Introduction
Immune thrombocytopenia (ITP) is an acquired
autoimmune disorder, which is characterized by excessive bleeding
(1). Although ITP is classified as
a benign disease, the quality of life of patients with ITP is lower
than that of patients with cancer. A comprehensive understanding of
the pathogenesis of ITP is a means of improving the quality of life
of these patients. To date, research on the pathogenesis of ITP has
been focused mainly on platelets or megakaryocyte apoptosis, which
are destroyed or functionally inhibited by humoral and cellular
immunity (2,3), with the latter receiving far less
attention than the former. In ITP patients, megakaryocytes
predominantly remain at the granule megakaryocyte stage, although
the fate of these cells is unclear. Programmed cell death can be
divided into several categories, including type I (apoptosis) and
type II (autophagic death) (4).
Houwerzijl et al (5)
demonstrated increased vacuoles, plasma membrane thickening and
chromatin condensation in the mitochondria and endoplasmic
reticulum (ER) of ITP megakaryocytes. This may result in
endoplasmic reticulum stress, leading to autophagy (6,7).
Autophagy is a biological process, where cytoplasmic macromolecules
self-degrade through the lysosomal pathway (8). Chloroquine (CQ) can affect the pH of
lysosomes and the ability of lysosomes to degrade proteins, as well
as block the fusion of autophagosomes and lysosomes, which
subsequently inhibits autophagy (9). In recent years, great progress has
been made in the study of the association between autophagy and
tumors, however, the role of autophagy in hematological diseases is
not yet clear. Previous studies have demonstrated that premature
death of megakaryocytes may induce ITP thrombocytopenia and
megakaryocytes undergo autophagic (10). Therefore, it was hypothesized that
megakaryocytes are likely to undergo autophagy in ITP patients. In
the present study, the effect of autophagy on megakaryocytes was
investigated to identify the mechanism of action in ITP using the
human megakaryoblast leukemic cell line MEG-01 and plasma obtained
from patients with ITP.
Materials and methods
Cell culture
The human megakaryoblast leukemic cell line MEG-01
was obtained from Guangzhou JENNIO Biological Technology
(Guangzhou, China). Cells were maintained in RPMI-1640 media
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% heat-inactivated fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin, and
cultured in an incubator at 37°C under 5% CO2 and 95%
humidity.
Patient information
A total of 14 patients [11 females and 3 males,
median age 49 years (range 23–68 years)] with ITP and 23 healthy
controls [16 females and 7 males, median age 46 years (range 22–66
years)] were recruited. All patients were newly diagnosed and
excluded from other autoimmune diseases and malignant tumors.
Venous blood samples (2 ml) were collected into sterilized plastic
tubes prior to any drug treatment by the Laboratory of Hematology
(Yongchuan Hospital of Chongqing Medical University, Chongqing,
China) between June and December 2016. Plasma was separated by
centrifugation at room temperature (2,000 × g for 10 min), and
aliquots were frozen at −80°C for subsequent experiments. The
present study was approved by the Research Ethics Board of the
Yongchuan Hospital of Chongqing Medical University and informed
consent was obtained from all participants.
Cell processing method
i) No plasma group: Meg-01 cells were cultured in
plasma-free 1640 medium. ii) Normal plasma group: Meg-01 cells were
co-cultured with normal control plasma. iii) ITP plasma group:
Meg-01 cells were co-cultured with ITP patient plasma. Each
experiment was repeated three times.
Morphological changes
i) Meg-01 cells were taken in the logarithmic growth
phase, centrifuged at 100 × g for 5 min at room temperature, and an
appropriate amount of serum-free 1640 culture solution was added,
mixed. The cell density was adjusted to 1×105/ml, to
inoculate a six-well plate. Each group was inoculated with 2 wells
and 1.8 ml cell suspension was added to each well. ii) According to
the experimental group, 200 µl serum-free medium, 200 µl normal
control plasma, and 200 µl ITP plasma were added to each well for
incubation for 36 h at 37°C in a humidified cell incubator
containing 5% CO2. iii) After 36 h, 2 ml cell suspension
was taken from each group and transferred to two 1.5 ml EP tubes
and centrifuged at 100 × g for 5 min, at room temperature. iv) The
cells were washed twice with PBS (at 400 × g for 5 min, at room
temperature and the cells were harvested to a cell concentration of
1×105/ml. v) The different groups of cells were placed
on labelled polylysine slides. vi) Slices were placed in a
ventilated room and allowed to dry naturally. Then the slide was
placed on the staining rack. vii) The remaining steps are the same
as in part i. viii) The slides were observed under oil by laser
scanning confocal microscope.
Evaluation of apoptosis by flow
cytometric analysis of Annexin V-FITC/propidium iodide (PI)
staining
To investigate cell apoptosis, MEG-01 cells
(2×105 cells/ml) were obtained post-treatment according
to the flow cytometry kit protocol. Following this, cells were
stained with Annexin V/PI (Beyotime Institute of Biotechnology,
Haimen, China), according to the manufacturer's protocol and then
analyzed with a FACStar PLUS™ (BD Biosciences, Franklin Lakes, NJ,
USA).
Evaluation of cell autophagy by
Lyso-Tracker Red/ansylcadaverine (MDC) assay and flow
cytometry
Cell death of MEG-01 cells (2×105
cells/ml) was investigated. Autophagosomes were marked with
Lyso-Tracker Red (Beyotime Institute of Biotechnology, Shanghai,
China) at 37°C for 30 min and MDC (Beijing Solarbio Science and
Technology Co., Ltd., Beijing, China) at room temperature for 30
min in the dark, according to the manufacturer's protocol.
Autophagy was detected under a fluorescence microscope using flow
cytometric analysis as described previously (11).
Western blot analysis
Following washing of the treated cells three times
using PBS, MEG-01 cells were harvested and lysed in RIPA buffer
(Sigma-Aldrich; Merck KGaA (Darmstadt, Germany) containing 0.1
mg/ml of phenylmethane sulfonyl fluoride on ice for 30 min.
Supernatants were subsequently isolated via centrifugation at
12,000 × g for 5 min at 4°C, Protein content was determined by BCA.
Following this, equal amounts of protein from cell lysates (30 µg
total protein per lane) were separated via 10% SDS-PAGE and then
transferred to polyvinylidene difluoride membranes. Following this,
membranes were blocked for 1 h at room temperature with 5% skim
milk powder in Tris-buffered saline containing 0.1% Tween-20
(TBST). Western blot analysis was performed according to standard
protocol using the following primary antibodies at room temperature
for 2 h: Anti-B-cell lymphoma (Bcl)-2 mouse monoclonal antibody
(cat. no. 15071; 1:1,000; CST Biological Reagents Co., Ltd.,
Shanghai, China) and anti-Bcl-associated X protein (Bax) rabbit
polyclonal antibody (cat. no. 2774; 1:1,000; CST Biological
Reagents Co., Ltd.); anti-Beclin-1 rabbit polyclonal antibody (cat.
no. ab207612; 1:1,000) and anti- active Caspase-3 rabbit monoclonal
antibody (cat. no. ab2302; 1:1,000; both Abcam, Cambridge, UK), and
anti-β-actin mouse monoclonal antibody (cat. no. AA128; 1:1,000;
Beyotime Institute of Biotechnology, Shanghai, China). Membranes
were subsequently washed three times for 10 min each with TBST;
incubated with horseradish peroxidase-conjugated secondary IgG
(goat anti-rabbit, cat. no. A16096; 1:10,000; Thermo Fisher
Scientific, Inc.; goat anti-mouse, cat. 1706516; 1:10,000; Bio-rad
Laboratories, Inc., Hercules, CA, USA) in TBST with 5% non-fat milk
(goat anti-rabbit IgG and goat anti-mouse IgG was purchased from
Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h at room
temperature; and then washed a further three times with TBST.
Following this, immunoblotting signals were visualized using an ECL
chemiluminescence substrate reagent kit (Bio-rad Laboratories,
Inc.), and band densities were quantified using Quantity One
software 3.0 (Bio-Rad Laboratories, Inc.). CQ was obtained from
Sigma-Aldrich; Merck KGaA.
Statistical analysis
Statistical analysis was performed using SPSS
version 16.0 for Windows (SPSS, Inc., Chicago, IL, USA).
Differences between groups were determined using the Student's
t-test or one-way analysis of variance, three or more groups
compared using ANOVA. The Scheffe test was used as the post-hoc
test. All data are presented as the mean ± standard deviation. All
assays were independently performed a minimum of three times.
P<0.05 was considered to indicate a statistically significant
difference.
Results
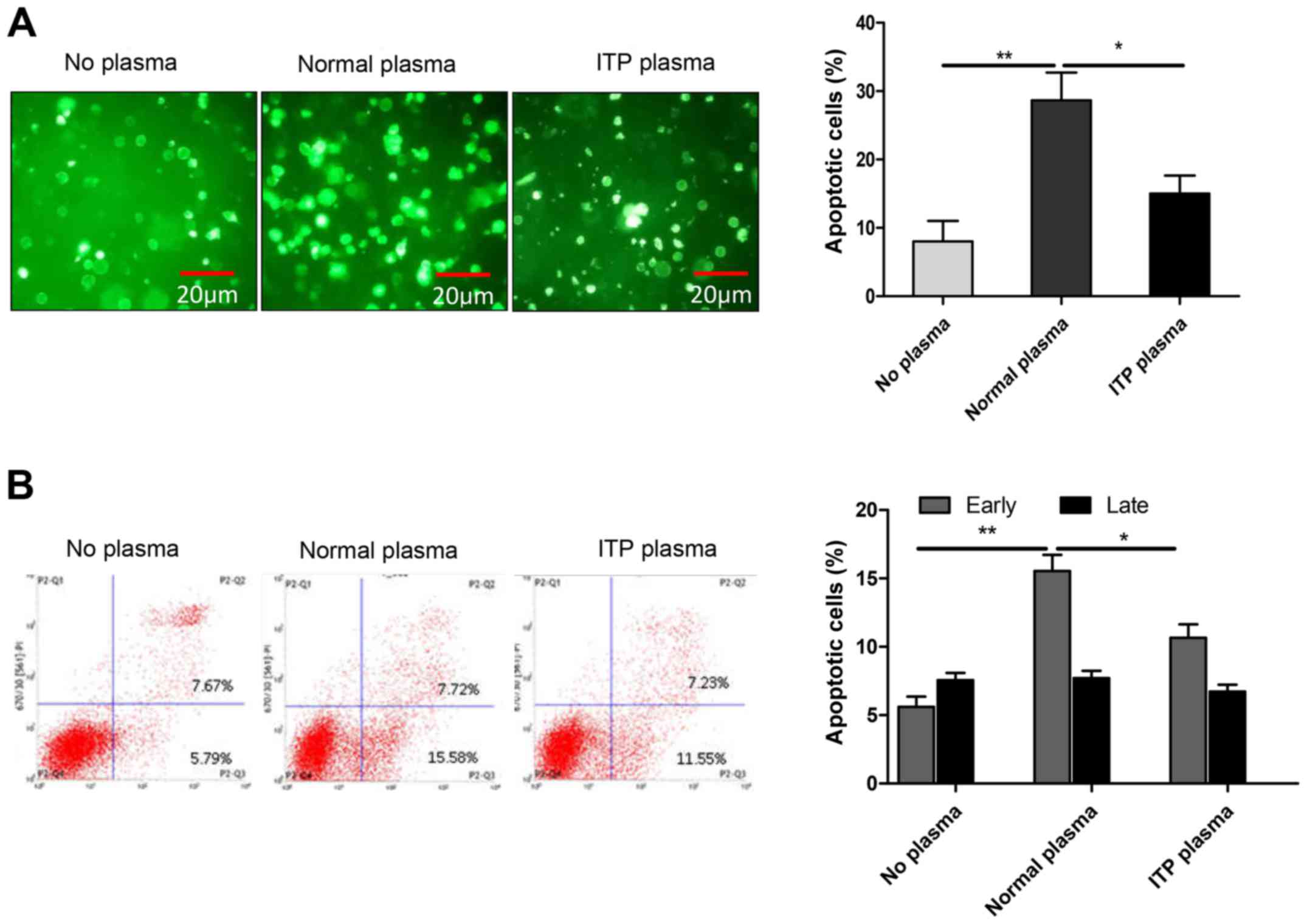
ITP plasma exhibits decreased rates of
apoptosis
In patients with ITP, the presence of apoptotic
abnormalities in megakaryocytes is the current diagnostic marker.
It is also accepted that megakaryocyte apoptosis in ITP patients is
dependent on caspase-3 activation and Bcl-2 (12). To investigate this theory, MEG-01
cells were treated with RPMI-1640 media, 10% normal plasma, or 10%
ITP plasma and apoptosis was evaluated by flow cytometric analysis
of Annexin V-FITC/PI staining. The results demonstrated that the
ITP plasma group exhibited decreased levels of apoptosis compared
with the normal plasma group (Fig.
1A). The rate of early-stage apoptosis in the ITP plasma group
was decreased compared with normal plasma group, but still
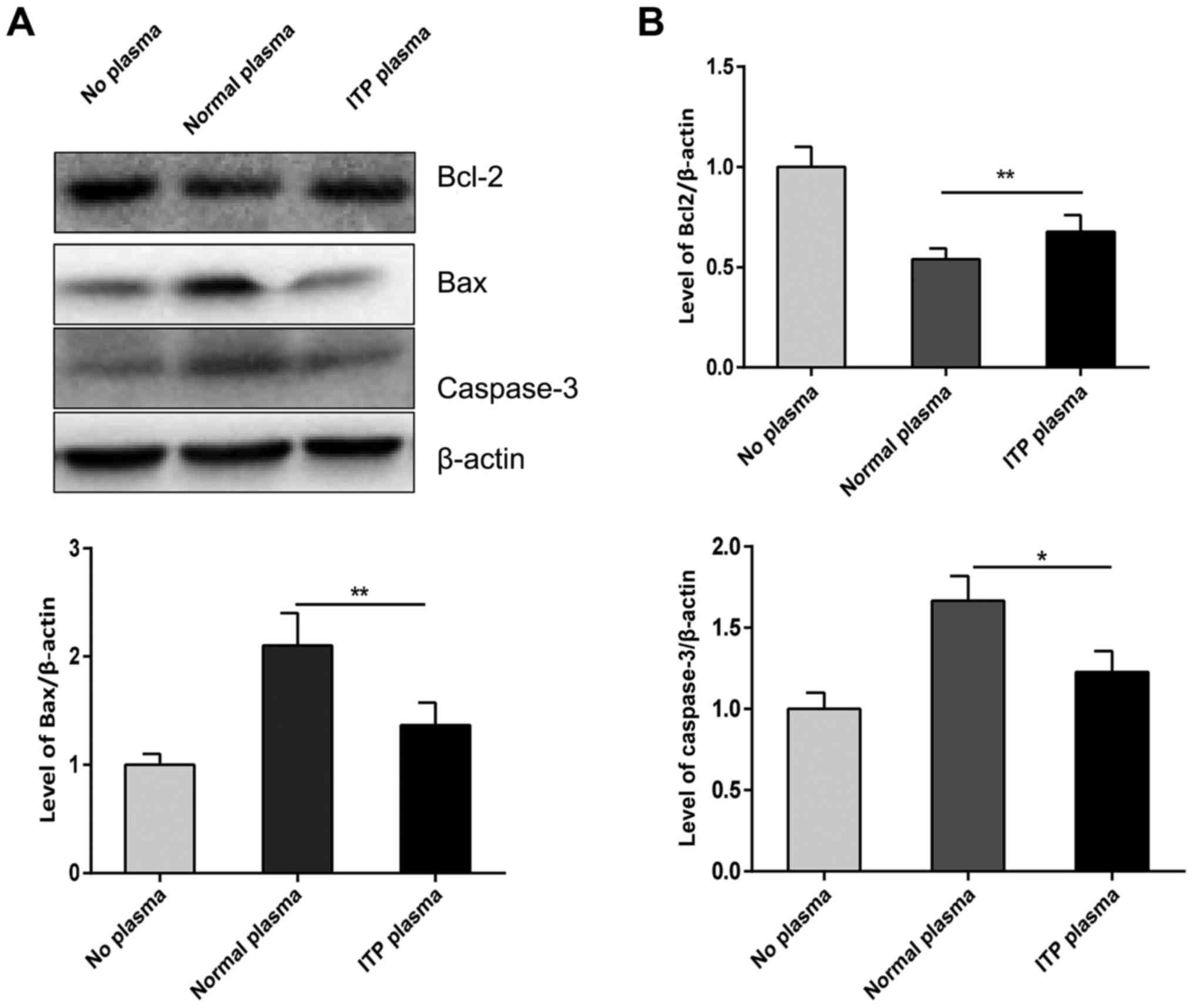
increased compared with no plasma group (Fig. 1B). Additionally, Fig. 2 revealed that cleaved caspase-3 and
Bax were downregulated in the ITP plasma group compared with the
normal plasma group; however, Bcl-2 was upregulated in the ITP
plasma group compared with the normal plasma group (Fig. 2). These results confirmed that
apoptosis abnormalities exist in ITP megakaryocytes.
Under normal conditions, the changes associated with
megakaryocyte differentiation occur mainly in the cytoplasm, with
few changes in the nucleus; however, initiation of nuclear lysis
was observed in the megakaryocytes. In the ITP plasma group,
vacuolization and nuclear condensation were observed in MEG-01
cells compared with the no plasma group; however, there were no
such morphological changes in the normal plasma group (Fig. 3).
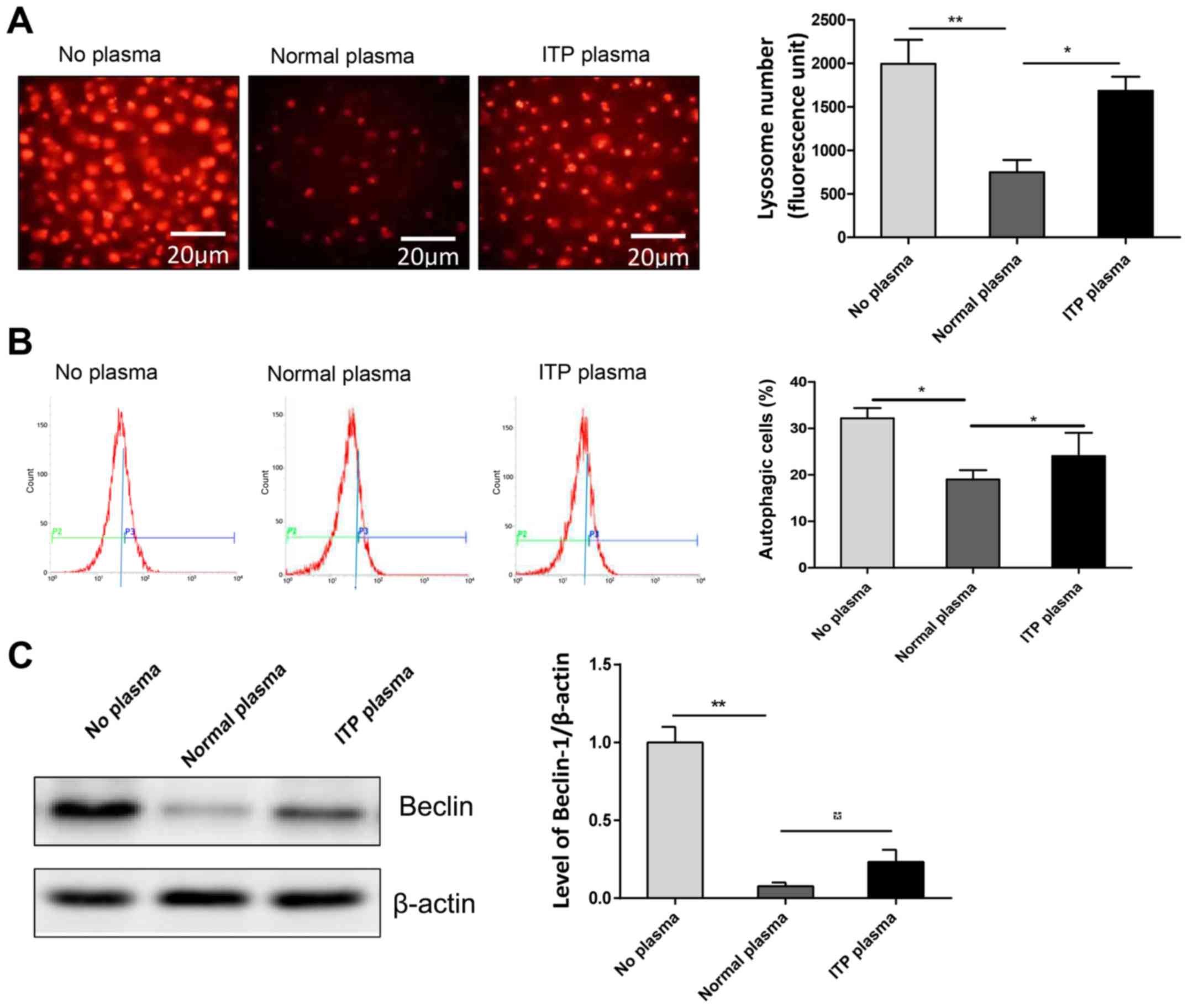
ITP plasma can induce autophagy
The causes of abnormal apoptosis in the
megakaryocytes in ITP patients remain unclear. A previous study
have demonstrated that apoptosis is closely associated with
autophagy, with apoptosis inhibiting autophagy, or vice versa
(13). Therefore, the occurrence
of autophagy in megakaryocytes of ITP patients was investigated by
Lyso-Tracker Red and ansylcadaverine analysis of MEG-01 cells as an
in vitro model. It was demonstrated that the number of
Lysosomes in ITP plasma group was increased compared with the
normal plasma group (Fig. 4A),
indirectly indicating that the proportion of autophagy in the ITP
plasma group was higher than that in the normal plasma group, but
still lower than that in the no plasma group (Fig. 4B). The western blot also confirmed
this result, revealing that ITP plasma exhibited increased levels
of Beclin-1 compared with the normal plasma group (Fig. 4C).
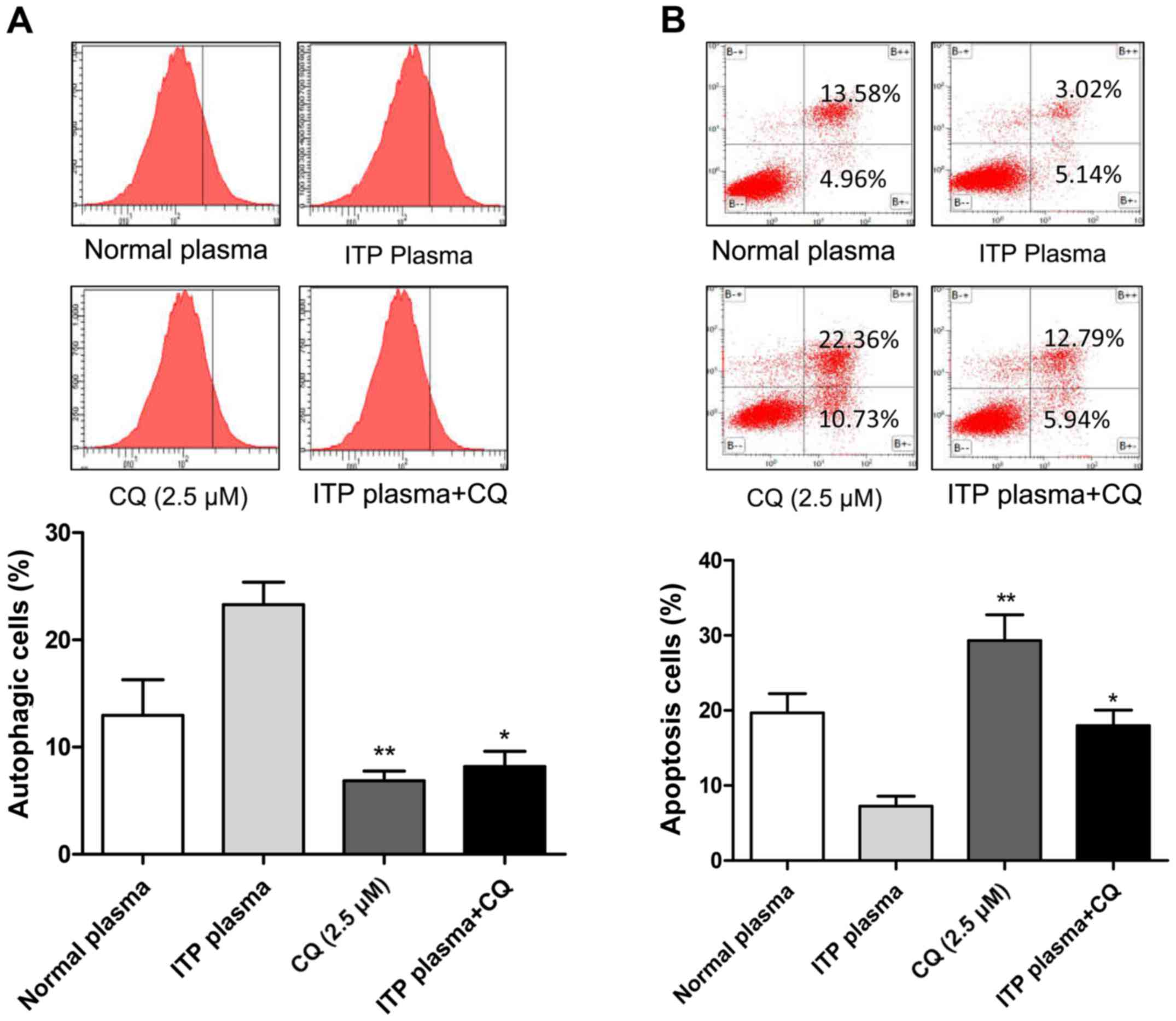
ITP plasma can induces autophagy and
suppresses apoptosis, which inhibited by CQ
CQ was used as an inhibitor of autophagy to
investigate whether ITP plasma suppresses apoptosis by autophagy in
MEG-01 cells. Western blotting indicated that when cells were
treated with CQ, the ITP plasma was unable to upregulate the
apoptosis-related inhibitory proteins (Fig. 5). Flow cytometry demonstrated
consistent results with western blotting, in that the ITP plasma
induces autophagy otherwise inhibited by CQ (Fig. 6A), which thereby suppresses
apoptosis compared with CQ group (Fig.
6B). Therefore, ITP plasma may serve a key role in development
of megakaryocyte autophagy.
Discussion
Investigations regarding the mechanism of autoimmune
diseases have focused mainly on the destruction of platelets,
decrease in platelet production and T lymphocyte immune imbalance
(3,14). Studies of the survival of
megakaryocytes in ITP patients are rare and few studies have
investigated the role of autophagy in ITP. Fabre et al
(15) revealed that many
autophagic vacuoles are present in the cytoplasm prior to
apoptosis, detected via transmission electron microscopy.
Houwerzijl et al (10)
demonstrated that patients with ITP exhibited mitochondrial and
endoplasmic reticulum formation in megakaryocytes, increased
vacuolization in the cytoplasm, thickening of the plasma membrane
and chromatin condensation in the nucleus; which may be due to
para-apoptosis or autophagic cell death. Platelet production
depends on compartmentalized caspase activation within
megakaryocytes, especially the activation of caspase-3, which leads
to apoptosis (16). The present
results indicated that caspase-3 activity decreased in the ITP
plasma group, which provides evidence of the apoptotic
abnormalities of megakaryocytes in ITP patients. Subsequent
investigations of morphological changes and Lyso-Tracker Red/MDC
assays confirmed the existence of autophagy in the ITP plasma
group. These findings indicated that caspase activation is
responsible for autophagy in megakaryocytes of ITP patients, thus
affecting the apoptotic process. The apoptotic pathways of
megakaryocytes are intrinsic and extrinsic, and both require Bcl-2
involvement (17). Furthermore,
recent studies have reported that Bcl-2 is a cross-over point
between autophagy and apoptosis in autoimmune disease, tumor, or
injury (7,18–20).
Furthermore, numerous studies have demonstrated the involvement of
Bcl-2 in both apoptosis and autophagy in diverse diseases (13,21).
This involvement depends on the formation of complexes containing
Bcl-2, Bax and Beclin-1 and their interactions, which determine
whether cells enter apoptosis or autophagy. Under conditions of
cellular stress, the reduction in Bcl-2 results in a decrease in
binding to Beclin-1, leading to an increased free Beclin-1, which
promotes autophagy. Alternatively, phosphorylation of Beclin-1
increases Beclin-1-Bcl-2 complexes, displacing Bax from Bcl-2 and
resulting in apoptosis (22). The
existence of such a change in ITP patients was investigated by
evaluation of alterations in cell morphology, apoptosis, autophagy,
and protein expression. The present results demonstrated that Bax
and Beclin-1 were downregulated, whereas Bcl-2 was upregulated in
ITP plasma compared with normal plasma, and this change may be
associated with ER stress; however, this requires further
investigation (23). When CQ was
used as an inhibitor of autophagy, this inhibited autophagy induced
by ITP. However, there are several limitations in the present
study. First, more case and functional verification assays in the
future studies are required. Furthermore, larger population-based
studies are needed in order to confirm the present results.
In conclusion, the present study demonstrated that
the ITP plasma induces autophagy and suppresses apoptosis.
Inhibition of autophagy may be a novel treatment strategy in ITP,
however this requires further investigation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
TM conceived and designed the present study. ZL
performed the experiments and TM performed data analysis and wrote
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Board of the Yongchuan Hospital of Chongqing Medical
University and informed consent was obtained from all
participants.
Patient consent for publication
Informed consent was obtained from all
participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li J, Van der Wal DE, Zhu G, Xu M,
Yougbare I, Ma L, Vadasz B, Carrim N, Grozovsky R, Ruan M, et al:
Desialylation is a mechanism of Fc-independent platelet clearance
and a therapeutic target in immune thrombocytopenia. Nat Commun.
6:77372015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou H, Hou Y, Liu X, Qiu J, Feng Q, Wang
Y, Zhang X, Min Y, Shao L, Liu X, et al: Low-dose decitabine
promotes megakaryocyte maturation and platelet production in
healthy controls and immune thrombocytopenia. Thromb Haemost.
113:1021–1034. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kashiwagi H and Tomiyama Y:
Pathophysiology and management of primary immune thrombocytopenia.
Int J Hematol. 98:24–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shimizu S, Kanaseki T, Mizushima N, Mizuta
T, Arakawa-Kobayashi S, Thompson CB and Tsujimoto Y: Role of Bcl-2
family proteins in a non-apoptotic programmed cell death dependent
on autophagy genes. Nat Cell Boil. 6:1221–1228. 2004. View Article : Google Scholar
|
|
5
|
Houwerzijl EJ, Blom NR, Van der Want JJ,
Esselink MT, Koornstra JJ, Smit JW, Louwes H, Vellenga E and de
Wolf JT: Ultrastructural study shows morphologic features of
apoptosis and para-apoptosis in megakaryocytes from patients with
idiopathic thrombocytopenic purpura. Blood. 103:500–506. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morishima N and Nakanishi K: Proplatelet
formation in megakaryocytes is associated with endoplasmic
reticulum stress. Genes Cells. 21:798–806. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee WS, Sung MS, Lee EG, Yoo HG, Cheon YH,
Chae HJ and Yoo WH: A pathogenic role for ER stress-induced
autophagy and er chaperone GRP78/BiP in T lymphocyte systemic lupus
erythematosus. J Leukoc Biol. 97:425–433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu L, Chen Y and Tooze SA: Autophagy
pathway: Cellular and molecular mechanism. Autophagy. 14:207–215.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Q, Luo XY, Jiang H, Yang MH, Yuan GH,
Tang Z and Wang H: Hydroxychloroquine facilitates autophagosome
formation but not degradation to suppress the proliferation of
cervical cancer SiHa cells. Oncol Lett. 7:1057–1062. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Houwerzijl EJ, Blom NR, Van der Want JJ,
Vellenga E and de Wolf JT: Megakaryocytic dysfunction in
myelodysplastic syndromes and idiopathic thrombocytopenic purpura
is in part due to different forms of cell death. Leukemia.
20:1937–1942. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xue E, Zhang Y, Song B, Xiao J and Shi Z:
Effect of autophagy induced by dexamethasone on senescence in
chondrocytes. Mol Med Rep. 14:3037–3044. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang L, Wang L, Zhao CH, Zhu XJ, Hou Y,
Jun P and Hou M: Contributions of TRAIL-mediated megakaryocyte
apoptosis to impaired megakaryocyte and platelet production in
immune thrombocytopenia. Blood. 116:4307–4316. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mariño G, Niso-Santano M, Baehrecke EH and
Kroemer G: Self-consumption: The interplay of autophagy and
apoptosis. Nat Rev Mol Cell Biol. 15:81–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou J, Zhou Y, Wen J, Sun X and Zhang X:
Circulating myeloid-derived suppressor cells predict disease
activity and treatment response in patients with immune
thrombocytopenia. Braz J Med Biol Res. 50:e56372017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fabre C, Carvalho G, Tasdemir E, Braun T,
Adès L, Grosjean J, Boehrer S, Métivier D, Souquère S, Pierron G,
et al: NF-kappaB inhibition sensitizes to starvation-induced cell
death in high-risk myelodysplastic syndrome and acute myeloid
leukemia. Oncogene. 26:4071–4083. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De Botton S, Sabri S, Daugas E, Zermati Y,
Guidotti JE, Hermine O, Kroemer G, Vainchenker W and Debili N:
Platelet formation is the consequence of caspase activation within
megakaryocytes. Blood. 100:1310–1317. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kile BT: The role of apoptosis in
megakaryocytes and platelets. Br J Haematol. 165:217–226. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mukhopadhyay S, Panda PK, Sinha N, Das DN
and Bhutia SK: Autophagy and apoptosis: Where do they meet?
Apoptosis. 19:555–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ruddy SC, Lau R, Cabrita MA, McGregor C,
McKay BC, Murphy LC, Wright JS, Durst T and Pratt MA: Preferential
estrogen receptor β ligands reduce Bcl-2 expression in
hormone-resistant breast cancer cells to increase autophagy. Mol
Cancer Ther. 13:1882–1893. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song DD, Zhang TT, Chen JL, Xia YF, Qin
ZH, Waeber C and Sheng R: Sphingosine kinase 2 activates autophagy
and protects neurons against ischemic injury through interaction
with Bcl-2 via its putative BH3 domain. Cell Death Dis.
8:e29122017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Su M, Mei Y and Sinha S: Role of the
crosstalk between autophagy and apoptosis in cancer. J Oncol.
2013:102710352013. View Article : Google Scholar
|
|
22
|
Li M, Gao P and Zhang J: Crosstalk between
autophagy and apoptosis: Potential and emerging therapeutic targets
for cardiac diseases. Int J Mol Sci. 17:3322016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhong JT, Xu Y, Yi HW, Su J, Yu HM, Xiang
XY, Li XN, Zhang ZC and Sun LK: The BH3 mimetic S1 induces
autophagy through ER stress and disruption of Bcl-2/Beclin 1
interaction in human glioma U251 cells. Cancer Lett. 323:180–187.
2012. View Article : Google Scholar : PubMed/NCBI
|