Introduction
Cisplatin is a highly effective chemotherapeutic
agent that has been approved for the treatment of testicular and
ovarian cancer, non-small cell lung cancer, and squamous cell
carcinoma of the head and neck (1); however, clinical limitations of this
treatment occur due to its nephrotoxicity, ototoxicity and
neurotoxicity (2). Renal toxicity
is enhanced by underlying host risk factors, including older age,
sex, congestive heart failure, cancer-induced nephrotic syndrome,
hepatic dysfunction, obstructive jaundice and acute or chronic
kidney disease (3). Currently,
recommendations for the prevention of cisplatin nephrotoxicity
include the maintenance of euvolemia by adequate hydration with
normal saline during cisplatin chemotherapy (4). Preventive efforts have been proposed;
however, oncologists commonly encounter acute cisplatin-associated
kidney damage. Therefore, novel therapeutic or preventive
approaches for cisplatin nephrotoxicity are required.
The sirtuin family comprises evolutionarily
conserved proteins with NAD+-dependent protein
deacetylase and ADP-ribosyltransferase activities (5,6).
There are seven homologous mammalian sirtuins (Sirts), Sirt1-7,
which function act as non-histone deacetylases (7). Sirt3 is highly expressed in
mitochondrion-rich tissues, including the brain, heart, liver,
brown adipose and the kidney. Sirt3 regulates global mitochondrial
lysine acetylation (8,9). The known roles of Sirt3 include the
regulation of mitochondrial biogenesis (10), redox status (11), fatty acid metabolism (12) and aging (13). In addition, impaired Sirt3 activity
may have an important role in myocardial hypertrophy and
dysfunction (14). In an obesity
and diabetic cardiomyopathy animal model, downregulation of Sirt3
in the heart was reported to be associated with metabolic stress,
and contribute to myocardial mitochondrial dysfunction and
oxidative stress (15,16). However, controversial results in
regulation of Sirt3 on cellular apoptosis have been reported
(17,18). Sirt3 has been demonstrated to serve
a pro-apoptotic role in B-cell lymphoma-2 (Bcl-2) or c-Jun
N-terminal kinase (JNK)-induced apoptosis within tumorigenic and
normal cells under non-stress conditions (17). In renal proximal tubular cells,
high glucose levels decrease Sirt3 mRNA and protein expression, but
increase cellular apoptosis (18).
Overexpression of Sirt3 in renal tubular cells reduces high
glucose-induced apoptosis by regulating oxidative stress and the
protein kinase B/forkhead box protein O signaling pathway (18). Koyama et al (19) reported that overexpression of Sirt3
decreased palmitate-induced lipotoxicity and reactive oxygen
species (ROS)-associated inflammation in renal tubular cells;
however, further investigation into the role of Sirt3 in cisplatin
nephrotoxicity via regulation of cellular apoptosis and
inflammation is required.
It has been suggested that cisplatin induces renal
tubular apoptosis and inflammation by activating the p53 tumor
suppressor protein, the nuclear factor-κB (NF-κB) signaling
pathway, and by inducing the production of ROS (20–22).
Our previous studies suggested that Sirt1 has a protective role in
cisplatin nephrotoxicity via deacetylation of p53 and NF-κB p65
(23,24). Morigi et al (25) suggested that Sirt3 exhibits
protective effects in acute kidney injury by modulating
mitochondrial dynamics. In the present study, whether Sirt3
exhibits anti-inflammatory and anti-apoptotic effects on cisplatin
nephrotoxicity were investigated in mice.
Materials and methods
Animal experiments
The animal experimental protocol was reviewed and
approved by the Institutional Animal Care and Use Committee of
Chonbuk National University (Jeonju, Korea; CBU 2014–0018).
Sirt3 knockout mice (129-Sirt3tm1.1Fwa/J;
n=30, 8–10 weeks-old) and wild type control mice (129S1/SvlmJ;
n=30, approximately 20–25 g) were purchased from the Jackson
Laboratory (Bar Harbor, ME, USA) and housed under controlled
conditions: Temperature (23±1°C), humidity (50±10%) under a 12 h
light/dark cycle with free access to water and standard chow. The
mice were divided into four groups: Vehicle-treated wild type (WT)
mice (n=15), vehicle-treated Sirt3 knockout (Sirt3KO) mice
(n=15), cisplatin-treated WT mice (n=15), and cisplatin-treated
Sirt3KO mice (n=15). The dose of cisplatin and duration of
treatment were selected based on our previous study (21). PBS was used as the vehicle
treatment and 200 µl of PBS was injected intraperitoneally. Maximal
renal injury was observed at 72 h after a single intraperitoneal
injection of cisplatin (20 mg/kg; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) by functional and histologic assessments as
described below. On the final experimental day, mice were
anesthetized with a mixture of ketamine (100 mg/kg; Huons Co.,
Ltd., Seoul, Korea) and xylazine (10 mg/kg; Bayer Korea Ltd.,
Seoul, Korea) via an intraperitoneal injection. A total of 800 µl
blood was collected by intracardiac puncture and the kidneys were
harvested to evaluate changes in renal morphology and degree of
tubular apoptosis at 72 h after treatment with vehicle or
cisplatin. Following collection of the blood and kidney samples,
mice were sacrificed by CO2 inhalation. For functional
analysis, blood urea nitrogen (BUN) and creatinine levels were
measured by an enzymatic assays using an automatic analyzer
(Hitachi 7180; Hitachi, Ltd., Tokyo, Japan).
Renal histologic examination
Kidneys were fixed in 4% paraformaldehyde for 24 h
at 4°C and embedded in paraffin. Blocks were cut into 5-µm sections
and stained with Periodic acid-Schiff (PAS) by using 0.5% Periodic
acid solution for 5 min and Schiff reagent for 15 min at room
temperature. Immunohistochemical staining was performed as
described previously (26). Tissue
sections were deparaffinized with xylene, rehydrated via washes
with graded ethanol in water, and rinsed in pure water. Following
the previously described heat-induced antigen retrieval process
(26) and treatment with blocking
buffer (Protein Block Serum-Free Ready-to-use; cat. no. X0909;
Dako; Agilent Technologies, Inc., Santa Clara CA, USA) for 10 min
at room temperature (26), slides
were incubated overnight at 4°C with rabbit anti-mouse monocyte
chemoattractant protein-1 (MCP-1; 1:100; cat. no. 70R50662;
Fitzgerald Industries International, Acton, MA, USA) and rat
anti-mouse lymphocyte antigen 6 complex, locus G (Gr-1; 1:50; cat.
no. 560453; BD Pharmingen; BD Biosciences, San Jose, CA, USA)
antibodies. Subsequently, polyclonal goat anti-rabbit
immunoglobulins/Biotinylated (1:500; cat. no E0432; Dako; Agilent
Technologies, Inc.) for MCP-1 and polyclonal goat anti-rat
immunoglobulins/Biotinylated (1:500; cat. no. E0468; Dako; Agilent
Technologies, Inc.) for Gr-1 and incubated for 1 h at room
temperature. The kidney sections were treated with chromogen (Dako
AEC + High Sensitivity Substrate Chromogen Ready-to-Use; cat. no.
K3469; Dako; Agilent Technologies, Inc.) to visualize
immunocomplexes for 10 min at room temperature and then
counterstained with 0.1% hematoxylin (Sigma-Aldrich; Merck KGaA)
for 1 min at room temperature.
For immunofluorescence staining, freshly frozen
renal tissues were fixed with 4% paraformaldehyde at 4°C for 24 h,
and embedded in optimum cutting temperature (OCT) compound
Tissue-Tek (Sakura Finetek, Tokyo, Japan) and cut into 10-µm
thickness on Superfrost (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) slide. The slides were permeabilized in 1% Triton X-100
for 10 min at room temperature and then incubated with a blocking
buffer (Protein Block Serum-Free Ready-to-use; cat. no. X0909;
Dako; Agilent Technologies, Inc.) for 10 min at room temperature.
The tissue samples were incubated with rat anti-mouse adhesion G
protein-coupled receptor E4 (F4/80; 1:200; cat. no. 14-4801-82;
eBioscience; Thermo Fisher Scientific, Inc.). Slides were exposed
to a goat anti-rat Cy3-labeled secondary antibody (1:1,000; cat.
no. A10522; Thermo Fisher Scientific, Inc.) for 1 h at room
temperature. Nuclear staining was performed using DAPI (1:1,000,
Molecular Probes; Thermo Fisher Scientific, Inc.) for 1 min at room
temperature.
Analysis was conducted by two researchers in a
blinded manner to evaluate the morphology of all slides using a
Zeiss Z1 microscope for light and fluorescence microscopy (Zeiss
AG, Oberkochen, Germany). For immunofluorescence, 543 nm was
applied for the detection of Cy3-labled F4/80 (+) macrophages.
Renal tubular injury and positive areas of immunohistochemical
staining was assessed as previously described (21,26).
Tubular injury was classified by a six-level scoring system based
on the magnitude of tubular epithelial cell loss, necrosis,
intratubular debris, and tubular cast formation in 10 randomly
selected, non-overlapping fields (magnification, ×200) under light
microscopy: 0, none; 0.5, <10%; 1, 10–25%; 2, 25–50%; 3, 50–75%;
and 4, >75% (21,26). The MCP-1-positive area was measured
in 10 randomly chosen, non-overlapping fields at a magnification of
×200 using ImageJ 1.47v software (National Institutes of Health,
Bethesda, MD, USA). The number of F4/80-positive macrophages and
Gr-1-positive neutrophils were counted in 10 randomly chosen,
non-overlapping fields (magnification, ×400).
Detection of apoptosis
Apoptosis was assessed by a terminal
deoxynucleotidyl transferase-mediated dUTP nick-end labeling
(TUNEL) assay, and the number of apoptotic cells, as defined by
chromatin condensation or nuclear fragmentation (apoptotic bodies),
was counted as previously described (21). Apoptosis in the specimens was
detected using the ApopTag Plus Peroxidase In Situ Apoptosis
Detection kit (EMD Millipore, Billerica, MA, USA) according to the
manufacturer's protocols. Samples were fixed and embedded as
aforementioned. The number of apoptotic cells in each section (n=10
sections per kidney) was calculated by counting the number of
TUNEL-positive apoptotic cells in 10 random selected,
nonoverlapping fields per slide (magnification, ×400).
Western blot analysis
Western blot analysis was performed as described
previously (21). Kidney tissues
were homogenized in radioimmunoprecipitation assay lysis and
extraction buffer (Thermo Fisher Scientific, Inc.) with a protease
inhibitor cocktail (EMD Millipore), and the protein concentration
was quantified by a Bradford protein assay. Samples (20–30 µg
protein per lane) were mixed with sample buffer, boiled for 7 min,
separated by (8–15%) SDS-PAGE and electroblotted onto a
nitrocellulose membrane (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The membrane was blocked with 5% non-fat dry milk in
Tris-buffered saline [25 mmol/l Tris (pH 7.5), 150 mmol/l NaCl]
with 0.1% Tween-20 buffer for 1 h at room temperature and then
incubated overnight at 4°C with rabbit anti-mouse Sirt3 (1:1,000;
cat. no. 5490, Cell Signaling Technology, Inc., Danvers, MA, USA)
and rabbit anti-mouse caspase-3 (1:1,000; cat. no. 9662; Cell
Signaling Technology, Inc.). Membranes were washed with PBS and
incubated with horseradish peroxidase-conjugated anti-rabbit IgG
antibodies (1:5,000; cat. no. 65-6120; Thermo Fisher Scientific,
Inc.) for 1 h at room temperature. Bands were visualized with a
chemiluminescent detection kit according to the manufacturer's
protocols (Amersham ECL Prime Western Blotting Detection Reagent,
GE Healthcare, Chicago, IL, USA). Membranes were then probed with
an anti-β-actin antibody (1:10,000; cat. no. A5316; Sigma-Aldrich;
Merck KGaA) to verify equal loadings of protein in each lane. All
signals were analyzed by densitometric scanner (LAS-3000 pro
software; FUJIFILM Corporation, Tokyo, Japan).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Graphs and statistical analyses were produced and conducted,
respectively, using the SigmaPlot program (version 11; Systat
Software, Inc., San Jose, CA, USA). Analysis was conducted in
triplicate. Multiple comparisons were performed to investigate
significant differences using one-way analysis of variance,
followed by an individual comparison with a Tukey post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of Sirt3 on cisplatin-induced
renal tubular damage
To address the role of Sirt3 in cisplatin-associated
nephrotoxicity, the present study investigated Sirt3 protein
expression following the administration of cisplatin. At 72 h
following intraperitoneal injection of cisplatin (20 mg/kg), renal
expression of Sirt3 was significantly decreased in
cisplatin-treated WT mice compared within the vehicle group
(Fig. 1A). Subsequently, Sirt3KO
mice were employed to evaluate the effects of Sirt3 on renal
function following treatment with cisplatin. Compared with in WT
mice, Sirt3KO mice exhibited significantly increased BUN and serum
creatinine levels at 72 h after the administration of cisplatin
(Fig. 1B). Consistent with the
aforementioned blood chemistry findings, renal histologic analysis
revealed that Sirt3KO mice accumulated more intratubular debris,
PAS-positive materials and cast formation in the tubular lumen;
loss of the brush border and tubular epithelial cells, tubular
dilatation, and inflammatory cell infiltration were also observed
at 72 h following cisplatin treatment compared with in cisplatin
treated WT mice (Fig. 1C). The
tubular injury score was determined at 72 h after treatment with
cisplatin in WT and Sirt3KO mice. The Sirt3KO mice that were
injected with cisplatin exhibited significantly higher tubular
injury scores than control WT and Sirt3KO mice (Fig. 1D). These data suggested that the
absence of Sirt3 may be associated with aggravation of
cisplatin-induced renal injury.
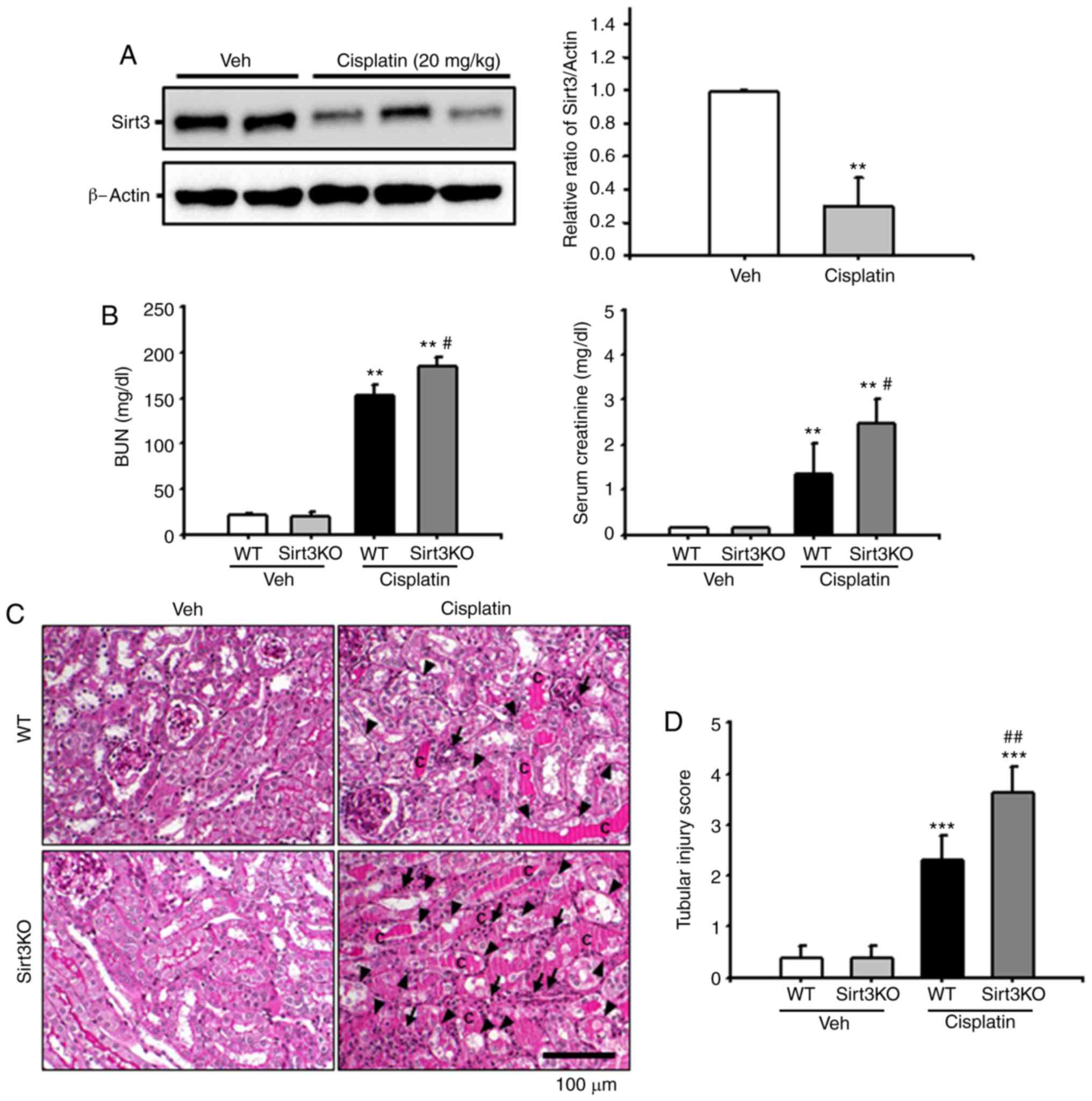 | Figure 1.Effects of Sirt3 on cisplatin-induced
renal tubular damage. (A) Sirt3 expression in kidney tissue from WT
mice injected with Veh or cisplatin (20 mg/kg body weight) was
evaluated by western blotting. Data obtained from densitometric
analysis are presented as the relative ratio of each protein to
β-actin. The relative ratio of protein measured in kidneys from
vehicle-injected mice is arbitrarily presented as 1. **P<0.01
vs. Veh. (B) Kidneys in Sirt3KO and WT mice were harvested 3 days
after treatment with cisplatin (20 mg/kg body weight). Blood
samples were collected 72 h following cisplatin treatment, and BUN
and serum creatinine levels were measured (n=10 in each group).
**P<0.01 vs. Veh; #P<0.05 vs. WT Cisplatin. (C)
Representative PAS-stained sections of kidneys from WT and Sirt3KO
mice treated with vehicle or cisplatin. Arrows indicate
inflammatory cells; arrowheads indicate tubular epithelial cell
loss and necrosis, and c represents intratubular debris and tubular
cast formation. Scale bar, 100 µm. (D) Histological damage in
PAS-stained kidney sections was scored by measuring the magnitude
of tubular epithelial cell loss, necrosis, intratubular debris and
tubular cast formation. ***P<0.001 vs. Veh,
##P<0.01 vs. WT Cisplatin. Data are expressed as the
mean ± standard deviation. PAS, periodic acid-Schiff; Veh, vehicle;
Sirt3, Sirtuin 3; BUN, blood urea nitrogen; WT, wild type; KO,
knockout. |
Effects of Sirt3 on cisplatin-induced
renal inflammation
The infiltration of inflammatory cells, including
neutrophils and macrophages, and MCP-1 expression were evaluated by
immunofluorescence and immunohistochemical analysis, respectively,
to investigate the effects of Sirt3 on cisplatin-induced renal
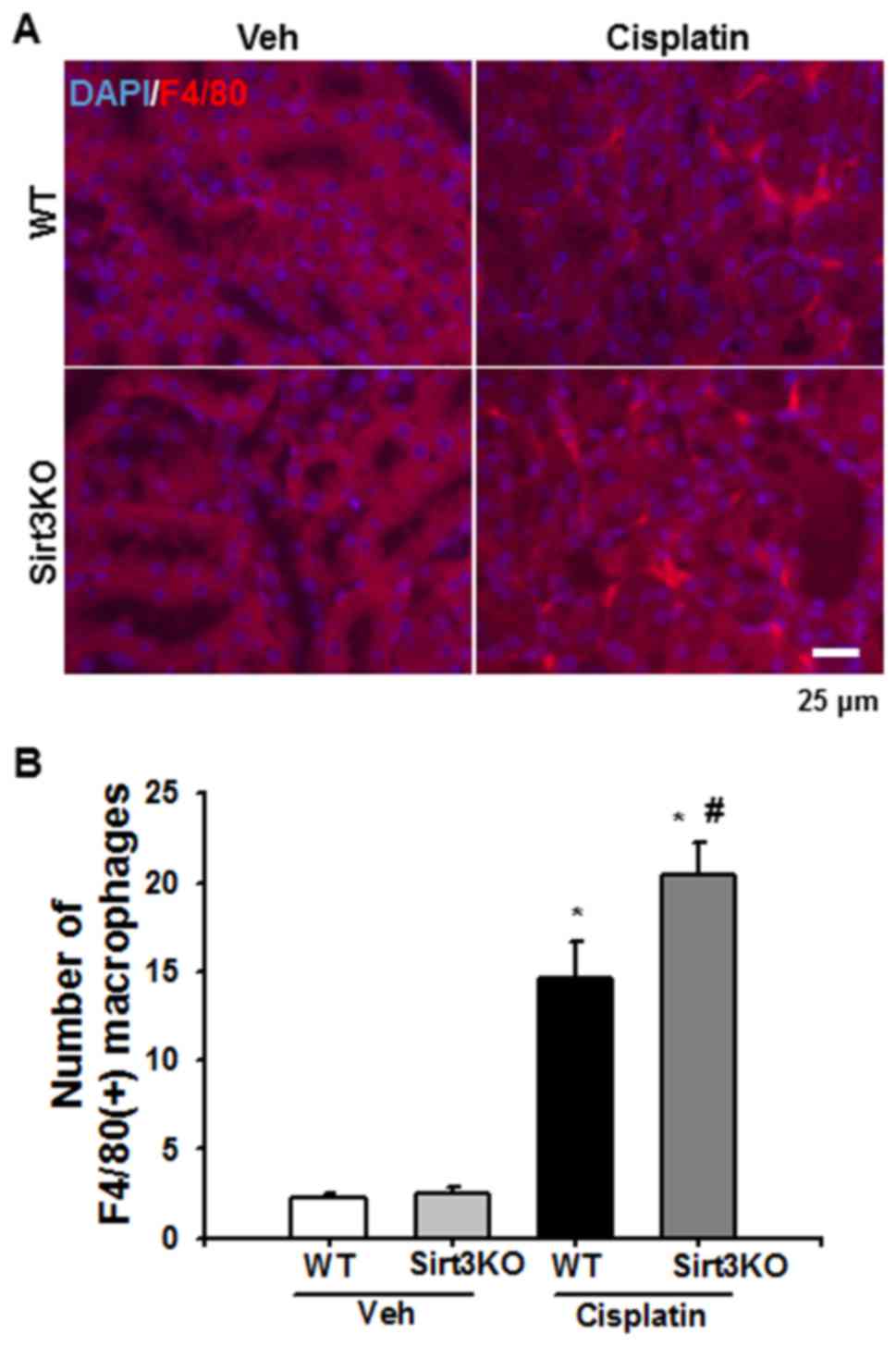
inflammation. F4/80-positive macrophages were observed in the
tubulointerstitial area at 72 h following cisplatin treatment in
the WT and Sirt3KO mice; the number of F4/80-positive macrophages
was significantly higher in Sirt3KO mice compared with WT mice
(Fig. 2A and B). Additionally,
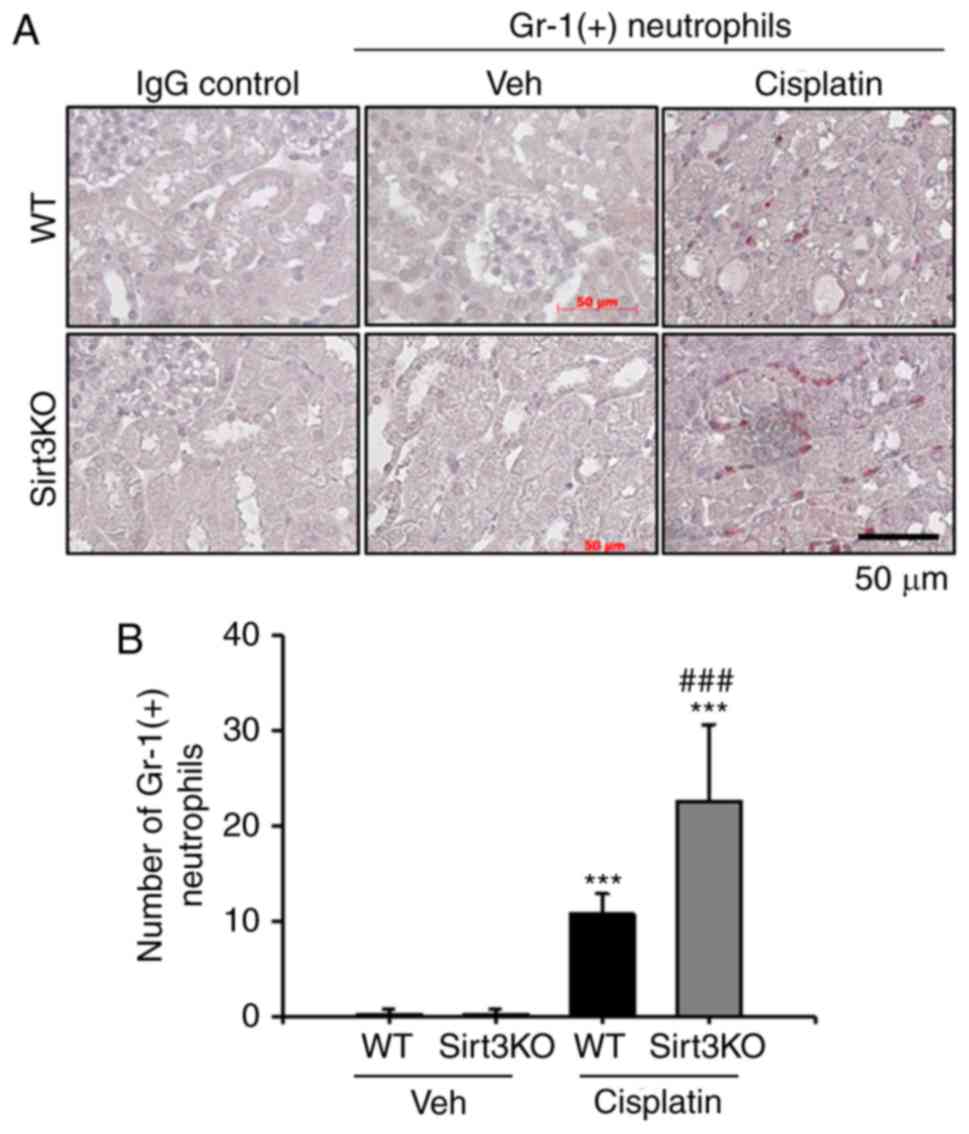
neutrophil infiltration following cisplatin-induced renal injury
was investigated in the present study. Gr-1-positive neutrophils
notably infiltrated the tubulointerstitial area at 72 h following
cisplatin treatment in WT and Sirt3KO mice compared with the
vehicle treated groups (Fig. 3A).
Cisplatin treated Sirt3KO mice exhibited significantly increased
neutrophil infiltration compared with WT mice with cisplatin
treatment (Fig. 3B). IgG control
probed slides were not stained in both WT and Sirt3KO mice
samples.
MCP-1 expression was significantly increased in the
renal tubulointerstitial area at 72 h after the administration of
cisplatin. The area of staining MCP-1 was significantly higher in
Sirt3KO mice than WT mice following treatment with cisplatin, as
well as that in the vehicle groups (Fig. 4A and B). IgG control probed slides
were not stained in both WT and Sirt3 KO mice samples. These data
suggested that the absence of Sirt3 may aggravate cisplatin-induced
renal inflammation by inducing an increase in the infiltration of
inflammatory cells.
Effects of Sirt3 on cisplatin-induced
renal tubular apoptosis
Renal tubular apoptosis is a suggested mechanism of
cisplatin-induced nephrotoxicity (27). Cisplatin inhibits mitochondrial
oxidative phosphorylation and membrane potentials, which results in
apoptotic cell death (28).
Therefore, cisplatin-induced renal tubular apoptosis was evaluated
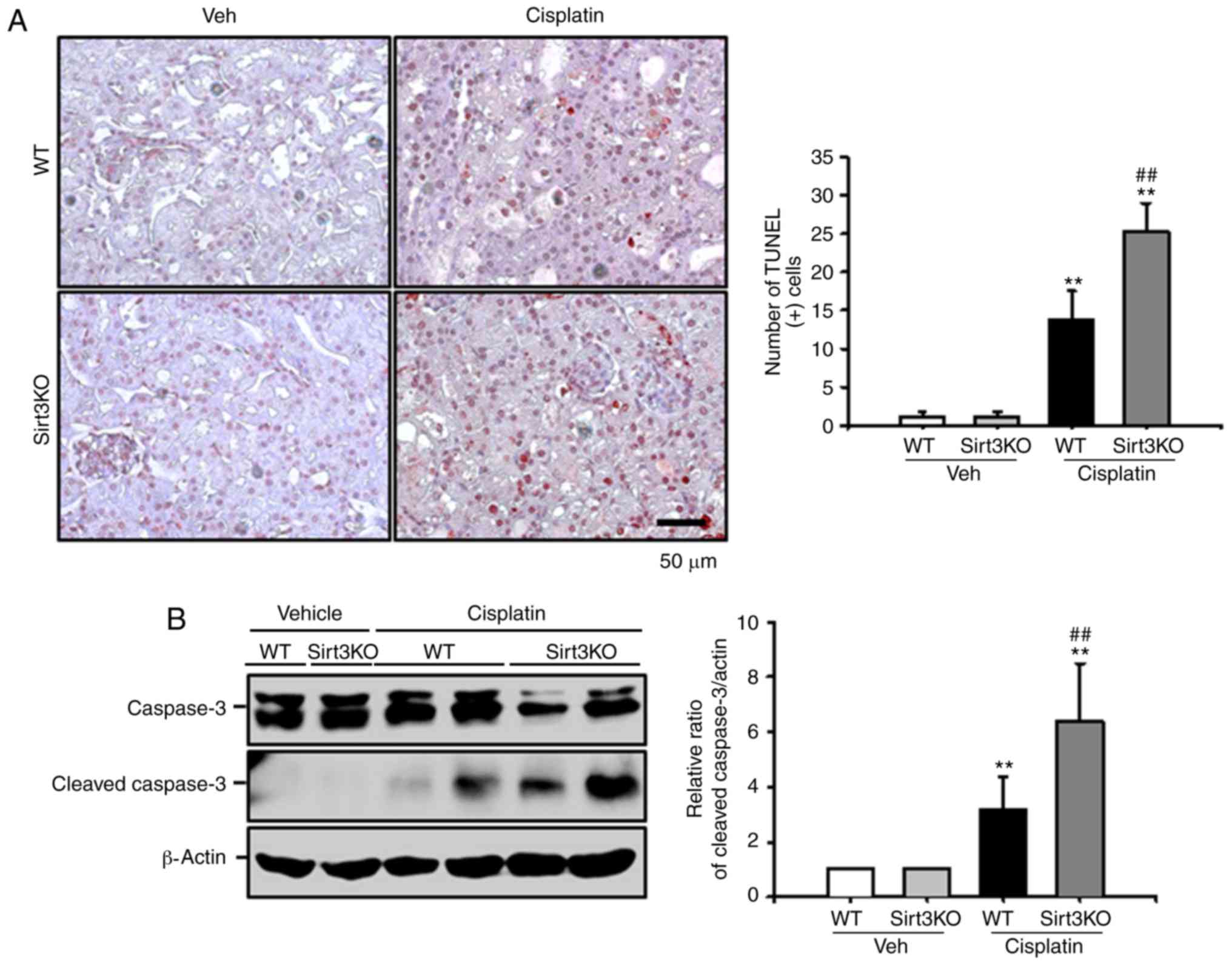
by a TUNEL assay in the present study. As presented in Fig. 5A, the number of TUNEL-positive
cells significantly increased in Sirt3KO mice compared with in WT
mice at 72 h after cisplatin treatment. Additionally, the number
TUNEL-positive cells in both cisplatin-treated groups compared with
the respective vehicle controls. The expression level of cleaved
caspase-3 was significantly increased at 72 h after cisplatin
treatment in Sirt3KO mice compared with in WT mice; a significant
difference was observed between both the cisplatin treatment groups
and the respective vehicle controls (Fig. 5B). These findings indicated that
Sirt3 may serve a critical role in cisplatin-induced tubular
apoptosis.
Discussion
Renal dysfunction is a common complication of
cancer; renal dysfunction may be associated with the cancer itself,
induced by cancer-associated treatment or due to secondary
complications, including sepsis, nephrotoxic drugs or radiocontrast
agent administration (29). Acute
kidney injury following chemotherapy has been associated with
increased morbidity and mortality in patients with cancer (3). As there are limited therapeutic
options for treating cisplatin nephrotoxicity, the underlying
mechanisms of toxicity require further investigation to identify
novel therapeutic agents or targets. In the present study, it was
demonstrated that Sirt3 may serve an essential role in protecting
the kidney from cisplatin-induced renal inflammation and tubular
apoptosis. In the current study, the absence of Sirt3 was been
associated with increased renal macrophage infiltration and tubular
MCP-1 expression in cisplatin-induced nephrotoxicity. In addition,
Sirt3KO mice exhibited increased cisplatin-induced tubular
apoptosis compared with WT mice in the present study.
Renal inflammation is an important mechanism of
cisplatin nephrotoxicity (22,27).
Several inflammatory mediators are involved in cisplatin
nephrotoxicity. We previously reported that, following cisplatin
injection, macrophages infiltrate the tubulointerstitial areas
(22). In addition to inflammatory
cell infiltration, cell adhesion molecules, including intercellular
adhesion molecule-1 and MCP-1 were expressed in peritubular
capillaries and injured tubules (21,22,30).
Therefore, regulation of the cisplatin-induced inflammatory
response may be important for the treatment and prevention of
cisplatin nephrotoxicity. The present study revealed that the
absence of Sirt3 in cisplatin-treated mice was associated with
increased induction of renal inflammatory responses compared with
in WT mice. These data support reports of the protective role of
Sirt3 in cisplatin nephrotoxicity by regulating inflammation.
The renal toxicity of cisplatin may be associated
with formation of DNA adducts, which can inhibit DNA replication
and transcription, resulting in altered protein synthesis,
mitochondrial injury, DNA damage and programmed cell death
(31). We previously reported that
cisplatin-induced activation of the p53 tumor suppression gene is
one mechanism of cisplatin-induced renal tubular apoptosis, which
was attenuated by treatment with luteolin (21). In the present study, it was
demonstrated that the absence of Sirt3 in cisplatin-treated mice
was associated with increased tubular apoptosis and cleaved caspase
3 expression levels compared with in cisplatin-treated WT mice.
These data indicated that Sirt3 may serve an important role in
cisplatin-induced renal tubular apoptosis.
Sirt3 is an NAD+-dependent deacetylase of
the sirtuin family of proteins and is localized to the
mitochondrial matrix (32). Sirt3
has a role in controlling respiratory chain activity, the
tricarboxylic acid cycle, fatty acid β-oxidation and the
antioxidant pathway (32). Sirt3
can regulate mitochondrial dynamics in cisplatin-induced proximal
tubular injury and alter the NF-κB dependent inflammatory pathway
and oxidative stress in proteinuric kidney disease (19,25).
The findings of the present study revealed that cisplatin treatment
was associated with downregulated Sirt3 expression in WT mice;
however, Sirt3KO mice exhibited aggravated cisplatin-induced renal
injury, potentially by increasing the induction of the inflammatory
response and tubular apoptosis.
The role of Sirt3 on cellular apoptosis remains
controversial. Allison and Milner (17) reported that Sirt3 is an essential
pro-apoptotic mediator for the Bcl-2/p53- and JNK2/JNK1-regulated
apoptosis signaling pathways in a colorectal carcinoma cell line.
In leukemic cell lines, kaempferol was reported to increase
auto-oxidation and generate ROS to induce cellular apoptosis by
increasing Bcl-2-associated-X (Bax) and Sirt3 expression levels,
and activating caspase-3 cascades (33). However, the results of the present
study suggested that the absence of Sirt3 increased
cisplatin-induced renal tubular apoptosis via increasing the
expression levels of active caspase-3. Sundaresan et al
(34) reported that Sirt3 augments
Ku70-Bax interactions to prevent Bax translocation to the
mitochondrial membrane, which protects cells from stress-induced
apoptosis. This discrepancy may be due to variations in cell types,
including tumorigenic and normal cells. Therefore, further
investigation is required to identify the specific role of Sirt3 in
cisplatin-associated nephrotoxicity.
Different pharmacological approaches have suggested
that potential Sirt3 activators may increase Sirt3 expression or
Sirt3 activity (14). Honokiol and
resveratrol are natural products that increase Sirt3 expression and
modulate Sirt3 activity, and have high bioavailability (14,35).
These agents possess anti-inflammatory, anti-apoptotic and
anti-oxidant properties, as well as AMP activated protein kinase
activation and induction of autophagy abilities, which exert
protective effects against cardiac hypertrophy, heart failure and
myocardial ischemia-reperfusion injury (36–39).
Therefore, a pharmacologic agent to activate Sirt3 may have
beneficial effects on cisplatin-induced renal injury. Alhazzazi
et al (40) reported that a
novel Sirt3 inhibitor, LC-0296, exhibited anticancer effects in
head and neck cancer via the regulation of cell proliferation and
apoptosis. This agent may also have synergistic effects on cancer
cells to increase sensitivity to radiation and cisplatin treatment.
Therefore, modulation of Sirt3 may benefit patients with cancer as
an anticancer strategy and preserve renal function.
A limitation of the present study was that a novel
Sirt3 activator or inhibitor for the prevention of
cisplatin-induced renal injury was not used. In. addition, the
present study did not establish a tumor model to evaluate the role
of a Sirt3 activator or inhibitor in chemotherapy-associated
toxicity in the kidney and other tissues. Therefore, further
investigation of novel Sirt3 activators and inhibitors for the
treatment of chemotherapy-associated complications are
required.
In summary, the present study reported that the
cisplatin nephrotoxicity induced by loss of Sirt3 may occur via
increased renal inflammation and tubular apoptosis. Therefore,
Sirt3 may serve an important role protecting against
cisplatin-induced nephrotoxicity; however, further investigation is
required to understand the molecular mechanisms underlying this
nephrotoxicity.
Acknowledgements
We thank Ms. Kieu Thi Thu Trang for the excellent
technical assistance.
Funding
The present study was supported by the National
Research Foundation of Korea funded by the Korean government (grant
nos. NRF-2015R1D1A3A03015653 to KPK and NRF-2017R1D1A3B03035494, to
SKP) and the research funds of Chonbuk National University Hospital
(grant no. CUH2013-0047, to KPK).
Availability of data and materials
Not applicable.
Authors' contributions
DK, WP and KPK designed and performed the experiment
of the present study. SL and WK contributed to the design of the
present study, the analysis of the results and the final version of
the manuscript. SKP and KPK made substantial contributions to the
conception and design of the present study, conducted data analysis
and writing of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Chonbuk National University
(Jeonju, Korea; CBU 2014-0018).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
dos Santos NA, Carvalho Rodrigues MA,
Martins NM and dos Santos AC: Cisplatin-induced nephrotoxicity and
targets of nephroprotection: An update. Arch Toxicol. 86:1233–1250.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arany I and Safirstein RL: Cisplatin
nephrotoxicity. Semin Nephrol. 23:460–464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perazella MA and Moeckel GW:
Nephrotoxicity from chemotherapeutic agents: Clinical
manifestations, pathobiology, and prevention/therapy. Semin
Nephrol. 30:570–581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Launay-Vacher V, Rey JB, Isnard-Bagnis C,
Deray G and Daouphars M: European Society of Clinical Pharmacy
Special Interest Group on Cancer Care: Prevention of cisplatin
nephrotoxicity: State of the art and recommendations from the
European society of clinical pharmacy special interest group on
cancer care. Cancer Chemother Pharmacol. 61:903–909. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bause AS and Haigis MC: SIRT3 regulation
of mitochondrial oxidative stress. Exp Gerontol. 48:634–639. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haigis MC and Guarente LP: Mammalian
sirtuins-emerging roles in physiology, aging, and calorie
restriction. Genes Dev. 20:2913–2921. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sack MN: The role of SIRT3 in
mitochondrial homeostasis and cardiac adaptation to hypertrophy and
aging. J Mol Cell Cardiol. 52:520–525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lombard DB, Alt FW, Cheng HL, Bunkenborg
J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D,
Murphy A, et al: Mammalian Sir2 homolog SIRT3 regulates global
mitochondrial lysine acetylation. Mol Cell Biol. 27:8807–8814.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dang W: The controversial world of
sirtuins. Drug Discov Today Technol. 12:e9–e17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahn BH, Kim HS, Song S, Lee IH, Liu J,
Vassilopoulos A, Deng CX and Finkel T: A role for the mitochondrial
deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad
Sci USA. 105:pp. 14447–14452. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qiu X, Brown K, Hirschey MD, Verdin E and
Chen D: Calorie restriction reduces oxidative stress by
SIRT3-mediated SOD2 activation. Cell Metab. 12:662–667. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hirschey MD, Shimazu T, Goetzman E, Jing
E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S,
Ilkayeva OR, et al: SIRT3 regulates mitochondrial fatty-acid
oxidation by reversible enzyme deacetylation. Nature. 464:121–125.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bellizzi D, Rose G, Cavalcante P, Covello
G, Dato S, De Rango F, Greco V, Maggiolini M, Feraco E, Mari V,
Franceschi C, et al: A novel VNTR enhancer within the SIRT3 gene, a
human homologue of SIR2, is associated with survival at oldest
ages. Genomics. 85:258–263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koentges C, Bode C and Bugger H: SIRT3 in
cardiac physiology and Disease. Front Cardiovasc Med. 3:382016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeng H, Vaka VR, He X, Booz GW and Chen
JX: High-fat diet induces cardiac remodelling and dysfunction:
Assessment of the role played by SIRT3 loss. J Cell Mol Med.
19:1847–1856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng H, He X, Hou X, Li L and Chen JX:
Apelin gene therapy increases myocardial vascular density and
ameliorates diabetic cardiomyopathy via upregulation of sirtuin 3.
American journal of physiology. Am J Physiol Heart Circ Physiol.
306:H585–H597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Allison SJ and Milner J: SIRT3 is
pro-apoptotic and participates in distinct basal apoptotic
pathways. Cell Cycle. 6:2669–2677. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiao X, Li Y, Zhang T, Liu M and Chi Y:
Role of Sirtuin3 in high glucose-induced apoptosis in renal tubular
epithelial cells. Biochem Biophys Res Commun. 480:387–393. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koyama T, Kume S, Koya D, Araki S, Isshiki
K, Chin-Kanasaki M, Sugimoto T, Haneda M, Sugaya T, Kashiwagi A, et
al: SIRT3 attenuates palmitate-induced ROS production and
inflammation in proximal tubular cells. Free Radic Biol Med.
51:1258–1267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramesh G and Reeves WB: TNF-alpha mediates
chemokine and cytokine expression and renal injury in cisplatin
nephrotoxicity. J Clin Invest. 110:835–842. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kang KP, Park SK, Kim DH, Sung MJ, Jung
YJ, Lee AS, Lee JE, Ramkumar KM, Lee S, Park MH, et al: Luteolin
ameliorates cisplatin-induced acute kidney injury in mice by
regulation of p53-dependent renal tubular apoptosis. Nephrol Dial
Transplant. 26:814–822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kang KP, Kim DH, Jung YJ, Lee AS, Lee S,
Lee SY, Jang KY, Sung MJ, Park SK and Kim W: Alpha-lipoic acid
attenuates cisplatin-induced acute kidney injury in mice by
suppressing renal inflammation. Nephrol Dial Transplant.
24:3012–3020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jung YJ, Lee JE, Lee AS, Kang KP, Lee S,
Park SK, Lee SY, Han MK, Kim DH and Kim W: SIRT1 overexpression
decreases cisplatin-induced acetylation of NF-κB p65 subunit and
cytotoxicity in renal proximal tubule cells. Biochem Biophys Res
Commun. 419:206–210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim DH, Jung YJ, Lee JE, Lee AS, Kang KP,
Lee S, Park SK, Han MK, Lee SY, Ramkumar KM, et al: SIRT1
activation by resveratrol ameliorates cisplatin-induced renal
injury through deacetylation of p53. Am J Physiol Renal Physiol.
301:F427–F435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morigi M, Perico L, Rota C, Longaretti L,
Conti S, Rottoli D, Novelli R, Remuzzi G and Benigni A: Sirtuin
3-dependent mitochondrial dynamic improvements protect against
acute kidney injury. J Clin Invest. 125:715–726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim D, Lee AS, Jung YJ, Yang KH, Lee S,
Park SK, Kim W and Kang KP: Tamoxifen ameliorates renal
tubulointerstitial fibrosis by modulation of estrogen receptor
alpha-mediated transforming growth factor-β1/Smad signaling
pathway. Nephrol Dial Transplant. 29:2043–2053. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pabla N and Dong Z: Cisplatin
nephrotoxicity: Mechanisms and renoprotective strategies. Kidney
Int. 73:994–1007. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Havasi A and Borkan SC: Apoptosis and
acute kidney injury. Kidney Int. 80:29–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fischler R, Meert AP, Sculier JP and
Berghmans T: Continuous renal replacement therapy for acute renal
failure in patients with cancer: A well-tolerated adjunct
treatment. Front Med (Lausanne). 3:332016.PubMed/NCBI
|
|
30
|
Sung MJ, Kim DH, Jung YJ, Kang KP, Lee AS,
Lee S, Kim W, Davaatseren M, Hwang JT, Kim HJ, et al: Genistein
protects the kidney from cisplatin-induced injury. Kidney Int.
74:1538–1547. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park MS, De Leon M and Devarajan P:
Cisplatin induces apoptosis in LLC-PK1 cells via activation of
mitochondrial pathways. J Am Soc Nephrol. 13:858–865.
2002.PubMed/NCBI
|
|
32
|
Perico L, Morigi M and Benigni A:
Mitochondrial Sirtuin 3 and Renal Diseases. Nephron. 134:14–19.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Marfe G, Tafani M, Indelicato M,
Sinibaldi-Salimei P, Reali V, Pucci B, Fini M and Russo MA:
Kaempferol induces apoptosis in two different cell lines via Akt
inactivation, Bax and SIRT3 activation, and mitochondrial
dysfunction. J Cell Biochem. 106:643–650. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sundaresan NR, Samant SA, Pillai VB,
Rajamohan SB and Gupta MP: SIRT3 is a stress-responsive deacetylase
in cardiomyocytes that protects cells from stress-mediated cell
death by deacetylation of Ku70. Mol Cell Biol. 28:6384–6401. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pillai VB, Samant S, Sundaresan NR,
Raghuraman H, Kim G, Bonner MY, Arbiser JL, Walker DI, Jones DP,
Gius D and Gupta MP: Honokiol blocks and reverses cardiac
hypertrophy in mice by activating mitochondrial Sirt3. Nat Commun.
6:66562015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Averett C, Arora S, Zubair H, Singh S,
Bhardwaj A and Singh AP: Molecular targets of honokiol: A promising
phytochemical for effective cancer management. Enzymes. 36:175–193.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kumar A, Kumar Singh U and Chaudhary A:
Honokiol analogs: A novel class of anticancer agents targeting cell
signaling pathways and other bioactivities. Future Med Chem.
5:809–829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zordoky BN, Robertson IM and Dyck JR:
Preclinical and clinical evidence for the role of resveratrol in
the treatment of cardiovascular diseases. Biochim Biophys Acta.
1852:1155–1177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen T, Li J, Liu J, Wang S, Liu H, Zeng
M, Zhang Y and Bu P: Activation of SIRT3 by resveratrol ameliorates
cardiac fibrosis and improves cardiac function via the TGF-β/Smad3
pathway. Am J Physiol Heart Circ Physiol. 308:H424–H434. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Alhazzazi TY, Kamarajan P, Xu Y, Ai T,
Chen L, Verdin E and Kapila YL: A Novel Sirtuin-3 Inhibitor,
LC-0296, inhibits cell survival and proliferation, and promotes
apoptosis of head and neck cancer cells. Anticancer Res. 36:49–60.
2016.PubMed/NCBI
|