Introduction
Articular cartilage has limited regenerative
capabilities following injury, due to the lack of vascularization,
reduced progenitor cell supply and the presence of few cells with
low mitotic activity (1,2). Cell-based therapies are considered
useful approaches for cartilage restoration (3,4).
Mesenchymal stem cells (MSCs) can be isolated from multitudinous
tissues, including bone marrow, adipose tissue and the placenta,
and are frequently used as a source of cartilage restoration due to
high yields, easy accessibility and the capability to differentiate
along the chondrogenic lineage (5). Bone-derived MSCs (BMSCs) appear to be
the most promising choice for cartilage regeneration, due to the
ease with which they can be acquired, and their high proliferative
capacity and chondrogenic differentiation ability (6). Growth factors, such as transforming
growth factor-β1, serve critical roles in the regulation the
chondrogenic differentiation of MSCs. Although conditioned medium
that regulates chondrogenesis of MSCs has been well established
(7), the high cost, short
half-life, versatility and functional heterogeneity of traditional
growth factors remain an obstacle for stem cell-based therapy.
In our previous study, it was revealed that a novel
growth factor, nerve growth factor (NGF) extracted from Chinese
cobra venom, specifically induces the chondrogenic differentiation
of MSCs and further promotes cartilage repair (8). The chondral specificity of NGF is
better than traditionally used growth factors, which may lead to
osteophyte formation instead of chondrogenesis during cartilage
regeneration (9–11). Other peptide neurotrophins, such as
Nel-like molecule-1, which is abundantly secreted in neural tissue,
are also reported to promote the proliferation of chondrocyte and
maintain its phenotype in vitro (12). Grassel (13) also demonstrated the roles of
sensory and sympathetic neurotransmitters on limb formation during
the process of embryonic skeletal development. Notably,
venom-derived NGF rarely induces BMSCs to differentiate into a
neuronal phenotype (14).
There are numerous sources of NGF, which has been
isolated from venom and body fluids, and formed by recombination.
Lipps (15,16) studied the bioactivity of NGF from
bee, scorpion and spider venoms, and NGF from body fluids,
including serum, saliva and urine, and compared these NGFs with
cobra venom-derived NGF (cvNGF). The findings of these previous
studies indicated that the biological activities of NGFs obtained
from human body fluids on PC12 cells were minor in comparison to
cvNGF, and the biological activity of bee NGF on PC12 cells was
1/10 that of cvNGF. It remains to be determined as to whether NGF
from other sources behaves the same as NGF extracted from natural
venom.
The present study compared the effects of
commercially purchased recombinant murine β-NGF (mNGF) and cvNGF on
the chondrogenic differentiation of MSCs by detecting cell
proliferation, glycosaminoglycan (GAG) synthesis and
cartilage-specific gene expression. The findings of this study may
aid in the clinical application of NGF for cartilage repair.
Materials and methods
Cell culture
BMSCs were isolated from bone marrow that was
extracted from two male Sprague-Dawley rats (weight, 8–10 g; age, 3
days), which were purchased from the Animal Experimental Center of
Guangxi Medical University (Nanning, China). The rats were housed
at a constant temperature (26°C) and relative humidity (60%) under
a reversed 12-h dark/light cycle (lights on until 8:00 p.m.), with
free access to a standard diet and water. All experiments were
conducted in accordance with the standard guidelines approved by
the Animal Care and Experiment Committee of Guangxi Medical
University (Guangxi, China; protocol number: 2014-12-3), and the
present study was approved by the ethics committee of Guangxi
Medical University. Briefly, the rats were sacrificed with an
injected overdose of pentobarbital, and bone marrow was collected
from the bilateral femur by flushing with α-modified Eagle's medium
(α-MEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 1% penicillin/streptomycin (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China). Following
density gradient centrifugation (1,100 × g at 25°C for 30 min), the
isolated BMSCs were cultured in α-MEM supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin. The culture medium was changed every 2
days. Characterization of isolated BMSCs was performed as described
previously (5).
Cell seeding
Two sources of NGF were added to the cell cultures.
mNGF was purchased from Peprotech, Inc. (Rocky Hill, NJ, USA), and
cvNGF was extracted and purified from the venom of Chinese cobra
(Naja naja atra) as described in our previous study
(8). Briefly, BMSCs were cultured
in chondrogenic medium including 50 µg/ml ascorbic acid (A7506;
Sigma-Aldrich; USA), 100 nM dexamethasone (D1756; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), 1% insulin-transferrin-selenium
solution (41400045; Gibco; Thermo Fisher Scientific, Inc.) and 0.06
µg/ml mNGF or 6 µg/ml vNGF. For each experiment, cells at passage 2
were used and the seeding density was 2×104
cells/cm2. Experiments were performed at 7, 14 and 21
days.
Cytotoxicity assay
NGF toxicity on BMSCs was detected using the MTT
assay (Gibco; Thermo Fisher Scientific, Inc.). BMSCs were seeded
onto 96-well plates and treated with various concentrations of mNGF
(0, 0.0075, 0.015, 0.03, 0.06, 0.12, 0.24, 0.48 and 0.96 µg/ml) and
cvNGF (0, 1, 1.5, 2, 3, 4, 6, 8, 12, 16, 24 and 32) and incubated
at 37°C. After 3 days, 20 µl MTT solution (5 mg/ml) was added and
incubated at 37°C for 4 h in the dark. After removing the medium,
the formazan crystals in each well were dissolved with 200 µl
dimethyl sulfoxide (Gibco; Thermo Fisher Scientific, Inc.). The
absorbance was measured at 570 nm using a microplate reader (Thermo
Fisher Scientific, Inc.).
Cell viability assay
Cell viability was determined by fluorescein
diacetate (FDA; GenWay Biotech, Inc., San Diego, CA, USA) and
propidium iodide (PI; Sigma-Aldrich; Merck KGaA) staining on days
7, 14 and 21. Briefly, after rinsing with PBS for three times, 0.5
µM FDA and 2 µM PI in 1 ml PBS was added to the cells and incubated
in the dark for 5 min. Images were captured under a laser scanning
confocal microscope (Olympus Corporation, Tokyo, Japan).
Cell morphological examination
The BMSCs were seeded onto 24-well-plates and were
cultured with mNGF (0.06 µg/ml) or cvNGF (6 µg/ml) for 7, 14 and 21
days. The cells were then washed three times with PBS and fixed
with 95% alcohol for 30 min at room temperature. Subsequently, the
cells were washed with PBS and stained with 10% hematoxylin and 2%
eosin (H&E; Nanjing Jiancheng Bioengineering Institute,
Nanjing, China) at room temperature for 5 min and 30 sec,
respectively. Finally, cells were observed and images were captured
under an inverted phase contrast microscope (Olympus
Corporation).
Cell proliferation analysis and
biochemical assay
After being cultured for 7, 14 or 21 days, cells
were washed with PBS and then digested with 0.25% trypsin/ETDA.
Cells were centrifuged at 200 × g for 5 min at room temperature,
and digested with proteinase K (20 mg/ml; Sigma-Aldrich; Merck
KGaA) for 16 h at 56°C. After staining with 10 µg/ml Hoechst 33258
dye at room temperature for 10 min (Sigma-Aldrich; Merck KGaA), the
DNA content was determined using a fluorescence microplate reader
(BioTek Instruments, Inc., Winooski, VT, USA) at 460 nm; calf
thymus DNA (Sigma-Aldrich; Merck KGaA) was used as a standard and
absorption of Hoechst 33258 dye was considered the baseline. The
total production of GAG was quantified by detecting absorbance at
525 nm, following treatment with 16 mg/l 1, 9-dimeth-ylmethylene
blue (DMMB; Sigma-Aldrich; Merck KGaA) at room temperature for 10
min; chondroitin sulfate (Sigma-Aldrich; Merck KGaA) was used as a
standard. The GAG content in each cell was normalized to the total
DNA content of all cells, which represented its biosynthetic
activity under various culture conditions.
Safranin O staining
Safranin O staining was performed to assess the
synthesis of GAG. After being fixed with 95% alcohol for 30 min, at
room temperature the cells were stained with 0.1% safranin O
(Sigma-Aldrich; Merck KGaA) for 10 min. Subsequently, the cells
were washed with water and sealed with neutral gum. Eventually,
cells were observed and images were captured under an inverted
phase contrast microscope (Olympus Corporation).
Immunohistochemical staining
Immunohistochemistry was performed to analyze the
expression levels of collagen type I α1 chain (COL1A1) and collagen
type II α1 chain (COL2A1). After 7, 14 and 21 days, cells were
washed three times with PBS, fixed with 95% alcohol at room
temperature for 30 min and treated with Triton X-100
(Sigma-Aldrich; Merck KGaA) at room temperature for 10 min. To
eliminate endogenous peroxidase activity, cells were incubated with
3% H2O2 at room temperature for 15 min and
blocked with goat serum (Beijing Solarbio Science & Technology
Co., Ltd.) for 15 min at room temperature. After incubating with
COL1A1 (1:200; Wuhan Boster Biological Technology, Ltd., Wuhan,
China) and COL2A1 (BA0533; 1:200; Wuhan Boster Biological
Technology Ltd.) primary antibodies overnight at 4°C, cells were
incubated with the secondary antibody (G1080, 1:200; Beijing
Solarbio Science & Technology Co., Ltd.) and followed by
biotin-labeled horseradish peroxidase (Zhongshanjinqiao
Biotechnology Inc., Wuhan, China) at room temperature for 15 min. A
DAB kit (Wuhan Boster Biological Technology, Ltd.) was used to
visualize antibody binding. Finally, images of the cells were
captured under an inverted phase contrast microscope (Olympus
Corporation).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
RT-qPCR was conducted to analyze the expression
levels of aggrecan (Acan), SRY-box 9 (Sox9),
Col2a1, Col1a1, runt-related transcription factor 2
(RUNX2) and enolase 2 (ENO2). The sequences of
primers used for RT-qPCR are presented in Table I. Total RNA was extracted using a
RNA isolation kit (Wuhan Megentec Biological Technology, Ltd.,
Wuhan, China), according to the manufacturer's protocol. RT of RNA
was performed using a RT kit (Fermentas; Thermo Fisher Scientific,
Inc.) and was carried out at 25°C for 5 min, 42°C for 60 min and
72°C for 5 min. RT-qPCR was performed on a qPCR Detection system
with Fast Start Universal SYBR Green Master Mix (Roche Diagnostics,
Basel, Switzerland) under the following conditions: 10 min at 95°C
for initial denaturation, 15 sec at 95°C and 1 min at 60°C for
final extension (40 cycles). The melting curve data were collected
to verify PCR specificity; each gene was analyzed in triplicate.
The relative mRNA expression levels were calculated using the
2−ΔΔCq (17) method
with β-actin as a reliable internal control.
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer | Reverse primer |
|---|
| Acan |
5′-GACAAGGACGAGTTCCCTGG-3′ |
5′-CTCCGGGGATGTGGCATAAA-3′ |
| Sox9 |
5′-TCCAGCAAGAACAAGCCACA-3′ |
5′-TCCAGCAAGAACAAGCCACA-3′ |
| Col2a1 |
5′-AGATGGCTGGAGGATTTGACG-3′ |
5′-TTTCCGGGCTTTCCAGCTT-3′ |
| Col1a1 |
5′-GATCCTGCCGATGTCGCTAT-3′ |
5′-GGGACTTCTTGAGGTTGCCA-3′ |
| RUNX2 |
5′-GTGGCCAGGTTCAACGATCT-3′ |
5′-TGAGGAATGCGCCCTAAATCA-3′ |
| ENO2 |
5′-TCAAGTCTCCTGCTGACCCT-3′ |
5′-AACGTGTCCTCGGTTTCTCC-3′ |
| β-actin |
5′-CCCATCTATGAGGGTTACGC-3′ |
5′-TTTAATGTCACGCACGATTTC-3′ |
Statistical analysis
Data are presented as the means ± standard deviation
from 4 repeated experiments. Data were analyzed using one-way
analysis of variance followed by Dunnett's post hoc test (SPSS
version 21; IBM Corp., Armonk, NY, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Cytotoxicity of NGF
An MTT assay was performed to detect the
cytotoxicity of NGF on BMSCs. Cells were treated with increasing
concentrations of mNGF (0–0.96 µg/ml) or cvNGF (0–32 µg/ml). As
shown in Fig. 1, mNGF at
concentrations ranging between 0.03 and 0.12 µg/ml and cvNGF at
1.5–16 µg/ml significantly accelerated cell growth (P<0.05). On
the basis of these results, 0.06 µg/ml mNGF (Fig. 1A) and 6 µg/ml cvNGF (Fig. 1B), which exhibited the most obvious
effect on BMSC proliferation, were chosen for further
investigation.
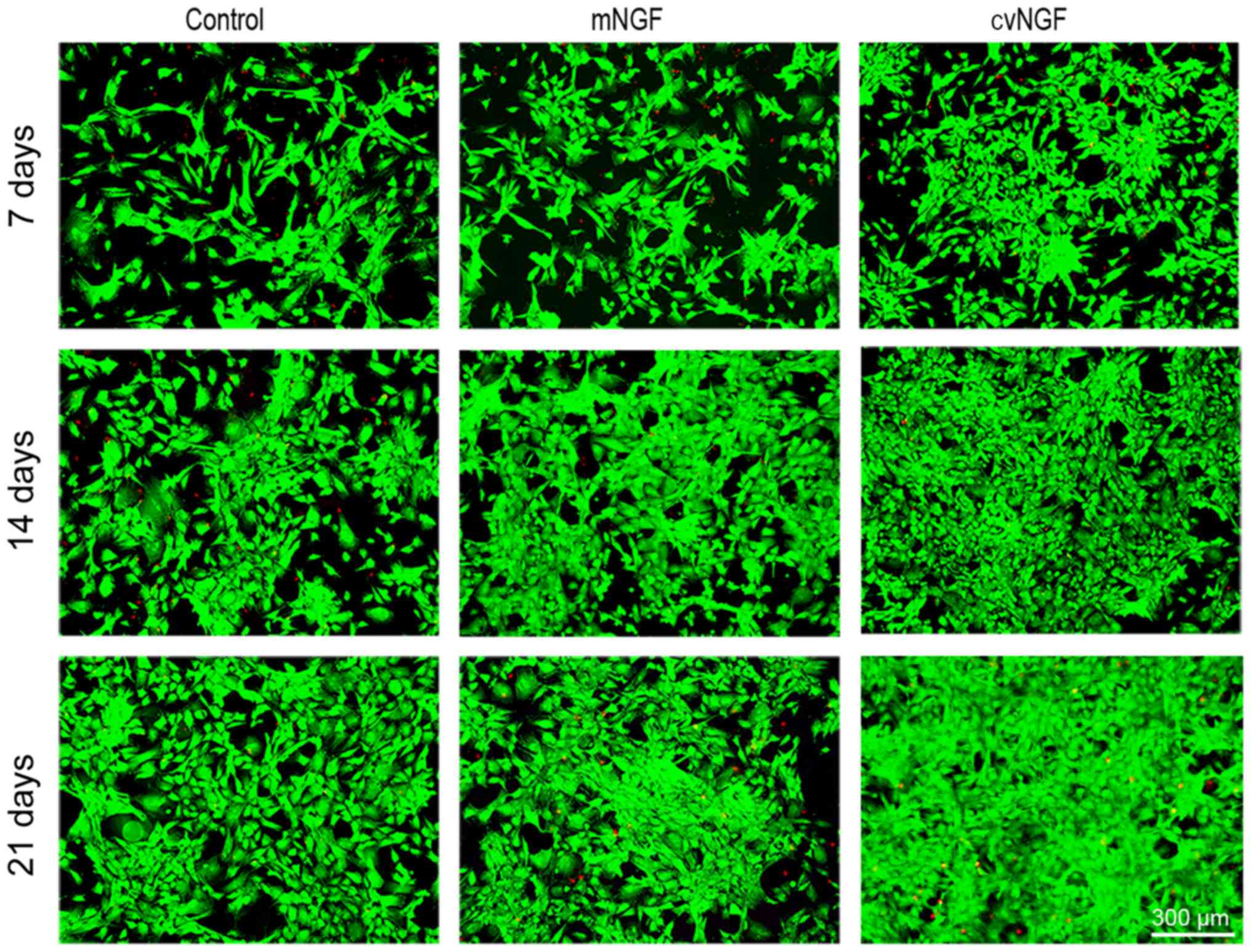
Cell viability
To evaluate the effects of NGF on cell viability, a
FDA/PI staining kit (Fig. 2) was
used. The majority of the cells in all groups were stained green,
which indicated a greater amount of viable cells. More viable cells
and fewer apoptotic cells (stained red) were detected in the cvNGF
group compared with the other groups.
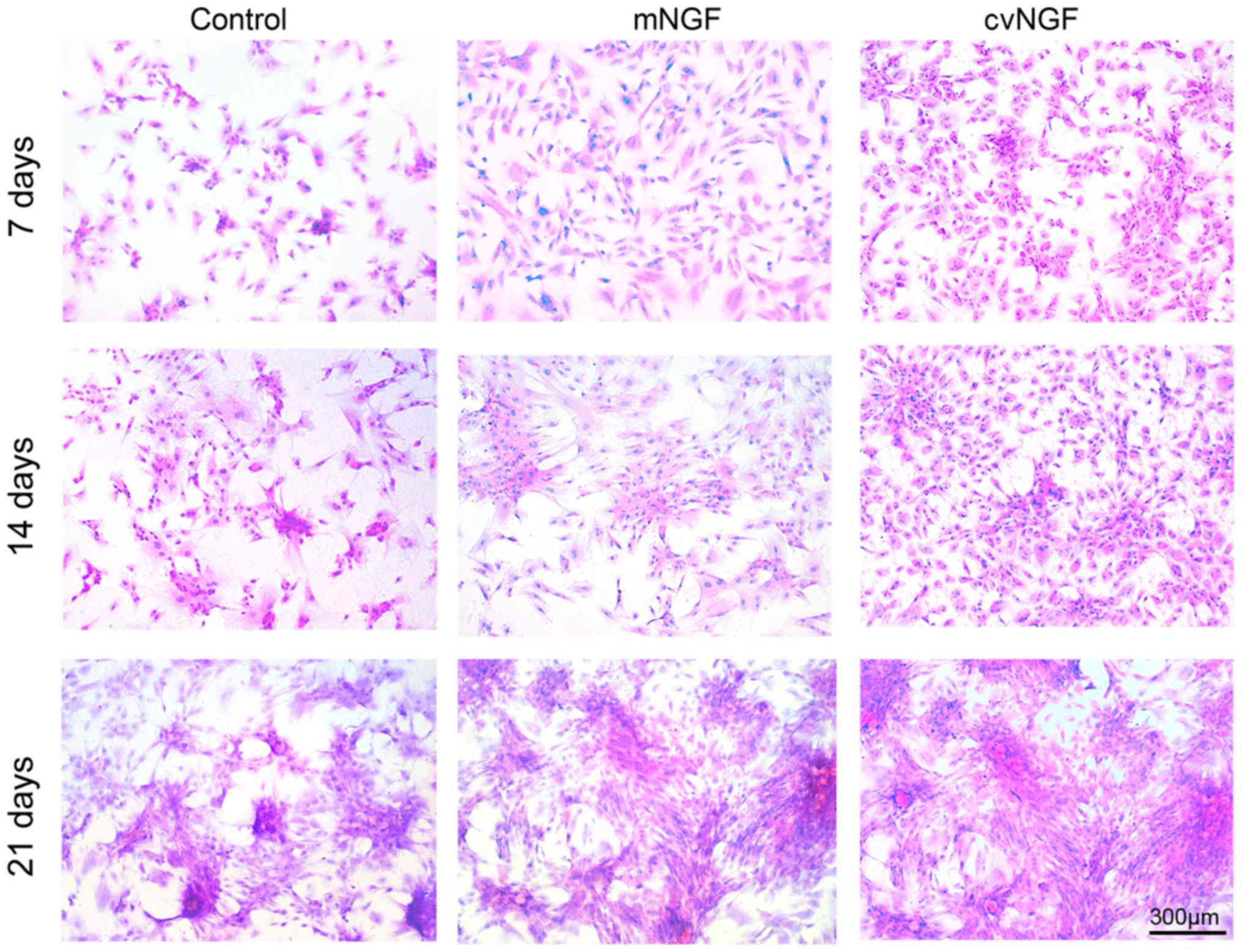
Cell morphology
Morphological alterations in BMSCs were detected
following treatment with NGF for 7, 14 and 21 days using H&E
staining. As shown in Fig. 3, more
cells displayed chondrocyte-like morphology in the cvNGF group at
the same culture period compared with the control and mNGF groups.
In addition, more cell colonies were observed in the cv-NGF
group.
Cell proliferation
The proliferation of NGF-treated BMSCs was analyzed
according to DNA content. The DNA content in all groups was
increased in a time-dependent manner (Fig. 4A). The growth rate of BMSCs was 126
and 59% higher in the cvNGF and mNGF groups at day 21,
respectively, compared with in the control group, as evidenced by
the significantly higher DNA content. In particular, cvNGF promoted
cell growth compared with the other two groups; these findings were
consistent with the results of the cell viability assay (Fig. 2) and H&E staining (Fig. 3).
 | Figure 4.Quantification of cell proliferation,
as detected by (A) DNA content and (B) matrix production by GAG.
(A) Proliferation of bone-derived mesenchymal stem cells cultured
alone (control) or with NGFs (mNGF, 0.06 µg/ml; cvNGF, 6 µg/ml) for
7, 14 and 21 days. (B) GAG (mg) normalized to DNA (mg). Data from
four independent experiments were evaluated, and are presented as
the means ± standard deviation. *P<0.05 vs. the control group;
#P<0.05, as indicated. cvNGF, cobra-venom-derived
NGF; GAG, glycosaminoglycan; mNGF, murine β-NGF; NGF, nerve growth
factor. |
GAG secretion
GAG deposition in BMSCs treated with NGFs for 7, 14
and 21 days was measured by DMMB assay and Safranin O staining. As
shown in Fig. 4B, GAG production
was gradually increased in a time-dependent manner. GAG synthesis
in NGF-treated BMSCs was significantly higher compared with in the
control group at the same timepoint. Compared with the control
group, cvNGF promoted GAG synthesis most effectively among all
groups with an increase of 60% at day 21. Safranin O staining also
detected intense staining in the NGF groups compared with in the
control group (Fig. 5). In
addition, more abundant and homogeneously distributed GAG was
detected in BMSCs in the cvNGF group.
Production of collagen type I and type
II
Immunohistochemical staining was used to detect the
expression of type I and type II collagen in BMSCs following
treatment with NGFs in vitro (Fig. 6). Strong type II collagen-positive
staining (Fig. 6B) and weaker type
I collagen staining (Fig. 6A) were
detected in the NGF groups after 7, 14 and 21 days of culturing,
particularly in the cvNGF group.
Gene expression
The mRNA expression levels of Acan, Sox9, Col2a1,
Col1a1, RUNX2 and ENO2 were detected by RT-qPCR. As
shown in Fig. 7,
cartilage-specific genes, Acan, Sox9 and Col2a1, were
significantly upregulated by cvNGF compared with the control and
mNGF groups. Conversely, Col1a1 was significantly
downregulated in the cvNGF group. The expression levels of
RUNX2, a specific gene associated with hypertrophy and
osteogenic differentiation, were similar to those of Col1a1.
Furthermore, ENO2, a specific gene marker for neural
differentiation, was not induced by NGF, as evidenced by reduced
expression compared with in the control group.
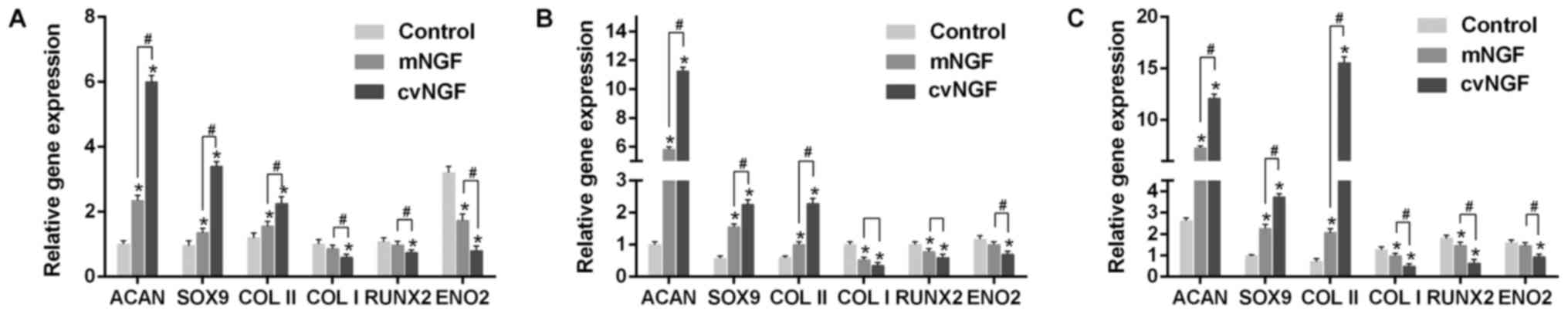 | Figure 7.Quantitative comparison of Acan,
Sox9, Col2a1, Col1a1, RUNX2 and ENO2 mRNA
expression, as detected by reverse transcription-quantitative
polymerase chain reaction. Bone-derived mesenchymal stem cells
cultured alone (control) or with NGFs (mNGF, 0.06 µg/ml; cvNGF, 6
µg/ml) for (A) 7, (B) 14 and (C) 21 days. Data are presented as the
means ± standard deviation from 3 repeated experiments. The mRNA
expression levels were analyzed using the 2−ΔΔCq method,
with β-actin as the internal control. *P<0.05 vs. the control
group; #P<0.05, as indicated. Acan, aggrecan;
Col2a1, collagen type II; Col1a1, collagen type I;
cvNGF, cobra-venom-derived NGF; ENO2, enolase 2; mNGF,
murine β-NGF; NGF, nerve growth factor; RUNX2, runt-related
transcription factor 2; Sox9, SRY-box 9. |
Discussion
Articular cartilage repair remains a huge challenge
for researchers and clinicians. MSC-based therapies have been
employed to tackle these obstacles, due to their high proliferation
rate, easy availability and capacity to differentiate into numerous
cell types (18). Growth factors
are involved in MSC-based therapy. In our previous study, NGF was
extracted from Chinese cobra venom using an improved three-step
chromatography method, and its specific chondrogenesis-promoting
effects on BMSCs were verified (8). Furthermore, cvNGF has been reported
to exhibit higher bioactivity compared with other natural sources
of NGF (15,16).
The present study focused on comparing the
chondrogenic effects of NGF from two sources, commercially
purchased recombinant mNGF and extracted cvNGF, on BMSCs. The
results of this study verified that cvNGF greatly accelerated the
survival and proliferation of BMSCs compared with the control and
mNGF groups, as evidenced by the results of cell viability,
morphological and proliferation analyses.
The results of an RT-qPCR analysis demonstrated that
the expression levels of Acan, Sox9 and Col2a1, which
are cartilage specific markers (19,20),
were significantly upregulated in the cvNGF group compared with in
the other two groups. SOX9 has been reported to act as a
chondrogenic transcription factor and is a marker of early
cartilage formation that has an important role in the process of
chondrogenisis though promoting the production of Acan and
activating co-expression with collagen type II (21,22).
Consistent with the upregulation of cartilage-specific genes, the
deposition of GAG, which serves a pivotal role in maintaining
cartilage load-bearing capacity (23), was markedly enhanced by cvNGF, as
indicated in the results of a biochemical assay and safranin O
staining. In addition, more collagen type II-positive staining was
detected in the cvNGF group compared with in the other two groups.
These results suggested that cvNGF may accelerate chondrocyte
proliferation and stimulate cartilage matrix secretion via
regulating the key activators of the chondrocyte-specific enhancer,
including Acan, Sox9 and Col2a1.
Collagen type I is a marker of fibrocartilage
(24), which was downregulated in
response to treatment with cvNGF, as evidenced by the results of
PCR and immunohistochemistry. When type II collagen and
cartilage-specific proteoglycan are lost and replaced by a complex
collagen phenotype, which is predominately associated with type I
collagen and a low level of proteoglycan synthesis,
dedifferentiation of chondrocytes ensues. The present study
indicated that cvNGF could maintain a phenotype of induced
chondrocytes, which was in accord with our previous study that
revealed cvNGF protected chondrocytes from differentiation in
vitro. Furthermore, RUNX2, a transcription factor for
chondrocyte terminal differentiation (25), was also downregulated in the cvNGF
group. ENO2, which has a role in neural differentiation, was
also suppressed by cvNGF (26)
compared with in the mNGF and control groups, thus indicating that
cvNGF did not induce BMSCs neural differentiation.
The present results indicated that 6 µg/ml cvNGF and
0.06 µg/ml mNGF resulted in increased cell proliferation. Although
the effective concentration of mNGF was lower than that of cvNGF,
the chondrogenic effects of cvNGF on BMSCs were superior to mNGF.
Furthermore, the high cost and sophisticated manufacturing process
of mNGF limits its clinical application. Conversely, cvNGF is
separated from natural venom, which is easily accessed and low
cost, and is therefore conducive to clinical use. The present
results suggested that cvNGF may be a promising growth factor for
cartilage reconstruction.
In conclusion, the present study compared the
chondrogenic effects of NGF from two sources, the commercially
purchased mNGF and the extracted cvNGF, on BMSCs in vitro.
The results suggested that cvNGF was more effective at inducing
chondrogenic differentiation of BMSCs compared with mNGF. The easy
acquirement and low cost of cvNGF indicated that it may be
considered a favorable growth factor for cartilage reconstruction
and beneficial for clinical applications. However, the present
study conducted only in vitro experiments; therefore, these
findings need to be further confirmed in vivo.
Acknowledgements
Not applicable
Funding
The National Natural Science Fund of China (grant
nos. 81760326 and 81460345), the Guangxi Science and Technology
Major Project (grant no. Guike AA17204085), the Distinguished Young
Scholars Program of Guangxi Medical University and the Key
Scientific Research Collaboration Program of Guangxi Biomedical
Collaborative Innovation Center (grant no. GCICB-SR-2017002), the
2018 Basic Ability Improvement Project for Middle-aged and Young
Teachers in Colleges and Universities in Guangxi and the 2017 Young
Creative Talents Training Plan of Guangxi Collaborative Innovation
Center of Biomedicine (grant no. GCICB-TC-2017005).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LZ and JZ designed the study and directed its
structure. ZM and ZL performed the experiments and contributed to
the writing and revision of the manuscript. SL contributed to the
revision of the manuscript, performed the PCR and processed the
data. DL, YH and HW were responsible for the statistical
analysis.
Ethics approval and consent to
participate
All experiments were conducted in accordance with
the standard guidelines approved by the Animal Care and Experiment
Committee of Guangxi Medical University (Guangxi, China; protocol
number: 2014-12-3), and the present study was approved by the
ethics committee of Guangxi Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Risbud MV and Sittinger M: Tissue
engineering: Advances in in vitro cartilage generation. Trends
Biotechnol. 20:351–356. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu YN, Yang Z, Hui JH, Ouyang HW and Lee
EH: Cartilaginous ECM component-modification of the micro-bead
culture system for chondrogenic differentiation of mesenchymal stem
cells. Biomaterials. 28:4056–4067. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakasa T and Ochi M: Cell based therapy
for articular cartilage injury. Clin Calcium. 21:890–895. 2011.(In
Japanese). PubMed/NCBI
|
|
4
|
Caldwell KL and Wang J: Cell-based
articular cartilage repair: The link between development and
regeneration. Osteoarthritis Cartilage. 23:351–362. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang T, Xu G, Wang Q, Yang L, Zheng L,
Zhao J and Zhang X: In vitro expansion impaired the stemness of
early passage mesenchymal stem cells for treatment of cartilage
defects. Cell Death Dis. 8:e28512017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Szychlinska MA, Stoddart MJ, D'Amora U,
Ambrosio L, Alini M and Musumeci G: Mesenchymal stem cell-based
cartilage regeneration approach and cell senescence: Can we
manipulate cell aging and function? Tissue Eng Part B Rev.
23:529–539. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Raghunath J, Salacinski HJ, Sales KM,
Butler PE and Seifalian AM: Advancing cartilage tissue engineering:
The application of stem cell technology. Curr Opin Biotechnol.
16:503–509. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu Z, Lei D, Jiang T, Yang L, Zheng L and
Zhao J: Nerve growth factor from Chinese cobra venom stimulates
chondrogenic differentiation of mesenchymal stem cells. Cell Death
Dis. 8:e28012017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Beuningen HM, Glansbeek HL, van der
Kraan PM and van den Berg WB: Differential effects of local
application of BMP-2 or TGF-beta 1 on both articular cartilage
composition and osteophyte formation. Osteoarthritis Cartilage.
6:306–317. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bakker AC, van de Loo FA, van Beuningen
HM, Sime P, van Lent PL, van der Kraan PM, Richards CD and van den
Berg WB: Overexpression of active TGF-beta-1 in the murine knee
joint: Evidence for synovial-layer-dependent chondro-osteophyte
formation. Osteoarthritis Cartilage. 9:128–136. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Davidson Blaney EN, Vitters EL, van
Beuningen HM, van de Loo FA, van den Berg WB and van der Kraan PM:
Resemblance of osteophytes in experimental osteoarthritis to
transforming growth factor beta-induced osteophytes: Limited role
of bone morphogenetic protein in early osteoarthritic osteophyte
formation. Arthritis Rheum. 56:4065–4073. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li C, Jiang J, Zheng Z, Lee KS, Zhou Y,
Chen E, Culiat CT, Qiao Y, Chen X, Ting K, et al: Neural EGFL-like
1 is a downstream regulator of runt-related transcription factor 2
in chondrogenic differentiation and maturation. Am J Pathol.
187:963–972. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grässel SG: The role of peripheral nerve
fibers and their neurotransmitters in cartilage and bone physiology
and pathophysiology. Arthritis Res Ther. 16:4852014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nojiri Y, Takeda S, Enomoto M, Nishitsuji
H, Masuda T, Sotome S and Shinomiya K: Co-overexpression of GDNF
and GFRalpha1 induces neural differentiation in neural progenitor
cells in comparison to bone marrow stromal cells. J Med Dent Sci.
55:121–128. 2008.PubMed/NCBI
|
|
15
|
Lipps BV: Detection of nerve growth factor
(NGF) in venoms from diverse source: Isolation and characterization
of NGF from the venom of honey bee (Apis melifera). J Nat Toxins.
9:13–19. 2000.PubMed/NCBI
|
|
16
|
Lipps BV: Isolation of nerve growth factor
(NGF) from human body fluids; saliva, serum and urine: Comparison
between cobra venom and cobra serum NGF. J Nat Toxins. 9:349–356.
2000.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Caplan AI: Adult mesenchymal stem cells
for tissue engineering versus regenerative medicine. J Cell
Physiol. 213:341–347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tew SR, Li Y, Pothacharoen P, Tweats LM,
Hawkins RE and Hardingham TE: Retroviral transduction with SOX9
enhances re-expression of the chondrocyte phenotype in passaged
osteoarthritic human articular chondrocytes. Osteoarthritis
Cartilage. 13:80–89. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Uebersax L, Merkle HP and Meinel L:
Insulin-like growth factor I releasing silk fibroin scaffolds
induce chondrogenic differentiation of human mesenchymal stem
cells. J Control Release. 127:12–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marshall OJ and Harley VR: Molecular
mechanisms of SOX9 action. Mol Genet Metab. 71:455–462. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tew SR and Clegg PD: Analysis of post
transcriptional regulation of SOX9 mRNA during in vitro
chondrogenesis. Tissue Eng Part A. 17:1801–1807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Robinson D, Ash H, Yayon A, Nevo Z and
Aviezer D: Characteristics of cartilage biopsies used for
autologous chondrocytes transplantation. Cell Transplant.
10:203–208. 2001.PubMed/NCBI
|
|
24
|
Zhang W, Chen J, Tao J, Jiang Y, Hu C,
Huang L, Ji J and Ouyang HW: The use of type 1 collagen scaffold
containing stromal cell-derived factor-1 to create a matrix
environment conducive to partial-thickness cartilage defects
repair. Biomaterials. 34:713–723. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu CF, Samsa WE, Zhou G and Lefebvre V:
Transcriptional control of chondrocyte specification and
differentiation. Semin Cell Dev Biol. 62:34–49. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoshii SR, Kuma A and Mizushima N:
Transgenic rescue of Atg5-null mice from neonatal lethality with
neuron-specific expression of ATG5: Systemic analysis of adult
Atg5-deficient mice. Autophagy. 13:763–764. 2017. View Article : Google Scholar : PubMed/NCBI
|