Introduction
Ischemic cerebrovascular disease is the most common
cause of cerebrovascular-related mortality (1). Previous studies have demonstrated
that ischemia-reperfusion (I/R)-induced cerebrovascular injury may
result in cerebral infarct, neurological dysfunction and neuronal
cell apoptosis (2,3). It has been widely recognized that
neuronal apoptosis serves important roles in I/R-induced
cerebrovascular injury (4–6). Inhibition of nuclear factor (NF)-κB
was reported to increase neuronal apoptosis, which may represent a
potential therapeutic target for the treatment of neurodegenerative
disorders and diseases (7,8). In addition, previous studies have
revealed that the myeloid differentiation primary response protein
MyD88 (MyD88) signaling pathway is associated with cellular
apoptosis through the regulation of oxidative stress (9,10).
Furthermore, previous studies on the mechanisms of the
toll-interleukin-1 receptor domain-containing adapter molecule 1
(TRIF)-induced NF-κB activation and apoptosis pathways have
suggested that NF-κB activation is important in the process of
apoptosis (11). Therefore, the
present study aimed to investigate whether NF-κB activation induces
neuronal apoptosis via the MyD88/TRIF signaling pathway in a rat
model of I/R-induced cerebrovascular injury.
Simvastatin is a statin drug that is used to
regulate blood cholesterol levels and to prevent the development of
cardiovascular and cerebrovascular diseases, resulting from
decreased 3-hydroxy-3-methylglutaryl coenzyme A reductase activity
(12,13). It has been demonstrated that when
administered for 1 week following cerebral injury, a combination of
simvastatin and atorvastatin improves neurological recovery,
decreases tissue loss and increases neurogenesis (14). Furthermore, systemic simvastatin
was reported to rescue retinal ganglion cells from optic nerve
injury by suppressing NF-κB activation (15). However, it has also been
demonstrated that simvastatin may inhibit the mevalonate cascade to
induce apoptosis in neuronal cells (16,17).
Therefore, the present study investigated the efficacy of
simvastatin to reduce neuronal apoptosis in a rat model of
I/R-induced cerebrovascular injury, as well as intracellular levels
of NF-κB activation in ischemic tissue. In addition, whether
decreased NF-κB expression attenuated brain damage and sustained
improvement in neurological outcomes was investigated. The
molecular mechanism underlying simvastatin-mediated
MyD88/TRIF/NF-κB signaling were investigated using neurons isolated
from cerebrovascular injury model rats.
Materials and methods
Ethics statement
The present study was performed in accordance with
the recommendations outlined in the Guide for the Care and Use of
Laboratory Animals and in accordance with the National Institutes
of Health (Bethesda, MD, USA), and was approved by the Committee on
the Ethics of Affiliated Hospital of Jiujiang University (Jiujiang,
China; 20160214AHJUN3).
Establishment I/R-induced
cerebrovascular injury rat model
Male 6–8 week old Sprague-Dawley rats (n=20; weight,
290–320 g) were purchased from Shanghai SLAC Laboratory Animal Co.,
Ltd. (Shanghai, China). All rats were housed in a
temperature-controlled facility at 23±1°C, a relative humidity of
50±5% and with a 12-h light/dark cycle, with free access to food
and water. The rat model of cerebrovascular injury was established
using a modified I/R method (18);
rats received right middle cerebral artery occlusion for 90 min and
reperfusion by withdrawal of the filament at 37°C during surgery
and post-surgery. I/R model rats were randomly divided into 2
groups (n=6/group) and each received an intravenous injection of
either simvastatin (I/R + simvastatin group; 10 mg/kg/day;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) or the same volume
of PBS (I/R group) (19).
Sham-operated rats received surgery without right middle cerebral
artery occlusion and were used as a control (Sham group; n=6). The
treatments were administered daily for 14 days.
Protein overexpression
Neuronal cells were isolated from mice in the three
experimental groups as previously described (20); cells (1×105 cells/well)
were cultured in 6-well plates until 85% confluence, the medium was
removed and plates were washed three times with PBS. Neuronal cells
were transfected with 100 pmol pLentivirus-NF-κB (pNF-κB),
pLentivirus-MyD88 (pMyD88), pLentivirus-TRIF (pTRIF) or pLentivirus
empty vector (control; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) using Lipofectamine® 2000 (Sigma-Aldrich; Merck
KGaA) for 48 h at 37°C, according to the manufacturer's protocol.
Cells overexpressing NF-κB, MyD88 or TRIF were treated with
simvastatin (2 mg/ml; Sigma-Aldrich; Merck KGaA) for 12 h at 37°C
for further analysis. NF-κB, MyD88 and TRIF mRNA expression levels
were detected by polymerase chain reaction (PCR), as previously
described (21). PCR was performed
using PCR cloning kit (cat. no. K270040; Thermo Fisher Scientific,
Inc.). Total RNA was extracted with a RNAeasy Mini kit (Qiagen
Sciences, Inc., Gaithersburg, MD, USA). cDNAs were synthesized with
ReverTra Ace-a-™ RT kit (Toyobo Life Science, Osaka, Japan) at 42°C
for 2 h. The sequences of the primers were as follows: NF-κB
forward, 5′-TAAGTGGGGCATCAAAGGA-3′ and reverse,
5′-TGGGAAAAGAGCCAAGAGAA-3′; MyD88 forward, 5′-GACCCAGCATTGGGC-3′
and reverse, 5′-TCAGGGCAGGGACAAGGCCTTGGCAAG-3′; TRIF forward,
5′-CTGCTTGGMGACTTCCTGAC-3′ and reverse,
5′-GTGGATGGTSCCGTTACTGAG-3′; β-actin forward,
5′-CGGAGTCAACGGATTTGGTC-3′ and reverse, 5′-AGCCTTCTCCATGGTCGTGA-3′.
The qPCR thermocycling conditions were as follows: 95°C for 2 min,
35 cycles of 95°C for 20 sec, 55.8°C for 20 sec and 72°C for 20
sec, followed by a final extension at 72°C for 5 min. The results
were expressed as the fold difference in expression compared with
the housekeeping gene (β-actin) (22). Subsequent experiments were
performed 72 h following transfection.
Western blot analysis
Neuronal cells were isolated from rats 14 days
following I/R-induced cerebrovascular injury (23). Cells and tissues were lysed at 4°C
for 10 min in mammalian protein extraction reagent (PER) or tissue
PER reagent, respectively (Thermo Fisher Scientific, Inc.). Protein
concentration was determined with a bicinchoninic acid protein
assay kit (Thermo Fisher Scientific, Inc). Protein samples (20 µg)
were separated by 12.5% SDS-PAGE and transferred to nitrocellulose
membranes. The membranes were incubated in blocking buffer (5%
non-fat milk) at 4°C for 12 h. The primary rabbit antibodies used
included: NF-κBp65 (1:1,200; cat. no. ab16502),
phosphorylated-NF-κB (1:1,200; cat. no. ab86299), P53 (1:1,200;
cat. no. ab26), matrix metalloproteinase-9 (MMP-9; 1:1,000; cat.
no. ab54230), caspase-3 (1:1,200; cat. no. ab2171), B-cell lymphoma
2 (Bcl-2; 1:1,000; cat. no. ab692), MyD88 (1:500; cat. no. ab2068),
TRIF (1:500; cat. no. ab13810) and β-actin (1:500; cat. no. ab8226;
all Abcam, Cambridge, UK). The membranes were subsequently
incubated with horseradish peroxidase-conjugated anti-rabbit
immunoglobulin G secondary antibody (1:5,000; cat. no.
172-1033-SDS; Bio-Rad Laboratories, Inc., Hercules, CA, USA) for 12
h at 4°C, and protein bands were detected using an Enhanced
Chemiluminescence assay system (Roche Diagnostics, Basel,
Switzerland). Densitometric quantification of the immunoblot data
was performed using the software of Quantity-One version 1.1
(Bio-Rad Laboratories, Inc.) and protein expression was normalized
to β-actin.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay
Apoptotic neuronal cells in the hippocampus of I/R
model rats were analyzed using the DeadEnd Colorimetric TUNEL
System (Promega Corporation, Madison, WI, USA) according to the
manufacturer's instructions. Cells were fixed with 10%
paraformaldehyde for 30 min at 37°C. Cells were subsequently washed
with PBS and stained with TUNEL reagent for 30 min at 37°C,
followed by DAPI staining for 15 min at 37°C. Cell damage was
indicated by the TUNEL-positive cell number. The cells were
analyzed using an Olympus Bx51 fluorescence microscope (Olympus
Corporation, Tokyo, Japan) in six random fields of view.
Behavioral tests
Three different behavioral tests were performed,
including neurological deficit, forelimb foot-fault and open-field
tests. Neurological deficit score were determined using a scoring
system (24). The forelimb
foot-fault-placing test was used to examine forelimb function, as
described previously (25).
Open-field tests (rearing time and locomotor activity) were
performed to investigate the efficacy of simvastatin administration
on I/R injury, as previously described (26).
Analysis of cerebral water content
(CWC)
Brain tissues were obtained from experimental mice
as previously described (27), and
the brain water content of I/R model rats was determined following
14 days treatment with simvastatin, as previously described
(28). Rat brains were isolated
and divided into two hemispheres. The two hemispheres were weighed
using an electronic analytical balance to obtain the wet weight.
Brain tissues were dried in an electric oven at 100°C for 24 h and
weighed to determine the brain water content using the following
formula: Water content (%)=[(wet weight-dry weight)/wet
weight]x100.
Quantitative analysis of blood-brain
barrier (BBB) permeability
BBB leakage was investigated using previously
described protocol (29), with a
slight modification. The experimental rats (simvastatin or saline
groups; n=4 in each group) were intravenously administered 100 µl
5% Evan's blue 14 days following I/R-induced injury. A total of 2 h
following Evan's blue injection, cardiac perfusion was performed
using 200 ml of saline under deep anesthesia to clear the cerebral
circulation of Evan's blue. The brain was then isolated using a
freezing microtome. Following this, the two hemispheres were
homogenized in 750 µl of N,N-dimethylformamide. Quantitative
analysis of BBB permeability was determined (620 nm and 680 nm)
using ultraviolet spectrophotometer (DR6000; Hach Company,
Loveland, CO, USA) via analysis of Evan's blue content.
Statistical analysis
Data were presented as mean ± standard deviation of
at least three replicated experiments. All data were analyzed using
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). Significant
differences were analyzed using one-way analysis of variance
followed by Tukey's honest significant difference test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Simvastatin improves cerebral water
content and BBB disruption in I/R model rats
To investigate the effects of simvastatin on I/R
injury, a rat model of cerebrovascular injury was established. Rats
in the I/R + simvastatin and Sham-operated groups exhibited a
significantly lower CWC compared with the untreated I/R rats
(Fig. 1A); no significant
difference in CWC was detected between the I/R + simvastatin rats
and the Sham rats. Furthermore, the results demonstrated that
simvastatin treatment significantly increased BBB disruption in
cerebrovascular injury rats compared with the I/R group (Fig. 1B). These results suggested that
simvastatin treatment significantly attenuated CWC and BBB
disruption in I/R injury rats.
Simvastatin improves cognitive
performance in I/R injury rats
The neurological effects of simvastatin in ischemic
rats were also investigated. Treatment with simvastatin
significantly decreased neurological deficit scores compared with
vehicle-treated I/R model rats (Fig.
2A). The results of the foot-fault-placing test revealed that
simvastatin significantly improved functional deficits of the left
forelimb in simvastatin-treated I/R rats compared with
vehicle-treated I/R rats (Fig.
2B). Furthermore, ipsilateral cerebral hemisphere volume and
motor functions were significantly improved in ischemic rats
following administration of simvastatin; I/R + simvastatin rats
exhibited significantly enhanced open-field activities, such as
locomotion and rearing behavior, compared with the vehicle-treated
I/R rats (Fig. 2C and D). These
results suggest that simvastatin exhibited beneficial effects
regarding I/R injury-induced behavioral dysfunction.
Simvastatin decreases I/R
injury-induced neuronal apoptosis
The effects of simvastatin administration on
neuronal apoptosis in I/R model rats were investigated. The results
demonstrated that the percentage of TUNEL-positive neuronal cells
was significantly decreased in I/R + simvastatin rats compared with
I/R rats (Fig. 3A). Simvastatin
treatment significantly increased Bcl-2 and P53 protein expression
levels in I/R rats compared with expression levels in the untreated
I/R group (Fig. 3B). MMP-9 and
caspase-3 expression levels were significantly decreased in I/R +
simvastatin rats compared with untreated I/R rats (Fig. 3C). Furthermore, simvastatin
treatment significantly lowered the I/R-induced NF-κB and
phosphorylated-NF-κB protein expression levels compared with
vehicle-treated I/R rats (Fig 3D).
These results suggested that simvastatin administration decreased
I/R injury-induced neuronal apoptosis.
Simvastatin reduces neuronal apoptosis
via suppression of NF-κB-regulated MyD88/TRIF signaling
pathway
To determine the mechanism of simvastatin-inhibited
neuronal apoptosis, the NF-κB signaling pathway induced by
MyD88/TRIF in cultured neuronal cells was investigated. Cells
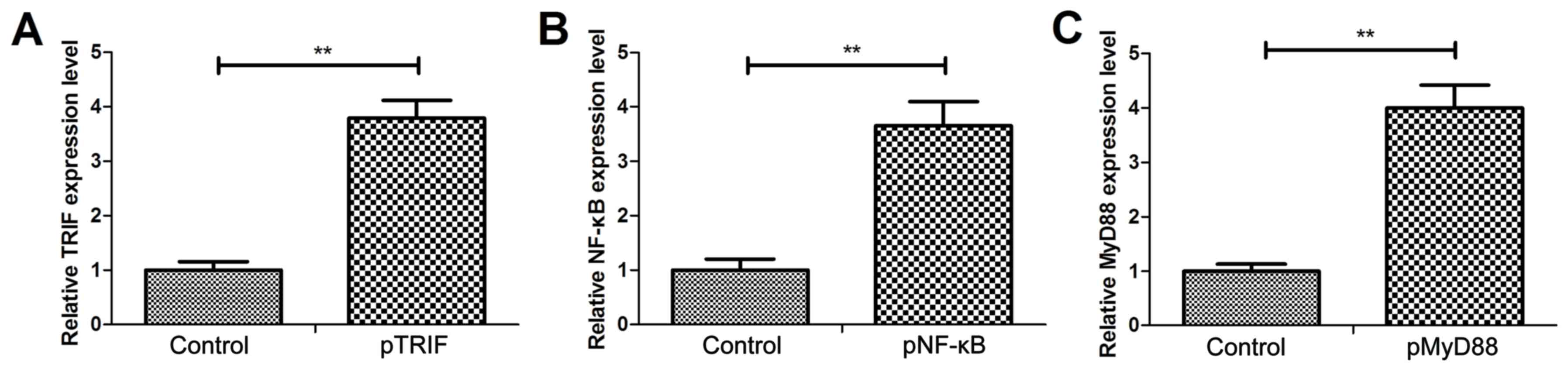
transfected with pTRIF, pNF-κB or pMyD88 were confirmed to exhibit
a significant increase in the levels of TRIF, NF-κB and MyD88 mRNA
expression, respectively, compared with the control cells (Fig. 4A-C). In I/R + simvastatin rat
neuronal cells, MyD88 and TRIF protein expression levels were
significantly reduced compared with untreated I/R rats (Fig. 5A). MyD88 overexpression suppressed
pMyD88-simvastatin-inhibited (pMyD88-SV) TRIF and NF-κB expression
in neuronal cells, compared with the control cells (Fig. 5B). TRIF overexpression also
suppressed pTRIF-SV NF-κB expression in neuronal cells (Fig. 5C). MyD88 or TRIF overexpression
increased neuronal apoptosis and canceled simvastatin-inhibited
neuronal apoptosis compared with control cells (Fig. 5D and E). NF-κB overexpression also
increased neuronal apoptosis and canceled simvastatin-inhibited
neuronal apoptosis compared with control cells (Fig. 5F). These results suggested that
administration of simvastatin suppresses neuronal apoptosis via
NF-κB activation regulated by the MyD88/TRIF signaling pathway.
Discussion
It has previously been demonstrated that simvastatin
exhibits anti-apoptotic effects and increases neuronal excitability
in the hippocampus (30,31). Previous studies have revealed that
the MyD88/TRIF signaling pathway is involved in NF-κB activation,
which may regulate apoptosis in inflammatory injury in
intracerebral hemorrhage-induced neurological deficits (32). In the present study, whether
simvastatin exhibited protective effects to neurons against
apoptosis through the regulation of MyD88/TRIF/NF-κB signaling was
investigated. The results suggested that simvastatin treatment
significantly improved CWC and BBB disruption and attenuated
neuronal apoptosis via suppression of the NF-κB-mediated MyD88/TRIF
signaling pathway in I/R model rats.
A previous study demonstrated that simvastatin may
be a therapeutic agent for treatment against ischemic brain injury,
and the protective effects of simvastatin may be partially due to
its ability to improve microvascular reperfusion. (33). The present study demonstrated that
simvastatin treatment not only improved neurological deficit, but
also improved motor function of I/R rats. HMG CoA reductase
inhibitors were previously reported to attenuate ischemic brain
injury in Wistar rats through the suppression of neuronal oxidative
stress (34). The results of the
present study demonstrated that simvastatin administration
significantly reduced the levels of I/R-induced neuronal apoptosis.
Similarly, Hadi et al (35)
suggested that administration of simvastatin attenuates myocardial
I/R injury in rats via suppression of apoptosis. In the present
study, it was demonstrated that simvastatin decreased the number of
TUNEL-positive neuronal cells in I/R rats compared with
vehicle-treated I/R rats. It was recently reported that a
combinatory treatment of simvastatin with tissue-type plasminogen
activator is safe for patients with acute ischemic stroke (36). The present study demonstrated that
simvastatin reduced neuronal apoptosis through the suppression of
the NF-κB-mediated MyD88/TRIF signaling pathway. Numerous studies
have demonstrated that simvastatin is beneficial in the treatment
of brain injury via different signaling pathways (37–40).
A previous study demonstrated that vascular recovery was enhanced
by administration of simvastatin following experimental cerebral
injury, as revealed by magnetic resonance imaging and histological
investigation (41). The present
study revealed that simvastatin exhibited an important
neuroprotective role against I/R injury-induced neuronal apoptosis
via suppression neuronal apoptosis and attenuation of neurological
dysfunctions. Zhao et al (42) demonstrated that simvastatin
protects human osteosarcoma cells from oxidative stress-induced
apoptosis by downregulating caspase-3 and caspase-9 activation, as
well as upregulating of Bcl-2 expression. Suppression of MMP-9
expression was also reported to reduce neuronal apoptosis following
I/R injury (43). In the present
study, it was revealed that simvastatin treatment may have
suppressed neuronal apoptosis via a NF-κB-mediated decrease in
MMP-9 and caspase-3 expression in I/R rats. The results suggested
that simvastatin may inhibit I/R injury-induced NF-κB expression,
which may contribute to the suppression of neuronal apoptosis.
Inhibition of NF-κB signaling may suppress apoptosis
and promote neuronal differentiation of medulloblastoma cells
(44). Cates et al
(45) demonstrated that MyD88
overexpression stimulates NF-κB activation and apoptosis. Results
of the present study demonstrated that simvastatin downregulated
NF-κB and MyD88 expression in neurons isolated from I/R model rats.
TRIF-induced apoptosis is dependent on NF-κB activity (46). The present study results
demonstrated that simvastatin treatment may suppress I/R-induced
neuronal apoptosis through inhibition of the NF-κB-mediated
MyD88/TRIF signaling pathway, which may have led to the,
improvements in neurological assessment performance and motor
function. These effects were sustained for 14 days post-I/R.
In conclusion, administration of simvastatin
exhibited neuroprotective effects by disrupting the
MyD88/TRIF-mediated NF-κB pathway following I/R-induced
cerebrovascular injury. Therefore, the NF-κB-mediated MyD88/TRIF
signaling pathway may represent a potential therapeutic target for
the treatment of cerebrovascular injury. These results suggested
that simvastatin may represent a novel therapeutic agent for the
prevention and treatment of I/R-induced cerebrovascular injury.
Acknowledgements
Not applicable.
Funding
This study was supported by Natural Science
Foundation of China 81660209.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZC performed the experiments. YX, BB, XW, ZX, JY and
HN analyzed the data. HN designed the study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the recommendations outlined in the Guide for the Care and Use of
Laboratory Animals and in accordance with the National Institutes
of Health, and was approved by the Committee on the Ethics of
Affiliated Hospital of Jiujiang University (20160214AHJUN3).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ueda P, Cnattingius S, Stephansson O,
Ingelsson E, Ludvigsson JF and Bonamy AK: Cerebrovascular and
ischemic heart disease in young adults born preterm: A
population-based Swedish cohort study. Eur J Epidemiol. 29:253–260.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Su D, Riley J, Armstead WM and Liu R:
Salvinorin A pretreatment preserves cerebrovascular autoregulation
after brain hypoxic/ischemic injury via extracellular
signal-regulated kinase/mitogen-activated protein kinase in
piglets. Anesth Analg. 114:200–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sorrentino E, Diedler J, Kasprowicz M,
Budohoski KP, Haubrich C, Smielewski P, Outtrim JG, Manktelow A,
Hutchinson PJ, Pickard JD, et al: Critical thresholds for
cerebrovascular reactivity after traumatic brain injury. Neurocrit
Care. 16:258–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang S, Yuan Y, Xia W, Li F, Huang Y, Zhou
Y and Guo Y: Neuronal apoptosis and synaptic density in the dentate
gyrus of ischemic rats' response to chronic mild stress and the
effects of Notch signaling. PLoS One. 7:e428282012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Atici A, Bozlu G, Turhan AH, Polat A,
Nayci A, Okuyaz C and Taskinlar H: The role of trapidil on neuronal
apoptosis in neonatal rat model of hypoxic ischemic brain injury.
Early Hum Dev. 84:243–247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao J, Pei DS, Zhang QG and Zhang GY:
Down-regulation Cdc42 attenuates neuronal apoptosis through
inhibiting MLK3/JNK3 cascade during ischemic reperfusion in rat
hippocampus. Cell Signal. 19:831–843. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sompol P, Xu Y, Ittarat W, Daosukho C and
St Clair D: NF-kappaB-associated MnSOD induction protects against
beta-amyloid-induced neuronal apoptosis. J Mol Neurosci.
29:279–288. 2006. View Article : Google Scholar
|
|
8
|
Kaltschmidt B, Uherek M, Wellmann H, Volk
B and Kaltschmidt C: Inhibition of NF-kappaB potentiates amyloid
beta-mediated neuronal apoptosis. Proc Natl Acad Sci USA.
96:9409–9414. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lv J, Jia R, Yang D, Zhu J and Ding G:
Candesartan attenuates Angiotensin II-induced mesangial cell
apoptosis via TLR4/MyD88 pathway. Biochem Biophys Res Commun.
380:81–86. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ha T, Hua F, Li Y, Ma J, Gao X, Kelley J,
Zhao A, Haddad GE, Williams DL, Browder IW, et al: Blockade of
MyD88 attenuates cardiac hypertrophy and decreases cardiac myocyte
apoptosis in pressure overload-induced cardiac hypertrophy in vivo.
Am J Physiol Heart Circ Physiol. 290:H985–H994. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han KJ, Su X, Xu LG, Bin LH, Zhang J and
Shu HB: Mechanisms of the TRIF-induced interferon-stimulated
response element and NF-kappaB activation and apoptosis pathways. J
Biol Chem. 279:15652–15661. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramadan WH and Kabbara WK:
Sitagliptin/Simvastatin: A first combination tablet to treat type 2
diabetes and hypercholesterolemia-a review of its characteristics.
Vasc Health Risk Manag. 11:125–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Taveira-DaSilva AM, Jones AM,
Julien-Williams PA, Stylianou M and Moss J: Retrospective review of
combined sirolimus and simvastatin therapy in
lymphangioleiomyomatosis. Chest. 147:180–187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karki K, Knight RA, Han Y, Yang D, Zhang
J, Ledbetter KA, Chopp M and Seyfried DM: Simvastatin and
atorvastatin improve neurological outcome after experimental
intracerebral hemorrhage. Stroke. 40:3384–3389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morishita S, Oku H, Horie T, Tonari M,
Kida T, Okubo A, Sugiyama T, Takai S, Hara H and Ikeda T: Systemic
simvastatin rescues retinal ganglion cells from optic nerve injury
possibly through suppression of astroglial NF-κB activation. PloS
One. 9:e843872014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park DS, So HS, Lee JH, Park HY, Lee YJ,
Cho JH, Yoon KH, Park C, Yun K and Park R: Simvastatin treatment
induces morphology alterations and apoptosis in murine cochlear
neuronal cells. Acta Otolaryngol. 129:166–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leitmeyer K, Glutz A, Setz C, Wieland L,
Egloff S, Bodmer D and Brand Y: Simvastatin results in a
dose-dependent toxic effect on spiral ganglion neurons in an in
vitro organotypic culture assay. Biomed Res Int. 2016:35803592016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao Y, Pan R, Li S, Luo Y, Yan F, Yin J,
Qi Z, Yan Y, Ji X and Liu KJ: Chelating intracellularly accumulated
zinc decreased ischemic brain injury through reducing neuronal
apoptotic death. Stroke. 45:1139–1147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Q, Zengin A, Deng C, Li Y, Newell KA,
Yang GY, Lu Y, Wilder-Smith EP, Zhao H and Huang XF: High dose of
simvastatin induces hyperlocomotive and anxiolytic-like activities:
The association with the up-regulation of NMDA receptor binding in
the rat brain. Exp Neurol. 216:132–138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Walker TL and Kempermann G: One mouse, two
cultures: Isolation and culture of adult neural stem cells from the
two neurogenic zones of individual mice. J Vis Exp.
25:e512252014.
|
|
21
|
Wu X, Gowda NM, Kawasawa YI and Gowda DC:
A malaria protein factor induces IL-4 production by dendritic cells
via PI3K-Akt-NF-κB signaling independent of MyD88/TRIF and promotes
Th2 response. J Biol Chem. 293:10425–10434. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park JS, Kim S, Han DK, Lee JY and Ghil
SH: Isolation of neural precursor cells from skeletal muscle
tissues and their differentiation into neuron-like cells. Exp Mol
Med. 39:483–490. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meyrat BJ, Tercier S, Lutz N, Rilliet B,
Forcada-Guex M and Vernet O: Introduction of a urodynamic score to
detect pre- and postoperative neurological deficits in children
with a primary tethered cord. Childs Nerv Syst. 19:716–721. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McBride DW, Nowrangi D, Kaur H, Wu G,
Huang L, Lekic T, Tang J and Zhang JH: A composite neurobehavioral
test to evaluate acute functional deficits after cerebellar
haemorrhage in rats. J Cereb Blood Flow Metab. 38:433–446. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gamberini MT, Rodrigues DS, Rodrigues D
and Pontes VB: Effects of the aqueous extract of Pimpinella anisum
L. seeds on exploratory activity and emotional behavior in rats
using the open field and elevated plus maze tests. J
Ethnopharmacol. 168:45–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Annunziata I, Patterson A and d'Azzo A:
Isolation of mitochondria-associated ER membranes (MAMs) and
glycosphingolipid-enriched microdomains (GEMs) from brain tissues
and neuronal cells. Methods Mol Biol. 1264:25–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hijioka M, Matsushita H, Hisatsune A,
Isohama Y and Katsuki H: Therapeutic effect of nicotine in a mouse
model of intracerebral hemorrhage. J Pharmacol Exp Ther.
338:741–749. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tong LS, Shao AW, Ou YB, Guo ZN, Manaenko
A, Dixon BJ, Tang J, Lou M and Zhang JH: Recombinant Gas6 augments
Axl and facilitates immune restoration in an intracerebral
hemorrhage mouse model. J Cereb Blood Flow Metab. 37:1971–1981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang G, Li M, Xu Y, Peng L, Yang C, Zhou
Y and Zhang J: Antioxidation effect of simvastatin in aorta and
hippocampus: A rabbit model fed high-cholesterol diet. Oxid Med
Cell Longev. 2016:69293062016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Metais C, Hughes B and Herron CE:
Simvastatin increases excitability in the hippocampus via a PI3
kinase-dependent mechanism. Neuroscience. 291:279–288. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin S, Yin Q, Zhong Q, Lv FL, Zhou Y, Li
JQ, Wang JZ, Su BY and Yang QW: Heme activates TLR4-mediated
inflammatory injury via MyD88/TRIF signaling pathway in
intracerebral hemorrhage. J Neuroinflammation. 9:462012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shabanzadeh AP, Shuaib A and Wang CX:
Simvastatin reduced ischemic brain injury and perfusion deficits in
an embolic model of stroke. Brain Res. 1042:1–5. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hayashi T, Hamakawa K, Nagotani S, Jin G,
Li F, Deguchi K, Sehara Y, Zhang H, Nagano I, Shoji M and Abe K:
HMG CoA reductase inhibitors reduce ischemic brain injury of Wistar
rats through decreasing oxidative stress on neurons. Brain Res.
1037:52–58. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hadi NR, Al-Amran F, Yousif M and Zamil
ST: Antiapoptotic effect of simvastatin ameliorates myocardial
ischemia/reperfusion injury. ISRN Pharmacol. 2013:8150942013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Montaner J, Bustamante A, Garcia-Matas S,
Martínez-Zabaleta M, Jiménez C, de la Torre J, Rubio FR, Segura T,
Masjuán J, Cánovas D, et al: Combination of thrombolysis and
statins in acute stroke is safe: Results of the STARS randomized
trial (stroke treatment with acute reperfusion and simvastatin).
Stroke. 47:2870–2873. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen G, Zhang S, Shi J, Ai J, Qi M and
Hang C: Simvastatin reduces secondary brain injury caused by
cortical contusion in rats: Possible involvement of TLR4/NF-kappaB
pathway. Exp Neurol. 216:398–406. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu H, Mahmood A, Lu D, Jiang H, Xiong Y,
Zhou D and Chopp M: Attenuation of astrogliosis and modulation of
endothelial growth factor receptor in lipid rafts by simvastatin
after traumatic brain injury. J Neurosurg. 113:591–597. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang H, Lynch JR, Song P, Yang HJ, Yates
RB, Mace B, Warner DS, Guyton JR and Laskowitz DT: Simvastatin and
atorvastatin improve behavioral outcome, reduce hippocampal
degeneration, and improve cerebral blood flow after experimental
traumatic brain injury. Exp Neurol. 206:59–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Balduini W, De Angelis V, Mazzoni E and
Cimino M: Simvastatin protects against long-lasting behavioral and
morphological consequences of neonatal hypoxic/ischemic brain
injury. Stroke. 32:2185–2191. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang D, Knight RA, Han Y, Karki K, Zhang
J, Ding C, Chopp M and Seyfried DM: Vascular recovery promoted by
atorvastatin and simvastatin after experimental intracerebral
hemorrhage: Magnetic resonance imaging and histological study. J
Neurosurg. 114:1135–1142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao XH, Xu ZR, Zhang Q and Yang YM:
Simvastatin protects human osteosarcoma cells from oxidative
stress-induced apoptosis through mitochondrial-mediated signaling.
Mol Med Rep. 5:483–488. 2012.PubMed/NCBI
|
|
43
|
Chaudhry K, Rogers R, Guo M, Lai Q, Goel
G, Liebelt B, Ji X, Curry A, Carranza A, Jimenez DF and Ding Y:
Matrix metalloproteinase-9 (MMP-9) expression and extracellular
signal-regulated kinase 1 and 2 (ERK1/2) activation in
exercise-reduced neuronal apoptosis after stroke. Neurosci Lett.
474:109–114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wen S, Li H, Wu ML, Fan SH, Wang Q, Shu
XH, Kong QY, Chen XY and Liu J: Inhibition of NF-κB signaling
commits resveratrol-treated medulloblastoma cells to apoptosis
without neuronal differentiation. J Neurooncol. 104:169–177. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cates EA, Connor EE, Mosser DM and
Bannerman DD: Functional characterization of bovine TIRAP and MyD88
in mediating bacterial lipopolysaccharide-induced endothelial
NF-kappaB activation and apoptosis. Comp Immunol Microbiol Infect
Dis. 32:477–490. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kaiser WJ and Offermann MK: Apoptosis
induced by the toll-like receptor adaptor TRIF is dependent on its
receptor interacting protein homotypic interaction motif. J
Immunol. 174:4942–4952. 2005. View Article : Google Scholar : PubMed/NCBI
|