Introduction
Leptin, an adipocytokine encoded by an
obesity-associated gene expressed in adipose tissue, affects
feeding behavior, thermogenesis and neuroendocrine status via
leptin receptors distributed in the brain, particularly in the
hypothalamus (1). Recently,
studies have investigated the role of leptin in non-hypothalamic
areas, including regions associated with learning and memory, and
cognitive function (2–7). An indicator that leptin may affect
cognitive function is that the leptin receptor is expressed
throughout the brain (2). Leptin
receptors, particularly M-type leptin receptors, are highly
expressed within the inner regions of the hypothalamus; however,
they are also expressed in other areas of the brain, including
regions associated with learning and memory, such as various
cortical regions and the hippocampus (3–7). A
potential regulatory role of leptin within these brain regions is
that it may be associated with the effects of diet and obesity on
cognitive function (8,9). Alterations in caloric intake or
dietary composition are associated with the dysregulated gene
expression profiles of the hippocampal and cortical areas, involved
in glycolysis, protein deacetylation, PGC-1α and mTor pathways,
suggesting that these brain regions may be associated with changes
in nutritional and metabolic status (8). Additionally, changes in nutritional
status can also alter cognitive function; obesity has been
associated with cognitive decline (9).
A number of studies investigating the role of leptin
in a variety of brain regions and signaling systems have provided
notable support for a neuroprotective role of the hormone (10–14);
however, the underlying mechanisms remain unclear. Previous studies
have demonstrated that leptin affects synaptic function at the
molecular and cellular levels, as well as neural structures,
suggesting that the peptide serves important diverse roles in the
brain (10). Numerous
investigations into the effects of leptin on the structure and
function of the hippocampus, cortex and other areas of the brain
have been conducted; such research has mainly focused on the
hypothalamus (3–7). Few studies have examined the role of
leptin signaling and resistance in non-hypothalamic regions.
Furthermore, a small number of studies have mainly focused on adult
neurocognitive diseases (11–14);
the neuroprotective effects of leptin on brain damage resulting
from premature development remain unknown.
Across 184 countries, the rate of preterm birth
(<37 weeks' gestation) ranges from 5 to 18% of total births,
with an estimated 15 million babies born preterm every year; this
number is increasing annually (15). Complications arising from preterm
birth are the leading cause of mortality among children under 5
years of age, and have been associated with almost 1 million cases
of mortality in 2013 (15). A
total of 75% of these babies may be saved with current,
cost-effective treatments; however, numerous survivors endure
lifelong disabilities, including learning disorders, and visual and
hearing issues (15). According to
the U.S. Centers for Disease Control and Prevention, very early
preterm births (<32 weeks' gestation) account for 16% of the
total number of preterm births, in the USA (16). It has been reported that ~10% of
the preterm births with gestational ages <32 weeks and birth
weights <1,500 g exhibit defects in locomotion, whereas ~60% of
infants possess neurocognitive disabilities and/or behavioral
problems (16,17). The most common defect in preterm
infants is periventricular leukomalacia (PVL) and the incidence of
PVL in preterm infants has been reported to be >50% (18,19).
At present, the mechanisms underlying premature brain damage are
unclear, yet hypothermic, stem cell-associated and other types of
therapeutic strategies have been reported (20); however, the majority of these
applications lack efficacy. Therefore, it is important to develop
novel, safe and effective treatment methods.
Hypoleptinemic patients have been reported to
exhibit impaired cognitive flexibility and decreased visuospatial
abilities when compared with their healthy counterparts (21–23).
Individuals with early Alzheimer's disease or mild cognitive
impairment with low plasma leptin levels may benefit from leptin
replacement therapy (24).
Elevated circulating leptin has been consistently detected in
childhood neurodevelopmental disorders, including autism spectrum
disorders and Rhett disorder (1).
Leptin treatment of neonates may reverse the hippocampal and
frontal cortical changes that occur in rats as observed within a
maternal deprivation model (25);
however, it remains unknown whether these biological effects of
leptin also manifest in the premature brain, or whether the preterm
brain requires leptin. The umbilical cord blood leptin can be
detected at the earliest 18 weeks of pregnancy and increase sharply
during the 34 weeks of pregnancy. The development of fetal adipose
tissue and the storage of fat are the determinants of leptin levels
in the fetus (26,27). The development of adipose tissue in
preterm infants may be delayed compared with in term infants,
potentially resulting in leptin deficiency. Veselá et al
(28) reported that the median
cord blood concentration of leptin was 3.07 µg/l and the median
cord blood concentration of leptin in infants at 32–33 weeks'
gestation was 2.89 µg/l, which was lower than the 3.13 µg/l
observed in preterm infants at 34–36 weeks' gestation. Nagasaki and
Ohta (29) revealed that the
median serum concentration of leptin at 33–38 weeks' gestation was
2.3 ng/ml (range: 1.0–3.9 ng/ml).
To investigate the potential protective effects of
leptin in the developing brain, the present study investigated the
neurocognitive and motor functional effects of leptin treatment in
a rat model of premature brain damage. In addition, Oomura et
al (30) reported that leptin
possesses an inverted-U-shaped dose-effect associated with spatial
learning and memory tasks with an optimal dose of 50 µg/kg.
Therefore, this particular dose was utilized in the present
study.
Materials and methods
Animals
The present study was approved by the Institutional
Animal Care and Use Committee of Southeast University (Nanjing,
China). Pregnant Sprague Dawley rats (days 18–19 of the estrous
cycle) were obtained from Nanjing Medical University of China
(Nanjing, China) and allowed to deliver (10–14 pups per dam). The
pups' birth weight was 5.5–7 g, with an average of 6.5 g. A total
of 41 pups were employed in the present study. The pups were housed
with their mothers under a constant 12-h dark/light cycle with free
access to food and water. Room temperature at 18~26°C, relative
humidity 40–70%, without noise, ammonia concentration below 20 ppm,
ventilation 8–12 times/h. Pups of each litter were randomly
assigned to all experimental groups, with 13 in sham group, 14 in
model and 14 in leptin group. A total of 36 rats were survival to
21-days-old and used in the behavioral experiments, with 6 male
rats and 6 female rats in each group, while 5 of them perished
following the model establishment.
Preterm brain damage model
Preterm human infants of 24–32 weeks' gestation are
at high risk of developing PVL (31). The oligodendrocytes in the white
matter 2–5 days following birth mainly constitutes late
oligodendrocytes, similar to the peak period of PVL in human
preterm infants (32). Therefore,
the present study used postnatal day 2 rat pups for the subsequent
experiments. The 2-day-old pups underwent permanent ligation of the
right common carotid artery under a shadowless lamp. Following
anesthesia via isoflurane inhalation for 1–2 min, rats were fixed
on the operating table, a midline incision length 1.0 cm was
performed on the neck. For both model and leptin groups, ligation
of the right common carotid artery was performed with a 5-0 suture,
followed by suturing of the skin. Surgery was conducted for <10
min; animals with excessive bleeding were excluded. The pups in the
model and leptin groups were then returned to their home cage with
their dam for 2 h, and thereafter removed from their dam and
exposed to hypoxia (94% N2/6% O2) for 2 h in
a sealed chamber partially submersed in a 37°C water bath (31). This procedure induced a major
lesion in the periventricular white matter. By the end of the
hypoxic treatment, pups were returned to their dam for recovery. In
sham-operated rats, the same surgery procedure was performed
without ligation or exposure to hypoxia.
Drug treatment
Following exposure to hypoxia, at the end of the
ventilation period that ensured hypoxia, leptin was administered to
the pups (2–5 days old) once daily as an intraperitoneal injection
of recombinant murine leptin (50 µg/kg/day, diluted with normal
saline, up to 0.1 ml/g; PeproTech EC Ltd., London, UK) for 4 days.
The model group was administered an equal volume of saline
following exposure to hypoxia, without leptin treatment. In
addition, the sham group was treated with an equal volume of saline
at the same time as the other two groups.
Observation of survival and monitoring
of body weight development
The survival rate was observed to 21-days-old.
Weight was measured on 0, 2, 3, 4, 5, 7, 10, 14, 17, 21 days of age
at 8:00 a.m., prior to the change of the padding materials and
feeding them. The weight was measured by the body weight meter,
accurate to 0.10 g, calibrated and zeroed before the
measurement.
Evaluation of neurocognitive motor
function
These experiments were conducted on postnatal days
27–30, as described below.
Resistance to capture
With sterile gloves, a researcher manually captured
the rats individually, in a gentle manner to observe its reaction.
The behavior of the rats (postnatal days 27–30) was then
subsequently scored. The scoring scale employed was as follows: 0,
easy to grab; 1, screaming or avoidance; 2, screaming and
avoidance; 3, escape; 4, escape and screaming; 5, bite or attempt
to bite the gloves; and 6, active jumps and attacks. The purpose of
this procedure was to observe the emotional behavior of the
animals.
Suspension test
This experiment was designed to assess the forelimb
grip of the rats. The rats (postnatal days 27–30) were allowed to
catch a level metal rod (diameter, 0.5 cm, length, 50 cm) with both
forelimbs, and the rod was then raised 45 cm above the ground. The
duration of grasp for each rat was then recorded.
Open field test
This experiment was performed to evaluate
exploratory behavior and anxiety in a new environment. The
experimental setup comprised a 45×45×45 cm carton without a lid.
The base of the box was divided into a grid of nine equal regions,
marked with black ink. Rats were placed in the central square and
covered with a small paper box. Subsequently, following 30 sec, the
box was lifted, and the rats were allowed to move freely for 90
sec. The frequency that more than half of the body was in an
adjacent square or when the animal was standing up on its hind legs
was recorded. In-house scoring criteria: More than half of the body
in an adjacent square, 1 point; every incidence of standing up on
hind legs, 1 point; and grooming and defecation, 1 point each. The
total score was then calculated for each animal.
Morris water maze test
The Morris water maze test was performed when the
rats were 21–28 days old and was used to evaluate spatial memory
learning ability. The Morris water maze test is the most objective
method of assessing learning and memory function currently
available (30). The maze consists
of a circular water tank and an automatic photographing and
analysis system. The automatic image acquisition and processing
system is comprised of a camera, computer and image monitor. As
soon as the animals are placed in the water, the monitoring device
that records the path of animal movement is activated; the analysis
of the relevant parameters is automatic. The experimental
procedures included the following: i) Place navigation, which was
used to measure the learning and memory ability of rats in the
water maze (this experiment lasted 5 days and included training the
animals to find the platform four times a day at a fixed time); and
ii) a spatial probe test, which was used to assess memory retention
of the spatial location following learning to find the platform. At
the end of the navigation experiment, the platform was removed, and
the animal was placed at the same point of entry into the water,
and the time of first arrival at the platform and the number of
crossings of the original platform location were recorded.
Statistical analysis
SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used for
all statistical analyses conducted in the present study. Numerical
data were presented with the mean ± standard and were analyzed
using one-way analysis of variance, followed by a
Student-Newman-Keuls (SNK-q) test, for pairwise comparisons. For
categorical data, the χ2 test was conducted for analysis;
multivariate linear regression was used for multivariate analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
General health observations
A total of 36 rats survived following the
establishment of the preterm brain damage model, with 12 in each
group. The mortality rate was 12.20% (36/41). Body weight was
examined up to 21 days of age. One-way analysis of variance was
used to compare developmental differences in body weight among the
groups. The average weight was higher in the model group when
compared with leptin treatment and the sham group, prior to 10 days
of age (Fig. 1). The differences
among the three groups prior to 2 days of age were not significant.
From 3–5 days of age, the weight in the leptin-treated group
decreased; however, after 5 days of age, weight in the
leptin-treated group increased. Additionally, at 10–14 days, the
average weight was higher in the leptin-treated group than in the
remaining groups (Fig. 1). It may
be suggested that leptin intervention could control the growth of
body weight after HI, and the growth accelerated following the
withdrawal of drug, leptin intervention had no adverse effect on
the long-term weight growth.
Morris water maze test
Latency and platform crossings
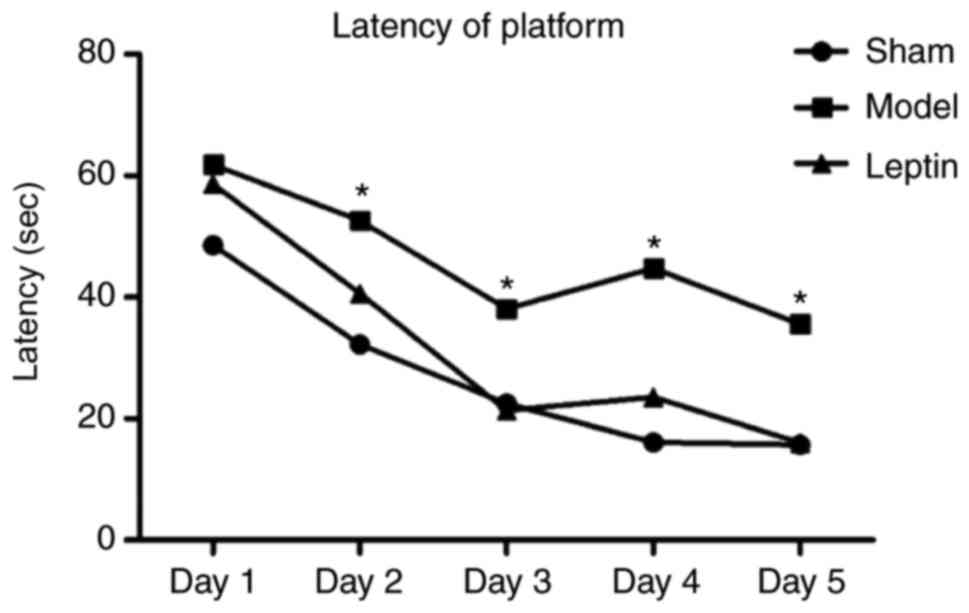
The average latency of finding the platform was
significantly longer in the model group than in the other groups
from day 2. The difference between the leptin-treated and the sham
groups was not significant (P>0.05) as determined by the SNK-q
test for pairwise comparisons (Fig.
2). The number of platform crossings was 1.33±0.41 in the
model, 3.17±0.32 in the leptin-treated and 4.00±0.61 in the sham
groups. The difference between the three groups was statistically
significant (F=8.306, P=0.004; Fig.
3A). The latency for finding the platform was 56.84±26.454 sec
in the model group, 14.27±9.167 sec in the leptin-treated group and
5.67±0.279 sec in the sham group. The difference between the three
groups was statistically significant (F=17.238, P<0.0001;
Fig. 3B). Additionally, the
typical trajectories of the three groups are presented in Fig. 4. Using the SNK-q test for pairwise
comparisons, the average number of crossings of the platform in the
leptin-treated group was significantly higher than that of the
model group (P=0.015; Fig. 3A).
The average latency to the platform was shorter in the
leptin-treated group than that of the model group (P<0.05;
Fig. 3B). The number of platform
crossings and latency to the platform in the leptin-treated group
were not significantly different when compared with in the sham
group.
Multivariate analysis
Regarding the latency to the platform as the
dependent variable, and including gender, treatment, weight on day
1 and age, multivariable linear regression analysis was performed
with an inclusion criterion of 0.05 and an exclusion criterion of
0.10. Latency was significantly correlated with treatment and age
(P<0.05). Latency to the platform was decreased in response to
leptin treatment and age (Table
I). It could be suggested that training could improve the
performance of the model group, but still lag behind the sham
group, intervention strategies, including drug therapy, is needed
to promote brain injury rehabilitation. Early intervention of
leptin and training can promote the recovery of brain injury and
make them tend to be normal.
 | Table I.Multivariate linear regression of
latency of rats in the platform. |
Table I.
Multivariate linear regression of
latency of rats in the platform.
| Variable | Assignment
method | Standard
coefficient | t-score | P-value | Non-standard
coefficient (95% CI) |
|---|
| Treatment | 1=Model | −0.607 | −3.854 | 0.001 | −13.201 (20.325,
−6.077) |
|
|
2=Interventiona |
|
|
|
|
|
| 3=Sham |
|
|
|
|
| Days old | Actual value | −0.903 | −5.734 | <0.0001 | −9.208 (−12.548,
−5.868) |
| Constants | – | – | 6.216 | <0.0001 | 315.338 (209.847,
420.829) |
Capture-resistance, suspension and
open field tests
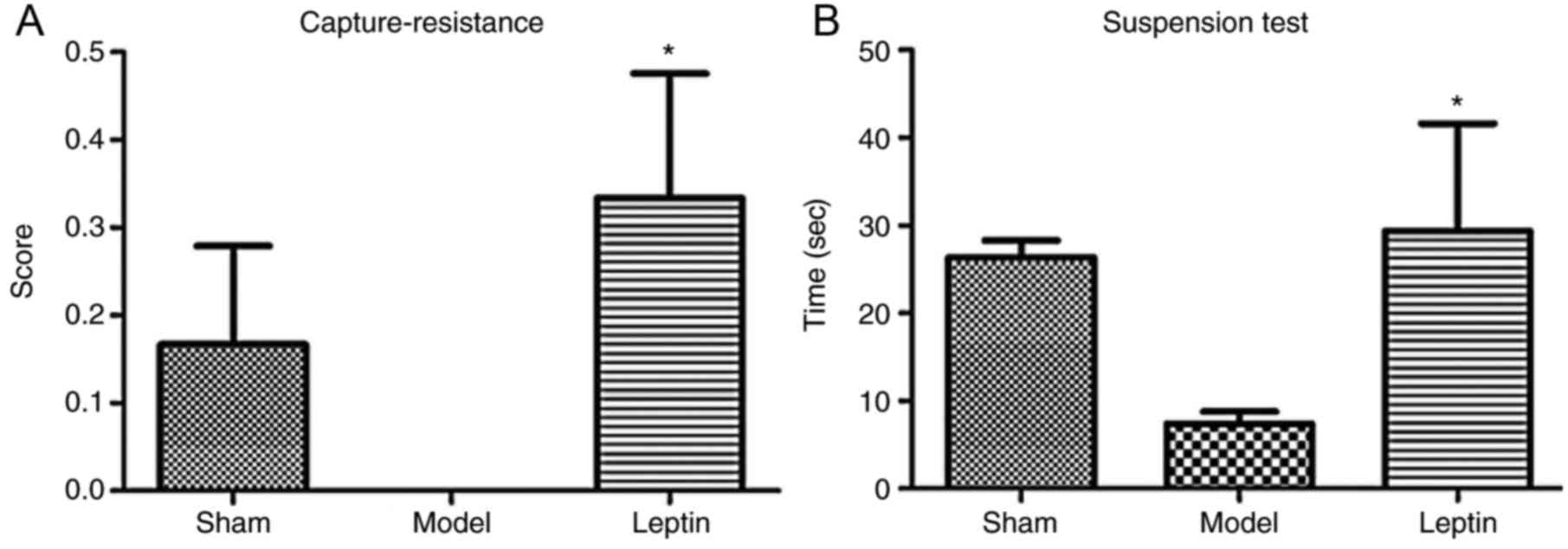
In the capture-resistance test, the differences in
capture-resistance scores among the three groups were not
statistically significant (F=2.174, P=0.131; Fig. 5A); in the forelimb suspension test,
the differences in grasp times among the three groups were also not
significant (F=2.267, P=0.120; Fig.
5B). In the open field test, the differences in defecation
among the three groups were also not significant (F=2.741, P=0.080;
Fig. 6A). The differences in grid
crossings among the three groups were also not significant
(F=2.265, P=0.121; Fig. 6B). In
addition, the differences in grooming (F=2.008, P=0.151; Fig. 6C), standing posture (F=1.172,
P=0.323; Fig 6D) and in total
scores (F=0.373, P=0.692; Fig. 6E)
among the three groups were not statistically significant.
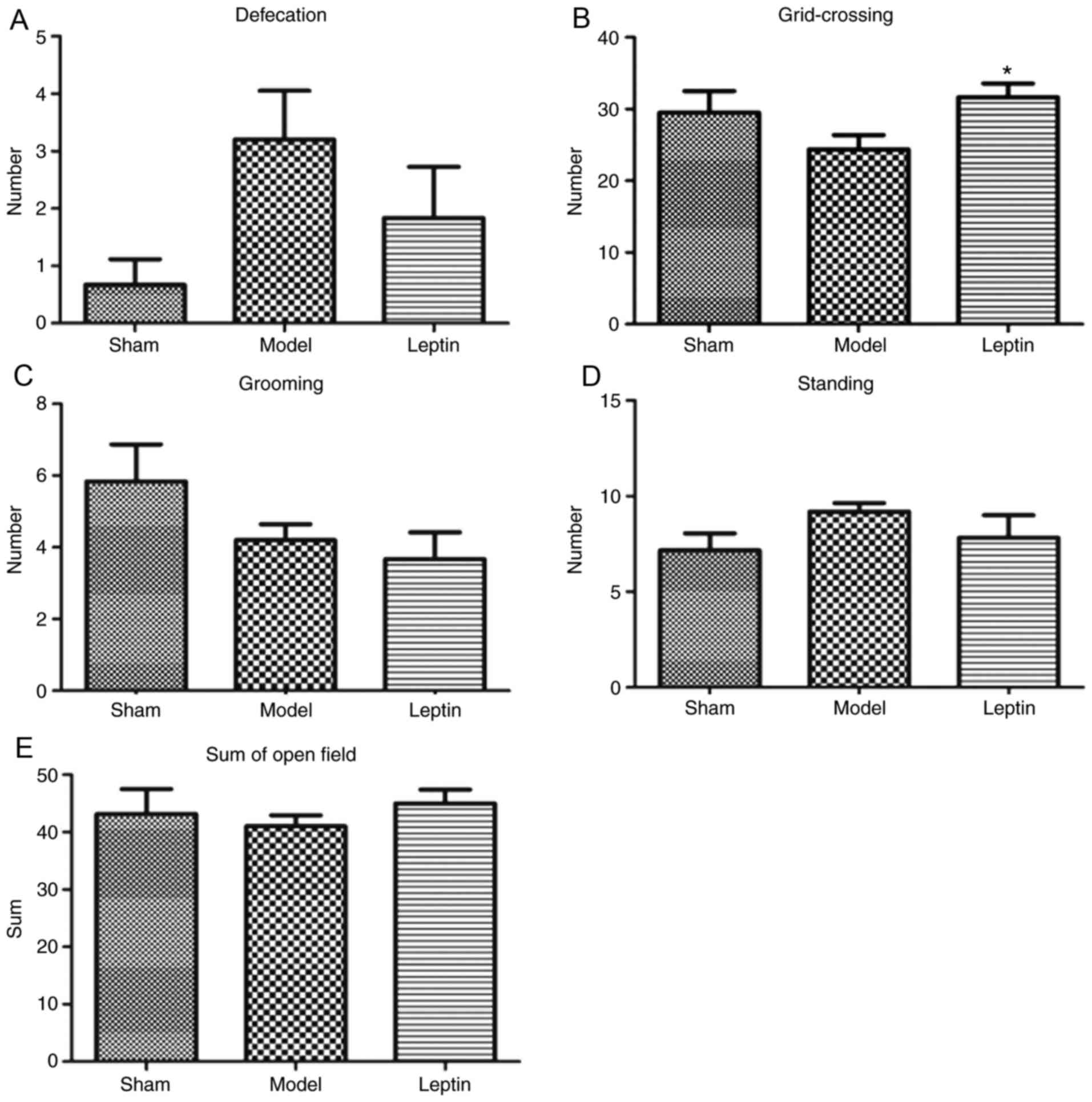 | Figure 6.Results of open field test between
the three groups. The results of (A) defecation, there was no
statistical significance among the three groups, (B) grid-crossing,
there was no statistical significance among the three groups, but a
trend (Student-Newman-Keuls analysis, P=0.045), (C) grooming, with
no statistical significance among the three groups, (D) standing,
with no statistical significance among the three groups and (E) sum
of the open field tests across the three groups, with no
statistical significance among the three groups. *P<0.05 vs.
model. |
The majority of the investigated parameters among
the three groups were not statistically significant; however,
similarities were observed in capture-resistance scores, duration
of suspension and grid crossings across the leptin-treated and sham
groups. Therefore, the SNK-q test for further pairwise comparison.
This analysis revealed that the average capture-resistance scores
were significantly higher in the leptin-treated group than in the
model group (P=0.046; Fig. 5A);
the duration of suspension was longer in the leptin-treated group
when compared with the model group (P=0.055; Fig. 5B). In addition, the number of grid
crossings was significantly higher in response to leptin treatment
when compared with the model group (P=0.045; Fig. 6B). It may be suggested that early
intervention of leptin may result in the recovery of forearm
grasping ability, even could close to normal, resulting to more
positive emotional reactions, more avoidance and exploratory
behavior.
Discussion
With advances in perinatal technologies, the
survival rates of premature infants, have increased; however,
cognitive impairments and behavioral issues still exist (15). The main pathological feature of
brain injury in preterm infants is periventricular leukomalacia,
particularly in infants born at 24–32 weeks' gestation (32). The model used in the present study
was based on the hypoxic-ischemic brain damage model developed by
Rice et al (31). In this
model, necrosis of the white matter was reported and was greater
ipsilaterally, originating and spreading from myelinogenic foci,
similar to the features of brain injury in preterm infants
(31). In the present study,
2-day-old neonatal Sprague Dawley rats were selected to establish
the preterm brain damage model as brain development in 2-day-old
rats is highly similar to that in severely preterm human infants
(24–32 weeks' gestation). Rats that are 21-days-old (approximately
equivalent to human childhood) are able to live without their
mother; at this point, the establishment of memory and emotional
responses is initiated (33).
The present study reported that leptin-treated rats
had lower weights during treatment, but their weights increased
following leptin withdrawal following the 4-days treatment, and the
leptin-treated group gained more weight. Leptin regulates energy
intake and expenditure (34). The
results of the present study suggested that leptin may serve a role
in growth control; however, leptin did not notably affect weigh.
This finding is consistent with those of Nagasaki and Ohta
(29), in which serum
concentrations of leptin were not correlated with body weight at
any time point in infants.
The protective effects of leptin on spatial memory
appeared to be more significant than the effects on emotion and
motor function. Differences in latency of navigation training
between the three groups were statistically significant from the
second day. In the water maze test, leptin significantly increased
the number of platform crossings and reduced the platform latency
when compared with the model group; the observations of leptin were
similar to those of the sham group. In addition, multivariate
analysis demonstrated that the spatial memory-enhancing effect of
leptin was independent of gender or weight, but was correlated with
age; however, whether spatial memory is similarly affected by
leptin in adult rats is unknown. Childhood and adolescence are
important periods for learning and memory development. Disruptions
during these periods may result in notable life-long adverse
effects (33). These results are
consistent with reports indicating that leptin treatment can
improve cognitive abilities (30,41);
however, Oomura et al (30)
observed this within 6–8-week-old rats without any disease. The
effects between 50 µg/kg leptin treatment and the control group
were significant from day 2 (30).
Rats treated with 50 µg/kg leptin exhibited a significantly shorter
duration in the goal area on test day, compared with the group that
received the vehicle control (30).
In the capture-resistance, suspension and open field
tests, there was significant differences between leptin and model
group, and the majority of tested parameters revealed similar
values of the leptin-treated and sham groups. This suggested that
leptin treatment partially, but not completely, alleviates motor
impairment. Furthermore, leptin may induce a more positive
emotional state (34). Diabetic
rats consistently exhibit greater anxiety-associated behavior and
lower leptin levels in the blood (35). Chronically stressed rats exhibit
reduced leptin levels and depression-like symptoms, which may be
reversed upon treatment with leptin (36). In present study, the leptin-treated
group had more evasive, anxious and even aggressive behavior than
the model group. These observations supported the findings of the
capture-resistance test.
Numerous studies on the role of leptin in various
brain regions and signaling systems provide strong support for a
neuroprotective role of leptin (37–43).
There is evidence that leptin promotes neurogenesis in the adult
hippocampus in vitro and in vivo (37–39).
Furthermore, the early use of leptin has been reported to promote
the proliferation of astrocytes in the hypothalamus (40). The leptin antagonist L39A/D40A/F41A
inhibits leptin-induced alterations in somatostatin receptors,
leptin signaling and cyclic adenosine monophosphate response
element binding protein activation (41). Chronic leptin treatment has been
reported to reverse Aβ-induced deficits in learning and memory, and
contributes to the maintenance of late phase-long term potentiation
(42). Leptin levels are decreased
in the neonatal hypoxic-ischemic rat brain, and leptin treatment
may improve neuronal density and decrease apoptosis in newborn rats
with hypoxic-ischemic brain injury (43).
The intracellular signaling mechanisms involved in
leptin signaling include the Janus kinase 2, signal transducer and
activator of transcription, phosphoinositide 3-kinase, protein
kinase B, mammalian target of rapamycin and extracellular
signal-regulated kinase signaling pathways (1). The mechanisms underlying the effects
of leptin on mood, psychiatric disorders and memory are unclear;
however, they may be associated with serotonin signaling, via the
inhibition of nitric oxide synthase (NOS) (44). The various isoforms of NOS may
affect learning and memory via different sites of action, such as
learning rate that is correlated positively with neuronal NOS
(nNOS) and negatively with inducible NOS (iNOS) level. It may also
be involved in the neuroprotective mechanism of leptin, which could
serve as a direction for further research on mechanism. (44). However, it has been reported that
the overall levels of hippocampal NOS were higher in rats with
reduced learning abilities due to increases in memory errors
(44). Thus, the inhibitory action
of leptin on NOS may promote memory within the hippocampus; further
investigation is required as numerous reports have revealed that
NOS and NO activities may promote learning and memory, and
deficient NOS and NO activities may be associated with Alzheimer's
disease and poorer memory function, underlying these observations
may be the vasodilating effects of NO, rather than the direct
effects on brain tissue (45,46).
Leptin has been demonstrated to be involved in the
organization and maturation of the nervous system (47). For instance, leptin may promote the
differentiation of glial cells in the brain during development, as
mice lacking leptin possess fewer functional glial cells later in
life due to improper differentiation during development (47). In addition, leptin also stimulates
the proliferation of neuroblastoma and prevents apoptotic cell
death via the regulation of apoptotic enzymes, including caspase-10
and the tumor necrosis factor-associated apoptosis-inducing ligand,
which are critical for brain development (48). Additionally, in obese mice that
lack leptin, myelination is impaired as it occurs less frequently
and myelination density is reduced; myelination only partially
recovers with post-natal leptin treatment and is not fully restored
to that of the wild type (49).
However, accumulating evidence has revealed that leptin serves an
important role in mood, and other cognitive and behavioral
disorders (34). Furthermore,
leptin has been reported to be critical for normal brain growth,
development and developmental maturation; however, the underlying
mechanism of leptin in these processes requires further
investigation. Additionally, whether the observed effects of leptin
on cognition and behavior are mediated by an effect within discrete
brain areas, including the hippocampus and cortex, or involve more
global alterations in neural structure or synaptic function and
plasticity is unknown. Future studies may be conducted to improve
understanding within these fields of research.
The present study demonstrated that leptin may
alleviate impairments in spatial memory resulting from premature
brain damage. The results reported in the present study suggested
that leptin may have therapeutic potential for the treatment of
preterm infants with brain damage, and alleviate neurocognitive
impairments and behavioral problems. However, prior to clinical
application, further understanding of the underlying mechanisms of
neuroprotection associated with leptin is required, as well as
determination of the optimal drug dose and administration
protocol.
Acknowledgements
Authors would like to thank Prof. Lijie Liu at the
department of physiology of the medical school of Southeast
University, for providing the laboratory to finish the experiment
of animal behavior.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81771628).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
LJ conceived and designed the study, and approved
the final version of the manuscript. ECF performed the experiments,
analyzed the data, and wrote the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Southeast University (Nanjing,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Farr OM, Tsoukas MA and Mantzoros CS:
Leptin and the brain: Influences on brain development, cognitive
functioning and psychiatric disorders. Metabolism. 1:114–130. 2015.
View Article : Google Scholar
|
|
2
|
Valleau JC and Sullivan EL: The impact of
leptin on perinatal development and psychopathology. J Chem
Neuroanat 61–62. 1–232. 2014.
|
|
3
|
Couce ME, Burguera B, Parisi JE, Jensen MD
and Lloyd RV: Localization of leptin receptor in the human brain.
Neuroendocrinol. 66:145–150. 1997. View Article : Google Scholar
|
|
4
|
Burguera B, Couce ME, Long J, Lamsam J,
Laakso K, Jensen MD, Parisi JE and Lloyd RV: The long form of the
leptin receptor (OB-Rb) is widely expressed in the human brain.
Neuroendocrinol. 71:187–195. 2000. View Article : Google Scholar
|
|
5
|
Huang XF, Koutcherov I, Lin S, Wang HQ and
Storlien L: Localization of leptin receptor mRNA expression in
mouse brain. Neuroreport. 7:2635–2638. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shioda S, Funahashi H, Nakajo S, Yada T,
Maruta O and Nakai Y: Immunohistochemical localization of leptin
receptor in the rat brain. Neurosci Lett. 243:41–44. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ur E, Wilkinson DA, Morash BA and
Wilkinson M: Leptin immunoreactivity is localized to neurons in rat
brain. Neuroendocrinol. 75:264–272. 2002. View Article : Google Scholar
|
|
8
|
Martin B, Pearson M, Brenneman R, Golden
E, Keselman A, Iyun T, Carlson OD, Egan JM, Becker KG, Wood W III,
et al: Conserved and differential effects of dietary energy intake
on the hippocampal transcriptomes of females and males. PLoS One.
3:e23982008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Farr SA, Yamada KA, Butterfield DA, Abdul
HM, Xu L, Miller NE, Banks WA and Morley JE: Obesity and
hypertriglyceridemia produce cognitive impairment. Endocrinol.
149:2628–2636. 2008. View Article : Google Scholar
|
|
10
|
Morrison CD: Leptin signaling in brain: A
link between nutrition and cognition? Biochim Biophys Acta.
5:401–408. 2009. View Article : Google Scholar
|
|
11
|
Alagiakrishnan K, Sankaralingam S, Ghosh
M, Mereu L and Senior P: Anti-diabetic drugs and their potential
role in treating mild cognitive impairment and Alzheimer's disease.
Discov Med. 16:277–286. 2013.PubMed/NCBI
|
|
12
|
Martins I, Gomes S, Costa RO, Otvos L,
Oliveira CR, Resende R and Pereira CM: Leptin and ghrelin prevent
hippocampal dysfunction induced by Aβ oligomers. Neuroscience.
241:41–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Platt TL, Beckett TL, Kohler K, Niedowicz
DM and Murphy MP: Obesity, diabetes, and leptin resistance promote
tau pathology in a mouse model of disease. Neuroscience.
315:162–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kerti L, Witte AV, Winkler A, Grittner U,
Rujescu D and Flöel A: Higher glucose levels associated with lower
memory and reduced hippocampal microstructure. Neurol.
81:1746–1752. 2013. View Article : Google Scholar
|
|
15
|
World Health Organization (WHO), . Preterm
birth Fact sheet no. 363. http://www.who.int/mediacentre/factsheets/fs363/en/September
10–2014
|
|
16
|
Burns YR, Danks M, O'Callaghan MJ, Gray
PH, Cooper D, Poulsen L and Watter P: Motor coordination
difficulties and physical fitness of extremely-low-birth-weight
children. Dev Med Child Neurol. 2:136–142. 2009. View Article : Google Scholar
|
|
17
|
Woodward LJ, Edgin JO, Thompson D and
Inder TE: Object working memory deficits predicted by early brain
injury and development in the preterm infant. Brain. 128:2578–2587.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
du Plessis AJ: Neurology of the newborn
infant. Preface. Clin Perinatol. 36:xi–xiii. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Volpe JJ: Brain injury in premature
infants: A complex amalgam of destructive and developmental
disturbances. Lancet Neurol. 1:110–124. 2009. View Article : Google Scholar
|
|
20
|
Rocha-Ferreira E and Hristova M:
Plasticity in the neonatal brain following hypoxic-ischaemic
injury. Neural Plast. 2016:49010142016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Favaro A, Santonastaso P, Manara R,
Bosello R, Bommarito G, Tenconi E and Di Salle F: Disruption of
visuospatial and somatosensory functional connectivity in anorexia
nervosa. Biol Psychiatry. 10:864–870. 2012. View Article : Google Scholar
|
|
22
|
Koyama KI, Asakawa A, Nakahara T, Amitani
H, Amitani M, Saito M, Taruno Y, Zoshiki T, Cheng KC, Yasuhara D
and Inui A: Intelligence quotient and cognitive functions in severe
restricting-type anorexia nervosa before and after weight gain.
Nutri. 28:1132–1136. 2012. View Article : Google Scholar
|
|
23
|
Sternheim L, Startup H, Pretorius N,
Johnson-Sabine E, Schmidt U and Channon S: An experimental
exploration of social problem solving and its associated processes
in anorexianervosa. Psychiatry Res. 200:524–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Johnston JM, Hu WT, Fardo DW, Greco SJ,
Perry G, Montine TJ, Trojanowski JQ, Shaw LM, Ashford JW and
Tezapsidis N: Alzheimer's: Low plasma leptin in cognitively
impaired ADNI subjects: Gender differences and diagnostic and
therapeutic potential. Curr Alzheimer Res. 11:165–174. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mela V, Díaz F, Borcel E, Argente J,
Chowen JA and Viveros MP: Long term hippocampal and cortical
changes induced by maternal deprivation and neonatal leptin
treatment in male and female rats. PLoS One. 10:e01372832015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Linnemann K, Malek A, Sager R, Blum WF,
Schneider H and Fusch C: Leptin production and release in the
dually in vitro perfused human placenta. J Clin Endocrinol Metal.
85:4298–4301. 2000. View Article : Google Scholar
|
|
27
|
Highman TJ, Friedman JE, Huston LP, Wong
WW and Catalano PM: Longitudinal changes in maternal serum leptin
concentrations, body composition and resting metabolic rate in
pregnancy. Am J Obstet Gynecol. 178:1010–1015. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Veselá PK, Kaniok R and Bayer M: Markers
of bone metabolism, serum leptin levels and bone mineral density in
preterm babies. J Pediatr Endocrinol. 1:27–32. 2015.
|
|
29
|
Nagasaki H and Ohta T: Extra-uterine
growth and adipocytokines in appropriate-for-gestational-age
preterm infants. Pediatr Int. Dec 30–2015.(Epub ahead of
print).
|
|
30
|
Oomura Y, Hori N, Shiraishi T, Fukunaga K,
Takeda H, Tsuji M, Matsumiya T, Ishibashi M, Aou S, Li XL, et al:
Leptin facilitates learning and memory performance and enhances
hippocampal CA1 long-term potentiation and CaMK II phosphorylation
in rats. Peptides. 27:2738–2749. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rice JE III, Vannucci RC and Brierley JB:
The influence of immaturity on hypoxic-ischemic brain damage in the
rat. Ann Neurol. 2:131–141. 1981. View Article : Google Scholar
|
|
32
|
Back SA, Han BH, Luo NL, Chricton CA,
Xanthoudakis S, Tam J, Arvin KL and Holtzman DM: Selective
vulnerability of late oligodendrocyte progenitors to
hypoxia-ischemia. J Neurosci. 22:455–463. 2006. View Article : Google Scholar
|
|
33
|
Wang W: Pediatrics. 8th. People's Health
Press; Beijing: 2013, View Article : Google Scholar
|
|
34
|
Farr OM, Tsoukas MA and Mantzoros CS:
Leptin and the brain: Influences on brain development, cognitive
functioning and psychiatric disorders. Metabolism. 64:114–130.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ates M, Dayi A, Kiray M, Sisman AR,
Agilkaya S, Aksu I, Baykara B, Buyuk E, Cetinkaya C, Cingoz S and
Uysal N: Anxiety- and depression-like behaviors were correlated
with leptin and leptin receptor expression in prefrontal cortex of
streptozotocin-induced diabetic rats. Biotech Histochem.
89:161–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Matarese G, La Rocca C, Moon HS, Huh JY,
Brinkoetter MT, Chou S, Perna F, Greco D, Kilim HP, Gao C, et al:
Selective capacity of metreleptin administration to reconstitute
CD4+ T-cell number in females with acquired hypoleptinemia. Proc
Natl Acad Sci USA. 9:pp. E818–E827. 2013;
|
|
37
|
Stiega MR, Sieversa C, Farrb O, Stallaa GK
and Mantzorosb CS: Leptin: A hormone linking activation of
neuroendocrine axes with neuropathology. Psychoneuroendocrinol.
51:47–57. 2015. View Article : Google Scholar
|
|
38
|
Garza JC, Guo M, Zhang W and Lu XY: Leptin
increases adult hippocampal neurogenesis in vivo and in vitro. J
Biol Chem. 283:18238–18247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang F, Wang S, Signore AP and Chen J:
Neuroprotective effects of leptin against ischemic injury induced
by oxygen-glucose deprivation and transient cerebral ischemia.
Stroke. 38:2329–2336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rottkamp DM, Rudenko IA, Maier MT,
Roshanbin S, Yulyaningsih E, Perez L, Valdearcos M, Chua S, Koliwad
SK and Xu AW: Leptin potentiates astrogenesis in the developing
hypothalamus. Mol Metab. 4:881–889. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Perianes-Cachero A, Burgos-Ramos E,
Puebla-Jiménez L, Canelles S, Frago LM, Hervás-Aguilar A, de Frutos
S, Toledo-Lobo MV, Mela V, Viveros MP, et al: Acute up-regulation
of the rat brain somatostatin receptor-effect or system by leptin
is related to activation of insulin signaling and may counteract
central leptin actions. Neuroscience. 252:289–301. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tong JQ, Zhang J, Hao M, Yang J, Han YF,
Liu XJ, Shi H, Wu MN, Liu QS and Qi JS: Leptin attenuates the
detrimental effects of β-amyloid on spatial memory and hippocampal
later-phase long term potentiation in rats. Horm Behav. 73:125–130.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang F, Wang S, Signore AP and Chen J:
Neuroprotective effects of leptin against ischemic injury induced
by oxygen-glucose deprivation and transient cerebral ischemia.
Stroke. 38:2329–2336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gökçek-Saraç Ç, Karakurt S, Adali O and
Jakubowska-Doğru E: Correlation between hippocampal levels of
neural, epithelial and inducible nos and spatial learning skills in
rats. Behav Brain Res. 235:326–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Paul V and Ekambaram P: Involvement of
nitric oxide in learning & memory processes. Indian J Med Res.
133:471–478. 2011.PubMed/NCBI
|
|
46
|
Lin AL, Zheng W, Halloran JJ, Burbank RR,
Hussong SA, Hart MJ, Javors M, Shih YY, Muir E, Solano Fonseca R,
et al: Chronic rapamycin restores brain vascular integrity and
function through no synthase activation and improves memory in
symptomatic mice modeling Alzheimer's disease. J Cereb Blood Flow
Metab. 33:1412–1421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Udagawa J, Nimura M and Otani H: Leptin
affects oligodendroglial development in the mouse embryonic
cerebral cortex. Neuro Endocrinol Lett. 27:177–182. 2006.PubMed/NCBI
|
|
48
|
Russo VC, Metaxas S, Kobayashi K, Harris M
and Werther GA: Antiapoptotic effects of leptin in human
neuroblastoma cells. Endocrinology. 145:4103–4112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hashimoto R, Matsumoto A, Udagawa J, Hioki
K and Otani H: Effect of leptin administration on myelination in
ob/ob mouse cerebrum after birth. Neuroreport. 1:22–29. 2013.
View Article : Google Scholar
|