, MMP-9 expression was
slightly increased in the 6-month-old mice.
Discussion
In the present study, significant hearing loss, but
not cognitive decline, was detected in 6-month-old C57BL/6J mice.
However, 15-month-old mice exhibited severe hearing loss and
obvious cognitive decline. Correlation analysis demonstrated that
cognitive decline was positively correlated with hearing loss in
C57BL/6J mice. These findings indicated the association between
age-related hearing loss and cognitive decline in C57BL/6J mice. In
addition, hearing impairment may occur earlier than cognitive
decline.
At present, the mechanisms underlying the
association between age-related hearing loss and cognitive decline
are unclear. It has been suggested that cognitive decline may be
the manifestation of fatigue from compensating for impaired
auditory input, which would be temporary and reversible. However,
permanent cognitive decline caused by hearing loss may be
associated with neuroplastic alterations that disadvantage general
cognition in favor of processes supporting speech perception
(2,7). It is well known that the hippocampus
is a major limbic region critical for learning and memory. The
hippocampus has been reported to receive direct or indirect neural
input from the central auditory system (26). In addition, there is a direct
connection between the CA1 region and the primary auditory cortex
in rats (19,26). It has previously been reported that
high intensity noise exposure not only damages the cochlea, but
also causes a significant and persistent decrease in hippocampal
neurogenesis, which may contribute to functional deficits in memory
(27). Cheng et al
indicated that the hippocampus may be more vulnerable to
environmental noise than the auditory cortex (28). The present study demonstrated that
the auditory cortex and the CA1 hippocampal region exhibited
significant pathological alterations and synaptic injury in
15-month-old C57BL/6J mice. These findings suggested that auditory
cortex-hippocampus interactions may be involved in the association
between age-related hearing loss and cognitive decline. In
addition, although significant hearing impairment was detected, no
obvious morphological alterations were observed in the auditory
cortex of 6-month-old mice. The hearing loss of 6-month-old mice
may be caused by pathological damage to the peripheral auditory
system; therefore, cognitive function was not significantly
affected by hearing loss in 6-month-old C57BL/6J mice.
MMP-9, which is also known as 92 kDa type IV
collagenase, 92 kDa gelatinase or gelatinase B, is a member of a
large family of zinc-dependent endopeptidases that can cleave
extracellular matrix and numerous cell surface receptors, allowing
for synaptic and circuit level reorganization (29). MMP-9 has been reported to serve a
crucial role in synaptic plasticity and the cognitive process
(30,31). Furthermore, a previous study
revealed that diabetes-associated cognitive deficits partially
stemmed from upregulation of hippocampal MMP-9 via activation of
the nuclear factor-κB signaling pathway (32). MMP-mediated proteolysis has an
important role in regulating nonpathological synaptic function and
plasticity in the mature hippocampus (33). Furthermore, MMP-9 serves various
roles in synaptic plasticity in different nuclei of the amygdala.
It was previously reported that overexpression of MMP-9 leads to an
increase in the strength of basal excitatory synaptic transmission
and impairs the long-term potentiation (LTP) maintenance phase in
the CA3-CA1 pathway in vitro (34). LTP maintenance in the hippocampal
mossy fiber-CA3 pathway requires fine-tuned MMP-9 activity and
raises the possibility that altered MMP-9 levels may be detrimental
for cognitive processes, as observed in some neuropathologies
(35). MMP-mediated extracellular
remodeling during LTP has an instructive role in establishing
persistent modifications in synapse structure and function of the
kind critical for learning and memory (36). The present study revealed that the
protein expression levels of MMP-9 in the hippocampus and auditory
cortex of C57BL/6J mice increased with age. Although no studies, to
the best of our knowledge, have reported on the role of MMP-9 in
age-related hearing loss, some reports have indicated that MMP-9
has a role in hearing function. MMP-9 may be involved in
degenerative processes in early auditory pathways. In addition,
indirect evidence has suggested that MMP-9 may have a role in
auditory critical period plasticity (29). A previous study also reported that
MMP-9 gene ablation was able to mitigate
hyperhomocystenemia-induced cognition and hearing dysfunction
(37). The findings of the present
study suggested that MMP-9 contributed to age-related hearing loss
and cognitive decline in C57BL/6J mice; therefore, MMP-9 may be
involved in the mechanism underlying the association between
age-related hearing loss and cognitive decline. It may be
hypothesized that elevated expression of MMP-9 induces cognitive
decline and hearing loss through destroying the extracellular
matrix and neural membranes, ultimately leading to neuronal
dysfunction in the auditory cortex and hippocampus.
In conclusion, age-related cognitive decline was
correlated with age-related hearing loss in C57BL/6J mice.
Alterations in the expression levels of MMP-9 in the auditory
cortex and hippocampus may contribute to mechanisms underlying the
association between age-related hearing loss and cognitive
decline.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Innovation
Program of Shanghai Municipal Education Commission (grant no.
14YZ064), the National Natural Science Foundation of China (grant
no. 81102695) and the Project of Shanghai Leading Academic
Discipline, Shanghai Education Committee (grant no. J50301).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YD performed the experiments, analyzed the data and
wrote the preliminary manuscript. CG, DC, SC, YP and HS performed
the experiments. JS designed the experiment, and wrote and
corrected the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This experiment was approved by the Ethics Committee
of Shanghai University of Traditional Chinese Medicine.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ABR
|
auditory brainstem response
|
|
SPL
|
sound pressure level
|
|
TEM
|
transmission electron microscopy
|
|
MMP-9
|
matrix metalloproteinase-9
|
|
LTP
|
long-term potentiation
|
References
|
1
|
Bernabei R, Bonuccelli U, Maggi S,
Marengoni A, Martini A, Memo M, Pecorelli S, Peracino AP, Quaranta
N, Stella R, et al: Hearing loss and cognitive decline in older
adults: Questions and answers. Aging Clin Exp Res. 26:567–573.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin FR, Yaffe K, Xia J, Xue QL, Harris TB,
Purchase-Helzner E, Satterfield S, Ayonayon HN, Ferrucci L and
Simonsick EM: Health ABC Study Group: Hearing loss and cognitive
decline in older adults. JAMA Intern Med. 173:293–299. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amieva H, Ouvrard C, Giulioli C, Meillon
C, Rullier L and Dartigues JF: Self-reported hearing loss, hearing
aids, and cognitive decline in elderly adults: A 25-year study. J
Am Geriatr Soc. 63:2099–2104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Castiglione A, Benatti A, Velardita C,
Favaro D, Padoan E, Severi D, Pagliaro M, Bovo R, Vallesi A,
Gabelli C and Martini A: Aging, cognitive decline and hearing loss:
Effects of auditory rehabilitation and training with hearing aids
and cochlear implants on cognitive function and depression among
older adults. Audiol Neurootol. 21 Suppl 1:S21–S28. 2016.
View Article : Google Scholar
|
|
5
|
Gates GA, Cobb JL, Linn RT, Rees T, Wolf
PA and D'Agostino RB: Central auditory dysfunction, cognitive
dysfunction, and dementia in older people. Arch Otolaryngol Head
Neck Surg. 122:161–167. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Surprenant AM and DiDonato R:
Community-dwelling older adults with hearing loss experience
greater decline in cognitive function over time than those with
normal hearing. Evid Based Nurs. 17:60–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wayne RV and Johnsrude IS: A review of
causal mechanisms underlying the link between age-related hearing
loss and cognitive decline. Ageing Res Rev. 23:154–166. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fortunato S, Forli F, Guglielmi V, De
Corso E, Paludetti G, Berrettini S and Fetoni AR: A review of new
insights on the association between hearing loss and cognitive
decline in ageing. Acta Otorhinolaryngol Ital. 36:155–166.
2016.PubMed/NCBI
|
|
9
|
Bao J and Ohlemiller KK: Age-related loss
of spiral ganglion neurons. Hear Res. 264:93–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xue T, Wei L, Zha DJ, Qiu JH, Chen FQ,
Qiao L and Qiu Y: miR-29b overexpression induces cochlear hair cell
apoptosis through the regulation of SIRT1/PGC-1α signaling:
Implications for age-related hearing loss. Int J Mol Med.
38:1387–1394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eckert MA, Cute SL, Vaden KI Jr, Kuchinsky
SE and Dubno JR: Auditory cortex signs of age-related hearing loss.
J Assoc Res Otolaryngol. 13:703–713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gröschel M, Hubert N, Müller S, Ernst A
and Basta D: Age-dependent changes of calcium related activity in
the central auditory pathway. Exp Gerontol. 58:235–243. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du Z, Yang Q, Zhou T, Liu L, Li S, Chen S
and Gao C: Dgalactose-induced mitochondrial DNA oxidative damage in
the auditory cortex of rats. Mol Med Rep. 10:2861–2867. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeng L, Yang Y, Hu Y, Sun Y, Du Z, Xie Z,
Zhou T and Kong W: Age-related decrease in the mitochondrial
sirtuin deacetylase Sirt3 expression associated with ROS
accumulation in the auditory cortex of the mimetic aging rat model.
PLoS One. 9:e880192014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Profant O, Balogová Z, Dezortová M,
Wagnerová D, Hájek M and Syka J: Metabolic changes in the auditory
cortex in presbycusis demonstrated by MR spectroscopy. Exp
Gerontol. 48:795–800. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiong H, Dai M, Ou Y, Pang J, Yang H,
Huang Q, Chen S, Zhang Z, Xu Y, Cai Y, et al: SIRT1 expression in
the cochlea and auditory cortex of a mouse model of age-related
hearing loss. Exp Gerontol. 51:8–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishitani N, Ikeda A, Nagamine T, Honda M,
Mikuni N, Taki W, Kimura J and Shibasaki H: The role of the
hippocampus in auditory processing studied by event-related
electric potentials and magnetic fields in epilepsy patients before
and after temporal lobectomy. Brain. 122:687–707. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moxon KA, Gerhardt GA, Bickford PC, Austin
K, Rose GM, Woodward DJ and Adler LE: Multiple single units and
population responses during inhibitory gating of hippocampal
auditory response in freely-moving rats. Brain Res. 825:75–85.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cenquizca LA and Swanson LW: Spatial
organization of direct hippocampal field CA1 axonal projections to
the rest of the cerebral cortex. Brain Res Rev. 56:1–26. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fetoni AR, Picciotti PM, Paludetti G and
Troiani D: Pathogenesis of presbycusis in animal models: A review.
Exp Gerontol. 46:413–425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Someya S, Xu J, Kondo K, Ding D, Salvi RJ,
Yamasoba T, Rabinovitch PS, Weindruch R, Leeuwenburgh C, Tanokura M
and Prolla TA: Age-related hearing loss in C57BL/6J mice is
mediated by Bak-dependent mitochondrial apoptosis. Proc Natl Acad
Sci USA. 106:19432–19437. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dong Y, Guo CR, Ding Y, Zhang Y, Song HY,
Peng YT, Zhang T and Shi JR: Effects of Erlong Zuoci decoction on
the age-related hearing loss in C57BL/6J mice. J Ethnopharmacol.
181:59–65. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu YF, Zhai F, Dai CF and Hu JJ: The
relationship between age-related hearing loss and synaptic changes
in the hippocampus of C57BL/6J mice. Exp Gerontol. 46:716–722.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bielefeld EC, Tanaka C, Chen GD and
Henderson D: Age-related hearing loss: Is it a preventable
condition? Hear Res. 264:98–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Franklin K and Paxinos G: The Mouse Brain
in Stereotaxic Coordinates. 2nd edition. Academic Press; San Diego,
CA: 2001
|
|
26
|
Kraus KS and Canlon B: Neuronal
connectivity and interactions between the auditory and limbic
systems. Effects of noise and tinnitus. Hear Res. 288:34–46. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kraus KS, Mitra S, Jimenez Z, Hinduja S,
Ding D, Jiang H, Gray L, Lobarinas E, Sun W and Salvi RJ: Noise
trauma impairs neurogenesis in the rat hippocampus. Neuroscience.
167:1216–1226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng L, Wang SH, Huang Y and Liao XM: The
hippocampus may be more susceptible to environmental noise than the
auditory cortex. Hear Res. 333:93–97. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang X, Zhu X, Ding B, Walton JP, Frisina
RD and Su J: Age-related hearing loss: GABA, nicotinic
acetylcholine and NMDA receptor expression changes in spiral
ganglion neurons of the mouse. Neuroscience. 259:184–193. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lei D, Gao X, Perez P, Ohlemiller KK, Chen
CC, Campbell KP, Hood AY and Bao J: Anti-epileptic drugs delay
age-related loss of spiral ganglion neurons via T-type calcium
channel. Hear Res. 278:106–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shen H, Matsui JI, Lei D, Han L,
Ohlemiller KK and Bao J: No dramatic age-related loss of hair cells
and spiral ganglion neurons in Bcl-2 over-expression mice or Bax
null mice. Mol Neurodegener. 5:282010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao Z, Huang G, Wang B and Zhong Y:
Inhibition of NF-kappaB activation by Pyrrolidine dithiocarbamate
partially attenuates hippocampal MMP-9 activation and improves
cognitive deficits in streptozotocin-induced diabetic rats. Behav
Brain Res. 238:44–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bozdagi O, Nagy V, Kwei KT and Huntley GW:
In vivo roles for matrix metalloproteinase-9 in mature hippocampal
synaptic physiology and plasticity. J Neurophysiol. 98:334–344.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wiera G, Szczot M, Wojtowicz T, Lebida K,
Koza P and Mozrzymas JW: Impact of matrix metalloproteinase-9
overexpression on synaptic excitatory transmission and its
plasticity in rat CA3-CA1 hippocampal pathway. J Physiol Pharmacol.
66:309–315. 2015.PubMed/NCBI
|
|
35
|
Wiera G, Wozniak G, Bajor M, Kaczmarek L
and Mozrzymas JW: Maintenance of long-term potentiation in
hippocampal mossy fiber-CA3 pathway requires fine-tuned MMP-9
proteolytic activity. Hippocampus. 23:529–543. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW,
Zhou Q and Huntley GW: Extracellular proteolysis by matrix
metalloproteinase-9 drives dendritic spine enlargement and
long-term potentiation coordinately. Proc Natl Acad Sci USA.
105:19520–19525. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bhargava S, Pushpakumar S, Metreveli N,
Givvimani S and Tyagi SC: MMP-9 gene ablation mitigates
hyperhomocystenemia-induced cognition and hearing dysfunction. Mol
Biol Rep. 41:4889–4898. 2014. View Article : Google Scholar : PubMed/NCBI
|