Introduction
Diabetes mellitus is the most prevalent endocrine
and metabolic disease worldwide and is characterized by
hyperglycemia caused by either impaired insulin secretion in the
pancreas or by insulin resistance (1). Many of the patients with diabetes
that fail to control their blood glucose levels may develop
hypertension and cardiovascular diseases, which may contribute to
the high morbidity and mortality rates in adults. In addition,
<50% insulin dependent patients achieve the recommended HB A1c
(4–6%) and exogenous insulin is unable to provide the same level of
glycemic control as insulin secreted by the pancreas (2,3). To
date, transplantation of intact pancreas or isolated pancreatic
islets appears to be a promising treatment for patients with type 1
diabetes. For example, it was previously reported that islet
transplantation significantly decreased the mean amplitude of
glycemic fluctuations in a study involving seven patients with type
1 diabetes (4). However, another
study reported that grafted islet function progressively decreased
following surgery and 44% of patients become insulin independent
after 3 years (5), which indicated
that diabetes may be successfully reversed in only a portion of
patients (6).
Mesenchymal stem cells (MSCs) are a subpopulation of
multipotent cells that were first identified in myeloid tissue
(7). Several studies have
demonstrated that co-transplantation with MSCs may improve islet
graft survival rate through various mechanisms, such as immune
modulation and enhancement of revascularization by cytokine
secretion, including vascular endothelial growth factor (VEGF),
hepatocyte growth factor, platelet-derived growth factor and matrix
metalloproteinases (MMPs) (8–15).
Nevertheless, previous studies have focused only on pancreatic
islet allografts as an experimental model in normoxic
microenvironmental conditions (8,16,17).
Conversely, in native bone marrow tissue, low oxygen tension (2%)
is considered an integral component of human MSC proliferation
(18). Furthermore, a hypoxic
environment was reported to have a close association with the
biological characteristics of MSCs. One study reported that
culturing MSCs under hypoxic conditions prior to transplantation
may improve their tissue regenerative ability (19). Hypoxia-stimulated MSCs may secret a
higher level of anti-apoptotic and antigenic related factors,
including interleukin (IL)-6, VEGF and monocyte chemoattractant
protein (MCP)-1. As a result, the hypoxia-cultured MSC conditioned
medium (hMSC-CM) may possess the ability to suppress apoptosis and
enhance angiogenesis of vascular endothelial cells (20). Therefore, pre-treating MSCs in a
hypoxic microenvironment may benefit pancreatic islet
transplantation graft survival rate.
The present study examined the proliferation rates
and the expression levels of several growth factors in
hypoxia-cultured MSCs. An in vivo co-transplantation model
was established using hMSCs and pancreatic islet allografts in
streptozotocin (STZ)-induced diabetic mice to evaluation the
transplantation effect. The present study also investigated
intravenous glucose tolerance and minimal islet number following
islet transplantation with hMSCs or MSCs, providing information
about the cross-protective effects of hMSCs with islet
transplantation.
Materials and methods
Bone marrow-derived cell (BMDC) and
MSC isolation, culture, adipose induction and oil red-O
staining
BMDCs, which contained MSCs, were isolated from
mouse bone marrow aspirates by adhesion to tissue culture-coated
plates in complete medium as previously described (21). Briefly, BMDCs were isolated from
bone marrow aspirates flushed from the femur and tibia of 20 female
BALB/c donor mice (age, 9–10 weeks, 21±1 g, Shanghai SLAC
Laboratory Animal Co., Ltd., Shanghai, China). Mice were kept at
18–29°C, with 40–70% humidity, 12-h light/dark cycle and free
access to food and water. Extracted BMDCs were cultured for 3–4
weeks using MSC medium composed of Iscove's modified Dulbecco's
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% (v/v) fetal calf serum (Gibco; Thermo Fisher
Scientific, Inc.), 10% (v/v) equine serum (HyClone; GE Healthcare
Life Sciences, Logan, UT, USA), 1% (v/v) L-glutamine and 1% (v/v)
antibiotic-antimycotic liquid (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The culture medium was replaced every 3–4
days. At 60–80% confluence, cells were treated with 0.25% (w/v)
trypsin containing 1 mmol/l EDTA (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and passaged until passage 7 at a cell
density of 2,500 cells/cm2. Cultures were maintained at
37°C and 5% CO2 with 2% (hypoxia) or 21% (normoxia)
O2 concentration. MSC adipose induction was performed
using an MSC adipogenic differentiation medium kit, (cat. no.
GUXMX-90031; Cyagen Biosciences Inc., Santa Clara, CA, USA)
following the manufacturer's protocols. Following the
differentiation of the cells, the MSC adipogenic differentiation
medium was removed from the wells which were rinsed with 1X
phosphate-buffered saline (PBS). The cells were fixed with 2 ml of
4% formaldehyde solution for 30 min at 37°C. Oil Red O
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) working solution
was prepared with saturated Oil Red O liquid (0.5 g Oil Red O
dissolved in 100 ml isopropanol) and distilled water with a 3:2
ratio. Staining was conducted with freshly prepared Oil Red O for
15 mins at room temperature, and rinsed with 60% isopropanol prior
to inverted microscope analysis.
Cell viability assay
Cell viability was measured by MTT (Sigma-Aldrich;
Merck KGaA) dye absorbance, according to the manufacturer's
protocol (Boehringer Mannheim; Roche Diagnostics GmbH, Mannheim,
Germany). Cells were incubated until desired density (60–80%
confluence). Formazan crystals were dissolved with DMSO and cell
viability was determined spectrophotometrically at a wavelength of
570 nm. Each experiment comprised five identical wells and was
repeated three times.
Hypoxic culture and MSC-CM
collection
An hypoxic environment was generated and maintained
using a hypoxia chamber (Stemcell Technologies, Inc., Vancouver,
BC, Canada), according to the manufacturer's protocol. Briefly,
cultures were enclosed in the chamber that was flushed with a
mixture of gasses (93% N2 and 5% CO2) for 3
min. Following the flushing period, the chamber was closed to
prevent the flow of exogenous normoxic air into the chamber. The
final level of hypoxia was 2% as specified by the manufacturer.
Serum-free IMDM medium was used during CM production. Prior to
culture, MSCs were counted and 3×104 cells were plated
in the different groups. Following 24 h culture at 37°C, the CM was
harvested and used in subsequent experiments; the CM was applied at
a 30:70 ratio with normal medium for islets culture.
Enzyme linked immunosorbent assay
(ELISA)
Cells was cultured until 70% confluence before CM
harvest. Competitive ELISA was performed as previously described
(22). Briefly, 96-well plate
Elisa kits were coated with monoclonal mouse antibodies against
VEGF (cat. no. VAL608), IL-6 (cat. no. VAL604), MCP-1 (cat. no.
MJE00B) and MMP-9 (cat no. MMPT90) (R&D Systems, R&D
Systems Inc, Minneapolis, USA). MSC-CM was harvested in 24, 48, 72
and 96 h following treatment. MSC-CM and standard test fluid
incubations were performed in duplicate, including serial dilutions
of the standard (0.005–500 pmol/ml) or MSCs CM for 4 h at room
temperature. Biotinylated secondary antibodies (B-2763; 1:2,000;
Invitrogen; Thermo Fisher Scientific, Inc.) were incubated at 37°C
for 30 min to reveal bound antibodies. The reaction was stopped
with HCl (1 mol/l) and the optical density was measured at 450 nm
using a multilabel plate reader (Hidex Oy, Turku, Finland).
Diabetes induction and
co-transplantation of MSCs and islets
A total of 80 Recipient female BALB/c mice (age, 8–9
weeks age, 21±1 g) were included, and each group contained 10 mice.
Mice were kept at 18–29°C, with 40–70% humidity, 12-h light/dark
cycle and free access to food and water. Mice were injected with
STZ (225 mg/kg) (Sigma-Aldrich; Merck KGaA; St. Louis, Missouri;
USA) to induce diabetes 5–7 days prior to islet transplantation and
were considered fit for transplantation if the non-fasting blood
glucose concentration was >11.1 mmol/l for 2 consecutive days.
Diabetic female BALB/c mice were anaesthetized (100 mg/kg ketamine
and 10 mg/kg xylazine) and a marginal mass of 250–400 islet
equivalents with or without 2.5×105 MSCs was centrifuged
(100 × g for 2 min at 37°C) and transplanted under the kidney
capsule.
Flow cytometry
MSC surface antigens were analyzed by flow
cytometry. MSCs were trypsinized, resuspended (1×106/ml)
in serum-free MSC medium and incubated at 4°C for 20 min with
fluorescein isothiocyanate (FITC)-conjugated anti-cluster of
differentiation CD34 (555822), anti-CD45 (550539), anti-CD29
(553715), and anti-CD90 (553016; BD Biosciences, Franklin Lakes,
NJ, USA). Cells were further incubated for 10 min at room
temperature with 7-aminoactinomycin D (7-AAD) and subsequently
washed and analyzed using a flow cytometer (BD Biosciences). The
islet apoptosis rate was determined by fluorescence-activated cell
sorting (FACS) flow cytometry and an Annexin V staining kit
(11858777001; Roche Diagnostics, Basel, Switzerland). After 48 h
incubation, the supernatant (floating apoptotic cells) was
collected and the adherent cells from each well trypsinized. The
collected cells were washed twice with PBS and centrifuged at 670 ×
g for 5 min at room temperature. Each pellet was re-suspended in
PBS (400 µl). The signal intensity was acquired by a FACS Calibur
(BD Biosciences) flow cytometer and analyzed with affiliated
software (BD CellQuest Pro Software version 5.1, BD Biosciences).
Propidium iodide (PI) negative and Annexin V negative were
considered healthy cells, PI negative and Annexin V positive were
considered apoptotic, and cells positive to both PI and Annexin V
considered necrotic.
In vitro insulin and release
quantitation
Cells were initially incubated for 3 h in
glucose-free IMDM medium. This was followed by incubation for 1 h
in medium containing 2.7, 11.1 or 27 mM glucose. The supernatant
was collected at the end of each incubation. The collected samples
were frozen at −70°C until assayed using an insulin ELISA kit
(EIA-3439; DRG Instruments GmbH, Marburg, Germany).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was prepared by using TRIzol reagent (Life
Technologies; Thermo Fisher Scientific, Inc.) from cells at ~80%
confluence. RNA purity and quantification assessment was performed
with an ultraviolet spectrophotometer and the 260OD/280OD
absorbance ratio was calculated (ratios of 1.8–2.0 indicated good
purity and quantification. For cDNA synthesis, random sequence
primers were used to prime the RT reactions, and synthesis was
carried out by SuperScript III Reverse Transcriptase (Invitrogen;
Thermo Fisher Scientific, Inc. USA). PCR cycling conditions were:
Enzyme activation, 95°C for 30 sec, one cycle; denaturation, 95°C
for 5 sec, 40 cycles; annealing, 55–60°C for 20 sec, 40 cycles.
Standard curves were represented for each target gene and for the
endogenous reference (GAPDH) in each sample. The quantification of
the samples was performed by the LightCycler 480 Real-Time PCR
System (Roche Diagnostics). Primer sequences were designed using
Primer premier version 6.0 software (Premier Biosoft International,
Palo Alto, CA, USA). Ideal primer sequences are listed below
(Table I). The 2−ΔΔCq
method was used to quantify gene expression (23). GAPDH was amplified in separate
wells as reference gene. Each experiment comprised five identical
wells and was repeated three times.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Sequence
(5′-3′) | Product size
(bp) | Annealing condition
(°C) |
|---|
| VEGF | F:
5-ATCTTCAAGCCGTCCTGTGTGC-3 | 120 | 60 |
|
| R:
5-TTGGCTTGTCACATTTTTCTGG-3 |
|
|
| IL-6 | F:
5-ATGAAGTTCCTCTCTGCAAGAGAC-3 | 108 | 60 |
|
| R:
5-CACTAGGTTTGCCGAGTAGATCTC-3 |
|
|
| MCP-1 | F:
5-TGTCTGGACCCCATTCCTTC-3 | 140 | 55 |
|
| R:
5-ACCAGCAAGATGATCCCAAT-3 |
|
|
| MMP-9 | F:
5-CCTGGAACTCACACGACATCTTC-3 | 132 | 55 |
|
| R:
5-TGGAAACTCACACGCCAGAA-3 |
|
|
| GAPDH | F:
5-GGAGAGAACCTGGTCCTCAG-3 | 300 | 55 |
|
| R:
5-ACCCAGAAGACTGTGGATGG-3 |
|
|
Apoptosis detection by fluorescein
diacetate (FDA)-propidium iodide (PI) viability staining
Islet preparations were assessed for islet cell
viability using cell membrane exclusion dyes FDA and PI both from
(Sigma-Aldrich; Merck KGaA; St. Louis, Missouri; USA) staining as
previously described (24). The
samples were examined using a fluorescent microscope, visualized
and photographed. Apoptotic cells were stained red and viable cells
were stained green. A total of 5 non-overlapping fields were imaged
and the red stained cells were counted out of the total number of
cells to determine the apoptosis rate.
mRNA microarray assay
Mice serum was collected and separated from blood
following sacrifice. Total serum RNA was extracted using TRIzol
reagent (Life Technologies; Thermo Fisher Scientific, Inc.). RNA
purity and quantification was assessed by ultraviolet
spectrophotometer scanning, 260OD/280OD suction photometric ratio
was calculated as described above using the Agilent Bioanalyzer
2100 (Agilent Technologies, Inc., Santa Clara, CA, USA). The
samples were cleaned and hybridized according to the Agilent 2100
expert software B.02.07 (Agilent Technologies, Inc.). Original data
were obtained using the mRNA Microarray Chip Mouse Gene 2.0 ST
Array and the Affymetrix Gene Chip Command Console version 4.0 and
analyzed with the Expression Console software version 1.1
(Affymetrix; Thermo Fisher Scientific, Inc. California, USA).
Ethical approval
All procedures performed using animals were in
accordance with the ethical standards of the institution and the
study was approved by the ethics committee of The Second Affiliated
Hospital of Zhejiang University (Hangzhou, China).
Statistical analysis
All experimental values were presented as the mean ±
standard deviation, and the differences of mean values were
statistically evaluated by one-way analysis of variance followed by
Student-Newman-Keuls post hoc test using SPSS software version 18
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
BMDC and MSC characterization
By the third passage, a homogeneous population of
fibroblast-like cells was obtained (Fig. 1A). Cells from passages 3–7 were
used throughout the present study. Characterization by flow
cytometry confirmed the absence of cells expressing the
hematopoietic markers CD34 (0.53±0.12%) and CD45 (1.03±0.14%).
BMDCs that expressed CD29 (36.32±1.42%) and CD90 (40.68±2.31%;
Fig. 1B), which is a typical
feature of cell group with large proportion of mesenchyme cells and
certain fibroblast cells (25,26),
were detected to identify MSCs.
Hypoxia treatment promotes MSC
proliferation and induces growth-related cytokine expression
To investigate the effects of hypoxia on MSCs, MSCs
were plated in 96-well plates with 15% fetal bovine serum and the
growth rate was measured every day. hMSCs were able to form a
greater number and larger-sized adipose globules compared with
those formed by normoxia-cultured MSCs (nMSCs), as determined by
adipose induction experiments (Fig.
2A). The results also revealed a higher proliferation rate in
hMSCs compared with nMSCs (Fig.
2B). MSCs secrete a number of soluble factors that are involved
in MSC growth, angiogenesis and autoimmune status (27). In the ELISA experiment, VEGFA,
IL-6, MCP-1 and MMP-9 mRNA expression levels were measured in nMSCs
and hMSCs. The average mRNA expression levels of VEGFA (1.73-fold
increase), IL-6 (1.31-fold increase), MCP-1 (2.13-fold increase)
and MMP-9 (1.63-fold increase) were significantly higher in hMSCs
compared with the nMSC group following 48 h hypoxia treatment
(P<0.01; Fig. 2C). In addition,
CM was collected and replaced at 24 h intervals until 96 h of
culture. ELISA revealed that VEGFA, IL-6, MCP-1 and MMP-9 secretion
was increased in hMSCs-CM compared with nMSCs-CM; VEGF, MCP-1 and
IL-6 demonstrated a secretion peak between 48 and 72 h, whereas the
highest secretion of MMP-9 was in the first 24 h. In addition, the
variation between the 4 factors was generally increased in the 24
to 96 h period under hypoxia induction compared with normoxia
condition (Fig. 2D). These results
suggested that hypoxic conditions may increase the secretion of
growth-related cytokines from MSCs to support graft vascularization
and to protect islet cells from apoptosis to avoid islet function
loss in the initial period of transplantation.
 | Figure 2.MSCs secrete more growth-promoting
related cytokines in hypoxic condition. (A) Adipogenesis was
examined by oil red-O staining of MSCs cultured in hypoxic and
normoxic environments. (B) Hypoxia induces proliferation increase
in MSCs compared with normoxia cultured MSCs, as demonstrated by
MTT assay (**P<0.01); absorbency was detected every day for 15
days. (C) Reverse transcription-quantitative polymerase chain
reaction was used to detect the mRNA expression levels of VEGF,
IL-6, MCP-1 and MMP-9 after 48 h hypoxia treatment (*P<0.05).
(D) MSCs secrete higher concentrations of VEGF, IL-6, MCP-1 and
MMP-9 in hypoxic conditions compared with normoxic-treated cells,
as detected by ELISA. IL, interleukin; MCP, monocyte
chemoattractant protein; MMP, matrix metallopeptidase; MSC,
mesenchymal stem cell; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; VEGF,
vascular endothelial growth factor (*P<0.05). |
hMSCs-CM protects islets from
hypoxia-induced apoptosis
For the induction of islet apoptosis, islets were
cultured in a low-oxygen environment. Subsequently, nMSC-CM or
hMSC-CM was introduced to aid islet survival rate in the hypoxic
condition. Microscopic evaluations of apoptosis were carried out on
the islets stained with FDA/PI; dead cells are stained red and
viable cells stained green (Fig.
3A). After 48 h of culture, apoptosis rates were significantly
increased in hypoxia-treated islets (77.3±5.7%) compared with
Control islets grown in normoxic conditions (4.8±2.1%; P<0.05);
hMSC-CM co-treatment significantly decreased the hypoxia-induced
apoptotic rates compared with the nMSCs-CM-treated group
(20.8±14.2% and 44.6±9.8%, respectively; P<0.05). Apoptotic
rates were also analyzed using flow cytometry with PI and Annexin
V-FITC labeling. All early and late apoptosis and necrotic cells
were identified as the non-survival group. Only Annexin
V-FITC)-/PI-cells were identified as the survival group. The
survival rate of islets in hypoxic conditions was significantly
reduced compared with those cultured in normoxic conditions
(42.3±6.1 and 93.8±2.1%, respectively; P<0.01; Fig. 3B). hMSC-CM treatment significantly
improved the survival rate of islets in hypoxic conditions compared
with the nMSC-CM treated group (77.3±3.2 and 62.4±3.8%,
respectively; P<0.01; Fig. 3B).
In addition, an in vitro glucose-stimulated insulin
secretion test was performed to examine the possible effects of
inducible MSC-CM on islet secretory function. Under additive
glucose stimulation, insulin secretion was increased in islets
co-cultured with the nMSC-CM and hMSC-CM groups in medium (11.1 mM)
and high (27 mM) glucose stimulation compared with the hypoxia
treatment group. HMSC-CM particularly increased insulin secretion
compared with the nMSC-CM group in medium (11.1 mM) and high (27
mM) glucose stimulation (P<0.05), whereas less difference was
detected in the low (2.7 mM) glucose concentration stimulation
group between the hMSC-CM and nMSC-CM groups (Fig. 3C).
 | Figure 3.MSC-CM protects islets from apoptosis
under hypoxia. (A) FDA/PI test for the islets in hypoxia condition
with nMSCs and hMSC CM culture. Scale bar=20 µm. Compared with
hypoxia mediated group, MSC-CM protected islets from hypoxia
induced impairment. hMSCs-CM better prevent islets from apoptosis
compared with nMSCs-CM group (**P<0.01). (B) Islet cells were
cultured with nMSC-CM or hMSC-CM for 48 h and apoptosis was
examined by Annexin V-FITC/PI staining and flow cytometry, Only
Annexin V-FITC)-/PI-cells were identified as the survival group
(**P<0.01). (C) Islets insulin secretion in different CM under
gradient glucose concentration (2.7, 11.1 and 27 mM) stimulation.
CM, conditioned medium; Crtl, control; FITC, fluorescein
isothiocyanate; h, hypoxia cultured; MSC, mesenchymal stem cell; n,
normoxia cultured; PI, propidium iodide (*P<0.05,
**P<0.01). |
Co-transplantation of hMSCs improves
the function of transplanted islets in vivo
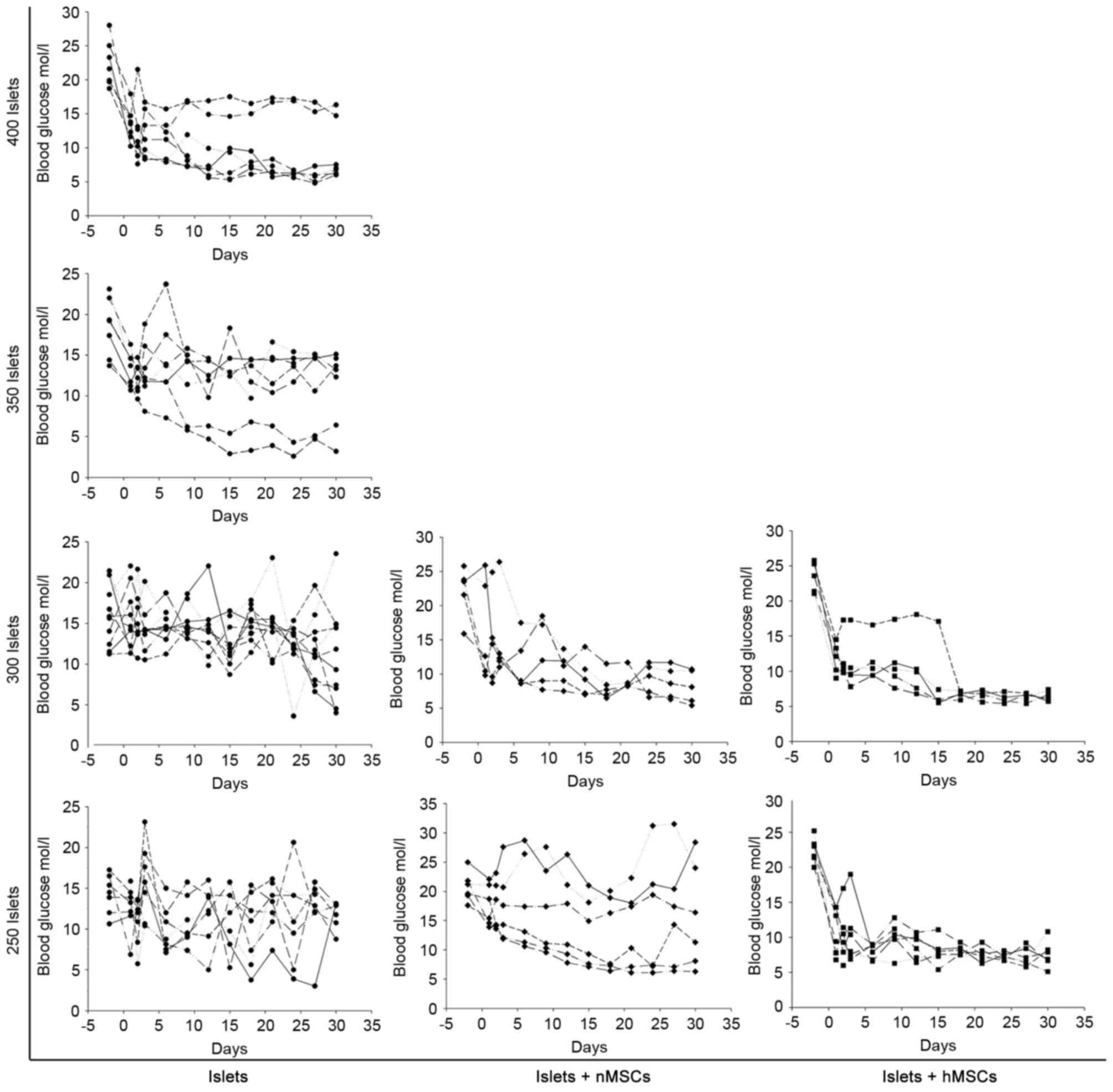
Mice were treated with STZ to induce diabetes. A
total of 250–400 islets were transplanted under the kidney capsule,
either alone or co-transplanted with nMSCs or hMSCs; blood glucose
levels were monitored every 3 days for 30 days. The minimal number
of islets required to reverse the impaired glucose tolerance
condition to reach normoglycemia was ~400 in mice in the Islet-only
group, ~300 in the Islet + nMSCs group and ~250 in the Islets +
hMSCs group (Fig. 4).
Co-transplantation of islets with
hMSCs reverses high postprandial blood glucose levels in diabetic
mice
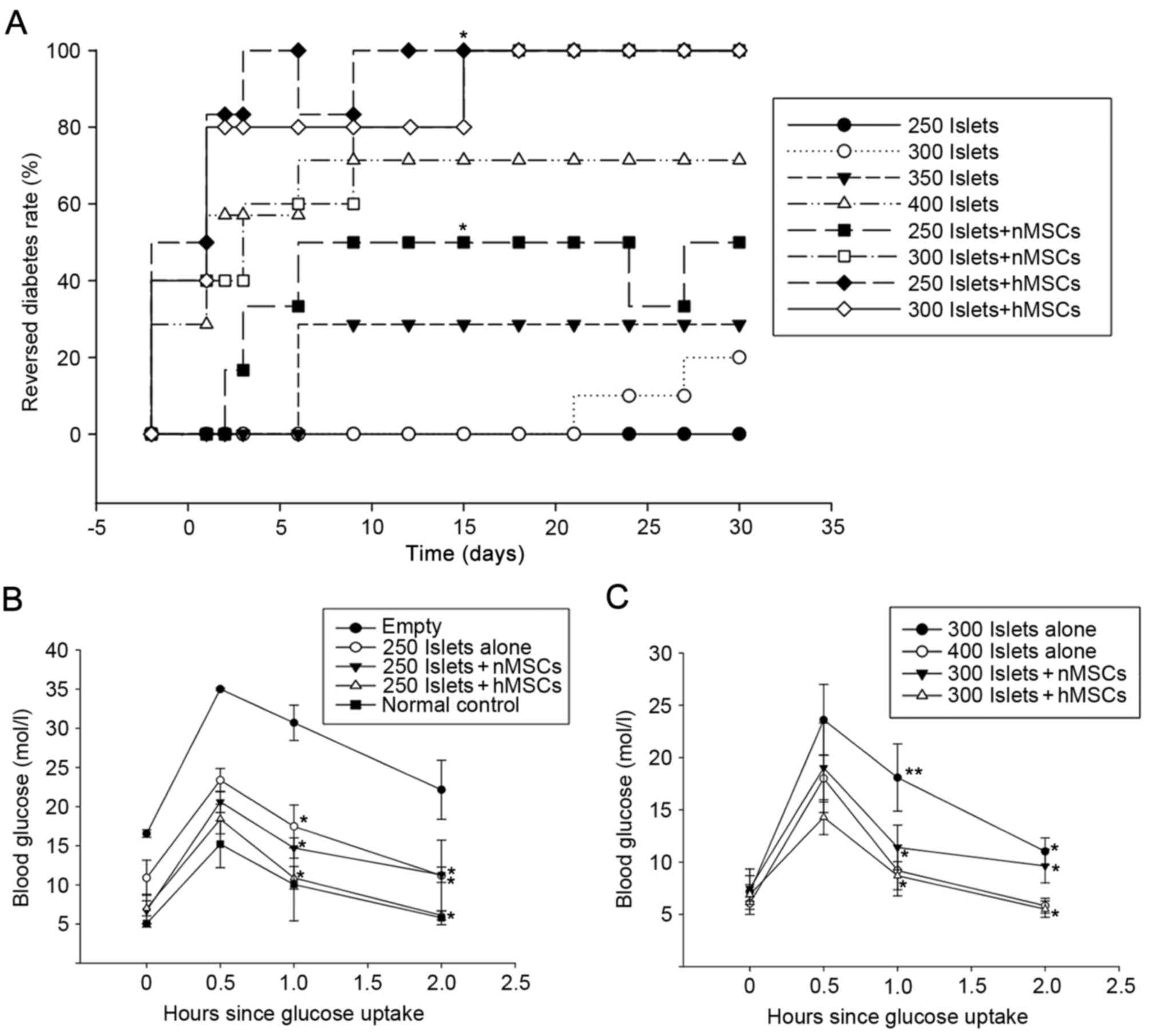
Cumulative diabetes reversal curves were described
in the transplant model following islets transplantation.
Post-operative transient hyperglycemia due to fasting and surgery
was observed in all groups. At 4 weeks following transplantation,
100% of mice in the 300 Islets + hMSCs, the 250 Islets + hMSCs and
the 300 Islets + nMSCs groups were restored to euglycemia compared
with 50% of mice in the 250 Islets + nMSCs, 72% in the 400 Islets,
28% in the 350 Islets, 20% in the 300 Islets and 0% in 250 Islets
groups (Fig. 5A). Mice in the two
Islets + hMSCs groups exhibited higher diabetes reversal ratios
compared with mice in the Islets-alone, Islets + nMSCs or empty (no
islet transplantation) groups. A glucose tolerance test was
performed to confirm the effects of Islets + hMSCs transplantation
on STZ-induced diabetic mice at 30 days following islet
implantation in 5 age-matched pairs of BALB/c mice. The normal
control group was mice without STZ treatment and the empty group
was STZ induced diabetes mice without any graft transplantation.
hMSCs co-transplantation significantly accelerated glycemic
utilization following glucose intake compared with MSCs and islets
only group (P<0.05; Fig. 5B and
C).
mRNA microarray data in islets
transplanted mice
Hierarchical clustering analysis of microarray data
revealed mouse serum mRNA expression of Islets + hMSCs, Islets +
nMSCs and the Islets-only control groups (n=3 mice/group).
Abnormally increased cytokines were detected between Islets + hMSCs
group and Islets + nMSCs and control groups (unaltered mRNAs not
shown; Fig. 6).
Discussion
Incomplete graft revascularization and hypoxic
conditions impede the clinical use of islet transplantation
(28–30). Early hypoxia-related islet death
following intramuscular transplantation is the major factor for
loss of islets and results in an increased rate of transplantation
failure (31,32). In previous studies, E26 avian
leukemia oncogene 1, solute carrier family 30 member 8 and zinc
transporter have been reported to improve the survival rate of
islets in hypoxic conditions (33,34);
however, maintenance of islet function remains unsatisfactory.
A previous study reported that co-transplantation of
islets with MSCs was associated with enhanced islet graft
vascularization and functional recovery (35). In another previous study, a number
of beneficial effects on blood glucose regulation were observed in
islets + nMSC transplanted mice (8). However, these observations did not
support the hypothesis that co-transplantation of islets with MSCs
is sufficient for a complete reversal of hyperglycemia in diabetic
mice. The present study pretreated MSCs in hypoxic conditions prior
to local kidney capsule transplantation in diabetic mice, to
evaluate their effects on islets and islet function. Hypoxia has a
strong effect on several aspects of cell biology, including
metabolism, angiogenesis, innate immunity and stemness induction
(36). In MSCs, stem cells
pre-cultured in hypoxic conditions could improve the potential of
their tissue regenerative function (19). Similarly, hypoxic conditions
induced MSCs pro-angiogenic and growth-related genes expression and
activated certain major receptors for hepatocyte growth factor
(HGF) and enhanced cMet signaling to improve their tissue
regenerative potential (19,37).
Therefore, the present study hypothesized that hMSCs may also
secrete increased levels of cytokines including VEGFA, IL-6, MCP-1
and MMP-9 to support graft vascularization, certain pro-angiogenic
and growth rate of MSCs.
In a previous study, VEGFA, known as a vital
angiogenesis-related factor in islets transplantation, was found to
improve intra-islet vascular reformation to protect islet survival
(38). IL-6 is a pleiotropic
cytokine with complex roles in inflammation and metabolism. During
islets transplantation, a previous study demonstrated that IL-6
robustly activated signal transducer and activator of transcription
3, which is involved in autophagy, and as a result directly
protected islet cells from apoptosis by stimulation of autophagy
(39). Several previous studies
have revealed that MCP-1 and MMP-9 possess various potential
clinical implications in islet transplantation. For example, one
study demonstrated that recipient C-C motif chemokine 2/MCP-1 may
be a major pharmacological target for the control of potentially
islet damaging reactions at the site of transplant (40). MMPs are proteolytic enzymes that
are involved in the breakdown of extracellular matrix proteins.
Inhibition of MMP-9 was reported to results in increased amyloid
deposition and apoptosis in mouse islets, which suggested that
MMP-9 may serve a physiological role in limiting islet amyloid
deposition and protects islets from amyloid-induced toxicity
(41). As a result, high secretion
of these cytokines in hMSCs may increase the concentration of these
protective factors around transplanted islet cells. In this
condition, a prospective environment suitable for improved islet
survival is created, which decreases the number of islets required
to reach the desired blood glucose concentration. This method
avoids the use of artificial gene delivery techniques to increase
the secretion of cytokines of transplanted cells in mouse
experiments and the risk of transfection toxicity (29,32).
Transplantation is preferable to the danger of biological
transfection cytotoxicity.
The present study demonstrated that hMSC-CM was able
to effectively protect the islets from apoptotic death and
increased the insulin secretion of the islet compared with nMSCs. A
notable reduction was observed in the minimal mass of islets
required to reverse diabetes in mice. In this respect, islet
function parameters were superior in the 250 Islets + hMSCs group
compared with the other treatment groups and was sufficient to
control the blood glucose concentration; previously, islets were
required from 2 to 4 donors to treat each recipient in order to
achieve a state of complete insulin independence (4). Clinical islet transplantation should
proceed towards the use of single donor organs instead of multiple
donors. Therefore, any improvements leading to the reduction of the
number of islets required in transplanted islets may be clinically
important. Insulin independence following islet transplantation
from a single donor and decreasing procedural complications
attributed to multiple islet infusions are of great importance.
In conclusion, results from the present study
demonstrated that the underlying mechanisms modulating pancreatic
islet viability may be attributable to the paracrine mediators
VEGFA, IL-6, MCP-1 and MMP-9 secreted by hMSCs; co-transplantation
with hMSCs may reduce the minimal islet mass required to reverse
diabetes in mice. In addition, a number of concerns about the use
of hMSC may be investigated in the context of allogeneic islet
transplantation for future proper glyco-metabolic control.
Acknowledgments
The authors would like to thank Dr Rashid. A.
Tabassum (The Second Affiliated Hospital of Zhejiang University,
Hangzhou, China) who provided language and writing guidance.
Funding
The present study was supported by The Scientific
Foundation of Zhejiang Province (grant no. LY14H160033).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QPX made substantial contributions to the concept
and design of the present study. CX performed the experiments,
wrote the paper and reviewed and edited the manuscript. Both
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed on animals were in
accordance with ethical standards and were approved by the ethics
committee of The Second Affiliated Hospital of Zhejiang University
(Hangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BMDC
|
bone marrow derived cell
|
|
CM
|
conditioned medium
|
|
IL
|
interleukin
|
|
MCP
|
monocyte chemoattractant protein
|
|
MSCs
|
mesenchymal stem cells
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
STZ
|
streptozotocin
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Abdel-Moneim A, Bakery HH and Allam G: The
potential pathogenic role of IL-17/Th17 cells in both type 1 and
type 2 diabetes mellitus. Biomed Pharmacother. 101:287–292. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koro CE, Bowlin SJ, Bourgeois N and Fedder
DO: Glycemic control from 1988 to 2000 among U.S. adults diagnosed
with type 2 diabetes: A preliminary report. Diabetes care.
27:17–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Badri N and Ghoneim MA: Mesenchymal
stem cell therapy in diabetes mellitus: Progress and challenges. J
Nucleic Acids. 2013:1948582013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shapiro AM, Lakey JR, Ryan EA, Korbutt GS,
Toth E, Warnock GL, Kneteman NM and Rajotte RV: Islet
transplantation in seven patients with type 1 diabetes mellitus
using a glucocorticoid-free immunosuppressive regimen. N Engl J
Med. 343:230–238. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barton FB, Rickels MR, Alejandro R, Hering
BJ, Wease S, Naziruddin B, Oberholzer J, Odorico JS, Garfinkel MR,
Levy M, et al: Improvement in outcomes of clinical islet
transplantation: 1999–2010. Diabetes Care. 35:1436–1445. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ricordi C: Islet transplantation: A brave
new world. Diabetes. 52:1595–1603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brandhorst H, Asif S, Andersson K, Mönch
J, Friedrich O, Rämsch-Günther N, Rämsch C, Steffens M, Lambrecht
J, Schräder T, et al: The effect of truncated collagenase class I
isomers on human islet isolation outcome. Transplantation.
90:334–335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ito T, Itakura S, Todorov I, Rawson J,
Asari S, Shintaku J, Nair I, Ferreri K, Kandeel F and Mullen Y:
Mesenchymal stem cell and islet co-transplantation promotes graft
revascularization and function. Transplantation. 89:1438–1445.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rackham CL, Chagastelles PC, Nardi NB,
Hauge-Evans AC, Jones PM and King AJ: Co-transplantation of
mesenchymal stem cells maintains islet organisation and morphology
in mice. Diabetologia. 54:1127–1135. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perez-Basterrechea M, Obaya AJ, Meana A,
Otero J and Esteban MM: Cooperation by fibroblasts and bone
marrow-mesenchymal stem cells to improve pancreatic rat-to-mouse
islet xenotransplantation. PLoS One. 8:e735262013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Berman DM, Willman MA, Han D, Kleiner G,
Kenyon NM, Cabrera O, Karl JA, Wiseman RW, O'Connor DH, Bartholomew
AM and Kenyon NS: Mesenchymal stem cells enhance allogeneic islet
engraftment in nonhuman primates. Diabetes. 59:2558–2568. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakata N, Goto M, Yoshimatsu G, Egawa S
and Unno M: Utility of co-transplanting mesenchymal stem cells in
islet transplantation. World J Gastroenterol. 17:5150–5155. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sordi V, Melzi R, Mercalli A, Formicola R,
Doglioni C, Tiboni F, Ferrari G, Nano R, Chwalek K, Lammert E, et
al: Mesenchymal cells appearing in pancreatic tissue culture are
bone marrow-derived stem cells with the capacity to improve
transplanted islet function. Stem Cells. 28:140–151. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Golocheikine A, Tiriveedhi V, Angaswamy N,
Benshoff N, Sabarinathan R and Mohanakumar T: Cooperative signaling
for angiogenesis and neovascularization by VEGF and HGF following
islet transplantation. Transplantation. 90:725–731. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ding Y, Xu D, Feng G, Bushell A, Muschel
RJ and Wood KJ: Mesenchymal stem cells prevent the rejection of
fully allogenic islet grafts by the immunosuppressive activity of
matrix metalloproteinase-2 and −9. Diabetes. 58:1797–1806. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karaoz E, Genc ZS, Demircan PC, Aksoy A
and Duruksu G: Protection of rat pancreatic islet function and
viability by coculture with rat bone marrow-derived mesenchymal
stem cells. Cell Death Dis. 1:e362010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie QP, Huang H, Xu B, Dong X, Gao SL,
Zhang B and Wu YL: Human bone marrow mesenchymal stem cells
differentiate into insulin-producing cells upon microenvironmental
manipulation in vitro. Differentiation. 77:483–491. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grayson WL, Zhao F, Izadpanah R, Bunnell B
and Ma T: Effects of hypoxia on human mesenchymal stem cell
expansion and plasticity in 3D constructs. J Cell Physiol.
207:331–339. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rosova I, Dao M, Capoccia B, Link D and
Nolta JA: Hypoxic preconditioning results in increased motility and
improved therapeutic potential of human mesenchymal stem cells.
Stem Cells. 26:2173–2182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hung SC, Pochampally RR, Chen SC, Hsu SC
and Prockop DJ: Angiogenic effects of human multipotent stromal
cell conditioned medium activate the PI3K-Akt pathway in hypoxic
endothelial cells to inhibit apoptosis, increase survival and
stimulate angiogenesis. Stem Cells. 25:2363–2370. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dao MA, Pepper KA and Nolta JA: Long-term
cytokine production from engineered primary human stromal cells
influences human hematopoiesis in an in vivo xenograft model. Stem
cells. 15:443–454. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
D'Amato F, Noli B, Brancia C, Cocco C,
Flore G, Collu M, Nicolussi P and Ferri GL: Differential
distribution of VGF-derived peptides in the adrenal medulla and
evidence for their selective modulation. J Endocrinol. 197:359–369.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ichii H, Wang X, Messinger S, Alvarez A,
Fraker C, Khan A, Kuroda Y, Inverardi L, Goss JA, Alejandro R and
Ricordi C: Improved human islet isolation using nicotinamide. Am J
Transplant. 6:2060–2068. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The international society for cellular
therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo JZ, Xiong F, Al-Homsi AS, Roy T and
Luo LG: Human BM stem cells initiate angiogenesis in human islets
in vitro. Bone Marrow Transplantat. 46:1128–1137. 2011. View Article : Google Scholar
|
|
28
|
Hajizadeh-Saffar E, Tahamtani Y, Aghdami
N, Azadmanesh K, Habibi-Anbouhi M, Heremans Y, De Leu N, Heimberg
H, Ravassard P, Shokrgozar MA and Baharvand H: Inducible VEGF
expression by human embryonic stem cell-derived mesenchymal stromal
cells reduces the minimal islet mass required to reverse diabetes.
Sci Rep. 5:93222015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sato Y, Endo H, Okuyama H, Takeda T,
Iwahashi H, Imagawa A, Yamagata K, Shimomura I and Inoue M:
Cellular hypoxia of pancreatic beta-cells due to high levels of
oxygen consumption for insulin secretion in vitro. J Biol Chem.
286:12524–12532. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Buchwald P: A local glucose-and oxygen
concentration-based insulin secretion model for pancreatic islets.
Theor Biol Med Model. 8:202011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Espes D, Lau J, Quach M, Banerjee U,
Palmer AF and Carlsson PO: Cotransplantation of Polymerized
Hemoglobin Reduces β-Cell Hypoxia and Improves β-Cell Function in
Intramuscular Islet Grafts. Transplantation. 99:2077–2082. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liljeback H, Grapensparr L, Olerud J and
Carlsson PO: Extensive loss of islet mass beyond the first day
after intraportal human islet transplantation in a mouse model.
Cell Transplant. 25:481–489. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qiao N, Xu C, Zhu YX, Cao Y, Liu DC and
Han X: Ets-1 as an early response gene against hypoxia-induced
apoptosis in pancreatic β-cells. Cell Death Dis. 6:e16502015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gerber PA, Bellomo EA, Hodson DJ, Meur G,
Solomou A, Mitchell RK, Hollinshead M, Chimienti F, Bosco D, Hughes
SJ, et al: Hypoxia lowers SLC30A8/ZnT8 expression and free
cytosolic Zn2+ in pancreatic beta cells. Diabetologia.
57:1635–1644. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sakata N, Chan NK, Chrisler J, Obenaus A
and Hathout E: Bone marrow cell cotransplantation with islets
improves their vascularization and function. Transplantation.
89:686–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Majmundar AJ, Wong WJ and Simon MC:
Hypoxia-inducible factors and the response to hypoxic stress. Mol
Cell. 40:294–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lampert FM, Kutscher C, Stark GB and
Finkenzeller G: Overexpression of Hif-1α in mesenchymal stem cells
affects cell-autonomous angiogenic and osteogenic parameters. J
Cell Biochem. 117:760–768. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mao D, Zhu M, Zhang X, Ma R, Yang X, Ke T,
Wang L, Li Z, Kong D and Li C: A macroporous heparin-releasing silk
fibroin scaffold improves islet transplantation outcome by
promoting islet revascularisation and survival. Acta Biomater.
59:210–220. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Linnemann AK, Blumer J, Marasco MR,
Battiola TJ, Umhoefer HM, Han JY, Lamming DW and Davis DB:
Interleukin 6 protects pancreatic beta cells from apoptosis by
stimulation of autophagy. FASEB J. 31:4140–4152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Melzi R, Mercalli A, Sordi V, Cantarelli
E, Nano R, Maffi P, Sitia G, Guidotti LG, Secchi A, Bonifacio E and
Piemonti L: Role of CCL2/MCP-1 in islet transplantation. Cell
Transplant. 19:1031–1046. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Meier DT, Tu LH, Zraika S, Hogan MF,
Templin AT, Hull RL, Raleigh DP and Kahn SE: Matrix
metalloproteinase-9 protects islets from amyloid-induced toxicity.
J Biol Chem. 290:30475–30485. 2015. View Article : Google Scholar : PubMed/NCBI
|