Introduction
Obesity is a life-threatening condition that affects
>600 million patients worldwide (1). Obesity has become a global public
health issue and is a major contributor to numerous diseases
(2). Over the past four decades,
global obesity prevalence displayed an increasing trend. If the
present trends continue, global obesity prevalence is expected to
reach 18% in men and surpass 21% in women by 2025 (1). Notably, the proportion of patients
with obesity in the north of the Northern hemisphere is greater
than in the south, with the highest proportion being observed in
the northeast region (1,3,4).
While there are numerous reasons such as diet (5) for the aforementioned geographical
variations, it is worth noting that the northern regions of the
Northern hemisphere [where the obesity incidence and body mass
index (BMI) are highest] are significantly colder than the southern
regions. These observations are consistent with Bergmann's rule,
which states that, within a polytypic warm-blooded species, body
size increases with decreasing the mean temperature of its habitat
(6).
In contrast to findings of higher obesity rates or
BMIs in colder climates, cold exposure has recently been proposed
as an intervention for the prevention and management of obesity.
This is associated with the effect of cold exposure to engage
metabolically active adipose tissue. There are three different
types of adipose tissue in humans and animals, namely white adipose
tissue (WAT), brown adipose tissue (BAT) and beige adipose tissue
(BeAT) (7). BeAT is sometimes
called brite adipose tissue (brown in WAT) or recruitable BAT
(rBAT), as it resembles classical brown adipocytes within
predominantly WAT and the fact quantity of BeAT in the body is
augmented by cold exposure (8).
BAT and BeAT are both able to use glucose and fatty acids for
non-shivering thermogenesis through the action of transmembrane
proteins in mitochondria that uncouple fuel oxidation by
maintaining a protein gradient producing heat, notably uncoupling
protein 1 (UCP-1). The action of UCP-1 in BAT and BeAT increases
body temperature while consuming energy. Thus, an increase in BAT
mass may prevent the development of obesity (9,10).
Cold exposure has been shown to increase the expression levels of
UCP-1 in BAT and BeAT in humans (11,12).
This change is associated with an increase in non-shivering
thermogenesis and energy expenditure. Cold exposure is a promising
method of reducing obesity while also reducing whole body insulin
resistance and improving glucose metabolism in humans (13,14).
These benefits have been proposed to occur via cold
stimulation-induced activation of sympathetic neural pathways
through BAT and BeAT (15).
Despite the aforementioned reports of cold exposure
in mice and humans leading to activation of BAT and BeAT, it is
also conceivable that cold exposure could lead to adverse metabolic
outcomes. Notably, subjecting the body to cold is a ‘stressful’
condition that activates the sympathetic nervous system (16). This not only activates BAT
(15) but also activates
feeding-related mechanisms (17).
For example, under cold stress and during fasting, agouti related
peptide (AgRP) neurons in the hypothalamic arcuate nucleus are
activated to promote feeding behavior (17). Such a mechanism could potentially
counteract the effect of increased energy expenditure to induce
weight loss by increasing food intake.
In order to clarify the effect of chronic cold
exposure on energy balance and glucose homeostasis, and to
elucidate the potential central mechanisms responsible for any of
these effects, the present study investigated the effects of
chronic cold exposure in mice subjected to daily cold exposure (1 h
standing in ice-cold water) on energy intake, body weight, adipose
tissue mass, glucose and insulin tolerance, as well as the
potential underlying neuronal pathways. The present neuronal
investigations were focused on the central amygdala and
hypothalamus, since the central amygdala is a key brain area
involved in the neural circuitry of stress responses (18), while the hypothalamus is the main
site of the brain modulating feeding behavior and energy
homeostasis, and both areas are interconnected (19). Particularly, the neurotransmitters
of interest were neuropeptide Y (NPY) and brain-derived neurotropic
factor (BDNF). NPY is prominently produced in the central amygdala
and the arcuate hypothalamic nucleus (ARC), while BDNF is mainly
expressed in the ventromedial hypothalamic nucleus (VMH). NPY
stimulates (20) while BDNF
reduces food intake (21,22). In addition, hypothalamic NPY was
considered a ‘stress molecule’ that is upregulated throughout
various regions of the brain, including the central amygdala and
hypothalamus, in response to cold stress (23,24).
The present study also investigated growth hormone releasing
hormone (GHRH), because it is known that neurons from the amygdala
project to GHRH neurons in the paraventricular nucleus (PVN), and
GHRH is an important regulator of peripheral glucose metabolism
(25). The present study
investigated mice that were fed either a standard chow diet or a
high-fat diet (HFD), since hypothalamic NPY expression is
upregulated not only by cold stress but also by HFD (26,27),
and overexpression of NPY in the brain affects peripheral
metabolism, including insulin resistance, thereby potentially
revealing possible changes in response to cold stress.
Materials and methods
Ethics statement and general animal
care
All animal experimental protocols were approved by
the Third Military Medical University Animal Care Committee
(Chongqing, China) and were performed according to the National
Institutes of Health (NIH) Guide for the Care and Use of Laboratory
Animals (NIH publication no. 8023). A total of 56 mice (C57BL/6)
were used in the study (28 mice for the in vivo
investigations of energy intake, body weight, adipose tissue mass,
glucose and insulin tolerance, and in situ hybridization
(Fig. 1A). In total, 28 mice were
used for investigations of central c-fos expression, and 28
transgenic mice expressing green fluorescent protein (GFP) under
the control of the NPY promoter (Npy-hrGFP mice, stock no. 006417,
which had been obtained from the Jackson Laboratory, Bar Harbor,
ME, USA and further bred in our facility.) were used to determine
whether NPY neurons in NPY-GFP mice were activated by cold exposure
and HFD. All mice were housed under conditions of controlled
temperature (22°C) and illumination (12 h light-dark cycle,
starting at 7 am) with ad libitum access to water and
standard chow (Gordon's Specialty Stock Feeds, Yanderra, New South
Wales, Australia). The standard chow diet provided 8% of energy
from fat, 21% from protein and 71% from carbohydrates, with a total
energy content of 10.88 kJ/g.
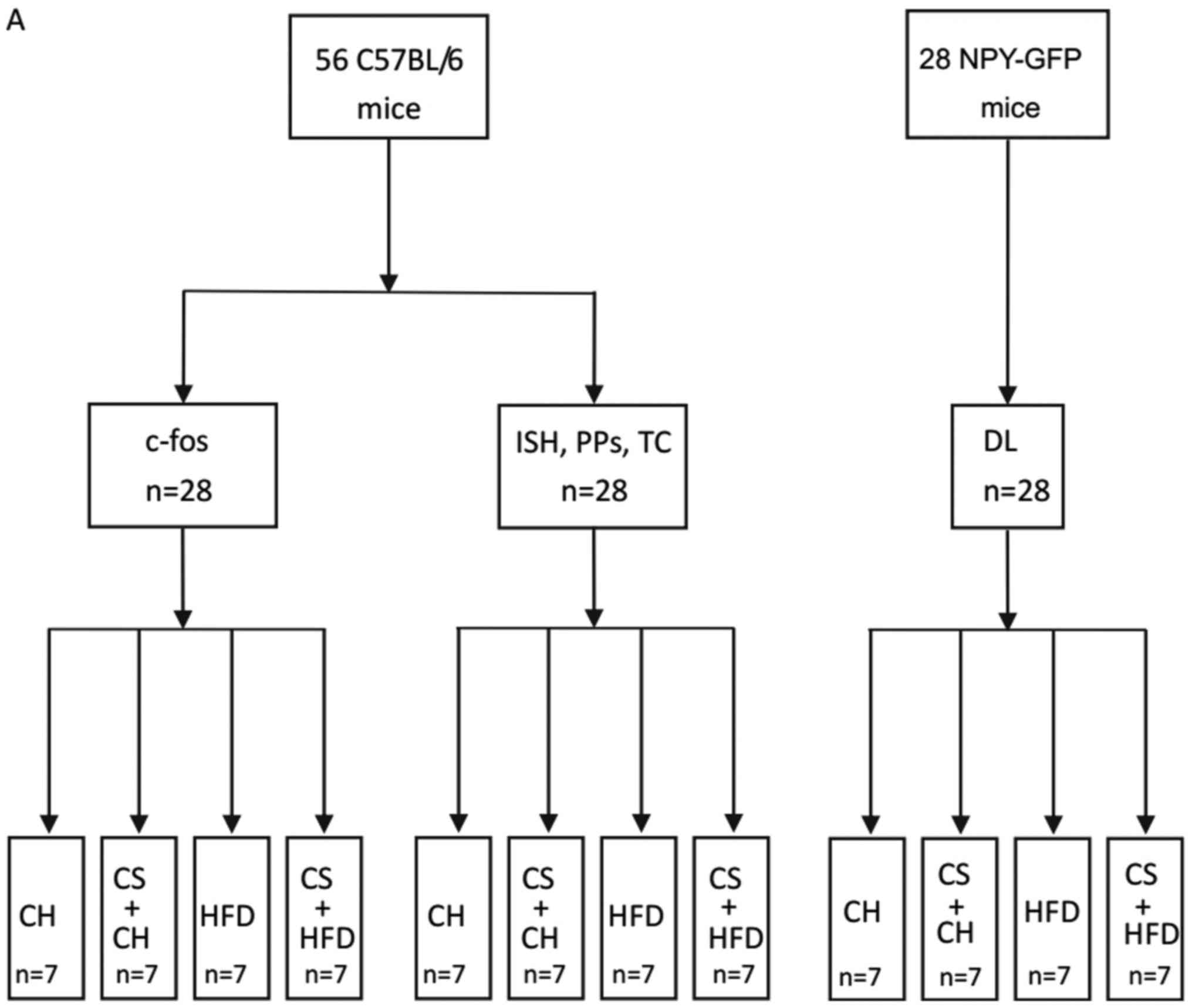 | Figure 1.Flowchart of the methodology employed
in the present study. (A) Animal groupings. (B) Timeline of the
experiments performed. CH, standard chow diet; CS, cold stress;
HFD, high-fat diet; c-fos, c-fos immunoreactivity test; ISH, in
situ hybridization; PPs: physiological parameters testing; TC,
tissue collection; BW, body weight; FI, food intake; GTT, glucose
tolerance test; ITT, insulin tolerance test. |
HFD, cold exposure, and measurement of
their effects on body weight and food intake
At 10 weeks of age, the 56 mice to be used in the
present study were transferred from housing conditions of 3–4 mice
per cage to individual cages. Half of the mice were fed a standard
chow diet, while the other half were fed a HFD (Gordon's Specialty
Stock Feeds), which provided 23.0% of energy from fat, 19.4% from
protein, 48.2% from carbohydrates, 4.7% from crude fiber, 4.7% from
acid detergent fiber, with a total energy content of 19.98 kJ/g.
Half of the mice on each diet were left to stand in ice-cold water,
which was 0.5 cm in depth, for 1 h per day for 7 weeks. Non-cold
exposed mice also stood on the water but at room temperature. 28 of
the mice were weighed at the same time of the day once per week.
Food intake was measured over 24 h when mice were 10, 12, 14 and 17
weeks of age (Fig. 1B). Energy
intake (kJ/day) was calculated as [(weight of food placed in the
hopper)-(weight of food remaining in the hopper after 24
h)]x(energy density of food in kJ/g)].
Measurement of effects of HFD and cold
exposure on glucose and insulin tolerance
At 16 weeks of age, i.e., 6 weeks after commencing
HFD and cold exposure interventions, the mice were fasted for 16 h
and injected intraperitoneally with a 10% D-glucose solution at a
dose of 1.0 g per kg body weight. Blood samples were collected from
the tip of the tail at 0, 15, 30, 60 and 90 min after glucose
administration, and blood glucose levels were measured using a
glucometer (Accu Check II; Roche, Castle Hill, New South Wales,
Australia). Two days later, the mice were fasted for 6 h from 8:00
a.m. and were injected intraperitoneally with insulin at a dose of
1.0 IU per kg body weight. Blood samples were obtained from the
tail tip at 0, 15, 30 and 60 min after insulin injection for
determination of glucose levels as stated above. Cold exposure was
performed subsequently to the glucose and insulin tolerance tests
on the same day.
Tissue collection upon HFD and cold
exposure
At 17 weeks of age, 5 days after the insulin
tolerance tests, a total of 28 of the mice from the four
experimental groups mentioned above were sacrificed by cervical
dislocation followed by decapitation. The brain was removed and
frozen on an aluminium plate on dry ice, and then stored at −70°C
until in situ hybridization. The interscapular BAT as well
as WAT depots (inguinal, epididymal, mesenteric and
retroperitoneal) were removed and weighed (17,28).
The weights of these WAT depots were summed together and expressed
as total WAT weight.
Determination of changes in c-fos
immunoreactivity in the central amygdala in response to HFD and
cold exposure
The remaining 28 mice from the four experimental
groups were used for this experiment (Fig. 1). Upon anesthesia with 100 mg/kg
ketamine and 20 mg/kg xylazine (Parke Davis-Pfizer, Sydney, New
South Wales, Australia and Bayer AG, Leverkusen, Germany,
respectively), mice were perfused via the left cardiac ventricle
with saline and then 4% paraformaldehyde. The brain was removed and
soaked in a solution of 30% sucrose overnight, prior to being cut
into 30-µm thick coronal sections using a microtome. Brain c-fos
immunoreactivity was detected via immunohistochemistry using
techniques described previously (29). The number of cells exhibiting
positive c-fos immunoreactivity was counted using a Zeiss Axioplan
light microscope (Carl Zeiss Imaging Solutions GmbH, Munich,
Germany).
Effect of HFD and cold exposure on
NPY-expressing neurons in the central amygdala
In order to investigate how NPY neurons in the
central amygdala respond to HFD and cold exposure, 28 transgenic
mice expressing GFP under the control of the mouse NPY promoter
(NPY-GFP mice) were used. The brain tissues from these mice, upon
exposure to ultraviolet light, displayed fluorescence emission in
all or the majority of known NPY neurons in the brain (30). For double labeling co-localization
experiments, 5 mice from the cold exposure with HFD group were
exposed to cold stress as described above for 1 h per day for 7
weeks, and were then sacrificed by cervical dislocation and
decapitation subsequent to perfusion. The brains were dissected,
placed overnight in 30% sucrose, and cut into coronal sections of
30 µm thickness using a microtome. Upon rinsing in PBS, the slides
were incubated with the primary antibody rabbit anti-mouse c-fos
(diluted at 1:2,000; Santa Cruz Biotechnology Inc., Dallas, TX,
USA). Next, the secondary antibody Alexa Fluor® 594 goat
anti-rabbit immunoglobulin G (A11037; Life Technologies; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) diluted at 1:250 was
added. Red c-fos stain in the c-fos immunohistochemistry test and
GFP-positive neurons in GFP-NPY transgenic mice were visualized and
counted under a Zeiss Axiophot microscope (Imaging Solutions
GmbH).
In situ hybridization to determine the
mRNA expression of Bdnf and Ghrh in the VMH and PVN, respectively,
in response to HFD and cold exposure
The frozen brain tissues collected were used for
radioactive in situ hybridization. For that purpose, DNA
oligonucleotides complementary to mouse Bdnf
(5′-CCGAACCTTCTGGTCCTCATCCAGCAGCTCTTCGATGACGTGCTCA-3′) and
Ghrh (5′-GCTTGTCCTCTGTCCACATGCTGTCTTCCTGGCGGCTGAGCCTGG-3′),
were used, which were labeled with [35S] thio-dATP
(Amersham Biosciences UK, Ltd., Little Chalfont, UK) using terminal
deoxynucleotidyl transferase (Roche, Mannheim, Germany). The mRNA
expression levels of Bdnf and Ghrh were evaluated by
measuring silver grain densities over individual neurons from
photo-emulsion-dipped sections, as described previously (31).
Statistical analysis
Data were presented as the mean ± standard error of
the mean. Two-way analysis of variance (ANOVA) was used for
analysis of body weight, energy intake, glucose levels, tissue
weight, BAT weight, c-fos, NPY, Bdnf mRNA and Ghrh
mRNA expression levels considering cold exposure and dietary
effects. Two-way repeated ANOVA was used to analysis body weight
and energy intake data. GraphPad Prism 6 software (GraphPad
Software, Inc., La Jolla, CA, USA) was used to analyze the levels
of mRNA expression. Bonferroni post hoc multiple comparison tests
were performed following ANOVA to identify differences among means.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Synergetic effect of cold exposure and
HFD on body weight and food intake
To evaluate the effect of different diets and cold
exposure, the body weight and food intake of mice were measured
weekly. During 7 weeks of treatment, all the mice gained weight but
at different rate. Chow-fed mice exposed to cold displayed a higher
weight gain than chow-fed mice at room temperature. Similarly, cold
exposure in HFD-fed mice led to more weight gain than exposure to
room temperature, which implied that cold exposure on HFD induced
weight gain in the mice. There is one obvious difference between
the two groups of mice that were exposed to cold condition for 1 h,
i.e., the body weight of HFD-fed mice was larger than the body
weight of chow-fed mice. In addition, among the four groups of
mice, the HFD-fed mice exposed to cold were the heaviest (Fig. 2A). Thus, HFD intensified the effect
of cold exposure on body weight in mice. By measuring food intake
of mice regularly, the weight growth curve of mice was similar to
the increasing curve of food intake, which suggested that weight
gain and food intake were positively correlated (Fig. 2B). The outcome suggested the extra
energy received by mice may increase energy storage as fat in the
body, thus increasing the body weight in these mice.
Cold exposure worsens glucose
metabolism in HFD-fed mice
The mice that suffered cold exposure stored more
energy than those without cold exposure. Combined with HFD, the
mice exposed to cold were prone to become obese. To investigate
whether cold exposure and HFD affected glucose metabolism and the
function of insulin, glucose and insulin tolerance tests were
carried out. In the standard chow-fed mice, the cold exposure group
exhibited significant slower glucose clearance ability than mice
without chronic cold exposure. Remarkably, the HFD-fed mice that
experienced cold displayed the worst glucose tolerance (Fig. 2C), which was consistent with the
areas under the glucose tolerance curves results (Table I). In addition, by measuring blood
glucose level following injection of insulin, it was observed that
there was no significant difference between mice exposed to cold
and mice without cold exposure on standard chow diet. However, the
blood glucose level of HFD-fed mice was notably higher than that of
standard chow-fed mice (Fig. 2D).
The areas under the insulin tolerance curves showed the same
results (Table I). These results
indicated that HFD made mice less sensitive to insulin.
 | Table I.Effect of cold exposure and HFD on
glucose metabolism. |
Table I.
Effect of cold exposure and HFD on
glucose metabolism.
| Area under the
curve | CH | CS+CH | HFD | CS+HFD |
|---|
| GTT (mmol/l/90
min) | 1,095.64±12.3 |
1,171.93±16.6a |
1,352.36±18.7a,b |
1,405.39±22.6a,b |
| ITT (mmol/l/60
min) | 428.40±12.7 | 422.20±7.9 |
560.25±14.9a,b |
573.88±10.6a,b |
Cold exposure and HFD result in
greater WAT weight in mice
Cold exposure and HFD have an additive effect on
body weight and energy intake of mice. To clarify if the extra
energy received by the mice was converted into chemical energy,
stored as fat in the body, and thus increase body weight in these
mice, we measured BAT and WAT depots (inguinal, epididymal,
mesenteric and retroperitoneal) upon dissection. The two groups of
mice subjected to cold exposure daily and HFD showed markedly
higher total WAT mass than standard chow-fed mice (Fig. 3A). The BAT masses did not differ
among the four groups (Fig.
3B).
 | Figure 3.Effect of cold stress and HFD on (A)
WAT and (B) BAT. Data are presented as the mean ± standard error of
the mean (n=7 mice per group). *P<0.05, **P<0.01 and
***P<0.001, as indicated. HFD, high-fat diet; BAT, brown adipose
tissue; WAT, white adipose tissue; WATi, inguinal WAT; WATe,
epididymal WAT; WATm, mesenteric WAT; WATr, retroperitoneal WAT;
WASum, total WAT mass. |
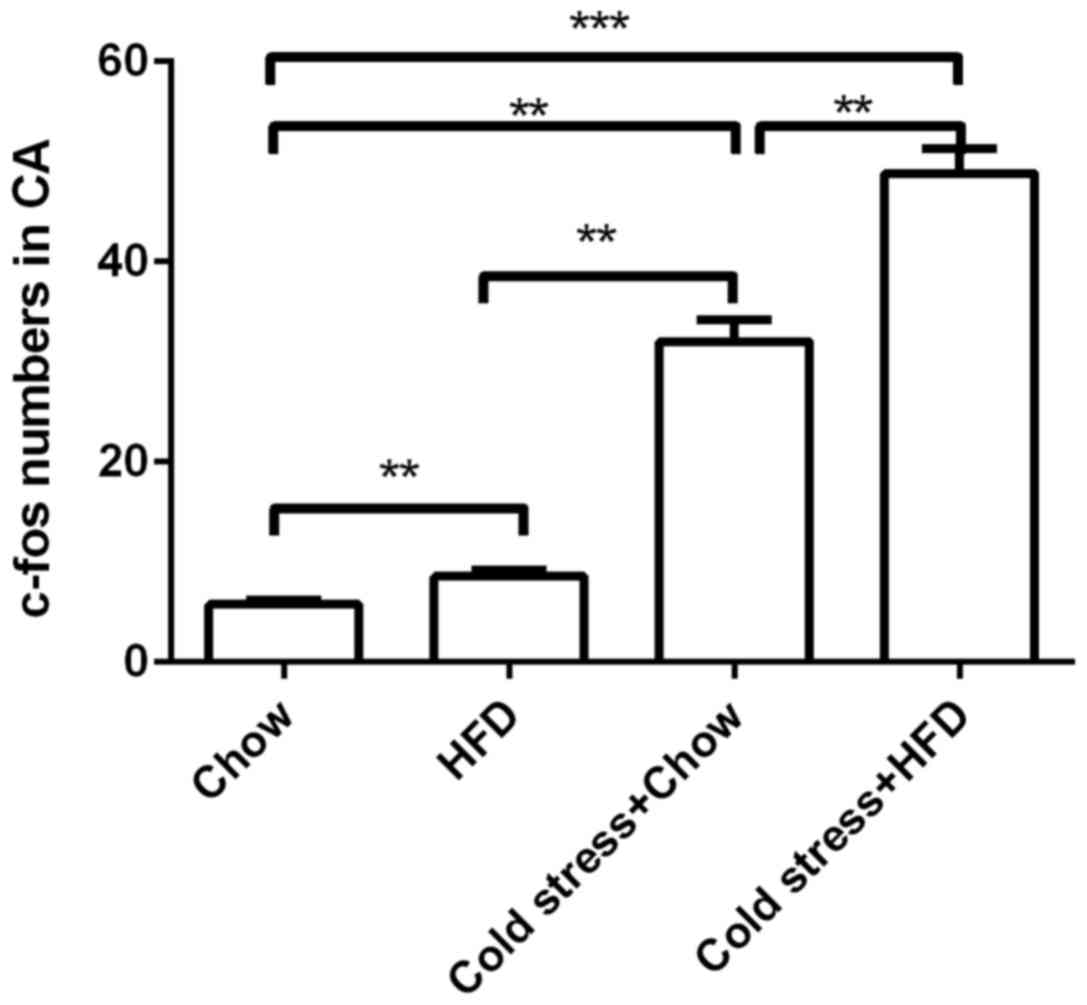
Chronic cold exposure activates
neurons in the central amygdala of mice on HFD
According to the results shown above, HFD and cold
exposure induced an adverse metabolic phenotype. To identify
possible mechanisms for this phenomenon, the present study
investigated the effects of HFD and cold exposure on the expression
of c-fos, an early marker of neuronal activation, in the central
amygdala, a stress-responsive region of the brain that is connected
with brain regions that influence energy homeostasis. In standard
chow-fed mice, the cold exposed group was observed to have
significantly more c-fos-positive neurons in the central amygdala
than the standard chow mice without cold exposure. Furthermore, in
the same region of the central amygdala, HFD-fed mice also
exhibited more labeled c-fos-positive neurons than that standard
chow-fed mice (Fig. 4). These
results suggested that cold exposure and HFD activated the central
neuronal system, which stimulated the central amygdala neurons at
an early stage.
NPY neurons in NPY-GFP mice are
activated by cold exposure and HFD
The results from examining c-fos activity verified
that cold exposure and HFD excited neurons in the central amygdala,
which suggested that the activated neurons excreted NPY, which
affected blood glucose metabolism. To confirm if NPY in mice
subjected to cold exposure and HFD was involved in regulating blood
glucose level, which subsequently resulted in obesity, the present
study investigated brain sections. The two groups without cold
exposure showed no difference in NPY neurons in the central
amygdala between HFD-fed mice and standard chow-fed mice (P=0.138).
For the two groups of mice fed with HFD, mice with cold exposure
displayed more NPY neurons than mice without cold exposure. It was
observed that the section of the central amygdala in the two cold
exposure mice group had more activated NPY neurons in the HFD-fed
mice than in the standard chow-fed mice (Fig. 5). Unexpectedly, the section of the
central amygdala in mouse brain showed that 61% of the
c-fos-positive neurons were positive for NPY (Fig. 6), which suggested that NPY neurons
in the central amygdala are highly activated by cold exposure and
HFD.
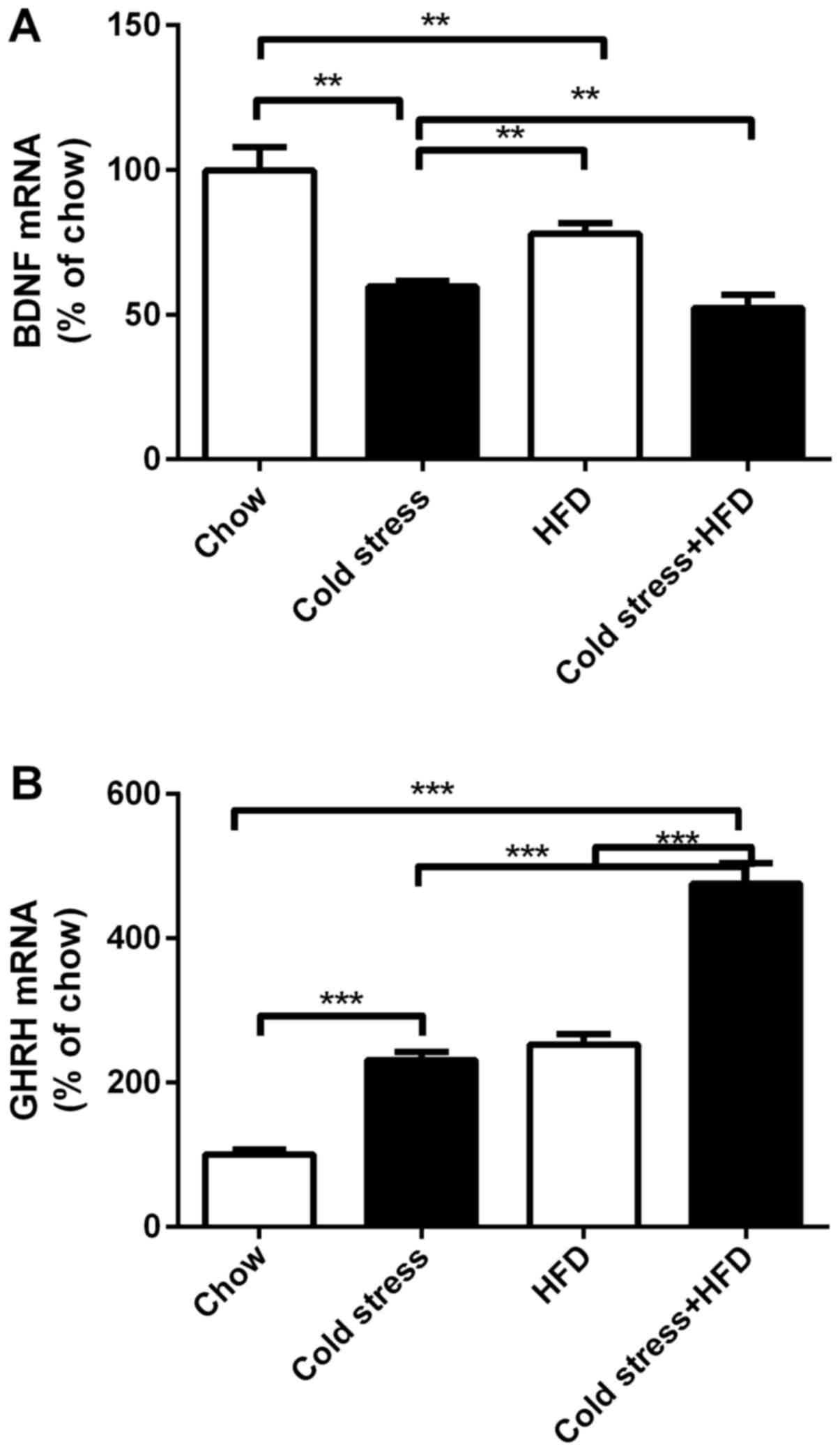
HFD and cold exposure suppresses
expression of Bdnf mRNA while inducing that of Ghrh mRNA
By visualizing the sliced sections of NPY-GFP mouse
brain, it was observed that the numbers of the central amygdala NPY
neurons increased in mice under cold exposure and HFD. It is well
known that central amygdala neurons project to their downstream
neurons in the VMH and PVN. To identify the mechanism of cold
exposure and HFD leading to obesity, the expression of Bdnf
mRNA and Ghrh mRNA was tracked. Compared to standard
chow-fed mice, neither cold exposure nor HFD suppressed the
expression of Bdnf mRNA. Furthermore, combining with HFD,
cold exposure led to the lowest level of Bdnf mRNA
expression (Fig. 7A). Regarding
the expression of Ghrh mRNA, mice that suffered cold
exposure or HFD showed higher expression than standard chow-fed
mice. Thus, the mice subjected to cold exposure together with HFD
displayed the highest mRNA expression levels of Ghrh
(Fig. 7B).
Discussion
In recent years, cold exposure has been investigated
as a potential means of combating obesity due to its ability to
activate BAT, leading to increased energy expenditure through
UCP-1-mediated non-shivering thermogenesis (32). However, the outcome of our study
was not consistent with the theory that cold exposure may
contribute to obesity reduction. Under conditions of cold exposure,
chow-fed mice received in more energy than mice maintained at room
temperature. Furthermore, cold exposure coupled with HFD induced a
more pronounced obese phenotype than either cold exposure or HFD
alone, which was consistent with the results of Kuo et al
(33).
Humans protect themselves from cold using
appropriate clothes and living in heated environments. Concerning
this self-protective behavior during our daily life, it is unlikely
that human beings are exposed to a cold surrounding for a long
period, but transmigrate from cold to warm environments in
occasions. Frequently, parts of the human body suffer from cold
exposure during winter. Stress has been demonstrated to change
feeding responses in a bidirectional pattern, with both increases
and decreases in intake observed. The effects of stress on food
intake regulation are dependent on the type of the stressor
(34,35). The present study observed that mice
fed with a HFD and subjected to conditions of cold showed
significant increases energy intake, and this corresponded to
significant increases in body weight and WAT mass compared to
standard chow mice. The present observations indicate that,
although previous work has shown that cold-induced BAT activation
increases energy expenditure (10,12),
increased energy intake having overcompensated for any increase in
energy expenditure related to cold exposure.
One region of the brain that is implicated in
exerting protective effects from stressors such as cold is the
amygdala, which is located in the medial temporal lobe and forms
part of the limbic system (36,37).
Animal studies have shown that the central amygdala, specifically,
is largely involved in stress-related responses (38,39).
This is in line with our current finding that cold exposure induced
an increase in c-fos immunoreactivity in the central amygdala of
mice. Furthermore, an increase in the dietary intake observed when
mice were fed with HFD during chronic conditions of cold exposure
could be due to the possibility that the central amygdala responses
in stressful conditions may occur via orexigenic neuropeptides
known to harbour abundant NPY neurons (40,41),
which are critically involved in regulating energy metabolism and
adiposity by promoting fat accumulation (42). Our observations showed that c-fos
immunoreactivity and NPY neuronal immunoreactivity were
co-localized within the central amygdala, suggesting that
activation of NPY neurons occurs in response to cold
acclimatization stress. This activation of NPY neurons in the
central amygdala could have contributed to the phenotype observed
in our HFD-fed cold-exposed mice. Cold exposure stimulates NPY
neurons in NPY-GFP mice to produce more NPY. As a feeding promoter,
the increased NPY enlarged the orexigenic effect. Mice subjected to
HFD combined with cold exposure, which is similar to modern human
lifestyle, are more likely to develop obesity.
Our observations of increased activity of NPY
neurons in the central amygdala of GFP mice supports the idea that
these neurons possibly cause stress-induced release of NPY, which
may be implicated in promoting angiogenesis and adipogenesis,
leading to exacerbation of diet-induced obesity and glucose
intolerance as observed by others (33,43,44).
In conjunction to affecting the NPY systems in the
central amygdala, it is important to note that central amygdala
projections innervate the regions of the hypothalamus, particularly
the VMH and the PVN (45). One of
the factors important in energy metabolism is the BDNF, which is
abundantly expressed in the PVN and VMH of the hypothalamus. It has
been previously reported that BDNF exerts hypophagic and weight
reducing effects in animals (46).
Furthermore, BDNF in the central nervous system can influence
glucose metabolism directly, leading to magnification of the
insulin function in peripheral tissues (47). Peripheral BDNF rapidly enhanced
insulin signal transduction in liver and performed hypoglycemic
action in diabetic mice (48).
BDNF is mainly expressed in the VMH, which were significantly
reduced in response to HFD and cold exposure, possibly contributing
to the increased energy intake and altered glucose homeostasis
observed in these mice. Another factor that is influenced by
neuronal projections from the central amygdala is GHRH, which is
abundantly expressed in the ARC, VMH and PVN regions of the
hypothalamus (49,50). It has been shown that GHRH can
stimulate the pituitary gland to release growth hormone (GH). GH
can promote the decomposition of glycogen (51), and inhibit glucose utilization by
muscle and adipose tissue (52).
In addition, GHRH actively modulates glucose homeostasis by
facilitating insulin action and normalizing glucose metabolism in
obese mice, irrespective of dietary intake (47). The present study observed that HFD
or cold exposure can independently and additively increase the
levels of Ghrh mRNA expression in the PVN. This change,
combined with reduced VMH Bdnf mRNA levels in response to
HFD and cold exposure, could have contributed to the hyperphagia,
weight gain and imbalances in glucose and insulin responses in the
mice of the present study.
In summary, while there previous reports have
suggested potential therapeutic values in manipulating BAT or BeAT
for the management of diabetes and obesity (53), it is important to note that the use
of cold exposure to activate BAT or BeAT needs to be carefully
considered, in light of our current findings that cold exposure
(with the current paradigm, at least), may cause undesired
physiological responses. Further studies are required using cold
exposure at different intensities and durations in order to reveal
the full association between cold exposure and energy/glucose
homeostasis. Potential adverse effects should be taken into
consideration when manipulating temperature to stimulate the
browning of white fat. Although our findings are consistent with an
earlier study showing that cold exposure exacerbates HFD-induced
obesity (43), we cannot rule out
the possibility that our observations are the net outcome of
ice-cold exposure and individual housing-induced stress, that is,
single housing in the current study may contribute to the ice-cold
stress-induced obesity in DIO (Diet induced obesity) mice. Further
studies using a different spectrum of cold exposure condition such
as 4°C in grouped housed mice will provide a better understanding
of the impacts of cold exposure on diet-induced obesity.
Acknowledgements
The authors would like to thank Professor Amanda
Sainsbury (The Boden Institute of Obesity, Nutrition, Exercise
& Eating Disorders Honorary Associate, School of Psychology,
the University of Sydney, Sydney, New South Wales, Australia) for
her critical review of the manuscript.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81570300, 81670402
and 81570395).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PZ and ZZ performed the experiments and drafted the
manuscript. XH, YS, NK, HY and SYL analyzed and interpreted the
data. XH and YS revised the manuscript. SL and ZS designed the
present study and revised the paper.
Ethics approval and consent to
participate
All animal experimental protocols were approved by
the Third Military Medical University Animal Care Committee
(Chongqing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
NCD Risk Factor Collaboration (NCD-RisC),
. Trends in adult body-mass index in 200 countries from 1975 to
2014: A pooled analysis of 1698 population-based measurement
studies with 19·2 million participants. Lancet. 387:1377–1396.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Must A, Spadano J, Coakley EH, Field AE,
Colditz G and Dietz WH: The disease burden associated with
overweight and obesity. JAMA. 282:1523–1529. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wan Y, Jiang X, He Y, Zhang Y, Liang Y,
Pan F, Xu Y and Shang L: Body mass index of young men in China:
Results from four national surveys conducted between 1955 and 2012.
Medicine (Baltimore). 95:e28292016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moon G, Quarendon G, Barnard S, Twigg L
and Blyth B: Fat nation: Deciphering the distinctive geographies of
obesity in England. Soc Sci Med. 65:20–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Batis C, Mendez MA, Gordon-Larsen P,
Sotres-Alvarez D, Adair L and Popkin B: Using both principal
component analysis and reduced rank regression to study dietary
patterns and diabetes in Chinese adults. Public Health Nutr.
19:195–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Watt C, Mitchell S and Salewski V:
Bergmann's rule; A concept cluster? Oikos. 119:89–100. 2010.
View Article : Google Scholar
|
|
7
|
Lidell ME, Betz MJ and Enerbäck S: Two
types of brown adipose tissue in humans. Adipocyte. 3:63–66. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abdullahi A and Jeschke MG: White adipose
tissue browning: A double-edged sword. Trends Endocrinol Metab.
27:542–552. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lidell ME, Betz MJ and Enerbäck S: Brown
adipose tissue and its therapeutic potential. J Intern Med.
276:364–377. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fedorenko A, Lishko PV and Kirichok Y:
Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat
mitochondria. Cell. 151:400–413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blondin DP, Labbé SM, Tingelstad HC, Noll
C, Kunach M, Phoenix S, Guérin B, Turcotte EE, Carpentier AC,
Richard D and Haman F: Increased brown adipose tissue oxidative
capacity in cold-acclimated humans. J Clin Endocrinol Metab.
99:E438–E446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van der Lans AA, Wierts R, Vosselman MJ,
Schrauwen P, Brans B and van Marken Lichtenbelt WD: Cold-activated
brown adipose tissue in human adults: Methodological issues. Am J
Physiol Regul Integr Comp Physiol. 307:R103–R113. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chondronikola M, Volpi E, Børsheim E,
Porter C, Annamalai P, Enerbäck S, Lidell ME, Saraf MK, Labbe SM,
Hurren NM, et al: Brown adipose tissue improves whole-body glucose
homeostasis and insulin sensitivity in humans. Diabetes.
63:4089–4099. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee P, Smith S, Linderman J, Courville AB,
Brychta RJ, Dieckmann W, Werner CD, Chen KY and Celi FS:
Temperature-acclimated brown adipose tissue modulates insulin
sensitivity in humans. Diabetes. 63:3686–3698. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trayhurn P: Recruiting brown adipose
tissue in human obesity. Diabetes. 65:1158–1160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuperman Y, Weiss M, Dine J, Staikin K,
Golani O, Ramot A, Nahum T, Kühne C, Shemesh Y, Wurst W, et al:
CRFR1 in AgRP neurons modulates sympathetic nervous system activity
to adapt to cold stress and fasting. Cell Metab. 23:1185–1199.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi SY, Zhang W, Luk CT, Sivasubramaniyam
T, Brunt JJ, Schroer SA, Desai HR, Majerski A and Woo M: JAK2
promotes brown adipose tissue function and is required for diet-
and cold-induced thermogenesis in mice. Diabetologia. 59:187–196.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wilson MA, Grillo CA, Fadel JR and Reagan
LP: Stress as a one-armed bandit: Differential effects of stress
paradigms on the morphology, neurochemistry and behavior in the
rodent amygdala. Neurobiol Stress. 1:195–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Loh K, Herzog H and Shi YC: Regulation of
energy homeostasis by the NPY system. Trends Endocrinol Metab.
26:125–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stanley BG, Magdalin W, Seirafi A, Nguyen
MM and Leibowitz SF: Evidence for neuropeptide Y mediation of
eating produced by food deprivation and for a variant of the Y1
receptor mediating this peptide's effect. Peptides. 13:581–587.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen P, Lin D, Giesler J and Li C:
Identification of urocortin 3 afferent projection to the
ventromedial nucleus of the hypothalamus in rat brain. J Comp
Neurol. 519:2023–2042. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu X, Zhu Z, Kalyani M, Janik JM and Shi
H: Effects of energy status and diet on Bdnf expression in the
ventromedial hypothalamus of male and female rats. Physiol Behav.
130:99–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Correll CM, Rosenkranz JA and Grace AA:
Chronic cold stress alters prefrontal cortical modulation of
amygdala neuronal activity in rats. Biol Psychiatry. 58:382–391.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eshkevari L, Permaul E and Mulroney SE:
Acupuncture blocks cold stress-induced increases in the
hypothalamus-pituitary-adrenal axis in the rat. J Endocrinol.
217:95–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van-Hover C and Li C: Stress-activated
afferent inputs into the anterior parvicellular part of the
paraventricular nucleus of the hypothalamus: Insights into
urocortin 3 neuron activation. Brain Res. 1611:29–43. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mormède P, Castagné V, Rivet JM, Gaillard
R and Corder R: Involvement of neuropeptide Y in neuroendocrine
stress responses. Central and peripheral studies. J Neural Transm
Suppl. 29:65–75. 1990.PubMed/NCBI
|
|
27
|
Lin S, Thomas TC, Storlien LH and Huang
XF: Development of high fat diet-induced obesity and leptin
resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord.
24:639–646. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi YC, Lin S, Wong IP, Baldock PA,
Aljanova A, Enriquez RF, Castillo L, Mitchell NF, Ye JM, Zhang L,
et al: NPY neuron-specific Y2 receptors regulate adipose tissue and
trabecular bone but not cortical bone homeostasis in mice. PLoS
One. 5:e113612010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin S, Shi YC, Yulyaningsih E, Aljanova A,
Zhang L, Macia L, Nguyen AD, Lin EJ, During MJ, Herzog H and
Sainsbury A: Critical role of arcuate Y4 receptors and the
melanocortin system in pancreatic polypeptide-induced reduction in
food intake in mice. PLoS One. 4:e84882009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van den Pol AN, Yao Y, Fu LY, Foo K, Huang
H, Coppari R, Lowell BB and Broberger C: Neuromedin B and
gastrin-releasing peptide excite arcuate nucleus neuropeptide Y
neurons in a novel transgenic mouse expressing strong Renilla green
fluorescent protein in NPY neurons. J Neurosci. 29:4622–4639. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin S, Boey D, Lee N, Schwarzer C,
Sainsbury A and Herzog H: Distribution of prodynorphin mRNA and its
interaction with the NPY system in the mouse brain. Neuropeptides.
40:115–123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McMillan AC and White MD: Induction of
thermogenesis in brown and beige adipose tissues: Molecular
markers, mild cold exposure and novel therapies. Curr Opin
Endocrinol Diabetes Obes. 22:347–352. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuo LE, Czarnecka M, Kitlinska JB, Tilan
JU, Kvetnanský R and Zukowska Z: Chronic stress, combined with a
high-fat/high-sugar diet, shifts sympathetic signaling toward
neuropeptide Y and leads to obesity and the metabolic syndrome. Ann
N Y Acad Sci. 1148:232–237. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pecoraro N, Reyes F, Gomez F, Bhargava A
and Dallman MF: Chronic stress promotes palatable feeding, which
reduces signs of stress: Feedforward and feedback effects of
chronic stress. Endocrinology. 145:3754–3762. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Maniam J and Morris MJ: The link between
stress and feeding behaviour. Neuropharmacology. 63:97–110. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Holzel BK, Carmody J, Evans KC, Hoge EA,
Dusek JA, Morgan L, Pitman RK and Lazar SW: Stress reduction
correlates with structural changes in the amygdala. Soc Cogn Affect
Neurosci. 5:11–17. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Haubensak W, Kunwar PS, Cai H, Ciocchi S,
Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM,
et al: Genetic dissection of an amygdala microcircuit that gates
conditioned fear. Nature. 468:270–276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Alheid GF: Extended amygdala and basal
forebrain. Ann N Y Acad Sci. 985:185–205. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Walker DL and Davis M: Double dissociation
between the involvement of the bed nucleus of the stria terminalis
and the central nucleus of the amygdala in startle increases
produced by conditioned versus unconditioned fear. J Neurosci.
17:9375–9383. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Redrobe JP, Dumont Y, Fournier A, Baker GB
and Quirion R: Role of serotonin (5-HT) in the antidepressant-like
properties of neuropeptide Y (NPY) in the mouse forced swim test.
Peptides. 26:1394–1400. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Husum H, Mikkelsen JD, Hogg S, Mathé AA
and Mørk A: Involvement of hippocampal neuropeptide Y in mediating
the chronic actions of lithium, electroconvulsive stimulation and
citalopram. Neuropharmacology. 39:1463–1473. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zukowska-Grojec Z: Neuropeptide Y. A novel
sympathetic stress hormone and more. Ann N Y Acad Sci. 771:219–233.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qi J, Zhang S, Wang HL, Wang H, de Jesus
Aceves Buendia J, Hoffman AF, Lupica CR, Seal RP and Morales M: A
glutamatergic reward input from the dorsal raphe to ventral
tegmental area dopamine neurons. Nature Commun. 5:53902014.
View Article : Google Scholar
|
|
44
|
Kuo LE, Kitlinska JB, Tilan JU, Li L,
Baker SB, Johnson MD, Lee EW, Burnett MS, Fricke ST, Kvetnansky R,
et al: Neuropeptide Y acts directly in the periphery on fat tissue
and mediates stress-induced obesity and metabolic syndrome. Nat
Med. 13:803–811. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Petrovich GD, Canteras NS and Swanson LW:
Combinatorial amygdalar inputs to hippocampal domains and
hypothalamic behavior systems. Brain Res Brain Res Rev. 38:247–289.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu B, Goulding EH, Zang K, Cepoi D, Cone
RD, Jones KR, Tecott LH and Reichardt LF: Brain-derived
neurotrophic factor regulates energy balance downstream of
melanocortin-4 receptor. Nat Neurosci. 6:736–742. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nakagawa T, Tsuchida A, Itakura Y,
Nonomura T, Ono M, Hirota F, Inoue T, Nakayama C, Taiji M and
Noguchi H: Brain-derived neurotrophic factor regulates glucose
metabolism by modulating energy balance in diabetic mice. Diabetes.
49:436–444. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tsuchida A, Nakagawa T, Itakura Y,
Ichihara J, Ogawa W, Kasuga M, Taiji M and Noguchi H: The effects
of brain-derived neurotrophic factor on insulin signal transduction
in the liver of diabetic mice. Diabetologia. 44:555–566. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bromek E, Wójcikowski J and Daniel WA:
Involvement of the paraventricular (PVN) and arcuate (ARC) nuclei
of the hypothalamus in the central noradrenergic regulation of
liver cytochrome P450. Biochem Pharmacol. 86:1614–1620. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lee SK, Ryu PD and Lee SY: Differential
distributions of neuropeptides in hypothalamic paraventricular
nucleus neurons projecting to the rostral ventrolateral medulla in
the rat. Neurosci Lett. 556:160–165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Balbis A, Bartke A and Turyn D:
Overexpression of bovine growth hormone in transgenic mice is
associated with changes in hepatic insulin receptors and in their
kinase activity. Life Sci. 59:1363–1371. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Thirone AC, Carvalho CR, Brenelli SL,
Velloso LA and Saad MJ: Effect of chronic growth hormone treatment
on insulin signal transduction in rat tissues. Mol Cell Endocrinol.
130:33–42. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mukherjee J, Baranwal A and Schade KN:
Classification of Therapeutic and experimental drugs for brown
adipose tissue activation: Potential treatment strategies for
diabetes and obesity. Curr Diabetes Rev. 12:414–428. 2016.
View Article : Google Scholar : PubMed/NCBI
|