Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common cause of cancer-associated mortality worldwide (1). It is a disease with a dismal outcome,
and the 5-year overall survival rate is <5% (2). Patients at the highest risk for
developing HCC are those with chronic liver disease. The
geographical variation in the incidence of HCC is explained by
disparity in the prevalence of the major risk factors, such as
hepatitis B virus (HBV) infection. For instance, the morbidity of
HCC is high in Eastern Asia and sub-Saharan Africa, where HBV
infection is endemic (3). There is
a range of diagnostic criteria for HCC, including index tumor
detection, staging of intrahepatic tumor and assessment of
extrahepatic metastasis (4).
However, there is still a lack of convenient and reliable tumor
markers in the diagnosis of HCC. α-fetoprotein (AFP) expression is
the most widely used tumor marker worldwide (5). An AFP level >200 ng/ml in patients
with cirrhosis has a very high positive predictive value for HCC
(6). Conversely, up to 40% of HCC
tissues never produce AFP, meaning that low AFP levels do not
exclude HCC (7). The lack of
knowledge regarding the molecular mechanisms of the development of
HCC lead to delayed diagnosis and a high probability of relapse
following treatment (8,9). Therefore, the identification of
reliable diagnostic markers for HCC is urgently required.
Long non-coding RNAs (lncRNAs) are molecules >200
bp long that do not encode protein products (10). Most of the currently known lncRNAs
exert their function by participating in the regulation of a broad
range of cellular processes at the epigenetic, transcriptional and
post-transcriptional levels (11).
A previous study has revealed that lncRNAs are frequently and
aberrantly expressed in various cancers, and may serve potential
roles as oncogenes and tumor suppressors (12). Notably, studies have also reported
that deregulation of lncRNAs has potential significance for cancer
diagnosis. Upregulated lncRNA cancer susceptibility candidate 15 is
associated with poor prognosis in patients with HCC (13). Ubiquitin specific peptidase 16 is
downregulated in HCC and functions as a tumor suppressor in HCC
pathogenesis (14). In addition,
serum UCA1 expression was identified as a noninvasive biomarker for
HCC screening and prognostic prediction (15). Although these studies reported the
crucial role of lncRNAs in the formation, progression and prognosis
of HCC, their expression profile and clinical significance in HCC
remain largely unknown (16).
Over the past few decades, microarray technology has
been widely used to screen genetic alterations at the genome level
(17). By performing lncRNA
microarray analysis, Tang et al (18) demonstrated that lncRNAs
RP11-160H22.5, XLOC_014172 and LOC149086 are upregulated in HCC,
and they may be used as potential predictive biomarkers for
tumorigenesis. Similarly, Cui et al (19) performed microarray analysis and
identified lncRNAs PVT1 and SNHG7, which may be involved in HCC
metastasis. The present study also used a lncRNA microarray assay
to determine the differentially expressed lncRNAs between HCC
tissues and corresponding adjacent normal tissues. The relationship
between aberrant lncRNA expression between tissues and plasma was
also analyzed. Expression levels of the lncRNA small nucleolar RNA
host gene 1 (SNHG1) in HCC tissues exhibited a good correlation
with those in plasma. Emerging evidence revealed that ectopic
expression of SNHG1 functions as an oncogene in various cancers,
including breast and lung cancer (20,21).
A recent study also identified that SNHG1 was upregulated in HCC
cells, and promoted HCC cells proliferation and cycle progression
(22). Moreover, although higher
SNHG1 expression in HCC tissues indicated a poorer prognosis
(22), circulating SNHG1 levels in
plasma in patients with HCC and its diagnostic properties remain
unclear. The present study aimed to investigate whether plasma
SNHG1 may serve as a biomarker for HCC using receiver operating
characteristic (ROC) curves and to further compare its diagnostic
value with AFP.
Materials and methods
Subjects
A total of 172 individuals were enrolled in the
present study, including 50 age- and sex-matched healthy subjects
(age range, 45–69; Control group), 50 patients with HBV-positive
chronic hepatitis and cirrhosis (age range, 39–73; HCH group) and
72 patients with HCC (age range, 42–71; HCC group) from the Third
Affiliated Hospital of Qiqihar Medical University (Qiqihar, China)
between January 2015 and December 2016. Clinical data were
collected, and the main demographic and clinical characteristics of
the studied subjects are provided in Table I. Blood samples from all subjects
prior to any medical interventions were collected into EDTA
anti-coagulation tubes and processed for plasma extraction within 2
h of collection (centrifuged at 3,000 × g for 10 min at 4°C). Blood
samples following surgery from patients with HCC were also
collected. The plasma was stored at −80°C in polypropylene tubes
for further analysis. Tumor tissues and adjacent normal tissues
from the 72 patients with HCC were collected during surgery at the
Third Affiliated Hospital of Qiqihar Medical University. All
procedures were conducted in accordance with the protocol that was
approved by the Ethics Committee of the Third Affiliated Hospital
of Qiqihar Medical University, and written informed consent was
obtained from each subject. Patients who underwent previous
preoperative chemotherapy or radiotherapy were excluded.
 | Table I.Clinicopathological characteristics of
subjects in the present study. |
Table I.
Clinicopathological characteristics of
subjects in the present study.
| Clinicopathological
features | HCC (n=72) | HCH (n=50) | Control (n=50) | P-value |
|---|
| Age (years) | 51.26±7.31 | 49.23±8.06 | 50.37±7.19 | 0.341 |
| Sex
(male/female) | 57/15 | 39/11 | 35/15 | 0.473 |
| Smoking | 46 | 33 | 27 | 0.408 |
| Alcoholism | 26 | 18 | 15 | 0.701 |
| ALT (U/l) |
70.12±45.72 |
79.56±63.46 |
24.52±10. 94 | <0.001 |
| AST (U/l) |
71.06±50.38 |
63.22±47.19 | 21.33±8.47 | <0.001 |
| Albumin (g/dl) | 37.80±4.66 | 38.25±6.71 | 39.72±5.14 | 0.154 |
| Total bilirubin
(mg/dl) |
23.73±10.16 |
29.57±48.36 | 15.24±4.60 | 0.031 |
| Glucose
(mmol/l) |
5.04±1.21 |
4.98±1.09 |
4.87±1.16 | 0.729 |
| AFP |
2,036.72±418.57 |
44.57±89.33 | 21.51±6.42 | <0.001 |
Biochemical analysis
The levels of alanine transaminase (ALT), aspartate
aminotransferase (AST), albumin, total bilirubin and glucose were
measured using an automated biochemistry analyzer (AU5800; Beckman
Coulter, Inc., Brea, CA, USA). The levels of plasma α-fetoprotein
(AFP) were detected with an enzyme-linked immunosorbent assay kit
(cat. no. XF00419B; Shanghai Xinfan Biotechnology Co., Ltd.,
Shanghai, China) according to the manufacturer's instructions.
Signals were determined by measuring the absorbance at 450 nm using
a spectraMax M series multi-mode microplate reader (Molecular
Devices, San Jose, CA, USA).
RNA isolation and microarray
analysis
Total RNA from six random tumor tissues and adjacent
normal tissues was extracted using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol (23). RNA
quality was confirmed by formaldehyde agarose gel electrophoresis
and quantified by NanoDrop ND-2000 (Thermo Fisher Scientific,
Inc.). RNA was used to synthesize double-stranded cDNA using
SuperScript Double-Stranded cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.), which was stored at −80°C for further use. The
RT reaction was performed at 95°C 15 sec, followed by 30 cycles of
95°C for 5 sec and 60°C for 1 min. cDNA was labeled with Cy3 or Cy5
fluorescent probes (Aksomics, Inc., Shanghai, China) and hybridized
to the Human LncRNA Expression Microarray v3.0 (format, 8×60 K;
Arraystar, Rockville, MD, USA), according to the manufacturer's
protocol. Following washing with a NimbleGen Wash kit (Roche
Diagnostics, Basel, Switzerland), the slides were scanned with an
Agilent Microarray Scanner (Agilent Technologies, Inc., Santa
Clara, CA, USA), and data were analyzed with ImaGene software
(version 9.0; BioDiscovery, Inc., El Segundo, CA, USA) on the
scanner and extracted as paired files. Significant differences were
calculated using the paired Student's t-test. Hierarchical
clustering was conducted to demonstrate the aberrantly expressed
lncRNAs via Cluster-TreeView software (version 3.0; Palo Alto, CA,
USA) (24).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). First strand cDNA was
synthesized from 72 tissue or plasma samples (50 samples in the
control group and HCH group, 72 samples in the HCC group) using a
PrimeScript RT Reagent Kit (Takara Biotechnology Co., Ltd., Dalian,
China), according to the manufacturer's protocol; the reaction was
performed at 16°C for 30 min, 42°C for 30 min and 85°C for 5 min.
qPCR was performed to quantify the expression level of lncRNA with
SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.) and the ABI
Prism 7900HT Fast Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.). PCR was performed at 95°C for 2 min,
followed by 40 cycles at 94°C (15 sec), 60°C (60 sec) and 72°C (30
sec). All experiments were carried out in triplicate. Relative
expression levels were calculated using the 2−ΔΔCq
method (25) and were normalized
to GAPDH. The primers used are provided in Table II.
 | Table II.Forward and reverse primer sequences
for reverse transcription-quantitative polymerase chain
reaction. |
Table II.
Forward and reverse primer sequences
for reverse transcription-quantitative polymerase chain
reaction.
| Gene | Primer sequence
(5′→3′) |
|---|
| BF896662 | F:
TGCACCAGTTCAGAGCCAGAG |
|
| R:
ACAGATGATGGTATGATGAC |
|
GSO_1539211_385 | F:
GATGTTCTTGCTGTGGTGGTT |
|
| R:
ACAGTCTCATCGGCTGATTG |
| ASLNC12707 | F:
CTCGCGAGAGACGACATTCG |
|
| R:
GGTAGGGAATCGAGGAGAATG |
| XLOC_002237 | F:
AGCCACTGGAGAAGTGTCACC |
|
| R:
CATTCGTTGGCCACGTCCATT |
| XLOC_014001 | F:
CTCGGCCAGCATGTCGT |
|
| R:
ATCGCTACACGATGCATACT |
| ASLNC12773 | F:
CTTAGAACACGGTCTAACGACTT |
|
| R:
GTTGCAACTCCTGGTCACCTGC |
| SNHG1 | F:
TAACCTGCTTGGCTCAAAGGG |
|
| R:
CAGCCTGGAGTGAACACAGA |
| BF899728 | F:
CTCAGACTGAGAGACATATCCAGGA |
|
| R:
GGATGTTCATCCGTCTTCCAGCAGC |
| ASLNC16612 | F:
GAAGCGTCGGGAAGTCATC |
|
| R:
GGCTTGCACACGCACTGACA |
| DGCR5 | F:
CACGAGTGTAGTGCCCAGTT |
|
| R:
GGTCAGGGACCTTTGTCGTT |
| GAPDH | F:
CTGGGCTACACTGAGCACC |
|
| R:
AAGTGGTCGTTGAGGGCAATG |
Statistical analysis
Statistical analysis was performed using SPSS 19.0
software (IBM Corp., Armonk, NY, USA) and GraphPad Prism 5.5
(GraphPad Software, Inc., La Jolla, CA, USA). Continuous variables
were expressed as the mean ± standard deviation, and categorical
variables were presented as frequencies. All enrolled subjects were
assigned to high and low SNHG1 groups, according to the mean value
of plasma SNHG1. The differences between SNHG1 expression and
clinical characteristics were analyzed by χ2, and a
paired Student's t-test was used to compare the differences between
plasma SNHG1 expression levels pre- and post-surgery. The
differences in SNHG1, ASLNC12773 and BF896662 expression levels
among the HCC, HCH and Control groups was analyzed by a one-way
analysis of variance, followed by the least significance difference
test. Correlation between plasma and tissue SNHG1 levels was
analyzed by Pearson correlation analysis. ROC curves were
constructed, and the area under the ROC curve (AUC) was generated
to assess the diagnostic performance of SNHG1. P<0.05 was
considered to indicate a statistically significant difference.
Results
SNHG1 expression is higher in tumoral
tissues and plasma of patients with HCC
To determine the lncRNAs profiling in HCC tissues,
lncRNA microarray analysis of six paired HCC and adjacent normal
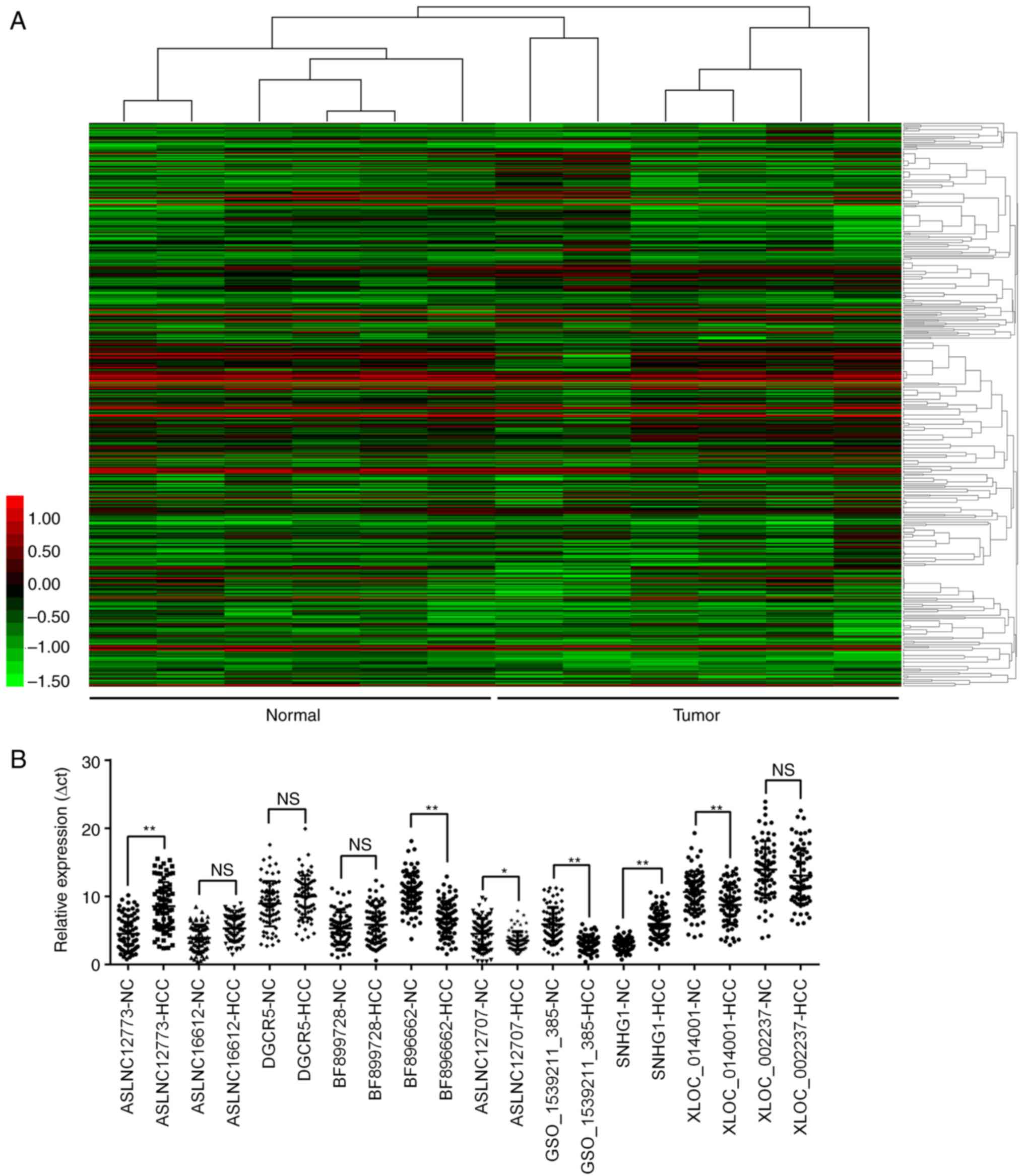
tissues was performed. Hierarchical clustering demonstrated
systematic variations in transcript expression levels between the
six paired tissues (Fig. 1A). A
total of 357 lncRNAs were identified to be differentially
expressed, and the top 5 upregulated and the top 5 downregulated
lncRNAs in HCC are listed in Table
III. These aberrantly expressed lncRNAs were further validated
in all 72 HCC and adjacent normal tissues using RT-qPCR, and the
results demonstrated that four of them demonstrated no significant
difference between the tissues (Fig.
1B). Additionally, the expression levels of six identified
lncRNAs were subsequently investigated. Among these, ASLNC12707,
GSO_1539211_385 and XLOC_014001 were not detectable in the plasma
and were excluded from further study. The relative expression
levels of SNHG1 and ASLNC12773 were significantly higher in
patients with HCH compared with the respective expression levels in
healthy Control patients (Fig. 2A and
B, respectively), but only SNHG1 was expressed at significantly
higher levels in HCC compared with HCH tissue (Fig. 2A). Conversely, the expression level
of BF896662 was lower in patients with HCH compared with healthy
Control individuals (Fig. 2C);
however, no statistically significant difference was identified
between patients with HCC and HCH. A positive correlation was
demonstrated between plasma and HCC tissue SNHG1 expression levels
(r=0.66; Fig. 2D). Owing to its
stability in plasma and its ability to differentiate HCC and HCH,
the plasma levels of SNHG1 were compared pre- and post-surgery
(Fig. 2E). Plasma SNHG1 expression
was notably reduced following surgery compared with expression
levels prior to surgery (Fig. 2E),
which suggested that SNHG1 may have been released from HCC tissues
into the bloodstream.
 | Table III.Top 5 upregulated and top 5
downregulated lncRNAs in hepatocellular carcinoma tissues
identified by microarray analysis. |
Table III.
Top 5 upregulated and top 5
downregulated lncRNAs in hepatocellular carcinoma tissues
identified by microarray analysis.
| Gene ID | Log2(FC) | P-value | Regulation |
|---|
| BF896662 | −2.433374357 | 0.00053002 | Down |
|
GSO_1539211_385 | −2.167898147 | 0.00015589 | Down |
| ASLNC12707 | −1.975377648 | 0.00008775 | Down |
| XLOC_002237 | −1.830784055 | 0.00020238 | Down |
| XLOC_014001 | −1.707527413 | 0.00010118 | Down |
| ASLNC12773 |
3.298459185 | 0.00050628 | Up |
| SNHG1 |
2.845947285 | 0.00023835 | Up |
| BF899728 |
2.541892168 | 0.00004255 | Up |
| ASLNC16612 |
2.503652367 | 0.00040132 | Up |
| DGCR5 |
2.348980639 | 0.00185469 | Up |
Association between SNHG1 expression
and clinicopathological characteristics
The relationship between plasma SNHG1 expression
level and the clinicopathological characteristics of the present
cohort was analyzed (Table IV).
High SNHG1 expression was significantly associated with tumor size
(P=0.047), TNM stage (P=0.012) and AFP level (P=0.024). By
contrast, SNHG1 expression did not demonstrate an association with
other clinical factors, including age, sex, smoking status,
cirrhosis and tumor number (all P>0.05).
 | Table IV.Clinicopathological characteristics
of the patients, divided into two groups according to the plasma
lncRNA SNHG1 levels in HCC. |
Table IV.
Clinicopathological characteristics
of the patients, divided into two groups according to the plasma
lncRNA SNHG1 levels in HCC.
|
|
| Plasma SNHG1
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | Total (n=72) | Low (n=39) | High (n=33) | P-value |
|---|
| Age |
|
|
| 0.605 |
|
<50 | 47 | 27 | 20 |
|
|
≥50 | 25 | 12 | 13 |
|
| Sex |
|
|
| 0.258 |
|
Male | 44 | 21 | 23 |
|
|
Female | 28 | 18 | 10 |
|
| Smoker |
|
|
| 0.401 |
|
Yes | 30 | 14 | 16 |
|
| No | 42 | 25 | 17 |
|
| Cirrhosis |
|
|
| 0.109 |
|
Yes | 33 | 14 | 19 |
|
| No | 39 | 25 | 14 |
|
| Tumor size
(cm) |
|
|
| 0.047 |
|
<5 | 32 | 22 | 10 |
|
| ≥5 | 40 | 17 | 23 |
|
| Tumor number |
|
|
| 0.722 |
|
Single | 61 | 32 | 29 |
|
|
Multiple | 11 | 7 | 4 |
|
| TNM stage |
|
|
| 0.012 |
|
I–II | 49 | 32 | 17 |
|
|
III–IV | 23 | 7 | 16 |
|
| AFP (µg/l) |
|
|
| 0.024 |
|
<200 | 46 | 30 | 16 |
|
|
≥200 | 26 | 9 | 17 |
|
Diagnostic performance of SNHG1 in
patients with HCC
ROC curves were constructed and the AUC was
generated to assess the diagnostic performance of SNHG1 in HCC. ROC
analyses demonstrated that the AUC values of plasma SNHG1 and AFP
were 0.92 [95% confidence interval (CI), 0.86–0.96] and 0.85 (95%
CI, 0.77–0.90), respectively, to distinguish HCC from controls
(Table V; Fig. 3A). The cut-off values of SNHG1 and
AFP were 2.54 and 187.88 µg/l, respectively. When distinguishing
between HCC and HCH, the AUC values of SNHG1 and AFP were 0.74 (95%
CI, 0.65–0.83) and 0.79 (95% CI, 0.71–0.86), respectively (Table V; Fig.
3B). Accordingly, the cut-off values of SNHG1 and AFP were 3.25
and 268.11 µg/l, respectively. In addition, the combination of
SNHG1 and AFP possessed higher AUC values for the discrimination
between HCC patients and controls as compared with SNHG1 alone,
with higher sensitivity (Table V;
Fig. 3C). The combination also
possessed a higher AUC values for discrimination between HCC and
HCH patients with higher specificity (Table V; Fig.
3D). These data indicated that the combination of SNHG1 and AFP
achieved a better diagnostic accuracy than AFP alone.
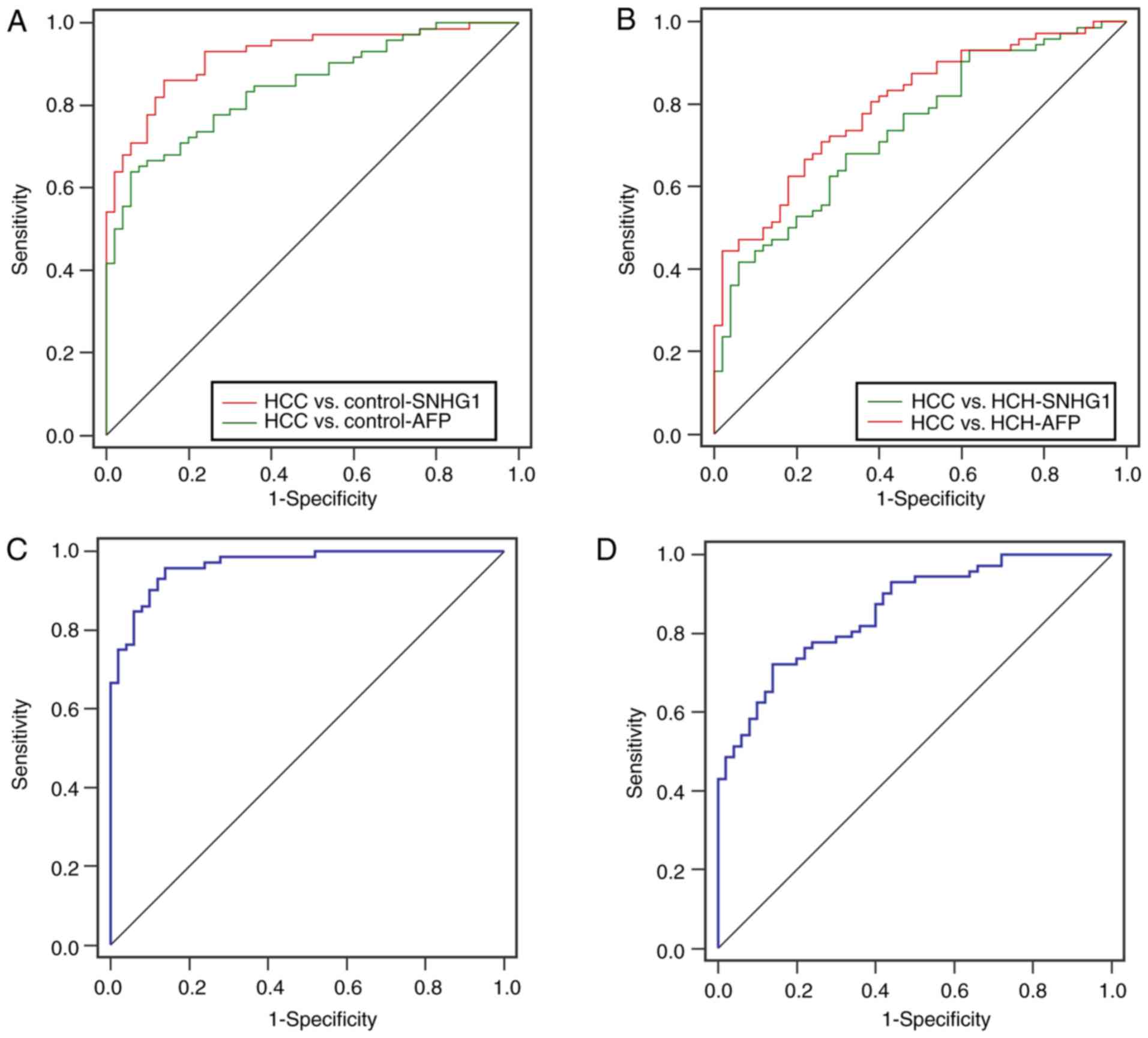 | Figure 3.Diagnostic performance of plasma SNHG1
and AFP. (A) ROC curve analysis of plasma SNHG1 and AFP expression
in differentiating patients with HCC (n=72) from healthy controls
(n=50). SNHG1, AUC = 0.92 (95% CI, 0.86–0.96), cut-off value =
2.54; AFP, AUC = 0.85 (95% CI, 0.77–0.90), cut-off value = 187.88
µg/l. (B) ROC analysis of plasma SNHG1 and AFP expression in
differentiating patients with HCC (n=72) from patients with HCH
(n=50). SNHG1, AUC = 0.74 (95% CI, 0.65–0.83), cut-off value =
3.25; AFP, AUC = 0.79 (95% CI, 0.71–0.86), cut-off value = 268.11
µg/l. (C) ROC analysis of combination of SNHG1 and AFP
differentiate patients with HCC from health Controls. (D) ROC
analysis of the combination of SNHG1 and AFP differentiate patients
with HCC from patients with HCH. AFP, α-fetoprotein; AUC, area
under the ROC curve; CI, confidence interval; HCC, hepatocellular
carcinoma; HCH, hepatitis B virus-positive chronic hepatitis and
cirrhosis; lncRNA, long non-coding RNA; NS, not significant; ROC,
receiver operating characteristic; SNHG1, small nucleolar RNA host
gene 1. |
 | Table V.Receiver operating characteristic
analysis of lncRNA in HCC patients. |
Table V.
Receiver operating characteristic
analysis of lncRNA in HCC patients.
| Group | AUC (95%CI) | P value | Sensitivity
(%) | Specificity
(%) |
|---|
| SHNG1 |
|
|
|
|
| HCC vs.
Control | 0.92
(0.86–0.96) | <0.001 | 87.3 | 86.0 |
| HCC vs.
HCH | 0.74
(0.65–0.83) | <0.001 | 70.1 | 68.2 |
| AFP |
|
|
|
|
| HCC vs.
Control | 0.85
(0.77–0.90) | <0.001 | 64.6 | 94.6 |
| HCC vs.
HCH | 0.79
(0.71–0.86) | <0.001 | 71.6 | 74.0 |
| Combined (SHNG1 +
AFP) |
|
|
|
|
| HCC vs.
Control | 0.97
(0.92–0.99) | <0.001 | 96.4 | 87.0 |
| HCC vs.
HCH | 0.86
(0.78–0.91) | <0.001 | 73.4 | 86.1 |
Discussion
Despite great advances in the diagnosing HCC, many
patients are still diagnosed with HCC at an advanced stage
(26), as techniques, such as
imaging and histology, only work at the late stages (27). To extend the time window for early
diagnosis, additional investigations should be made into
circulating biomarker testing. AFP has been extensively studied as
a biomarker for diagnosis and prognosis in patients with HCC, but
it has low accuracy and a high rate of false positives (28). A number of previous studies have
reported that lncRNAs may be used as biomarkers for predicting
survival and metastasis, or diagnosis, in a number of diseases,
including HCC. For example, the lncRNA differentiation antagonizing
non-protein coding RNA (DANCR) was determined to be higher in the
plasma of patients with HCC compared with DANCR levels in healthy
individuals and non-HCC patients, and subsequent diagnostic
evaluation identified DANCR as a reliable biomarker for HCC
diagnosis (29). Another study
identified circulating lncRNA SPRY4-intronic transcript 1 as a good
diagnostic value for HCC and its diagnostic property was increased
when in combination with AFP (30). In addition, LINC00152,
RP11-160H22.5 and XLOC014172 were reported as fingerprints for the
early identification of HCC (31).
The above findings provided the evidence that lncRNAs may be a
promising diagnostic target for early HCC diagnosis.
In the present study, six aberrantly expressed
lncRNAs in paired HCC tissues and adjacent normal ones were
investigated, through a microarray and RT-qPCR. We The present
study focused on the top five up and downregulated lncRNAs
identified in the collected HCC tissues. Their expression levels
were investigated in the plasma of patients with HCC and two other
groups of patients without HCC to evaluate their diagnostic values.
Apart from those undetectable in the plasma and which demonstrated
no significant differences among groups, only plasma SNHG1
expression was higher in HCC compared with HCH and healthy Control
group plasma. High SNHG1 expression in HCC tissues has been
reported in previous studies, and it was demonstrated to function
as an oncogene in HCC (32), and
the clinical prognostic significance of SNHG1 in HCC has been
demonstrated (22). However, the
expression profile of SNHG1 in plasma and its diagnostic value
remained unclear. Consistent with previous findings, the present
study revealed a significant increase of SNHG1 in HCC tissues, in
comparison with its level in non-tumoral tissues, and plasma SNHG1
expression was positively correlated with SNHG1 expression in
tissues; SNHG1 expression was demonstrated to be decreased
following surgery, which suggested that circulating SNHG1 may have
originated from HCC tissues. These results also demonstrated that
SNHG1 may serve a role in monitoring recurrence following
surgery.
Subsequently, the diagnostic value of SNHG1 was
investigated using ROC analysis. The results indicated that SNHG1
may have an excellent diagnostic ability to differentiate between
patients with HCC and healthy controls, which was better than AFP.
However, when it comes to distinguishing HCC from HCH, SNHG1
demonstrated a moderate diagnostic performance, similar to AFP.
Certain patients with HCC may have developed the disease from HBV
infection, and many may be suffering with chronic hepatitis or
liver cirrhosis (33). Biomarkers
for distinguishing HCC from HCH may be a useful clinical approach.
Therefore, both features were used in the diagnostic procedure. As
expected, the combination of SNHG1 with AFP achieved a better
diagnostic accuracy.
However, the present study has limitations. Firstly,
the sample size was small and these preliminary findings should be
validated in trials with more subjects. Secondly, although SNHG1
was detectable and stable in plasma, the mechanisms underlying its
secretion and transport to the circulation are poorly understood.
Thirdly, the biological functions of SNHG1 in HCC were not
investigated in vitro. However, it has been previously
demonstrated that SNHG1 promotes HCC cell proliferation, cell cycle
progression and inhibited apoptosis in vitro (22,32).
Finally, because the prognostic value of SNHG1 in tissues was
reported by other studies (34–36),
and the present study demonstrated that increased plasma SNHG1 was
correlated with tumor size and TNM stage, it was hypothesized that
plasma SNHG1 may serve as a biomarker for monitoring HCC following
surgery.
In conclusion, the present study data are the first,
to the best of our knowledge, to demonstrate that plasma SNHG1 was
significantly higher in patients with HCC compared with expression
levels in patients with HCH or healthy individuals. Increased
plasma SNHG1 expression may be a valuable biomarker for HCC
diagnosis. However, prospective studies with larger sample sizes
are required to confirm these findings.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Science and
Technology Project of Qiqihar City (grant no. SFGG-201652).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
XX provided the funding and designed the study. YW
and WZ collected the samples from the subjects and performed
reverse transcription-quantitative polymerase chain analysis. SG
and XW performed the microarray analysis. SG and YW performed the
statistical analysis. SG and XX wrote the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Third Affiliated Hospital of Qiqihar Medical
University (Qiqihar, China), and written informed consent was
obtained from each participant prior to enrolment in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
lncRNA
|
long non-coding RNA
|
|
SNHG1
|
small nucleolar RNA host gene 1
|
|
AFP
|
α-fetoprotein
|
|
HBV
|
hepatitis B virus
|
|
HCH
|
HBV-positive chronic hepatitis and
cirrhosis
|
|
ROC
|
receiver operating characteristic
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dhanasekaran R, Limaye A and Cabrera R:
Hepatocellular carcinoma: Current trends in worldwide epidemiology,
risk factors, diagnosis, and therapeutics. Hepat Med. 4:19–37.
2012.PubMed/NCBI
|
|
3
|
Wang CH, Wey KC, Mo LR, Chang KK, Lin RC
and Kuo JJ: Current trends and recent advances in diagnosis,
therapy, and prevention of hepatocellular carcinoma. Asian Pac J
Cancer Prev. 16:3595–3604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arslanoglu A, Seyal AR, Sodagari F, Sahin
A, Miller FH, Salem R and Yaghmai V: Current guidelines for the
diagnosis and management of hepatocellular carcinoma: A comparative
review. AJR Am J Roentgenol. 207:W88–W98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Jing W, Ma W, Liang C, Chai H and
Tu J: Down-regulation of long non-coding RNA GAS5-AS1 and its
prognostic and diagnostic significance in hepatocellular carcinoma.
Cancer Biomark. 2018. View Article : Google Scholar
|
|
6
|
Tateishi R, Yoshida H, Matsuyama Y, Mine
N, Kondo Y and Omata M: Diagnostic accuracy of tumor markers for
hepatocellular carcinoma: A systematic review. Hepatol Int.
2:17–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferenci P, Fried M, Labrecque D, Bruix J,
Sherman M, Omata M, Heathcote J, Piratsivuth T, Kew M, Otegbayo JA,
et al: World gastroenterology organisation guideline.
Hepatocellular carcinoma (HCC): A global perspective. J
Gastrointestin Liver Dis. 19:311–317. 2010.PubMed/NCBI
|
|
8
|
Earl TM and Chapman WC: Conventional
surgical treatment of hepatocellular carcinoma. Clin Liver Dis.
15(353–370): vii–x. 2011.
|
|
9
|
Li L, Lei Q, Zhang S, Kong L and Qin B:
Screening and identification of key biomarkers in hepatocellular
carcinoma: Evidence from bioinformatic analysis. Oncol Rep.
38:2607–2618. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Isin M and Dalay N: LncRNAs and neoplasia.
Clin Chim Acta. 444:280–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He T, Zhang L, Kong Y, Huang Y, Zhang Y,
Zhou D, Zhou X, Yan Y, Zhang L, Lu S, et al: Long non-coding RNA
CASC15 is upregulated in hepatocellular carcinoma and facilitates
hepatocarcinogenesis. Int J Oncol. 51:1722–1730. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng ZK, Pang C, Yang Y, Duan Q, Zhang J
and Liu WC: Serum long noncoding RNA urothelial
carcinoma-associated 1: A novel biomarker for diagnosis and
prognosis of hepatocellular carcinoma. J Int Med Res. 46:348–356.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sui J, Yang X, Qi W, Guo K, Gao Z, Wang L
and Sun D: Long non-coding RNA linc-USP16 functions as a tumour
suppressor in hepatocellular carcinoma by regulating PTEN
expression. Cell Physiol Biochem. 44:1188–1198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hao QQ, Chen GY, Zhang JH, Sheng JH and
Gao Y: Diagnostic value of long noncoding RNAs for hepatocellular
carcinoma: A PRISMA-compliant meta-analysis. Medicine (Baltimore).
96:e74962017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu J, Liu S, Ye F, Shen Y, Tie Y, Zhu J,
Jin Y, Zheng X, Wu Y and Fu H: The long noncoding RNA expression
profile of hepatocellular carcinoma identified by microarray
analysis. PloS One. 9:e1017072014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang J, Jiang R, Deng L, Zhang X, Wang K
and Sun B: Circulation long non-coding RNAs act as biomarkers for
predicting tumorigenesis and metastasis in hepatocellular
carcinoma. Oncotarget. 6:4505–4515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cui H, Zhang Y, Zhang Q, Chen W, Zhao H
and Liang J: A comprehensive genome-wide analysis of long noncoding
RNA expression profile in hepatocellular carcinoma. Cancer Med.
6:2932–2941. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
You J, Fang N, Gu J, Zhang Y, Li X, Zu L
and Zhou Q: Noncoding RNA small nucleolar RNA host gene 1 promote
cell proliferation in nonsmall cell lung cancer. Indian J Cancer. 3
Suppl 51:e99–e102. 2014. View Article : Google Scholar
|
|
21
|
Yu F, Bracken CP, Pillman KA, Lawrence DM,
Goodall GJ, Callen DF and Neilsen PM: p53 Represses the oncogenic
sno-MiR-28 derived from a snoRNA. PloS One. 10:e01291902015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang M, Wang W, Li T, Yu X, Zhu Y, Ding
F, Li D and Yang T: Long noncoding RNA SNHG1 predicts a poor
prognosis and promotes hepatocellular carcinoma tumorigenesis.
Biomed Pharmacother. 80:73–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu M, Xing LQ and Liu YJ: A three-long
noncoding RNA signature as a diagnostic biomarker for
differentiating between triple-negative and non-triple-negative
breast cancers. Medicine (Baltimore). 96:e62222017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Page RD: TreeView: An application to
display phylogenetic trees on personal computers. Comput Appl
Biosci. 12:357–358. 1996.PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chaiteerakij R, Addissie BD and Roberts
LR: Update on biomarkers of hepatocellular carcinoma. Clin
Gastroenterol Hepatol. 13:237–245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Benson AB III, Abrams TA, Ben-Josef E,
Bloomston PM, Botha JF, Clary BM, Covey A, Curley SA, D'Angelica
MI, Davila R, et al: NCCN clinical practice guidelines in oncology:
Hepatobiliary cancers. J Natl Compr Canc Netw. 7:350–391. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kong FY, Xu BY, Du Y, Xu JJ and Chen HY: A
branched electrode based electrochemical platform: Towards new
label-free and reagentless simultaneous detection of two
biomarkers. Chem Commun (Camb). 49:1052–1054. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma X, Wang X, Yang C, Wang Z, Han B, Wu L
and Zhuang L: DANCR acts as a diagnostic biomarker and promotes
tumor growth and metastasis in hepatocellular carcinoma. Anticancer
Res. 36:6389–6398. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jing W, Gao S, Zhu M, Luo P, Jing X, Chai
H and Tu J: Potential diagnostic value of lncRNA SPRY4-IT1 in
hepatocellular carcinoma. Oncol Rep. 36:1085–1092. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yuan W, Sun Y, Liu L, Zhou B, Wang S and
Gu D: Circulating LncRNAs serve as diagnostic markers for
hepatocellular carcinoma. Cell Physiol Biochem. 44:125–132. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang H, Zhou D, Ying M, Chen M, Chen P,
Chen Z and Zhang F: Expression of long non-coding RNA (lncRNA)
small nucleolar RNA host gene 1 (SNHG1) exacerbates hepatocellular
carcinoma through suppressing miR-195. Med Sci Monit. 22:4820–4829.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang SL, Liu LP, Yang S, Liu L, Ren JW,
Fang X, Chen GG and Lai PB: Preoperative serum α-fetoprotein and
prognosis after hepatectomy for hepatocellular carcinoma. Br J
Surg. 103:716–724. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sahu D, Hsu CL, Lin CC, Yang TW, Hsu WM,
Ho SY, Juan HF and Huang HC: Co-expression analysis identifies long
noncoding RNA SNHG1 as a novel predictor for event-free survival in
neuroblastoma. Oncotarget. 7:58022–58037. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang J, Cao L, Wu J and Wang Q: Long
non-coding RNA SNHG1 regulates NOB1 expression by sponging miR-326
and promotes tumorigenesis in osteosarcoma. Int J Oncol. 52:77–88.
2018.PubMed/NCBI
|
|
36
|
Zhang Y, Jin X, Wang Z, Zhang X, Liu S and
Liu G: Downregulation of SNHG1 suppresses cell proliferation and
invasion by regulating notch signaling pathway in esophageal
squamous cell cancer. Cancer Biomark. 21:89–96. 2017. View Article : Google Scholar : PubMed/NCBI
|