Introduction
Cardiopulmonary bypass (CPB) refers to the use of
artificial channels to connect the circulatory system of the body
with a heart-lung machine. Venous blood is drawn from the large
vein (or the right atrium) in vitro and oxygenated blood is
injected into the arterial system via the blood pump. During CPB,
aortic block, cardiac arrest and resuscitation may lead to
myocardial ischemia-reperfusion injury, which may result in
postoperative malignant arrhythmia and low cardiac output syndrome
(1). It has been previously
estimated that ~25% of postoperative mortality is associated with
malignant cardiovascular events (2). During CPB, the main causes of
myocardial injury include myocardial ischemia-reperfusion injury,
systemic inflammatory response and mechanical injury (3). Therefore, it is important to
determine the pathophysiological changes of myocardial injury
during CPB in order to develop clinical myocardial protection
strategies.
Ohsawa et al (4) demonstrated that hydrogen can
effectively remove oxygen free radicals and attenuate cerebral
ischemia and reperfusion injury. Hydrogen-rich solution (HRS) can
be obtained by dissolving hydrogen under specific pressurized
conditions to physiological saline. HRS is a good antioxidant, and
has a high hydrogen content. It is weakly alkaline has a negative
potential and contains the small molecule water (5). HRS has been demonstrated to be safe
and non-toxic, and exhibits strong anti-inflammatory,
anti-oxidative stress and anti-apoptotic characteristics (6). HRS exerts a protective effect on the
brain, liver and intestines against ischemia-reperfusion and
myocardial injury; however, the underlying therapeutic mechanism
remains to be determined, thus restricting its further development
and potential clinical application.
Aquaporin (AQP) is a membrane protein responsible
for the transport of water and small molecules between cells. A
recent study demonstrated that AQP has an important role in
regulating the transport in membranes and intracellular water
content. At present, 13 types of AQP proteins (AQP-0-AQP-12) have
been identified in mammals (7).
AQP-1 is concentrated in microvascular endothelial cells and
cardiomyocytes in myocardium (8).
Furthermore, AQP-1 is highly expressed in cardiomyocytes and
vascular endothelial cells in rabbits with chronic myocardial
ischemia (9). In addition, AQP-4
protein also has an important role in myocardial edema (10). A previous study demonstrated that
AQP-4 expression was downregulated following myocardial injury,
thus having a protective effect (11).
The phosphatidylinositol 3′-kinase (PI3K)/protein
kinase B (Akt) signaling pathway is an important signaling pathway
for the regulation of cell survival, growth and proliferation, and
has an important role in the regulation of the myocardial
ischemia-reperfusion injury protective mechanism (12–15).
Akt is in the central regulator of the PI3K/Akt pathway, and
affects numerous downstream effector molecules which manage
anti-ischemia-reperfusion injury (16–18).
The present study aimed to investigate the effects of HRS on
CPB-induced myocardial injury, AQP expression and the underlying
mechanism of the PI3K/Akt signaling pathway, thus providing a novel
approach for the investigation into myocardial therapeutic
strategies.
Materials and methods
Animals and cells
A total of 24 male Sprague Dawley rats, weighing
350–400 g, 10-weeks-old were obtained from the Experimental Animal
Center of China Medical University [Shanyang, China; production
license no. SCXK (Liao)-2013-0001; application license no. SYXK
(Liao)-2013-0007]. The present study was approved by the China
Medical University Laboratory Animal Welfare and Ethics Committee
and adhered to the guidelines of The Institutional Animal Care and
Use Committee; no. 2015048R. Rat myocardial H9C2 cells were
purchased from the Cell Bank of Type Culture Collection of Chinese
Academy of Sciences cell repository (Shanghai, China).
Hydrogen-rich water preparation
Hydrogen-rich water (HRS) was prepared as previously
reported (19): Under high
pressure (0.8 MPa), hydrogen was dissolved in saline for 24 h at
room temperature to reach saturation level and stored in a medical
vacuum bag at 4°C until further use.
Experimental protocols
Rats were randomly divided into 3 groups: i) Sham
operation group (n=8); ii) CPB group (n=8); and iii) CPB+HRS group
(n=8). In the sham group, intubation and mechanical ventilation
were performed on the right femoral artery only, and the right
internal jugular vein was catheterized without bypass. In the CPB
group, the CPB model was established; CPB+HRS group was treated
with HRS (6 ml/kg) via the tail vein. The sham and CPB groups were
injected with 0.9% NaCl solution (6 ml/kg).
H9C2 cells were divided into the following
experimental groups: i) CPB group; ii) HRS + CPB group (HRS group),
iii) LY294002 (cat. no. PHZ1144, Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), a PI3K inhibitor + CPB (PI3K
group); and iv) LY294002 + CPB + HRS (LHRS group). In the CPB
group, CPB was simulated by hypoxia and reoxygenation; the HRS
group was treated with 0.8 mM/l HRS for 3 days and a further HRS
dose was administered prior to hypoxia and during reoxygenation. In
the PI3K group, 40 µM/l PI3K inhibitor, LY294002 was added to H9C2
cells. In the LHRS group, both HRS and PI3K inhibitor, LY294002 (40
µM/l) were added.
Establishment of CPB models
Following anesthesia via an intraperitoneal
injection of pentobarbital sodium, mechanical ventilation was
performed using a small-animal ventilator (HX-100E, Shanghai xinman
scientific equipment Co., Ltd.) with tracheal intubation by
transparent method (20). ECG,
deep rectal temperature and arterial pressure were monitored in
real time using the Datex-Ohmeda S/5 Entropy Module (DRE, Inc.,
Louisville, KY, USA). CPB was performed using a venous drainage
tube, a blood container, a constant flow peristaltic pump, a
membrane oxygenator (extracorporeal membrane oxygenation), an
arterial infusion tube, a warming blood device and a filter. The
blood container was placed in front of the peristaltic pump and
connected to the right internal jugular vein drainage tube. The
membrane oxygenator was set up behind the pump and connected to the
perfusion end of right femoral artery via the warming blood device
with a pipe. There was a bypass pipe between the blood container
and membrane oxygenator (ideograph is presented in Fig. 1A). When activated clotting time
reached 400–500 sec, CPB began. At the beginning of CPB, the flow
rate was 35 ml·kg−1·min−1, and this gradually
increased to 100–120 ml·kg−1 min−1. The blood
volume was maintained at 1–2 ml. When CPB began, the respirator was
terminated. Oxygen was provided by an oxygenator
(FiO2=1.0). α-stat blood gas management was performed
using a small-animal ventilator. Following 1 h of CPB, mechanical
ventilation was restored. The flow rate was gradually reduced and
finally terminated, and each pipe was then removed from the heart.
Mechanical ventilation was continuous, and rectal temperature was
maintained at 36.5–37.5°C. The remaining blood from the blood
container was slowly infused, and stable circulation was
maintained. Arterial blood (0.2 ml) was collected at the following
time intervals during the surgery for blood gas analysis: Prior to
CPB (T0), time of aortic occlusion (T1), time
of restoring aorta (T2), time of CPB termination
(T3) and 2 h following CPB (T4). Blood loss
during blood gas analysis was supplemented with 6% hydroxyethyl
starch.
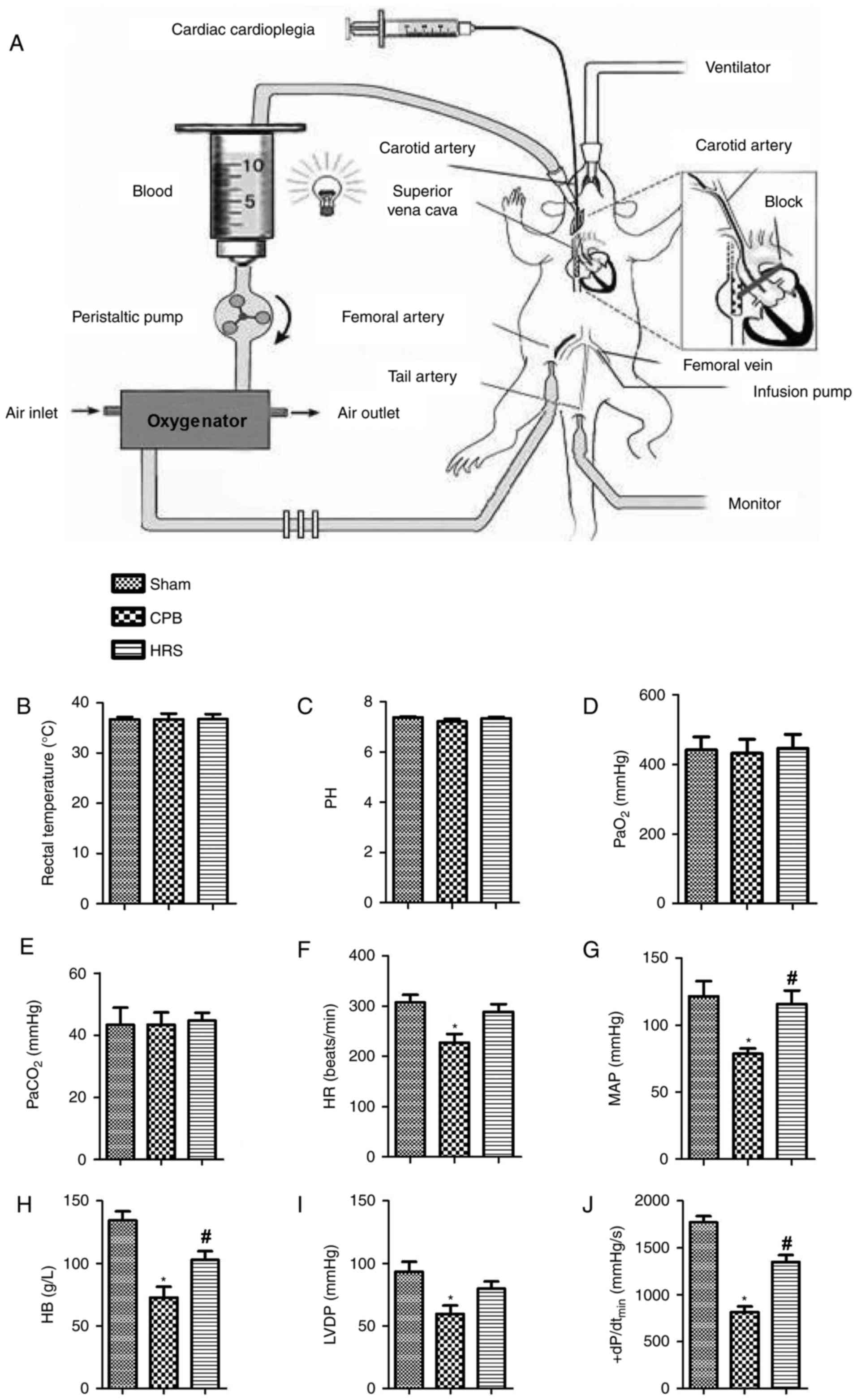 | Figure 1.Establishment of the CPB rat model
and the determination of hemodynamic changes. (A) Establishment of
the CPB rat model. Hemodynamic changes exhibited by the sham, CPB
and HRS groups were categorized as (B) rectal temperature, (C) pH,
(D) PaO2, (E) PaCO2, (F) HR, (G) MAP, (H) HB,
(I) LVDP and (J) +dP/dtmax. Data between two groups were
compared using the Student's t-test. Data among groups were
compared using one-way analysis of variance. *P<0.05 vs. sham
group; #P<0.05 vs. CPB group. CPB, cardiopulmonary
bypass; HRS, hydrogen rich solution; +dP/dtmax, the
highest rate of change of pressure development; PaO2,
oxygen partial pressure; PaCO2, carbon dioxide partial
pressure; HR, heart rate; MAP, mean arterial pressure; HB,
hemoglobin; LVDP, left ventricular diastolic pressure. |
Hemodynamic changes
All rats had awakened 60–90 min following
anesthesia. Hemodynamics were detected using the Datex-Ohmeda S/5
Entropy Module (DRE, Inc.). Rectal temperature, pH, arterial
CO2 partial pressure (PaCO2), oxygen partial
pressure (PaO2), heart rate (HR), mean arterial pressure
(MAP), left ventricular diastolic pressure (LVDP), the highest rate
of change of pressure development (+dP/dtmax) and
hemoglobin (Hb) were recorded.
Specimen collection and
processing
Arterial and venous blood samples were respectively
collected at CPB for 6 h following the sacrifice of rats via
administration of an overdose of anesthesia of sodium
pentobarbital. Venous blood was sterilely obtained, anticoagulated
with heparin and then centrifuged at 1,000 × g for 5 min at 4°C.
Blood plasma was isolated, packed separately, and stored at −80°C
for further use. Following this, the myocardial tissue was isolated
from the rat and one part was fixed in 4% paraformaldehyde and the
other part was stored at −80°C for subsequent western blotting and
polymerase chain reaction (PCR) analysis. The sera were separated
by centrifugation at 1,000 × g for 10 min at 4°C, and then stored
at −80°C.
Determination of myocardial water
content
The moisture on the surface of left ventricle (~100
mg) was blotted, hydrated at 100°C for 24 h, and then baked for
weighing. According to the Ellis formula: Myocardial water
content=(wet weight-dry weight)/wet weight ×100%.
Establishment of hypoxia-reoxygenation
model
The hypoxic solution was used to simulate the CPB
process in H9C2 myocardial cells. The hypoxic solution (pH 6.5)
consisted of: NaCl (137 mM), KCl (12 mM), MgCl2 (0.49
mM), CaCl2−2H2O (0.9 mM),
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (4 mM),
deoxyglucose (10 mM), sodium sulfite (0.75 mM) and sodium lactate
(20 mM). During hypoxia, the cells were placed in 95% N2
and 5% CO2 for 2 h at 37°C. During reoxygenation, the
cells were incubated in normal culture medium for 4 h at 37°C.
Myocardial morphology as observed by
hematoxylin & eosin (H&E) staining
Myocardium was washed with 0.9% physiological
saline, immersed in 10% neutral formaldehyde for 48 h at room
temperature, dehydrated, embedded in wax and then sliced into 5
mm-thick sections using a ultramicrotome (LKB-8800, GE Healthcaew,
Chicago, IL, USA). Following H&E staining (with 10% hematoxylin
for 10 min, differentiated with 1% hydrochloric acid and ethanol
for 3–5 sec, stained with 0.5% eosin for 1 min) at room
temperature. Each tissue was observed under 4–6 randomly selected
visual fields using a light microscope (magnification, ×200, Lecia
DM5500B; Lecia Microsystems GmbH, Wetzlar, Germany).
Masson staining
Paraffin sections (5 µm) were dewaxed using
distilled water and then stained using 5% Regaud dye hematoxylin
staining with Masson stain for 5–10 min (All staining treatments
were carried out at room temperature). Sections were then washed
and stained using 0.7% Ponceau Fuchsin acid solution for 5–10 min;
using 2% acetic acid aqueous solution soak for a moment, samples
were differentiated in 1% phosphomolybdic acid aqueous solution for
3–5 min. The sections were directly stained with 2% aniline blue
for 5 min. Following dehydration with a ethanol series, cleaning
with xylene and mounting with neutral resins, digital images were
captured using a light microscope (maginification, ×200, Lecia
DM5500B; Lecia Microsystems GmbH).
ELISA detection
The expression of the following markers of
myocardial injury were determined in myocardial tissue using ELISA
kits against the following proteins in accordance with the
manufacturer's protocol: Cardiac troponin I (cTnI; cat. no.
SEA478Ra; Cloud-Clone Corp., Wuhan, China), lactate dehydrogenase
(LDH; cat. no. SEB864Ra; Us Cloud-Clone Corp.), creatine kinase MB
(CK-MB; cat. no. SEA479Ra; Cloud-Clone Corp.), brain natriuretic
peptide (BNP; cat. no. CEA541Ra; Cloud-Clone Corp.); inflammatory
cytokines: interleukin (IL)-1β (cat. no. SEA563Ra; Cloud-Clone
Corp.), IL-6 (cat. no. SEA079Ra; Cloud-Clone Corp.), tumor necrosis
factor α (TNF-α; cat. no. SEA133Ra; Cloud-Clone Corp.); oxidative
stress: superoxide dismutase (SOD; cat. no. SES134Ra; Cloud-Clone
Corp.), malondialdehyde (MDA; cat. no. CEA597Ge; Cloud-Clone Corp.)
and myeloperoxidase (MPO; cat. no. SEA601Ra; Cloud-Clone Corp.).
Diluted standard substance (50 µl, of the aforementioned ELISA
kits), detected samples (50 µl) and biotin-labeled antibody (50 µl)
were added to 96-well plates and incubated at 37°C for 1 h, washed
with washing buffer from the aforementioned ELISA kits and then
shaken for 30 sec. This process was repeated three times.
Streptavidin-horseradish peroxidase (HRP) was then added to each
well and incubated at 37°C for 30 min, washed and shaken for 30
sec. This process was repeated three times. Substrates A and B
(each 50 µl) were added to each well, shaken and incubated at 37°C
for 10 min in the dark. The microplate was then removed and the
reaction was terminated. Optical density (OD) values were then
determined at 450 nm in each well.
Western blot assay
The samples underwent ultrasonic focalization
decomposition and centrifuged in a pre-chilled tissue lysate at
16,000 × g for 30 min at 4°C. Subsequently, the supernatant was
collected and protein quantification was performed by bicinchoninic
acid assay (cat. no. 23230, Pierce; Thermo Fisher Scientific,
Inc.), and equal amounts of protein lysate (40 µg) were separated
by 12% SDS-PAGE. The protein was semi-dried prior to polyvinylidene
difluoride membrane transfer. Following this, membranes were
blocked for 2 h at room temperature in TBS with 20% Tween-20 (TBST)
buffer and then incubated overnight at 4°C with antibodies against:
Bcl-2 (1:500; cat. no. ab59348; Abcam, Cambridge, MA, USA), Bax
(1:1,000; cat. no. ab32503; Abcam), caspase-3 (1:500; cat. no.
ab13847; Abcam), AQP-1 (1:1,000; cat. no. ab15080; Abcam), AQP-4
(diluted to 1 µg/ml, cat. no. ab46182; Abcam), PI3K (1:1,000; cat.
no. ab191606; Abcam), P-PI3K (1:500; cat. no. ab138364; Abcam), Akt
(1:10,000; cat. no. ab179463; Abcam), p-Akt (1:500; cat. no.
ab131443; Abcam) heme oxygenase-1 (HO-1; diluted to 4 µg/ml, cat.
no. ab13248; Abcam) and GAPDH (1:2,500; cat. no. ab9485; Abcam).
Following this, membranes were washed with TBST buffer three times
and then incubated with Goat anti-Mouse HRP IgG H&L (1:2,000;
cat. no. ab6789, Abcam) for HO-1 and Goat anti-Rabbit HRP IgG
H&L (1:2,000; cat. no. ab205718, Abcam) to for others for 1 h
at 4°C. Following four washes with tris-buffered saline with 0.1%
Tween-20, cells were developed using a Novex™ ECL Chemiluminescent
Substrate Reagent kit (cat. no. WP20005 Invitrogen; Thermo Fisher
Scientific, Inc.) and gel imaging system. Gray value was determined
using Quantity One software (ImageJ, v1.8.0, National Institutes of
Health).
Cell transfection
H9C2 cells were transfected with a plasmid
containing JAK2 small interfering (si)RNA (10 µM, sc-270385, Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) and then seeded into
6-well culture plates at a density of 2×105 cells/well
with high-glucose Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.) in a 5% CO2 incubator for 24 h
at 37°C. A total of 100 µl transfection medium mixed with 20–80 pM
JAK2 siRNA (solution A) and then mixed with 2–8 µl siRNA
transfection reagent of Lipofectamine® 2000 (Thermo
Fisher Scientific, Inc.). Following mixing, solutions A and B
solution were incubated for 45 min at room temperature, cells were
rinsed with 2 ml siRNA transfection medium, and then 0.8 ml siRNA
transfection medium was added to the mixture of solutions A and B,
and cells were incubated in a CO2 incubator for 7 h at
37°C. Following a 24 h incubation at 37°C, cells were used for
further experiments. In the HRS group, cells were treated with HRS
at hypoxia for 2 h at room temperature and then given reoxygenation
for 4 h at room temperature.
MTT colorimetry
The cells in logarithmic growth phase were
inoculated into 96-well culture plates at a density of 2,000–5,000
cells/well and 100 µl cells/well. A total of 20 µl of MTT solution
(5 mg/ml) was added to each well, and cells were cultured for a
further 4 h at 37°C. Following this, the supernatant was removed,
cells were shaken for 10 min and then the crystals were fully
dissolved using dimethyl sulfoxide (150 µl). Optical density (OD)
at 450 nm was determined via ELISA assay, and the cell survival and
inhibition rates were investigated using the respective formulae:
Cell survival rate=(OD value of the intervention group/OD value of
the normal control group) ×100; inhibition rate=1-OD value of the
intervention group/OD value of the control group.
Annexin V/PI staining
The Annexin V-PI (BD 556547, USA) method was used to
detect the apoptosis rate of H9C2 cells via flow cytometry. Cells
were harvested with 0.05% trypsin, washed three times with cold PBS
(4°C), and collected by centrifugation at 110 × g for 5 min at 4°C.
Following this, cells were resuspended in 200 µl binding buffer and
incubated with Annexin V (10 µg/ml) and PI (10 µg/ml) in the dark
for 15 min at room temperature. Then cells were detected with a
flow cytometer (BD, USA).
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay
Apoptotic rates of heart tissues were investigated
using the In situ cell death detection kit-POD
(Sigma-Aldrich; Merck KGaA; cat. no. 11684817910) according to the
manufacturer's instructions. Heart tissues were fixed in 10%
formaldehyde for 24 h at room temperature, and then dehydrated,
embedded, sliced and incubated with 0.9% NaCl for 5 min at room
temperature. Samples were then rinsed twice with PBS, mixed with
biotinylated nucleotides and terminal deoxynucleotidyl transferase,
covered with plastic coverslips and then incubated at 37°C for 60
min. A total of 50 µl of TUNEL reaction mixture was added to the
sections and then incubated for 60 min at 37°C in a humidified
atmosphere in the dark. The slides were rinsed three times in PBS
for 5 min at room temperature. Samples were then mounted with PBS
and then analyzed using fluorescence microscope (magnification,
×200).
4′,6-diamidino-2-phenylindole (DAPI)
staining
Heart tissues were fixed in 10% formaldehyde for 24
h at room temperature, and then dehydrated, embedded and sliced
into 5 µm sections. DAPI dye solution (1 mg/ml, cat. no. C0060;
Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) was applied to samples and stained for 10 min at room
temperature. The dye solution was rinsed off and filter paper was
used to remove excess water; a drop of fluorescent sealing solution
(Anti-Fluorescence Attenuation Envelope Containing DAPI, cat. no.
S2110, Beijing Solarbio Science & Technology Co. Ltd.) was
added and sample were analyzed using a fluorescence microscope and
an excitation wavelength of 360 nm (magnification, ×200).
Reverse transcription-quantitative PCR
(RT-qPCR)
Primers were designed according to the sequences of
Bax, Bcl-2 and caspase 3 reported in Genbank (National Center for
Biotechnology, Bethesda, MD, USA), and were synthesized by Sangon
Biotech Co., Ltd. (Shanghai, China). Total RNA was isolated from
myocardial samples or H9C2 cells using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) and reverse transcribed into cDNA
using 5X PrimeScript RT Master Mix 2 µl (RR036A, Takara
Biotechnology Co., Ltd., Dalian, China) and 8 µl total RNA. The RT
reaction was conducted at: 37°C for 15 min and then at 85°C for 5
sec. cDNA was then stored at 4°C until use. A SYBR®
Premix Ex Taq™ kit (RR820A; Takara Biotechnology Co., Ltd.) was
used for detection. The following thermocycling conditions were
used for RT-qPCR: Initial denaturation at 95°C for 30 sec; 40
cycles of 95°C for 5 sec and 60°C for 30 sec. Relative gene
expression data were analyzed using the 2−∆∆Cq method
(21). The primers used for qPCR
were as follows: Bax forward, 5′-GTGGATACAGACTCCCCC-3′ and reverse,
5′-AGCGGCTGTTTGTCTGGA-3′; Bcl-2 forward, 5′-TGATAACCGGGAGATCGT-3′
and reverse, 5′-TCTCTGAAGACGCTGCTC-3′; caspase-3 forward,
5′-TGAATGGAAACAACCAGT-3′ and reverse, 5′-TCAAGCACCTGACCCTTA-3′;
AQP-1 forward, 5′-CTGAGGAAAGGCAGCTAGA-3′ and reverse,
5′-TCTGGACTCAAGCTTTCTGG-3′; AQP-4 forward, 5′-TTAAGATCAGGGTGCTCC-3′
and reverse, 5′-AATGTGCCCCACTATTCC-3′; and GAPDH forward,
5′-AACTTTGGCATTGTGGAA-3′ and reverse, 5′-CACATTGGGGGTAGGAAC-3′.
Statistical analysis
Data were expressed as the mean ± standard deviation
and analyzed using SPSS version 13.0 software (SPSS, Inc., Chicago,
IL, USA). Mean values were compared among groups using one-way
analysis of variance followed by a Tukey's post-hoc test. Bivariate
analysis was determined using linear regression and Pearson's
correlation analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Changes in rat hemodynamics
All rats were awake 60–90 min following anesthesia.
The hemodynamics were detected by the Datex-Ohmeda S/5 Entropy
Module (DRE, Inc.). During CPB, levels of rectal temperature
(Fig. 1B), pH (Fig. 1C), PaCO2 (Fig. 1D) and were stable, and
PaO2 (Fig. 1E) did not
exhibit a significant difference compared with the sham group.
Furthermore, levels of HR (Fig.
1F), MAP (Fig. 1G), LVDP
(Fig. 1I), +dP/dtmax
(Fig. 1J) and Hb (Fig. 1H) exhibited a significant decrease
compared with the sham group; however, following treatment with
HRS, these parameters were significantly increased when compared
with the sham group (P<0.05).
HRS protects against myocardial injury
in CBP rats
To investigate the effects of HRS treatment during
CPB, myocardial cells were stained with H&E. H&E staining
revealed that myocardial cells were arranged in order in the sham
group, with clear boundaries and intact nuclei (Fig. 2A). Myocardial cells in the CPB
group were arranged disorderly, with unclear boundaries, myofiber
ruptures and nuclei degradation (Fig.
2A). In the HRS group, HRS ameliorated myocardial injury.
Masson trichrome staining of myocardial cells revealed myocardial
fibrosis and marked damage in the CPB group, and myocardial fiber
damage was attenuated in the HRS group (Fig. 2B). The levels of LDH, CK-MB and
cTnI were significantly increased in CPB group compared with the
sham group (P<0.05; Fig. 2C-E),
HRS treatment reversed the LDH, CK-MB and cTnI back toward normal
levels. There was no significant difference in BNP levels (Fig. 2F). These findings suggest that HRS
protects myocardial cells from CPB.
HRS attenuates mitochondrial oxidative
stress and secretion of inflammatory factors in CPB rats
The results of the ELISA analyses demonstrated that
levels of MDA and MPO were significantly enhanced in the CPB group
compared with the sham group (P<0.05; Fig. 3A and B); whereas the level of SOD
was significantly reduced in the CPB group compared with the sham
group (Fig. 3C). Following HRS
treatment, the levels of MDA and MPO were significantly reduced
when compared with the CPB group (P<0.05; Fig. 3A and B). The expression levels of
inflammatory factors (IL-1β, IL-6 and TNF-α) were significantly
increased in the CPB group compared with the sham group (P<0.05;
Fig. 3D-F); whereas following HRS
treatment, the levels of IL-1β, IL-6 and TNF-α were significantly
reduced (Fig. 3D-F). Therefore,
the current findings suggest that treatment with HRS attenuates
mitochondrial oxidative stress and secretion of inflammatory
factors in the CPB rat.
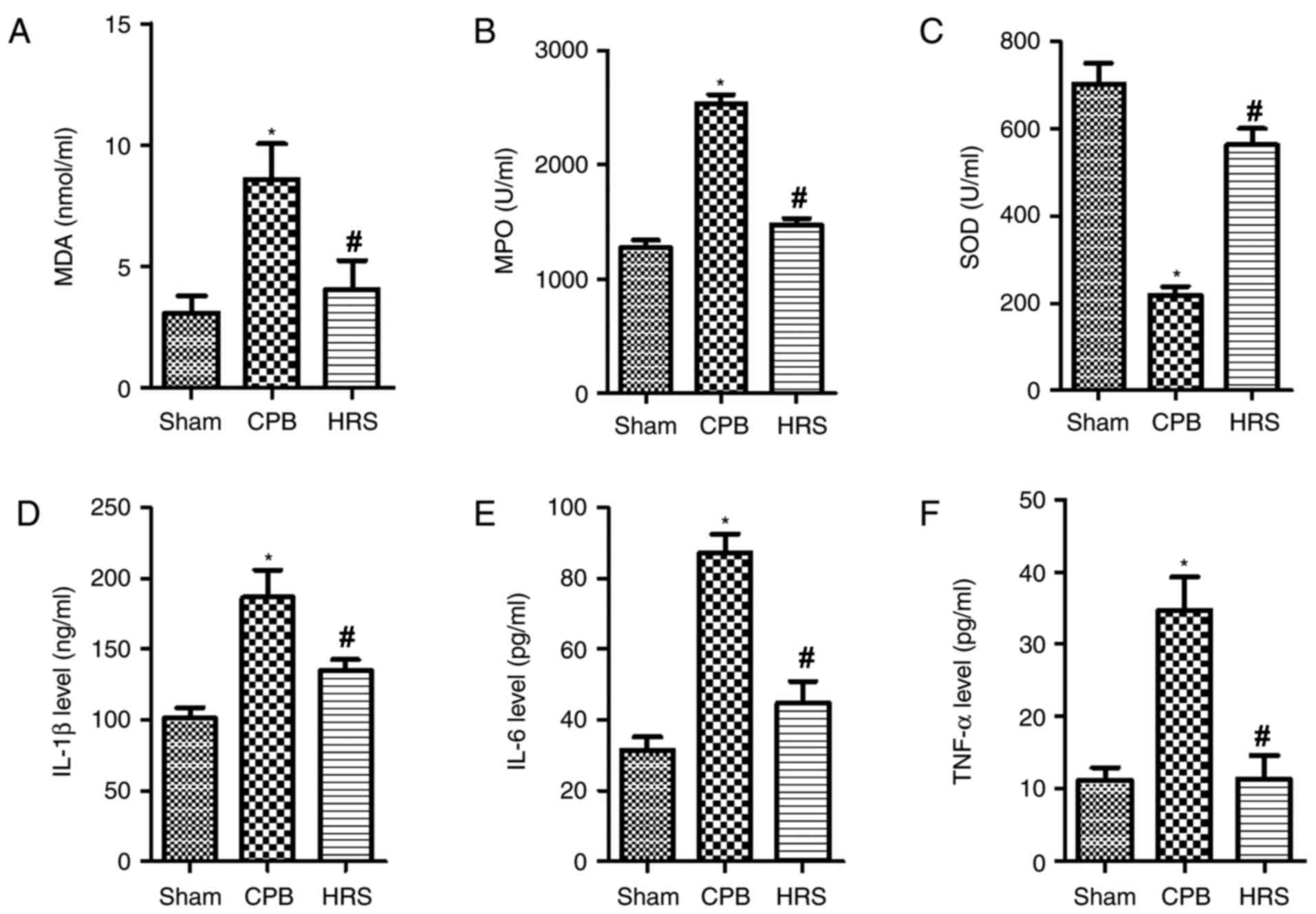 | Figure 3.HRS attenuated mitochondrial
oxidative stress and secretion of inflammatory factors in CPB rats.
Following HRS treatment, rats serum was collected, ELISA was
performed to determine the expression levels of (A) MDA, (B) MPO,
(C) SOD, (D) IL-1β, (E) IL-6 and (F) TNF-α. Data among groups were
compared using one-way analysis of variance. *P<0.05 vs. sham
group; #P<0.05 vs. CPB group. CPB, cardiopulmonary
bypass; HRS, hydrogen rich solution; MDA, malondialdehyde; MPO,
myeloperoxidase; SOD, superoxide dismutase; IL, interleukin; TNF-α,
tumor necrosis factor-α. |
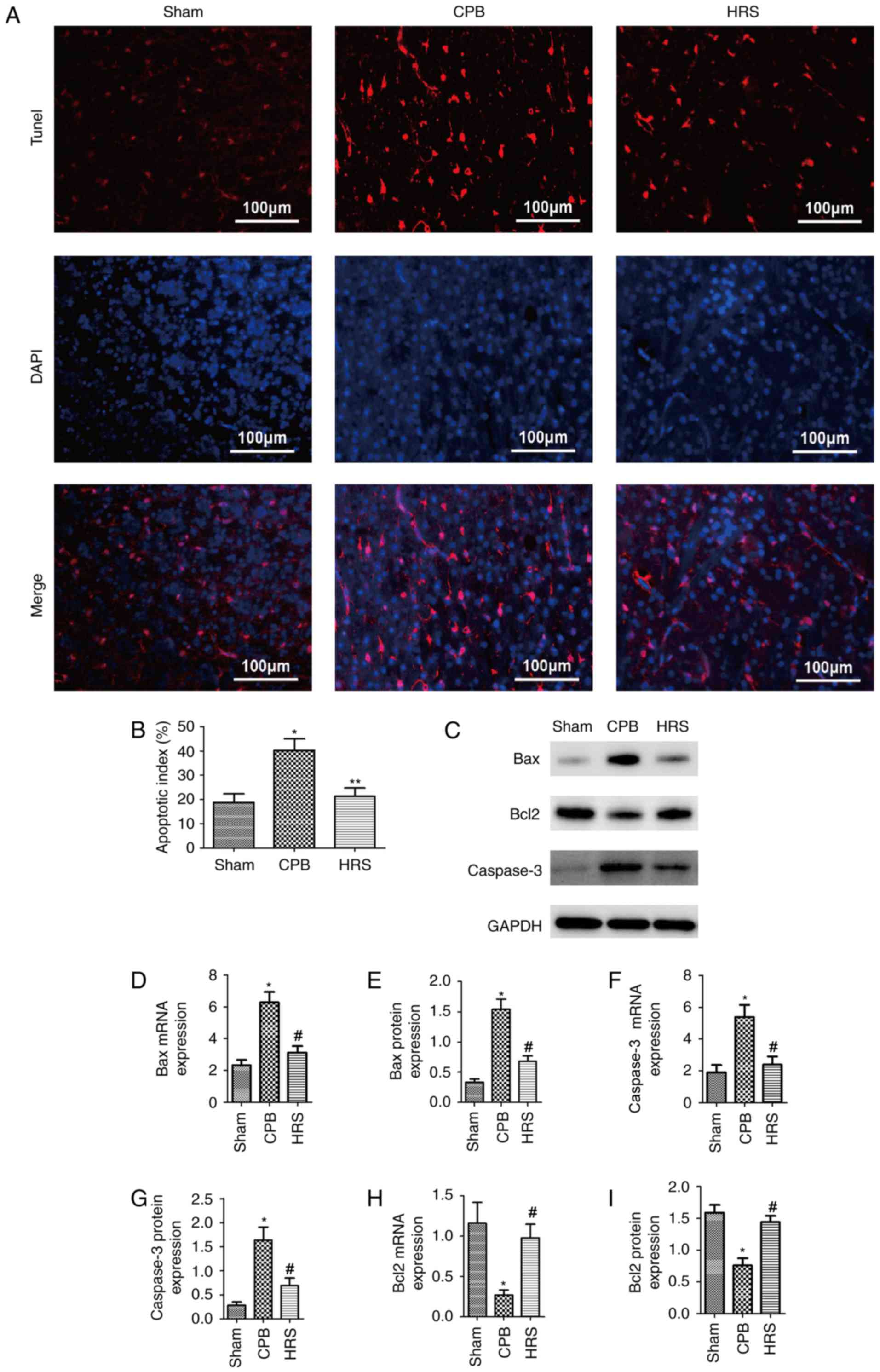
HRS protects the heart from
CPB-induced apoptosis
To analyze the effects of HRS on apoptosis levels in
the CPB group, TUNEL assays were performed, and the results
demonstrated that the number of TUNEL-positive cells was
significantly greater in the CPB group compared with the sham
group, and the number of TUNEL-positive cells significantly
decreased following HRS intervention compared with the CPB group
(P<0.05; Fig. 4A and B).
Furthermore, the expression levels of apoptosis-associated proteins
were investigated. The levels of Bax and caspase-3 in heart tissue
were significantly higher following CPB (P<0.05; Fig. 4C-G); whereas Bcl-2 expression was
significantly suppressed in the CPB group compared with the sham
group (P<0.05; Fig. 4C, H and
I). Following HRS treatment, Bax and caspase-3 expression
levels were significantly reduced (P<0.05; Fig. 4C-G), and the expression level of
Bcl-2 was increased following HRS treatment (P<0.05; Fig. 4C, H and I). Therefore, these
findings suggest that CPB induces apoptosis in heart tissue;
however, HRS treatment attenuates this effect.
HRS inhibits AQP protein expression
following CPB in rats
In order to verify the mRNA and protein expression
levels of AQP-1 and AQP-4 following CPB, western blot assays and
RT-qPCR analyses were performed. Results of western blot assays
were consistent with those obtained by the RT-qPCR analyses.
Compared with sham group, AQP-1 and AQP-4 mRNA and protein
expression levels significantly increased following CPB treatment
compared with the sham group (P<0.05; Fig. 5). Compared with the CPB group,
AQP-1 and AQP-4 mRNA and protein expression levels were suppressed
following treatment with HRS (P<0.05; Fig. 5). Thus, the results suggest that
administration of HRS can inhibit myocardial edema otherwise
induced by CPB.
HRS inhibits apoptosis via the
PI3K/Akt signaling pathway following CPB in rats
To investigate whether the PI3K/Akt signaling
pathway regulates apoptosis, the levels of several important
factors in the signaling pathway were determined. The results
revealed that PI3K, p-Akt and HO-1 levels were increased in the CPB
group (P<0.05); however, treatment with HRS significantly
attenuated these effects (Fig. 6A and
B). The results suggest that HRS protects against CPB-induced
cell apoptosis, and this may be regulated via the PI3K/Akt
signaling pathway.
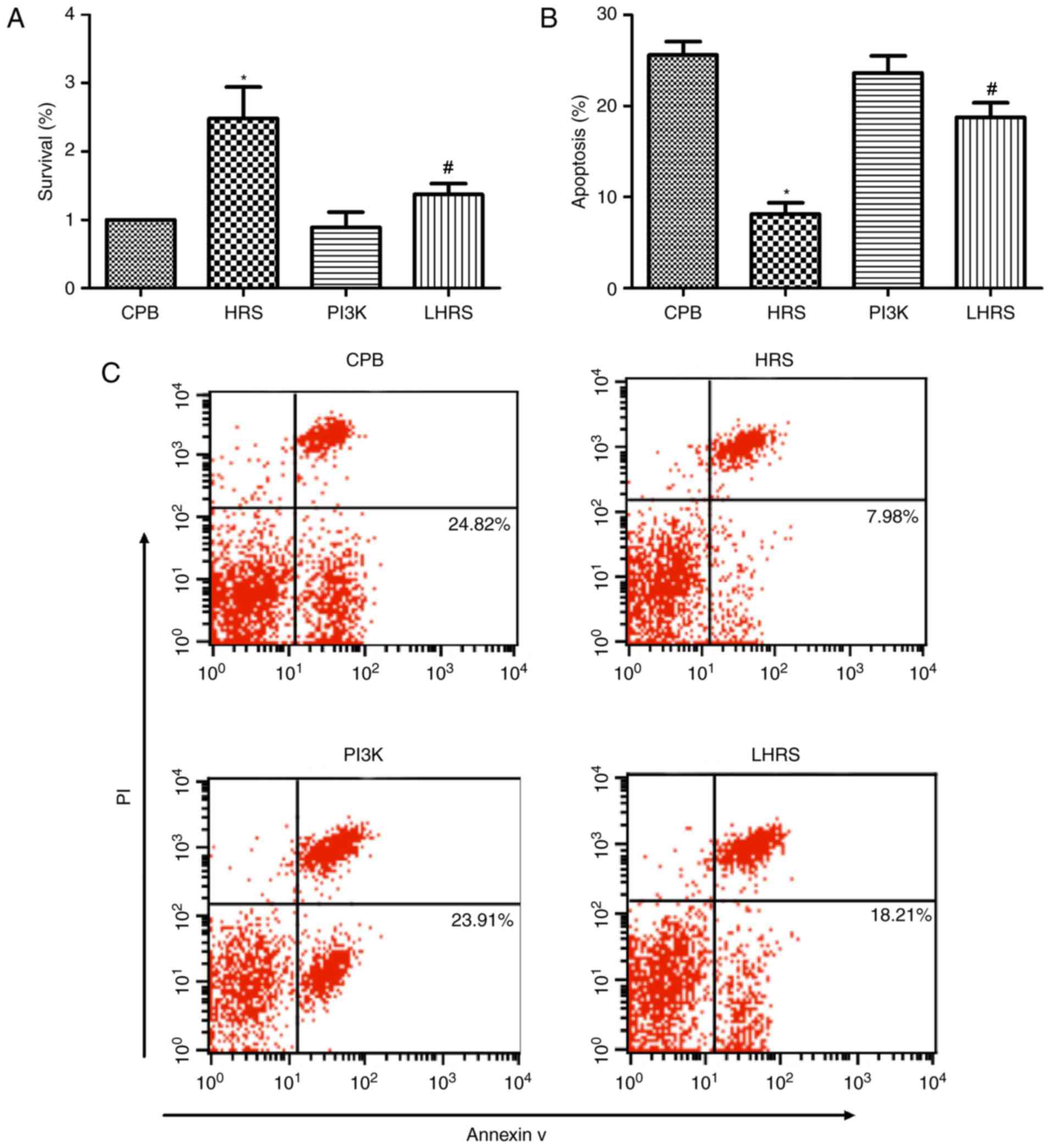
HRS enhances viability of myocardial
cells
The cell viability in each group was investigated
via MTT assays following reoxygenation. The results of the MTT
assays revealed that HRS treatment increased the viability of
myocardial cells following HRS treatment (P<0.05; Fig. 7A). Therefore, HRS was demonstrated
to enhance the viability of myocardial cells.
HRS protects myocardial cells from
apoptosis in vitro
The Annexin V-PI method was used to investigate the
apoptosis rate of myocardial cells. As revealed in Fig. 7B and C, HRS treatment significantly
suppressed the apoptotic rate of myocardial cells following HRS
treatment (P<0.05). Furthermore, western blot analyses revealed
that Bax and caspase-3 expression levels were suppressed (Fig. 7D-F), and the expression of Bcl-2
protein was enhanced in the HRS group (Fig. 7D and G). These results suggest that
HRS can protect myocardial cells from undergoing apoptosis.
HRS inhibits myocardial cell apoptosis
via the PI3K/Akt signaling pathway
The mechanism underlying the anti-apoptotic effect
of HRS treatment was further investigated. The expression levels of
Akt were similar to that of the CPB group. Additionally, HRS may
promote the phosphorylation of Akt. In the LHRS group, the levels
of p-Akt were significantly reduced compared with the HRS group
(Fig. 8A and B). Furthermore, the
expression of the downstream regulatory gene HO-1 was significantly
reduced in the LHRS group compared with the HRS group (Fig. 8A and C). Therefore, the results
suggest that HRS can suppress hypoxia/reoxygenation-induced heart
injury via the PI3K/Akt signaling pathway.
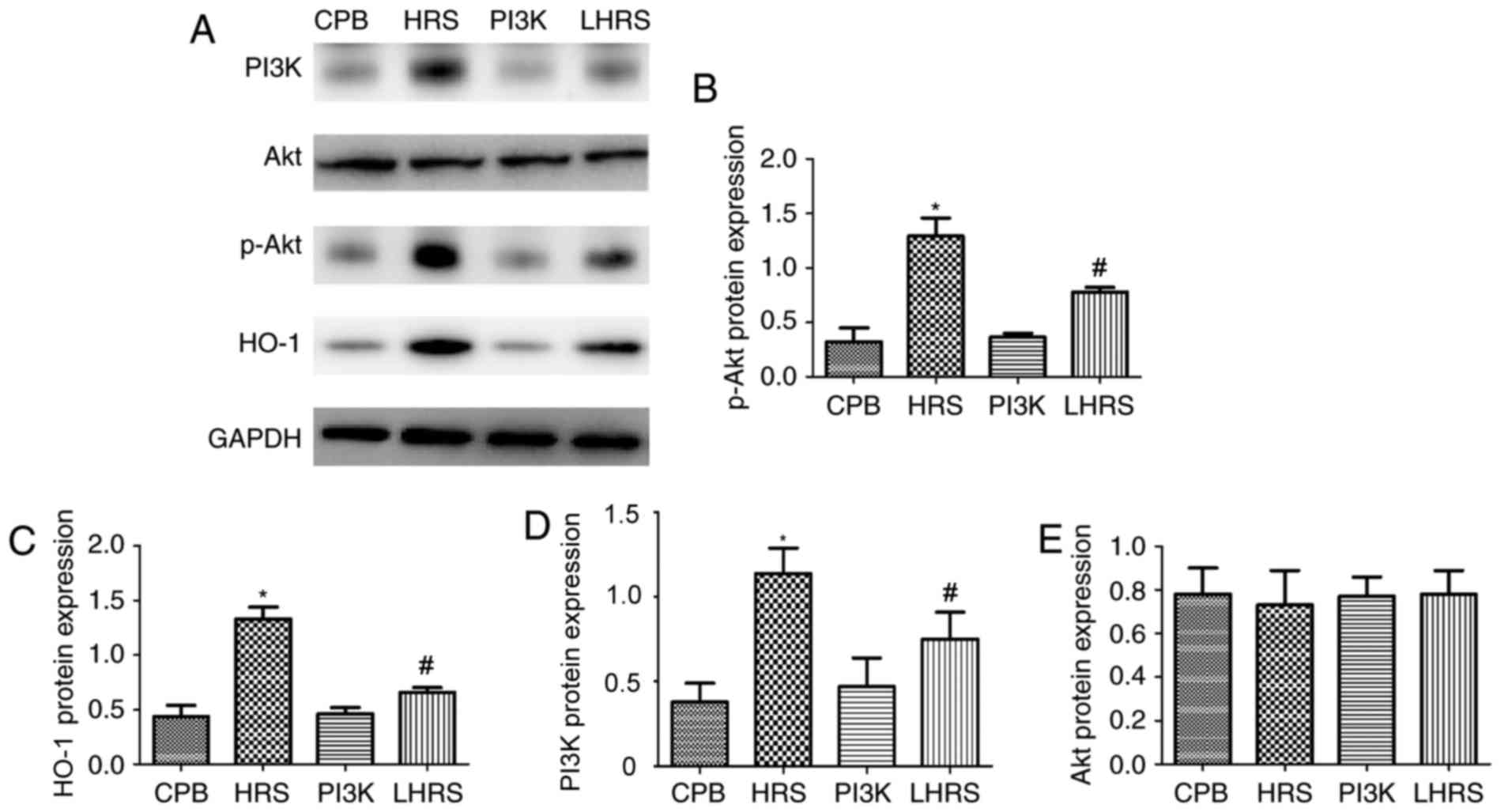 | Figure 8.HRS suppressed myocardial cell
apoptosis via the PI3K/Akt signaling pathway. (A) Western blot
assays were performed. The levels of (B) p-Akt, (C) HO-1, (D) PI3K
and (E) Akt were quantified. Data among multiple groups were
compared using one-way analysis of variance. *P<0.05 vs. CPB
group; #P<0.05 vs. PI3K group. PI3K group, LY294002 +
CPB; LHRS group, LY294002 + CPB + HRS; CPB, cardiopulmonary bypass;
HRS, hydrogen rich solution; PI3K, phosphatidylinositol 3-kinase;
Akt, proein kinase B; p-, phosphrylated; HO-1, heme oxygenase
1. |
Discussion
Myocardial injury is one of the common complications
following CPB, inducing a decrease in the diastolic and systolic
functions of myocardium, arrhythmia, myocardial energy metabolism
disturbance and microcirculation disorder (3). Myocardial injury may lead to heart
failure, thus severely threatening the postoperative recovery of
patients. The PI3K/Akt pathway has an important role in the
oxidative stress and apoptosis of myocardial cells, and HRS
inhibits myocardial injury via the PI3K/Akt pathway (22–24).
The incidence of complications and mortality following open heart
surgery with CPB is closely associated with the severity of
myocardial injury during surgery. HRS has antioxidant stress,
anti-apoptosis and anti-inflammation effects (25,26).
In the present study, a CPB model was established in vivo
and in vitro to investigate the protective effect of HRS.
The results of the present study revealed that HRS suppressed the
expression of inflammatory factors in the rats, reduced the
apoptotic rate of myocardial cells and inhibited the expression of
aquaporins, through the PI3K/Akt signaling pathway.
Previous studies investigating ischemia-reperfusion
injury during CPB have focused on the liver, kidney, intestine and
brain, without cardiac arrest or cardiac resuscitation, and could
not study heart and lung injury (27–30).
In accordance with previous studies (31,32),
the present study made a number of modifications, and established
rat models of CPB. Thus, the present study of myocardial protection
with CPB is more in line with clinical practice.
Non-physiological blood circulation causes systemic
inflammatory response syndrome during CPB (33). Furthermore, organ
ischemia-reperfusion injury and surgical trauma are also important
triggering factors of inflammatory response (34,35).
Various inflammatory factors are produced during CPB, which may
induce myocardial injury directly or indirectly (36). A previous study demonstrated that
TNF-α levels increased following myocardial ischemia, and further
increased following reperfusion, thereby aggravating myocardial
injury (37). Another previous
study revealed that suppression of TNF-α expression may attenuate
myocardial injury (38). IL-6 is
associated with reperfusion injury, and its expression is
associated with the severity of left ventricular dysfunction and
low cardiac output following thrombolytic therapy for myocardial
infarction (39,40). The results of the present study
suggested that CPB significantly increased plasma TNF-α and IL-6
levels. CPB induces systemic inflammation, and myocardial water
content increased following the initiation of CPB; thus, suggesting
that CPR aggravated myocardial injury and affected cell membrane
permeability. Furthermore, energy metabolism disorders may lead to
cardiomyocyte edema.
Hydrogen is a therapeutic antioxidant, which can
selectively suppress OH-free radicals, and its effect is
predominantly dependent on the antioxidant properties of hydrogen
for the protection of organs from oxidative damage (4). The present study demonstrated that
hydrogen may inhibit the release of cell adhesion molecules and
inflammatory cytokines, as well as increase the level of
anti-inflammatory factors. HRS is associated with metabolism,
thereby regulating cell detoxification, cell hydration, and the
immune system (41–43). Animal experiments and clinical
trials have previously confirmed that HRS significantly inhibits
heart, liver, lung and intestinal ischemia-reperfusion injury; as
well as inhibiting inflammatory responses and apoptotic rates
(6,26,44).
The present study suggested that HRS may inhibit CPB-induced
myocardial injury by reducing LDH, CK-MB, IL-1β, IL-6, TNF-α, MDA
and MPO levels; enhancing the release of SOD and decreasing the
expression of proteins associated with apoptosis. These results
suggest that HRS has a protective effect on CPB-induced myocardial
injury, and the mechanism underlying its protective effect is via
anti-inflammatory and anti-apoptotic effects.
Cardiomyocyte edema is a predominant pathological
change associated with myocardial injury (45). AQP-mediated transport of water
molecules accounts for approximately 1/3 of total water transport
in cardiomyocytes (46). Under
pathological conditions, the transmembrane transport of water
molecules in cardiomyocytes predominantly depends upon the
transport of AQPs (11). In the
AQPs family, AQP-1 is most widely distributed among cardiomyocytes
(47). Ding et al (9) demonstrated that AQP-1 expression is
upregulated in cardiomyocytes and vascular endothelial cells in
rabbit models of chronic myocardial ischemia. Myocardial ischemia
and the severity of myocardial edema are consistent with AQP-1
expression (48), thus suggesting
that a possible regulatory role of AQP-1 associated with myocardial
edema induced by chronic myocardial ischemia. AQP-4 is another
important AQP in the heart, and is predominantly distributed in
intercalated discs, endothelial cells, sarcolemma and serosa in the
heart (49,50). When myocardial edema induced by
myocardial infarction and water content in cardiomyocytes is
increased, AQP-4 expression is upregulated in cardiomyocytes
(51). AQP-4 mRNA and protein
expression has been revealed to be associated with the area of
myocardial infarction, thus suggesting that AQP-4 is involved in
myocardial edema following myocardial infarction, and that the
permeability of AQP-4 to water is greater than AQP-1 (52). In the present study, AQP-1 and −4
expression level were increased following the initiation of CPB,
and markedly increased following CPB. HRS was revealed to suppress
the expression of AQPs, thus suggesting that HRS can inhibit CPB
induced myocardial edema.
In the present study of the myocardial IR model, the
PI3K/Akt pathway has an important role in myocardial injury, such
as inflammation and apoptosis. Soy isoflavone has a protective role
in myocardial ischemia-reperfusion injury in ovariectomized rats
via activation of the estrogen receptor in the Pl3K/Akt/eNOS
signaling pathway (53).
Therefore, the present study hypothesized that HRS may increase the
activity of the PI3K/Akt pathway and induce the transcription and
expression of the HO-1 gene. Firstly, the levels of p-Akt were
investigated in the myocardium of each group, which is an important
marker of PI3K/Akt activity. Consistent with previous studies HRS
significantly enhanced the activity of PI3K/Akt (54,55).
Following this, a cell hypoxia-reoxygenation model, a simulated CPB
model and a PI3K inhibitor model were established. The results
demonstrated that LY294002 not only reversed the protective effect
of HRS on myocardial injury following CPB, but also suppressed the
inhibition of AQP1, AQP4 and HO-1. These results demonstrated that
the mechanism underlying the protective effect of HRS on myocardium
and the inhibition of AQP protein expression may be associated with
the activation of the PI3K/Akt pathway.
In conclusion, the present study revealed that the
PI3K/Akt signaling pathway has an important role in the mechanism
of CPB-induced myocardial injury. Furthermore, the results suggest
that HRS may attenuate CPB-induced mitochondrial oxidative stress
injury and apoptosis via the PI3K/Akt signaling pathway, thus
leading to protective effects against myocardial injury.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Liaoning Province (grant no. 2014020063) and
the Natural Science Foundation of China (grant nos. 81471121 and
3120175).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XL performed the reverse transcription-quantitative
polymerase chain reaction and collected data. YG, YJ, GZ and TZ
conducted the collection of samples. KY collected fresh samples. YJ
and LP also contributed to acquisition of funding support. LP
designed the study. DD conceived and designed the study, acquired
data, interpreted the results and drafted the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the China Medical
University Laboratory Animal Welfare and Ethics Committee and
adhered to the guidelines of The Institutional Animal Care and Use
Committee; no. 2015048R.
Consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing
interests.
References
|
1
|
Suleiman MS, Zacharowski K and Angelini
GD: Inflammatory response and cardioprotection during open-heart
surgery: The importance of anaesthetics. Br J Pharmacol. 153:21–33.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Steuer J, Granath F, de Faire U, Ekbom A
and Stahle E: Increased risk of heart failure as a consequence of
perioperative myocardial injury after coronary artery bypass
grafting. Heart. 91:754–758. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Hert S and Moerman A: Myocardial injury
and protection related to cardiopulmonary bypass. Best Pract Res
Clin Anaesthesiol. 29:137–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ohsawa I, Ishikawa M, Takahashi K,
Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S
and Ohta S: Hydrogen acts as a therapeutic antioxidant by
selectively reducing cytotoxic oxygen radicals. Nat Med.
13:688–694. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qian L, Li B, Cai J and Gao F: The
hypothesis of an effective safe and novel radioprotective agent:
Hydrogen-rich solution. West Indian Med J. 59:122–124.
2010.PubMed/NCBI
|
|
6
|
Noda K, Shigemura N, Tanaka Y, Kawamura T,
Lim Hyun S, Kokubo K, Billiar TR, Bermudez CA, Kobayashi H and
Nakao A: A novel method of preserving cardiac grafts using a
hydrogen-rich water bath. J Heart Lung Transplant. 32:241–250.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagaraju GP, Basha R, Rajitha B, Alese OB,
Alam A, Pattnaik S and El-Rayes B: Aquaporins: Their role in
gastrointestinal malignancies. Cancer Lett. 373:12–18. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jonker S, Davis LE, van der Bilt JD,
Hadder B, Hohimer AR, Giraud GD and Thornburg KL: Anaemia
stimulates aquaporin 1 expression in the fetal sheep heart. Exp
Physiol. 88:691–698. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ding FB, Yan YM, Huang JB, Mei J, Zhu JQ
and Liu H: The involvement of AQP1 in heart oedema induced by
global myocardial ischemia. Cell Biochem Funct. 31:60–64. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Butler TL, Au CG, Yang B, Egan JR, Tan YM,
Hardeman EC, North KN, Verkman AS and Winlaw DS: Cardiac aquaporin
expression in humans, rats, and mice. Am J Physiol Heart Circ
Physiol. 291:H705–H713. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rutkovskiy A, Stenslokken KO, Mariero LH,
Skrbic B, Amiry-Moghaddam M, Hillestad V, Valen G, Perreault MC,
Ottersen OP, Gullestad L, et al: Aquaporin-4 in the heart:
Expression, regulation and functional role in ischemia. Basic Res
Cardiol. 107:2802012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chaudhuri S, Singh MK, Bhattacharya D,
Datta A, Hazra I, Mondal S, Faruk Sk, Md O, Ronsard L, Ghosh TK and
Chaudhuri S: T11TS immunotherapy repairs PI3K-AKT signaling in
T-cells: Clues toward enhanced T-cell survival in rat glioma model.
J Cell Physiol. 233:759–770. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu X, Huang H, Zhang J, Liu H, Ao R, Xiao
M and Wu Y: The anticancer effects of Cucurbitacin I inhibited cell
growth of human non-small cell lung cancer through PI3K/AKT/p70S6K
pathway. Mol Med Rep. 17:2750–2756. 2018.PubMed/NCBI
|
|
14
|
Zhang B, Liu Y, Li Y, Zhe X, Zhang S and
Zhang L: Neuroglobin promotes the proliferation and suppresses the
apoptosis of glioma cells by activating the PI3K/AKT pathway. Mol
Med Rep. 17:2757–2763. 2018.PubMed/NCBI
|
|
15
|
Wu MP, Zhang YS, Zhou QM, Xiong J, Dong YR
and Yan C: Higenamine protects ischemia/reperfusion induced cardiac
injury and myocyte apoptosis through activation of β2-AR/PI3K/AKT
signaling pathway. Pharmacol Res. 104:115–123. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu S, Zhang Y, Zhang M, Guo Y, Yang P,
Zhang S, Simsekyilmaz S, Xu JF, Li J, Xiang X, et al: Aloperine
protects mice against ischemia reperfusion (IR)-induced renal
injury by regulating PI3K/AKT/mTOR signaling and AP-1 activity. Mol
Med. Nov 3–2015.(Epub ahead of print). View Article : Google Scholar
|
|
17
|
Yang C, Cao Y, Zhang Y, Li L, Xu M, Long
Y, Rong R and Zhu T: Cyclic helix B peptide inhibits ischemia
reperfusion-induced renal fibrosis via the PI3K/Akt/FoxO3a pathway.
J Transl Med. 13:3552015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun Y, Jiang C, Jiang J and Qiu L:
Dexmedetomidine protects mice against myocardium
ischaemic/reperfusion injury by activating an AMPK/PI3K/Akt/eNOS
pathway. Clin Exp Pharmacol Physiol. 44:946–953. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Wu Q, Song S, Wan Y, Zhang R, Tai
M and Liu C: Effect of hydrogen-rich water on acute peritonitis of
rat models. Int Immunopharmacol. 21:94–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stark RA, Nahrwold ML and Cohen PJ: Blind
oral tracheal intubation of rats. J Appl Physiol Respir Environ
Exerc Physiol. 51:1355–1356. 1981.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu F, Yu H, Liu J and Cheng L:
αB-crystallin regulates oxidative stress-induced apoptosis in
cardiac H9c2 cells via the PI3K/AKT pathway. Mol Biol Rep.
40:2517–2526. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Su D, Zhou Y, Hu S, Guan L, Shi C, Wang Q,
Chen Y, Lu C, Li Q and Ma X: Role of GAB1/PI3K/AKT signaling high
glucose-induced cardiomyocyte apoptosis. Biomed Pharmacother.
93:1197–1204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen K, Wang N, Diao Y, Dong W, Sun Y, Liu
L and Wu X: Hydrogen-rich saline attenuates brain injury induced by
cardiopulmonary bypass and inhibits microvascular endothelial cell
apoptosis via the PI3K/Akt/GSK3β signaling pathway in rats. Cell
Physiol Biochem. 43:1634–1647. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li J, Hong Z, Liu H, Zhou J, Cui L, Yuan
S, Chu X and Yu P: Hydrogen-rich saline promotes the recovery of
renal function after ischemia/reperfusion injury in rats via
anti-apoptosis and anti-inflammation. Front Pharmacol. 7:1062016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shigeta T, Sakamoto S, Li XK, Cai S, Liu
C, Kurokawa R, Nakazawa A, Kasahara M and Uemoto S: Luminal
injection of hydrogen-rich solution attenuates intestinal
ischemia-reperfusion injury in rats. Transplantation. 99:500–507.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li T, Luo N, Du L, Zhou J, Zhang J, Gong L
and Jiang N: Tumor necrosis factor-α plays an initiating role in
extracorporeal circulation-induced acute lung injury. Lung.
191:207–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim J, Yin T, Shinozaki K, Lampe JW and
Becker LB: DHA-supplemented diet increases the survival of rats
following asphyxia-induced cardiac arrest and cardiopulmonary
bypass resuscitation. Sci Rep. 6:365452016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cai DS, Jin BB, Pei L and Jin Z:
Protective effects of penehyclidine hydrochloride on liver injury
in a rat cardiopulmonary bypass model. Eur J Anaesthesiol.
27:824–828. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aregger F, Pilop C, Uehlinger DE,
Brunisholz R, Carrel TP, Frey FJ and Frey BM: Urinary proteomics
before and after extracorporeal circulation in patients with and
without acute kidney injury. J Thorac Cardiovasc Surg. 139:692–700.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou J, Zhou N, Wu XN, Cao HJ, Sun YJ,
Zhang TZ, Chen KY and Yu DM: Role of the Tolllike receptor 3
signaling pathway in the neuroprotective effect of sevoflurane
pre-conditioning during cardiopulmonary bypass in rats. Mol Med
Rep. 12:7859–7868. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peterss S, Guenther S, Kellermann K,
Jungwirth B, Lichtinghagen R, Haverich A, Hagl C and Khaladj N: An
experimental model of myocardial infarction and controlled
reperfusion using a miniaturized cardiopulmonary bypass in rats.
Interact Cardiovasc Thorac Surg. 19:561–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baehner T, Boehm O, Probst C, Poetzsch B,
Hoeft A, Baumgarten G and Knuefermann P: Cardiopulmonary bypass in
cardiac surgery. Anaesthesist. 61:846–856. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Prondzinsky R, Knupfer A, Loppnow H,
Redling F, Lehmann DW, Stabenow I, Witthaut R, Unverzagt S, Radke
J, Zerkowski HR and Werdan K: Surgical trauma affects the
proinflammatory status after cardiac surgery to a higher degree
than cardiopulmonary bypass. J Thorac Cardiovasc Surg. 129:760–766.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Balogh AL, Petak F, Fodor GH, Sudy R and
Babik B: Sevoflurane relieves lung function deterioration after
cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 31:2017–2026.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tavener SA, Long EM, Robbins SM, McRae KM,
Van Remmen H and Kubes P: Immune cell Toll-like receptor 4 is
required for cardiac myocyte impairment during endotoxemia. Circ
Res. 95:700–707. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu WB, Han XH, Guo YY, Zhang DM, Tang FJ,
Zhao L, Ji LL and Guo FM: Effects of tumor necrosis factor and
E-selectin on coronary artery flow. Eur Rev Med Pharmacol Sci.
21:1843–1849. 2017.PubMed/NCBI
|
|
38
|
Sadeghzadeh J, Vakili A, Bandegi AR,
Sameni HR, Khorasani Zahedi M and Darabian M: Lavandula reduces
heart injury via attenuating tumor necrosis factor-alpha and
oxidative stress in a rat model of infarct-like myocardial injury.
Cell J. 19:84–93. 2017.PubMed/NCBI
|
|
39
|
Sonnino C, Christopher S, Oddi C, Toldo S,
Falcao RA, Melchior RD, Mueller GH, Abouzaki NA, Varma A, Gambill
ML, et al: Leukocyte activity in patients with ST-segment elevation
acute myocardial infarction treated with anakinra. Mol Med.
20:486–489. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ikonomidis I, Athanassopoulos G, Lekakis
J, Venetsanou K, Marinou M, Stamatelopoulos K, Cokkinos DV and
Nihoyannopoulos P: Myocardial ischemia induces interleukin-6 and
tissue factor production in patients with coronary artery disease:
A dobutamine stress echocardiography study. Circulation.
112:3272–3279. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cui W, Gao C, Fang P, Lin G and Shen W:
Alleviation of cadmium toxicity in Medicago sativa by hydrogen-rich
water. J Hazard Mater. 260:715–724. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aoki K, Nakao A, Adachi T, Matsui Y and
Miyakawa S: Pilot study: Effects of drinking hydrogen-rich water on
muscle fatigue caused by acute exercise in elite athletes. Med Gas
Res. 2:122012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhao S, Yang Y, Liu W, Xuan Z, Wu S, Yu S,
Mei K, Huang Y, Zhang P, Cai J, et al: Protective effect of
hydrogen-rich saline against radiation-induced immune dysfunction.
J Cell Mol Med. 18:938–946. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Takahashi M, Chen-Yoshikawa TF, Saito M,
Tanaka S, Miyamoto E, Ohata K, Kondo T, Motoyama H, Hijiya K,
Aoyama A and Date H: Immersing lungs in hydrogen-rich saline
attenuates lung ischaemia-reperfusion injury. Eur J Cardiothorac
Surg. 51:442–448. 2017.PubMed/NCBI
|
|
45
|
Schulz-Menger J: Myocardial edema in acute
ischemic injury. JACC Cardiovasc Imaging. 4:279–281. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rutkovskiy A, Valen G and Vaage J: Cardiac
aquaporins. Basic Res Cardiol. 108:3932013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Netti VA, Vatrella MC, Chamorro MF, Rosón
MI, Zotta E, Fellet AL and Balaszczuk AM: Comparison of
cardiovascular aquaporin-1 changes during water restriction between
25- and 50-day-old rats. Eur J Nutr. 53:287–295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rutkovskiy A, Bliksoen M, Hillestad V,
Amin M, Czibik G, Valen G, Vaage J, Amiry-Moghaddam M and
Stensløkken KO: Aquaporin-1 in cardiac endothelial cells is
downregulated in ischemia, hypoxia and cardioplegia. J Mol Cell
Cardiol. 56:22–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu Y, Pan CY, Guo CZ, Dong ZJ, Wu Q, Dong
HM and Zhang W: Expression of aquaporin 1 and 4 in rats with acute
hypoxic lung injury and its significance. Genet Mol Res.
14:12756–12764. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tie L, Wang D, Shi Y and Li X: Aquaporins
in cardiovascular system. Adv Exp Med Biol. 969:105–113. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang HZ, Kim MH, Lim JH and Bae HR:
Time-dependent expression patterns of cardiac aquaporins following
myocardial infarction. J Korean Med Sci. 28:402–408. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Warth A, Eckle T, Kohler D, Faigle M, Zug
S, Klingel K, Eltzschig HK and Wolburg H: Upregulation of the water
channel aquaporin-4 as a potential cause of postischemic cell
swelling in a murine model of myocardial infarction. Cardiology.
107:402–410. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tang Y, Li S, Zhang P, Zhu J, Meng G, Xie
L, Yu Y, Ji Y and Han Y: Soy isoflavone protects myocardial
ischemia/reperfusion injury through increasing endothelial nitric
oxide synthase and decreasing oxidative stress in ovariectomized
rats. Oxid Med Cell Longev. 2016:50574052016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hong Y, Shao A, Wang J, Chen S, Wu H,
McBride DW, Wu Q, Sun X and Zhang J: Neuroprotective effect of
hydrogen-rich saline against neurologic damage and apoptosis in
early brain injury following subarachnoid hemorrhage: Possible role
of the Akt/GSK3β signaling pathway. PLoS One. 9:e962122014.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wu D, Liang M, Dang H, Fang F, Xu F and
Liu C: Hydrogen protects against hyperoxia-induced apoptosis in
type II alveolar epithelial cells via activation of PI3K/Akt/Foxo3a
signaling pathway. Biochem Biophys Res Commun. 495:1620–1627. 2018.
View Article : Google Scholar : PubMed/NCBI
|