Introduction
Li-chloride-pilocarpine induced inflammation in the
brain cortex reaction by igniting model of temporal lobe and
hippocampus of epileptic seizure. In order to achieve our
experiment, we demand model rat must occur in the process of model
making status epilepticus. Status epilepticus (SE) is a prolonged
self-perpetuating seizure which requires prompt intervention to
prevent its injury and mortality and is usually treated initially
with a benzodiazepine, such as diazepam (1). However, if SE lasts >30–40 min, it
becomes progressively more refractory to these agents (2). Therefore, applying novel efficient
therapies for treating SE is required to reduce
Li-pilocarpine-induced inflammation and suppress neuronal damage.
In the present study, SE rat models in the present study were
identified as demonstrating SE >10 min with good survival
outcomes; epileptic seizures must achieve Racine stage IV-Vusing
Racine's scale (3). In addition,
Ghrelin, as an anti-inflammatory treatment, was administrated prior
to the administration of pilocarpine to induce SE and its efficacy
on inflammation was observed.
Ghrelin is the endogenous ligand of the growth
hormone secretagogue receptor (GHSR), discovered in 1999 by Kojima
et al (4). In addition to
its involvement in appetite regulation and glucose and lipid
metabolism (5), ghrelin has a
protective function in multiple systems, including the
cardiovascular and immune systems (6). The association between ghrelin and
inflammation has previously been examined: Dixit et al
(7) proposed that ghrelin
functions as an important anti-inflammatory factor, and is involved
in endocrinal regulation of the immune system. However, ghrelin has
also previously been reported to mediate nuclear factor (NF)-κB to
stimulate interleukin (IL)-8 production and function as a
pro-inflammatory factor in the colon (8). This inconsistency across systems
suggests that the function of ghrelin merits further study.
Epilepsy is a group of neurological diseases
characterized by abnormal excessive discharge of neurons in the
brain, bringing about a temporary impediment of brain function
(9). Although the exact
pathogenesis is unclear, previous studies have demonstrated that T
lymphocytes are involved in the epileptic immune response and
cell-mediated immunity disorders in patients with epilepsy
(10,11). Based on the previous evidence
supporting the involvement of ghrelin in the regulation of
inflammation, the present study investigated the effect of ghrelin
on NF-κB and tumor necrosis factor (TNF)-α gene and protein
expression levels in epileptic rats with pilocarpine-induced
cerebral cortex inflammation, and explored the anti-inflammatory
effect of ghrelin on the cerebral cortex, providing a novel target
for the prevention and treatment of epilepsy in children.
Materials and methods
Materials
Pilocarpine hydrochloride was purchased from
Sigma-Aldrich; Merck Millipore (Darmstadt, Germany); ghrelin was
obtained from Phoenix Pharmaceuticals, Inc. (Burlingame, CA, USA);
NF-κB, TNF-α and β-actin antibodies were purchased from Santa Cruz
Biotechnology, Inc. [Dallas, TX, USA; cat. nos. NF-κB P-65
(sc-109), TNF-α (sc-52791); TRIzol reagent was purchased from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA); reverse
transcription kit (NF-κB p65, sc-8008), Taq DNA Polymerase
and DNA Marker DL2000 were purchased from Takara Bio. Inc. (Otsu,
Japan); and SP-9000 Histostain-Plus kits were obtained from OriGene
Technologies, Inc. (Rockville, MD, USA)].
Animals
Healthy male Wistar rats (60 in total; ~3 weeks old;
40–50 g) were obtained from Shengjing Hospital Laboratory Animal
Centre of China Medical University (Shenyang, China). The present
study was approved by the ethics committee Qingdao Municipal
Hospital. Individuals were kept at 19±2°C in a quiet environment on
a diurnal cycle, and were breast-fed. Unweaned rats and young rats
were bred together with 12-h light/dark cycle. Rats were divided at
random into the normal control group (n=8), pilocarpine group
(n=8), pilocarpine + saline group (n=8) and pilocarpine + ghrelin
group (n=8).
Administration method
At 9:00 a.m., 3 mg/kg lithium chloride was injected
into the peritoneal cavity. The control group rats were
administered with physiological saline only. The following day at
8:30 a.m., 80 µg/kg ghrelin or saline was injected
intraperitoneally, then 30 min later 30 mg/kg pilocarpine was
injected to SE. The behavioral manifestations were observed and a
resultant epileptic seizure of Racine stage IV or V was used as the
inclusion criteria for further analysis (3). Seizure severity was ranked using
Racine's scale: 1, seizure consisted of immobility and occasional
facial clonus; 2, head nodding; 3, bilateral forelimb clonus; 4,
rearing; 5, rearing and falling. In addition to the four groups,
the experiments were performed with young mice, a group of young
mice, and their mothers could continue to have mice. These mice as
well as rats were kept at 19±2°C in a quiet environment on a
diurnal cycle, and were breast-fed. Unweaned rats and young rats
were kept together with 12-h light/dark cycle. Diazepam (10 mg/kg)
was injected 60 min following the epileptic seizure. Epileptic rats
were administered diazepam intraperitoneally to reduce the
mortality of these rats when undergoing an epileptic seizure.
Lithium chloride combined with pilocarpine was employed to produce
the epileptic rat model.
Immunohistochemistry
After 24 h following the onset of epileptic seizure,
under 2% lidocaine (~4.0–4.5 mg/kg, intraperitoneal injection)
abdominal anesthesia, the chest was opened and the heart exposed.
The ascending aorta of the left ventricle was intubated and 50 ml
cold saline followed by 50 ml 4% paraformaldehyde PBS was perfused
to fix the brain tissue at 4°C overnight. Fixed brain tissue was
placed in 20% sucrose for 12 h, and 30% sucrose for a further 12 h.
Following washing with PBS, structural parts of the cerebral cortex
were cut into 5-µm coronal sections, dried at room temperature for
6 min and then preserved at −20°C. A total of 9 sections were cut
from each rat cortex in each group (n=8). Brain tissue fixed in 4%
paraformaldehyde for 24 h, conventional materials and 20% sucrose
solution for 48 h and then embedded in paraffin. Sections of
samples were cleaned with 0.01 mol/l PBS 100 ml three times for 5
min. Paraffin-embedded sections were treated with a solution of
methanol and 0.3% hydrogen peroxide (methanol + 0.01 mol/l PBS 100
ml 80 ml + 30% hydrogen peroxide) for 30 min at room temperature
after three washes with 0.01 mol/l PBS for 5 min in room
temperature. Subsequently, paraffin-embedded sections were treated
with 0.3% Triton X-100 (30% Triton X-100 + 100 ml 0.01 mol/l PBS)
at 20°C for 30 min, followed by three washes with 0.01 mol/l PBS
for 5 min. Primary antibodies were used at the following dilutions:
NF-κB P-65 (cat. no. SC-372:1), 1:200 and TNF-α, (cat. no.
SC-8301:1) 1:300 and sections were incubated in a wet box at room
temperature for 1 h, then 4°C overnight; subsequently, the
temperature was increased to 37°C after 45 min out of the fridge.
Following incubation, the sections were incubated with secondary
antibodies NF-κB P-65 (cat. no. SC-8008:2), 1:250 and TNF-α, (cat.
no. SC-358919:2), 1:300 at 37°C at room temperature for 2 h then
washed with 0.01 mol/l PBS, three times, three min each
time. Immunohistochemical staining reagent. (Vector Laboratories,
Inc., Burlingame, CA, USA; cat. no. PK-6103) was used.
Immunohistochemical staining reagent was added, incubated at room
temperature for 30 min, and washed with PBS three times, each time
for 3 min. Then washed with distilled water for three times of 2
min each. Dehydrate: Put the slices in 50, 70, 80, 90, 95 and, 100%
ethanol for two min. Transparent: Place the slices in 100% xylene
for 10 min. Neutral resin 50 µl seal, room temperature
preservation.
Primary and secondary antibodies were replaced with
PBS for a blank control. NF-κB and TNF-α
immunohistochemically-positive cells were counted using dark-field
microscopy (CX31-lv320; Olympus Corporation, Tokyo, Japan) and
quantified using Image-Pro Plus image analysis software version 6.0
(Media Cybernetics, Inc., Rockville, MD, USA; Table I). A total of 5 views per field
were analyzed.
 | Table I.Effect of ghrelin on TNF-α and NF-κB
protein expression levels in the cortex of a pilocarpine-induced
epileptic rat model expressed as gray values via
immunohistochemical analysis (n=8). |
Table I.
Effect of ghrelin on TNF-α and NF-κB
protein expression levels in the cortex of a pilocarpine-induced
epileptic rat model expressed as gray values via
immunohistochemical analysis (n=8).
| Group | TNF-α | NF-κB |
|---|
| Normal control
group | 0.175±0.032 | 0.145±0.006 |
| Pilocarpine
group |
0.606±0.042a | 0.420±0.012 |
| Pilocarpine + saline
group |
0.595±0.150a | 0.474±0.056 |
| Pilocarpine + ghrelin
group |
0.254±0.061b |
0.275±0.026a |
Semi-quantitative reverse
transcription polymerase chain reaction (RT-PCR)
Surgical dislocation was used to remove head and
brain 24 h following the onset of epileptic seizure. Seizures
following 24 h, with 2% lidocaine (~4–4.5 mg/kg) abdomen
anesthesia, then the cerebral cortex of the brain was isolated.
Total RNA was extracted using TRIzol reagent. Following measurement
of RNA concentration with a UV spectrophotometer, a 25 µl RT
reaction was set up according to the kit instructions: 2 µg RNA was
added to 5 µl 5× loading buffer, 2.5 µl MgSO4 (40
mmol/l), 2.5 µl dNTP (2.5 mmol/l), 1 µl oligo (dT) primer, 40 µl
RNase inhibitor, 30 µl UAMV and Rnase-free H2O to 25 µl.
Following mixing, cDNA was synthesized at 94°C for 60 sec, 37°C for
60 sec and 120 sec at 72°C; 25 cycles. PCR primers were designed to
amplify TNF-α, NF-κB and β-actin cDNA by referring to previous
literature (12) and are
identified in Table II.
Amplification products were electrophorised on 1.5% agarose gels
(10 µl per lane) containing 20 g/l TAE buffer. UV camera systems
were used to acquire images and analysis software (SPSS version
19.0, IBM Corp., Armonk, NY, USA) was used to multiply the size and
strength of the strips as PCR product content. TNF-α and NF-κB mRNA
expression levels were calculated relative to β-actin.
 | Table II.TNF-α, NF-κB and β-actin primer
sequences. |
Table II.
TNF-α, NF-κB and β-actin primer
sequences.
| Gene | Forward primers
(5′-3′) | Reverse primers
(5′-3′) | Product size
(bp) |
|---|
| NF-κB |
TGCCGAGTGAACCGAAAC |
TGGAGACACGCACAGGAGC | 318 |
| TNF-α |
GGTGCCTATGTCTCAGCCTCTT |
GCTCCTCCACTTGGTGGTTT | 326 |
| β-actin |
AAATCGTGCGTGACATTAA |
CTCGTCATACTCCTGCTTG | 473 |
Statistical analysis
Data were analyzed using SPSS version 19.0 software
(SPSS, Inc., Chicago, IL, USA). Each set of data obtained were the
average values of 6 repeated experiments, and all data were
expressed as the mean ± standard deviation. One-way analysis of
variance was used to compare parameters between the different
groups, and Fisher's Least Significant Difference method was used
for pair comparison. P<0.05 was considered to indicate a
statistically significant difference.
Results
Ghrelin affects NF-κB and TNF-α
protein expression in the cerebral cortex of young rats with
pilocarpine-induced epilepsy
Compared with the cerebral cortex of young rats in
the normal control group (Fig. 1A
and Table II), TNF-α protein
expression was significantly increased 24 h subsequent to induction
of pilocarpine-induced SE (P<0.05; Fig. 1B and Table III). TNF-α protein expression was
also significantly increased (compared with control) 24 h
subsequent to induction of pilocarpine-induced SE when an i.p.
injection of saline was administered 30 min prior to pilocarpine
(P<0.05; Fig. 1C and Table III). However, in rats who
received an i.p. injection of ghrelin 30 min prior to pilocarpine
induction of SE, significantly fewer TNF-α positive cells were
observed in the cytoplasm of cerebral cortical neurons compared
with the pilocarpine and pilocarpine + saline groups (both
P<0.05; Fig. 1D and Table III).
 | Table III.Effect of ghrelin on TNF-α and NF-κB
protein expression levels in the cortex of a pilocarpine-induced
epileptic rat model, expressed as optical density values. |
Table III.
Effect of ghrelin on TNF-α and NF-κB
protein expression levels in the cortex of a pilocarpine-induced
epileptic rat model, expressed as optical density values.
| Group | TNF-α | NF-κB |
|---|
| Normal control
group | 0.175±0.032 | 0.145±0.006 |
| Pilocarpine
group |
1.098±0.043a | 0.920±0.512 |
| Pilocarpine + saline
group |
1.305±0.050a | 0.850±0.086 |
| Pilocarpine + ghrelin
group |
0.855±0.081b |
0.265±0.023b |
In addition, compared with the cerebral cortex of
young rats in the normal control group (Fig. 2A and Table II), NF-κB protein expression was
increased 24 h subsequent to induction of pilocarpine-induced SE
(P<0.05; Fig. 2B and Table III). NF-κB protein expression was
also increased (compared with control) 24 h subsequent to induction
of pilocarpine-induced SE when an i.p. injection of saline was
administered 30 min prior to pilocarpine (P<0.05; Fig. 2C and Table III). However, in rats who
received an i.p. injection of ghrelin 30 min prior to pilocarpine
induction of SE, significantly fewer NF-κB positive cells were
observed in the cytoplasm of cerebral cortical neurons compared
with the pilocarpine and pilocarpine + saline groups (both
P<0.05; Fig. 2D and Table III).
Ghrelin affects NF-κB and TNF-α mRNA
expression in the cerebral cortex of young rats with
pilocarpine-induced epilepsy
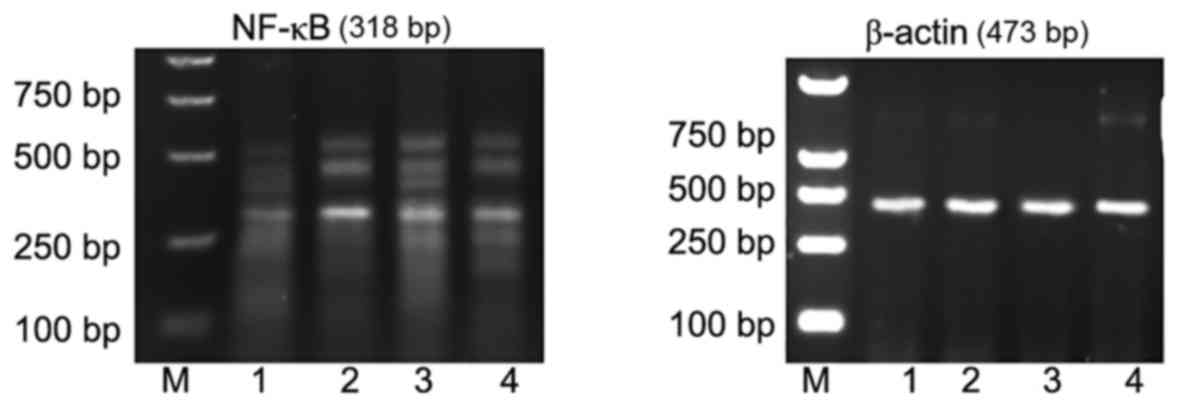
A260/A280 nm of extracted RNA was 1.8–2.0,
indicating that the total RNA purity was high, with no protein
pollution. Compared with the cerebral cortex of young rats in the
normal control group (Fig. 3, lane
1 and Table IV), TNF-α mRNA
expression was significantly increased 24 h subsequent to induction
of pilocarpine-induced SE (P<0.05; Fig. 3, lane 2 and Table IV). TNF-α mRNA expression was also
significantly increased (compared with the control) 24 h subsequent
to induction of pilocarpine-induced SE when an i.p. injection of
saline was administered 30 min prior to pilocarpine (P<0.05;
Fig. 3, lane 3 and Table IV). However, in rats who received
an i.p. injection of ghrelin 30 min prior to pilocarpine induction
of SE, TNF-α mRNA expression levels were significantly reduced in
the cytoplasm of cerebral cortical neurons compared with the
pilocarpine and pilocarpine + saline groups (P<0.05 and
P<0.05, respectively; Fig. 3,
lane 4 and Table IV).
 | Table IV.Effect of ghrelin on TNF-α and NF-κB
mRNA expression levels in the cortex of a pilocarpine-induced
epileptic rat model, expressed as optical density values. |
Table IV.
Effect of ghrelin on TNF-α and NF-κB
mRNA expression levels in the cortex of a pilocarpine-induced
epileptic rat model, expressed as optical density values.
| Group | TNF-α | NF-κB |
|---|
| Normal control
group | 0.907±0.023 | 0.747±0.069 |
| Pilocarpine
group |
1.667±0.016a |
1.907±0.075a |
| Pilocarpine +
saline group |
1.684±0.032a |
1.658±0.113a |
| Pilocarpine +
ghrelin group |
0.787±0.014b |
0.622±0.024b |
In addition, compared with the cerebral cortex of
young rats in the normal control group (Fig. 4, lane 1 and Table IV), NF-κB mRNA expression was also
significantly increased 24 h subsequent to induction of
pilocarpine-induced SE (P<0.05; Fig. 4, lane 2 and Table IV). NF-κB mRNA expression was also
significantly increased (compared with control) 24 h subsequent to
induction of pilocarpine-induced SE when an i.p. injection of
saline was administered 30 min prior to pilocarpine (P<0.05;
Fig. 4, lane 3 and Table IV). However, in rats who received
an i.p. injection of ghrelin 30 min prior to pilocarpine induction
of SE, NF-κB mRNA expression levels were significantly reduced in
the cytoplasm of cerebral cortical neurons compared with the
pilocarpine and pilocarpine + saline groups (both P<0.05;
Fig. 4, lane 4 and Table IV).
Discussion
Inflammation is an important contributor to the
pathophysiological mechanisms of epileptogenesis (13). Reducing inflammation helps to
reduce the extent and scope of epilepsy, reduce neuronal necrosis
and improve nerve cell recovery (10). Ghrelin is a peptide discovered in
the gastric tissue of rats by Kojima et al (4), and is a biologically active
endogenous ligand of GHSR, which promotes the secretion of growth
hormone. A further previous study revealed that NF-κB is a core
component of the inflammatory response, and ghrelin acts in an
anti-inflammatory manner by inhibiting TNF-α-inducing NF-κB
pathways (14). It has previously
been reported that 50% of newborn rats suffer from temporal lobe
hippocampal neuron loss and glial cell hyperplasia following
lasting epileptic seizure (15).
Therefore, inhibition of the inflammatory reaction following
seizures, to reduce neuronal death and fibrous tissue regeneration,
may be important to prevent excessive damage caused by epilepsy in
children and adults. The present study observed the effect of
ghrelin on inflammatory factors following pilocarpine-induced
seizure in immature rats with epilepsy.
Seizures result in inflammation of the central
nervous system, and in the rodent brain NF-κB, cytokines,
chemokines, cell adhesion molecules and inflammatory molecules,
including complement molecules, are expressed (16). When ghrelin was incubated with
mononuclear cells or T cells in the presence of inflammatory
stimuli, the inflammatory stimuli-induced secretion and expression
of IL-6 and TNF-α, two pro-inflammatory cytokines, was reduced
(17). This inhibition of
inflammatory cytokines has been confirmed in a murine model of
LPS-induced endotoxemia (7). TNF-α
enhances the expression of endothelial adhesion molecules and
increases capillary permeability, resulting in the infiltration of
inflammatory cells to the site of infection and eventual tissue
necrosis (18). TNF-α also
stimulates and facilitates the release of other cytokines,
including IL-1, IL-6 and IL-8. IL-6 is a glycoprotein with a
molecular weight of 21–26 kDa and is primarily derived from
monocytes, macrophages and endothelial cells, which are an
important source of pro-inflammatory cytokines. Upon entering the
circulatory system IL-6 initiates the hepatic synthesis of acute
phase proteins, stimulates thousands of bone marrow cells, B cell
generation and conversion, and promotes the activation of
inflammatory cells, among other inflammatory responses (19). Using a pilocarpine-induced
epileptic rat model, mRNA and protein expression levels of NF-κB
and TNF-α expression were measured using immunohistochemistry and
semi-quantitative RT-PCR in the present study. NF-κB and TNF-α
expression was inhibited by ghrelin treatment, and this result
indicates that ghrelin reduces seizure-induced inflammation in
young rats with pilocarpine-induced epilepsy by reducing NF-κB and
TNF-α. Further studies are required to determine whether reduction
of seizure-induced cortical damage results in improved disease
prognosis.
Two major types of feedback adjustment apply to the
NF-κB activation process in vivo, one of which occurs via
extracellular positive feedback: TNF-α and IL-lβ expression induces
the activation of NF-κB, while activation of NF-κB increases TNF-α
and IL-lβ gene transcription, resulting in increased TNF-α and
IL-lβ production and release which continues to activate NF-κB
(20). In the central nervous
system, NF-κB is expressed in cell types including neurons,
astrocytes, microglia, oligodendrocytes and brain vascular
endothelial cells (21). Activated
NF-κB combines with appropriate target sequences in the nucleus,
regulating transcription of target gene activity, including genes
involved in inflammation, the immune response and the cell
apoptosis process (22). NF-κB in
brain vascular endothelial cells and glial cells is activated
during brain ischemia, and promotes the transcription of TNF-α,
IL-6 and intercellular adhesion molecule-1 (ICAM-l)
pro-inflammatory genes (23).
Following brain injury, NF-κB, pro-inflammatory
cytokines and the inflammatory response form a complex network that
increases the severity of brain injury (24). NF-κB activation in the central
nervous system stimulates the expression of inflammatory cytokines
including TNF-α and IL-6, and acts on ICAM-1 expression to promote
leukocyte adhesion and migration to the brain, causing long-term
pathological changes following traumatic brain injury (25). The pilocarpine-induced epilepsy
model used in the present study, which demonstrated increases in
TNF-α and NF-κB expression 24 h following epileptic seizure,
embodied this response well. Despite not entirely suppressing this
increase, ghrelin intervention significantly reduced the increase
of these three indicators. The anti-inflammatory effect of ghrelin
is apparent in these results.
To summarize, ghrelin treatment reduced levels of
pro-inflammatory indicators, TNF-α, IL-6 and NF-κB, in immature
rats with pilocarpine-induced epilepsy 24 h following seizure. This
effect may inhibit seizures caused by inflammatory necrosis of
cortical neurons following brain injury, reducing overall brain
damage. Further ghrelin-focused research will provide a deeper
understanding of its protective effect against seizure-induced
brain injury, and may open up a novel therapeutic approach for the
treatment of epilepsy in children.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Qingdao Key
Health Discipline Development Fund and the Qingdao Outstanding
Health Professional Development Fund (2017).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KH was involved in the design of the experiment,
drafted and revised the manuscript, collected and processed the
specimens, was responsible for the collection and analysis of the
data and supervised QYW, RYZ, CXW, SYL, YW and WPT. QYW, RYZ, CXW,
SYL, YW and WPT were involved in the design of the experiment,
revised the important technical and theoretical content and
provided final approval of the version to be published. All the
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Qingdao Municipal Hospital (Qingdao, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen J, Naylor DE and Wasterlain CG:
Advances in the pathophysiology of status epilepticus. Acta Neurol
Scand Suppl. 186:7–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jones DM, Esmaeil N, Maren S and Macdonald
RL: Characterization of pharmacoresistance to benzodiazepines in
the rat Li-pilocarpine model of status epilepticus. Epilepsy Res.
50:301–312. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Racine RJ: Modification of seizure
activity by electrical stimulation II. Motor seizure.
Electroencephalogr Clin Neurophysiol. 32:281–294. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kojima M, Hosoda H, Date Y, Nakazato M,
Matsuo H and Kangawa K: Ghrelin is a growth-hormone-release in
gacylated peptide from stomach. Nature. 402:656–660. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Gils C and Cox PA: Ethnobotany of
nutmeg in the spice islands. J Ethnopharmacol. 42:117–124. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang X and Yang X: Different products of
nutmeg essential oil GC-MS analysis. China J Chin Mater Med.
32:1669–1675. 2007.(In Chinese).
|
|
7
|
Dixit VD, Schaffer EM, Pyle RS, Collins
GD, Sakthivel SK, Palaniappan R, Lillard JW Jr and Taub DD: Ghrelin
inhibits leptin- and activation-induced proinflammatory cytokine
expression by human monocytes and T cells. J Clin Invest.
114:57–66. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hattor IM, Yang XW, Miyashiro H and Namba
T: Inhibitory effects of monomeric and dimericphenylpropanoids from
mace on lipid peroxidation in vivo and in vitro. Phytother Res.
7:395–401. 1993. View Article : Google Scholar
|
|
9
|
Kong F, Li J and Chen Y: Significance of
aegis monitoring in diagnosis of epilepsy shaped. Chin Gen Pract.
8:19732005.(In Chinese).
|
|
10
|
Fang ML, Ren SS, Wu CY, et al: Children
with epilepsy T correlation analysis of cellular immune function
and its risk factors. J Clin Exp Med. 6:43–44. 2007.
|
|
11
|
Li G, Bauer S, Nowak M, et al: Cytokines
and epilepsy. Seizure. 20:249–256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao S, Li H, Yao X, Li L, Jiang L, Zhang
Q, Zhang J, Liu D and Lu H: Enzymatic characterization of two
acetyl-CoA synthetase genes from Populus trichocarpa. Springerplus.
5:8182016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vezzani A and Granata T: Brain
inflammation in epilepsy: Experimental and clinical evidence.
Epilepsia. 46:1724–1743. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li WG, Gavrila D, Liu X, Wang L,
Gunnlaugsson S, Stoll LL, McCormick ML, Sigmund CD, Tang C and
Weintraub NL: Ghrelin inhibits proinflammatory responses and
nuclear factor-kappaB activation in human endothelial cells.
Circulation. 109:2221–2226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dunleavy M, Shinoda S, Schindler C, Ewart
C, Dolan R, Gobbo OL, Kerskens CM and Henshall DC: Experimental
neonatal status epilepticus and the development of temporal lobe
epilepsy with unilateral hippocampal sclerosis. Am J Pathol.
176:330–342. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De Simoni MG, Perego C, Ravizza T, Moneta
D, Conti M, Marchesi F, De Luigi A, Garattini S and Vezzani A:
Inflammatory cytokines and related genes are induced in the rat
hippocampus by limbic status epilepticus. Eur J Neurosci.
12:2623–2633. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stasi C and Milani S: Functions of ghrelin
in brain, gut and liver. CNS Neurol Disord Drug Targets.
15:956–963. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hughes CB, El-Din AB, Kotb M, Gaber LW and
Gaber AO: Calcium channel blockade inhibits release of TNF alpha
and improves survival in a rat model of acute pancreatitis.
Pancreas. 13:22–28. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leser HG, Gross V, Scheibenbogen C,
Heinisch A, Salm R, Lausen M, Rückauer K, Andreesen R, Farthmann EH
and Schölmerich J: Elevation of serum interleukin-6 concentration
precedes acute-phase response and reflects severity in acute
pancreatitis. Gastroenterology. 101:782–785. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liou HC: Regulation of the immune system
by NF-kappaB and IkappaB. J Biochem Mol Biol. 35:537–546.
2002.PubMed/NCBI
|
|
21
|
Mattson MP and Camandola S: NF-kappaB in
neuronal plasticity and neurodegenerative disorders. J Clin Invest.
107:247–254. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Z and Andersson R: NF-kappaB
activation and inhibition: A review. Stroke. 18:99–106. 2002.
|
|
23
|
Clemens JA: Cerebral ischemia: Gene
activation, neuronal injury, and the protective role of
antioxidants. Free Radic Biol Med. 28:1526–1531. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hang CH, Shi JX, Li JS, Wu W and Yin HX:
Concomitant upregulation of nuclear factor-κB activity,
proinflammatory cytokines and ICAM-1 in the injured brain after
cortical contusion trauma in a rat model. Neurol India. 53:312–317.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim EJ, Kwon KJ, Park JY, Lee SH, Moon CH
and Baik EJ: Effects of peroxisome proliferator-activated receptor
agonists on LPS-induced neuronal death in mixed cortical neurons:
Associated with iNOS and COX-2. Brain Res. 941:1–10. 2002.
View Article : Google Scholar : PubMed/NCBI
|