Introduction
Psoriasis is a chronic inflammatory skin disease,
which may severely impact the quality of life of patients and
manifests as erythematous plaques covered with silvery-white scales
(1). Psoriatic lesions are
characterized by epidermal hyperplasia with parakeratosis, loss of
the granular layer, acanthosis, aberrant differentiation,
proliferation of keratinocytes and marked infiltration of immune
cells into the dermis or epidermis (2). The pathogenesis of psoriasis is
complex, and the exact underlying mechanism of the factors involved
remains elusive. The abnormal proliferation of keratinocytes is a
key feature of psoriasis, which results in epidermal hyperplasia
and the morphological characteristics of psoriasis (3). It is widely accepted that the
abnormal growth dynamics of keratinocytes are due to the
dysregulation of cytokines and growth factors, which are secreted
by infiltrated immune cells in the skin lesions (4,5).
Among these, interferon-γ (IFN-γ), interleukin (IL)-17A and IL-22
have been demonstrated to be increased and to serve important roles
in the development of the skin lesions observed in patients with
psoriasis (6–10). However, the molecular mechanisms
involved in this process remain unclear.
The Wilms' tumor 1 (WT1) gene, which maps to
chromosome 11p13 and contains 10 exons, encodes a DNA-binding
transcription factor that is involved in the regulation of human
cell growth and differentiation (11). This gene locus is frequently
mutated in patients with Wilms' tumor. In addition, alterations in
this gene have been identified in a variety of cancer types,
including breast cancer, renal cell cancer, ovarian cancer, lung
cancer, melanoma and acute leukemia (12–18).
In these types of cancer, WT1 acts as either an oncogene or a tumor
suppressor gene, depending on the different cellular
characteristics (19–21). However, to the best of our
knowledge, there have been no previous studies focusing on the
expression and role of WT1 in the formation of psoriatic skin
lesions.
The present study detected the expression of WT1 in
the non-lesional skins and skin lesions from patients with
psoriasis vulgaris (PV) and an imiquimod (IMQ)-induced
psoriasis-like mouse model. The effect of WT1 on the proliferation
and apoptosis of keratinocytes was subsequently investigated. It
was revealed that WT1 expression was significantly increased in
non-lesional skin tissues and psoriatic skin lesions.
Overexpressing WT1 promoted keratinocyte proliferation and
inhibited apoptosis. In addition, certain inflammatory cytokines
upregulated WT1 in keratinocytes. These findings indicated that WT1
may serve an important role in the formation of skin lesions
associated with PV.
Materials and methods
Human subjects
A total of 20 psoriatic patients who were diagnosed
with PV by pathological examination were recruited from outpatient
clinics at the Second Xiangya Hospital of Central South University
(Changsha, China). Psoriasis disease activity was assessed using
psoriasis area and severity index (PASI) scores (22), and blood samples and lesional skins
were collected. Non-lesional skin tissues were obtained from 10 of
the patients simultaneously. Patient information is presented in
Table I. Blood samples were
collected from 20 sex- and age-matched healthy controls who were
recruited from the medical staff at the Second Xiangya Hospital.
Normal skin tissues were obtained from the outpatient operating
room at the Department of Dermatology at the Second Xiangya
Hospital. The information of all healthy controls is presented in
Table II. The present study was
approved by the Ethics Committee of the Second Xiangya Hospital of
Central South University. Written informed consent was obtained
from all subjects.
 | Table I.Information on patients with
psoriasis vulgaris. |
Table I.
Information on patients with
psoriasis vulgaris.
| Sample ID | Age/sex | PASI score |
|---|
| 1 | 36/F | 0.7 |
| 2 | 25/F | 9.5 |
| 3 | 24/F | 8.6 |
| 4 | 54/M | 10.6 |
| 5 | 29/M | 2.4 |
| 6 | 45/M | 5.9 |
| 7 | 46/M | 10.2 |
| 8 | 18/F | 2.4 |
| 9 | 30/M | 0.2 |
| 10 | 45/M | 10.7 |
| 11 | 38/F | 8.1 |
| 12 | 22/M | 18.1 |
| 13 | 45/M | 8.0 |
| 14 | 30/M | 4.0 |
| 15 | 42/F | 4.5 |
| 16 | 48/M | 8.8 |
| 17 | 27/M | 8.9 |
| 18 | 30/M | 12.5 |
| 19 | 40/M | 11.8 |
| 20 | 20/M | 10.4 |
 | Table II.Information on healthy controls. |
Table II.
Information on healthy controls.
| A, Skin tissue |
|---|
|
|---|
| Sample ID | Age/sex |
|---|
| 1 | 16/Male |
| 2 | 31/Female |
| 3 | 35/Female |
| 4 | 24/Female |
| 5 | 19/Female |
| 6 | 25/Male |
| 7 | 32/Male |
| 8 | 28/Male |
| 9 | 50/Female |
| 10 | 55/Female |
| 11 | 26/Female |
| 12 | 35/Male |
| 13 | 34/Female |
| 14 | 54/Female |
| 15 | 39/Female |
| 16 | 25/Male |
| 17 | 37/Female |
| 18 | 29/Male |
| 19 | 48/Female |
| 20 | 35/Female |
|
| B, Blood
samples |
|
| Sample
ID | Age/sex |
|
| 1 | 39/Male |
| 2 | 45/Male |
| 3 | 37/Male |
| 4 | 26/Male |
| 5 | 22/Female |
| 6 | 38/Female |
| 7 | 42/Male |
| 8 | 47/Male |
| 9 | 29/Male |
| 10 | 25/Female |
| 11 | 38/Male |
| 12 | 32/Male |
| 13 | 35/Male |
| 14 | 29/Female |
| 15 | 43/Male |
| 16 | 36/Male |
| 17 | 27/Female |
| 18 | 35/Male |
| 19 | 44/Male |
| 20 | 31/Male |
Assessment of PASI scores
For determining the severity and extent of
psoriasis, PASI scoring was used (22,23).
In the four regions of the body, namely the head (h), upper
extremities (u), lower extremities (l) and torso (t), the
characteristics of the disease, including erythema (E),
infiltration (I) and desquamation (D), were evaluated with a score
of 1–4, and the involved area (A) of psoriatic lesions was
evaluated with a score of 1–6 (Table
III). PASI total scores ranges between 0 and 72. Higher scores
indicate greater psoriasis severity. The formula used to calculate
the total PASI score is as follows:
PASI=(Eh+Ih+Dh)xAhx0.1+(Eu+Iu+Du)xAux0.2+(Et+It+Dt)xAtx0.3+(El+Il+Dl)xAlx0.4
 | Table III.Psoriasis area and severity index
evaluation criteria. |
Table III.
Psoriasis area and severity index
evaluation criteria.
|
| Grade |
|---|
|
|
|
|---|
| Factor | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
|---|
| Erythema (E) | None | Mild | Medium | Severe | Very severe | – | – |
| Infiltration
(I) |
|
|
|
|
|
|
|
| Desquamation
(D) |
|
|
|
|
|
|
|
| Involved area of
the psoriatic lesions (A) % | 0 | <10 | 10–29 | 30–49 | 50–69 | 70–89 | 90–100 |
IMQ-induced psoriasis-like mouse
model
Female BALB/c mice (age, 6–8 weeks; 19.0–20.5 g)
were purchased from Shanghai SLAC Laboratory Animal Co., Ltd.
(Shanghai, China). All mice were maintained in specific
pathogen-free conditions (20–24°C; relative humidity, 50–55%; 12 h
light/dark cycle) with free access to food and water. The
IMQ-induced psoriasis-like mouse model was established as
previously described (24). The
mice were treated with a daily topical dose of 62.5 mg 5% IMQ cream
(cat. no. H20030128; Sichuan Med-Shine Pharmaceutical Co., Ltd.,
Chengdu, China) on their shaved backs for 7 consecutive days. The
control mice were treated with the same dose of vehicle cream. All
procedures were approved and supervised by the Animal Care and Use
Committee of the Second Xiangya Medical School of Central South
University.
Cell isolation and culture
Peripheral blood mononuclear cells (PBMCs) were
separated from the peripheral blood of healthy controls and
patients with psoriasis by density gradient centrifugation at 18°C
and 600 × g for 30 min (GE Healthcare, Chicago, IL, USA). The cells
were cultured in RPMI 1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; HyClone; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C
in 5% CO2, or collected directly for subsequent
experiments. HaCaT cells (cat no. BNCC101683; BeNa Culture
Collection, Beijing, China), which were stored in liquid nitrogen,
were revived and cultured in Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS at 37°C in 5% CO2. The medium was refreshed every 2
days and the cells were subcultured when 90% confluence was
reached.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells or skin
tissues using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), and a NanoDrop spectrophotometer (ND-2000;
Thermo Fisher Scientific, Inc.) was used for RNA quality control.
The mRNA was reverse-transcribed using a PrimeScript® RT
reagent kit with gDNA Eraser (Takara Biotechnology Co., Ltd.,
Dalian, China). Each test used 1 µg total RNA and was performed
according to the manufacturer's protocol. qPCR was subsequently
performed using the SYBR Premix Ex Taq II (Tli RnaseH Plus; Takara
Biotechnology Co., Ltd.) using a LightCycler® 96 (Roche
Diagnostics, Basel, Switzerland) thermocycler. The thermocycling
conditions were as follows: Initial denaturation at 95°C for 30
sec, followed by 45 cycles of 95°C for 5 sec and 60°C for 20 sec,
and a final extension (95°C for 1 sec, 65°C for 15 sec and 95°C for
1 sec). The relative expression of the target genes was calculated
using the 2−ΔΔCq method (25) and normalized against the GAPDH
internal control. Detailed information on the primers used is
summarized in Table IV.
 | Table IV.Primer sequences, product sizes and
annealing temperatures. |
Table IV.
Primer sequences, product sizes and
annealing temperatures.
| Gene name | Primer
sequence | Annealing
temperature, °C | Product size,
bp |
|---|
| Human GAPDH-F |
5′-ATGGGGAAGGTGAAGGTCG-3′ | 60 | 108 |
| Human GAPDH-R |
5′-GGGGTCATTGATGGCAACAATA-3′ |
|
|
| Human WT1-F |
5′-TTGAATGCATGACCTGGAAT-3′ | 60 | 147 |
| Human WT1-R |
5′-CCTGAATGCCTCTGAAGACA-3′ |
|
|
| Mouse GAPDH-F |
5′-AGGTCGGTGTGAACGGATTTG-3′ | 60 | 123 |
| Mouse GAPDH-R |
5′-TGTAGACCATGTAGTTGAGGTCA-3′ |
|
|
| Mouse WT1-F |
5′-GAGAGCCAGCCTACCATCC-3′ | 60 | 128 |
| Mouse WT1-R |
5′-GGGTCCTCGTGTTTGAAGGAA-3′ |
|
|
Western blot analysis
The cells or skin tissues were lysed in
radioimmunoprecipitation assay buffer supplemented with protease
and phosphatase inhibitors (Beyotime Institute of Biotechnology,
Haimen, China). The proteins were quantified using a Bradford assay
(Pierce; Thermo Fisher Scientific, Inc.) and 100 µg protein from
each sample was loaded for 8% SDS-PAGE. Following the transfer of
proteins onto a polyvinylidene fluoride membrane, the membrane was
blocked with 5% skim milk in PBS with 0.1% Tween-20 at room
temperature for 1 h. Rabbit anti-WT1 (1:1,000; cat no. ab89901;
Abcam, Cambridge, UK) and goat anti-GAPDH (1:2,000; cat no. ab9483;
Abcam) primary antibodies were incubated with the membrane at 4°C
overnight. Horseradish peroxidase (HRP) goat anti-rabbit
immunoglobulin (Ig)G (H+L) (1:5,000; cat no. AS014; ABclonal
Biotech Co., Ltd., Woburn, MA, USA) and HRP donkey anti-goat IgG
(H+L) (1:5,000; cat no. A00178; GenScript, Piscataway, NJ, USA)
secondary antibodies were incubated at room temperature for 2 h.
The data was analyzed using a GE-ImageQuant LAS 4000 mini (GE
Healthcare). The quantification of WT1 was normalized against GAPDH
by densitometric analysis with Image-Pro Plus 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA). The images were cropped for
presentation.
Immunohistochemistry
Skin tissues were fixed in formalin at room
temperature overnight and embedded in paraffin. The 6-µm-thick
sections were stained with hematoxylin for 10 min, and then stained
with eosin for 2 min at room temperature. For immunohistochemistry,
the sections were stained with rabbit anti-WT1 (1:200; cat no.
ab89901; Abcam) or rabbit anti-Ki67 polyclonal antibodies (1:100;
cat no. ab15580; Abcam) at 4°C overnight, according to the
manufacturer's protocol. Image analysis was performed using a DMI
4000B microscope (magnification, ×100) and Leica Qwin Std analysis
software version 3 (both Leica Microsystems GmbH, Wetzlar,
Germany).
Small interfering (si)RNA and plasmid
transfection of HaCaT cells
A total of 12 h prior to transfection, the HaCaT
cells were seeded in a 6-well cell culture plate at
6×103 cells/well. The cells were transfected with 20 nM
WT1 siRNA, 5 µg WT1 plasmid or their corresponding controls using
Lipofectamine® 2000 (Invitrogen, Thermo Fisher
Scientific, Inc., USA) for 6 h in Opti-minimum essential medium
(Opti-MEM; Gibco; Thermo Fisher Scientific, Inc.). The Opti-MEM was
removed and replaced with DMEM supplemented with 10% FBS for cell
culture. The WT1 expression plasmid and the empty control plasmid
were purchased from Vigene Biosciences, Inc. (Rockville, MD, USA).
The WT1 and negative control siRNAs (sequences unavailable) were
purchased from Invitrogen (Thermo Fisher Scientific, Inc.). The WT1
siRNA consisted of a mixture of two oligos:
5′-AAATATCTCTTATTGCAGCCTGGGT-3′ and
5′-TTTCACACCTGTATGTCTCCTTTGG-3′.
Cell Counting Kit (CCK)-8 assay
HaCaT cells were seeded in 96-well plates in
triplicate and transfected with WT1 siRNA or the WT1 overexpression
plasmid as described above. The cells were cultured in 100 µl DMEM
with 10% FBS for 24, 48, 72, 96 or 120 h. The CCK-8 kit (Beyotime
Institute of Biotechnology) was used to evaluate cell
proliferation. A total of 10 µl CCK-8 solution was added to each
well and the cells were incubated for 3 h at 37°C in 5%
CO2. The cell viability was detected at 450 nm using an
EnSpire Multimode Plate Reader (PerkinElmer, Inc., Waltham, MA,
USA).
Cellular apoptosis assay
HaCaT cells were seeded in 24-well plates in
triplicate and transfected with WT1 siRNA or the WT1 overexpression
plasmid as described above. After 24 or 48 h, the level of cellular
apoptosis was detected using a Fluorescein Isothiocyanate Annexin V
Apoptosis Detection kit II (BD Pharmingen; BD Biosciences, Franklin
Lakes, NJ, USA), according to the manufacturer's protocol. The data
were acquired using a flow cytometer (BD Canto II; BD Biosciences)
and analyzed using FlowJo software version 10 (FlowJo LLC, Ashland,
OR, USA).
Cytokine stimulation of HaCaT
cells
HaCaT cells were stimulated with 0, 10, 20 or 50
ng/ml IL-17A, IFN-γ or IL-22 (all PeproTech, Inc., Rocky Hill, NJ,
USA). The cells were collected after 24 h for RT-qPCR analysis.
Statistical analysis
Data are presented as the mean ± standard error of
the mean of at least three experiments. Statistical analysis was
performed using GraphPad Prism 6.0 software (GraphPad Software,
Inc., La Jolla, CA, USA) and P<0.05 was considered to indicate a
statistically significant difference. The data were assessed for
normality of distribution and a similar variance between groups. A
two-tailed unpaired Student's t-test was used for comparisons
between two groups and one-way analysis of variance with the
corresponding post-hoc test (Bonferroni or Dunnett's) were used for
the comparison of multiple groups. When the data were not normally
distributed or there were not equal variances between two groups, a
two-tailed Mann-Whitney U-test was used for statistical analysis.
Correlation analysis was performed using a Spearman's r test.
Results
WT1 expression is elevated in
non-lesional and lesional skins of patients with psoriasis and
IMQ-induced psoriasis-like model mice
To examine the role of WT1 in the pathogenesis of
psoriasis, the mRNA expression level of WT1 was initially
determined in 10 non-lesional skins and 20 lesional skins taken
from patients with psoriasis and skin samples taken from 20 normal
human controls using RT-qPCR. The results demonstrated that the
mRNA expression of WT1 was significantly increased in non-lesional
skins and lesional skins from patients with PV, compared with the
normal controls (Fig. 1A).
Similarly, the protein expression of WT1 in non-lesional skins and
lesional skins from patients with PV was also increased compared
with the normal controls (Fig. 1B and
C). The results suggested that WT1 mRNA and protein expression
levels in lesional skins were slightly increased compared with
those in non-lesional skins from patients with psoriasis, although
there were no statistical differences (Fig. 1A-C). Notably, the WT1 mRNA
expression level in the psoriatic skin lesions was positively
correlated with the PASI scores of the patients (Fig. 1D). In addition,
immunohistochemistry with anti-WT1 antibodies was used to detect
the expression and location of WT1 in the skin tissues. It was
confirmed that the expression of WT1 was primarily enhanced in the
epidermis of the psoriatic skin lesions (Fig. 1E). However, no significant
differences were observed in the mRNA and protein expression levels
of WT1 in the PBMCs of patients with PV compared with the normal
controls (Fig. 1F-H).
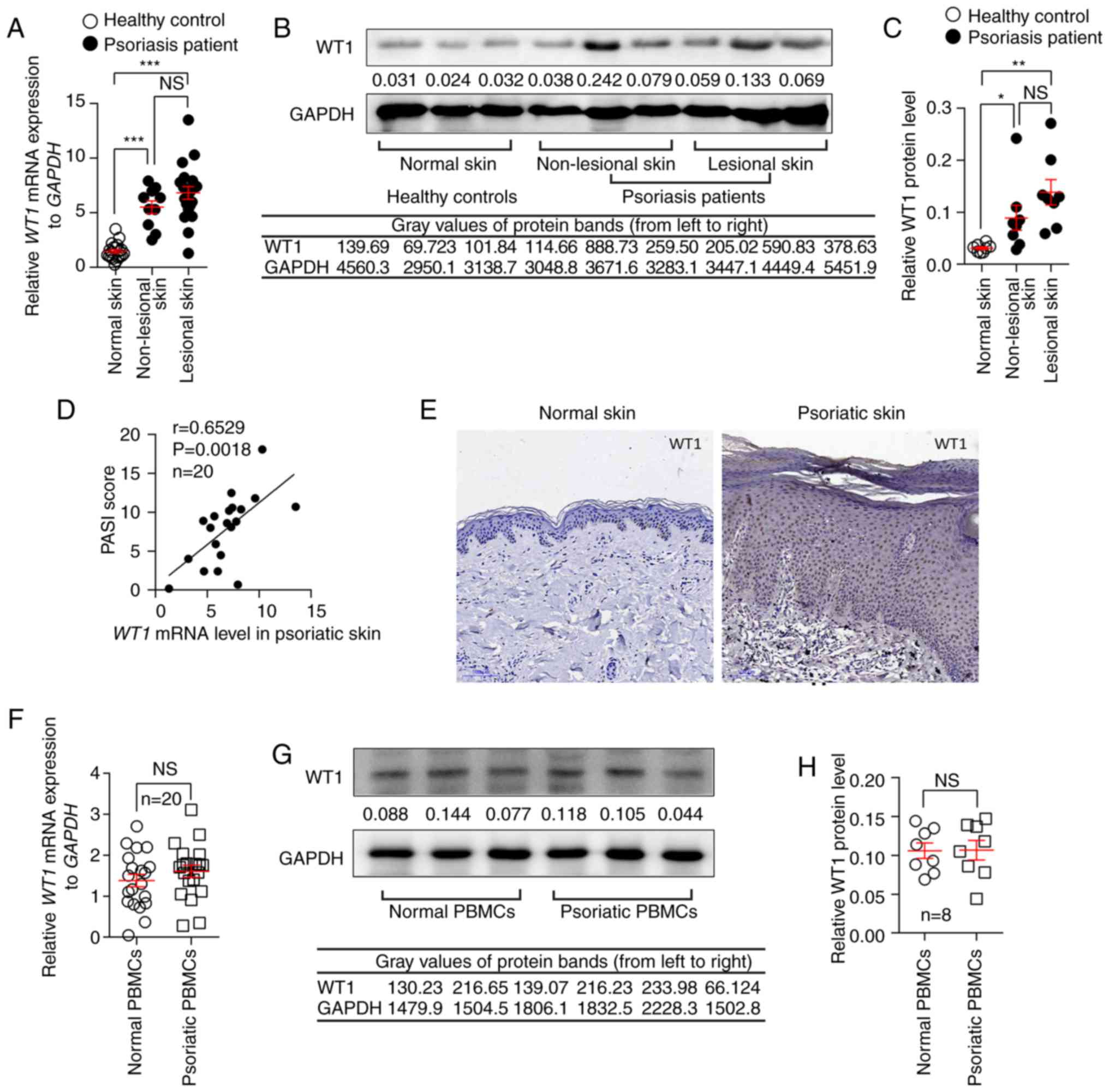 | Figure 1.WT1 expression is elevated in
non-lesional and lesional skins from patients with psoriasis. (A)
The mRNA expression of WT1 in non-lesional (n=10) and lesional
skins (n=20) from patients with psoriasis and normal skin samples
(n=20) from healthy controls (non-lesional skin vs. normal skin,
P<0.0001; lesional skin vs. normal skin, P<0.0001;
non-lesional skin vs. lesional skin, P=0.2510). The protein
expression of WT1 in non-lesional and lesional skins from psoriasis
patients (n=8) and normal skin samples from healthy controls (n=8)
was assessed. (B) A representative image of the western blotting
and (C) statistical analysis of the data for WT1 protein expression
(non-lesional skin vs. normal skin, P=0.0291; lesional skin vs.
normal skin, P=0.0060; non-lesional skin vs. lesional skin,
P=0.1690). (D) Correlation between WT1 mRNA expression in psoriatic
skin lesions and the PASI scores of patients with psoriasis
(r=0.6529; P=0.0018; n=20). (E) Immunostaining of WT1 in skin
lesions from psoriasis patients and normal skin samples from
healthy controls (n=6; magnification, ×100). (F) The mRNA
expression levels of WT1 in PBMCs from patients with psoriasis and
normal controls (n=20; P=0.2982). The protein expression of WT1 in
PBMCs from patients with psoriasis and normal controls (n=8). (G) A
representative image of the western blotting and (H) statistical
analysis of the data (P=0.9634) for WT1 protein expression. Data
are pooled from two independent experiments (A, D and F) or are
representative of three independent experiments (B, C, E, G and H).
Data are presented as the mean ± standard error of the mean.
*P<0.05, **P<0.01, ***P<0.001. NS, not significant.
One-way analysis of variance with Bonferroni post hoc test (A and
C), Spearman's r test (D) or two-tailed unpaired Student's t-test
(F and H) were used for analysis. PASI, psoriasis area and severity
index; PBMC, peripheral blood mononuclear cell; WT1, Wilms' tumor
1. |
Furthermore, the present study detected the WT1
expression in an IMQ-induced psoriasis-like mouse model, which
closely resembles the human psoriasis phenotype, according to a
previously published study (24).
IMQ cream was applied to the shaved backs of BALB/c mice for 7
consecutive days to establish this model. As expected, the
IMQ-treated mice developed typical psoriasis-like lesions with
evident clinical and histological alterations (Fig. 2A and B). The results demonstrated
that the expression of Ki67, a marker exclusively associated with
cell proliferation, was significantly increased in the IMQ-treated
mice compared with the controls, which indicated that the excessive
proliferation of keratinocytes was induced by the IMQ (Fig. 2C). Consistent with the results of
the human samples, the mice exposed to IMQ expressed significantly
higher levels of WT1 mRNA and protein in their non-lesional skins
and skin lesions compared with the vehicle-exposed mice (Fig. 2D-G).
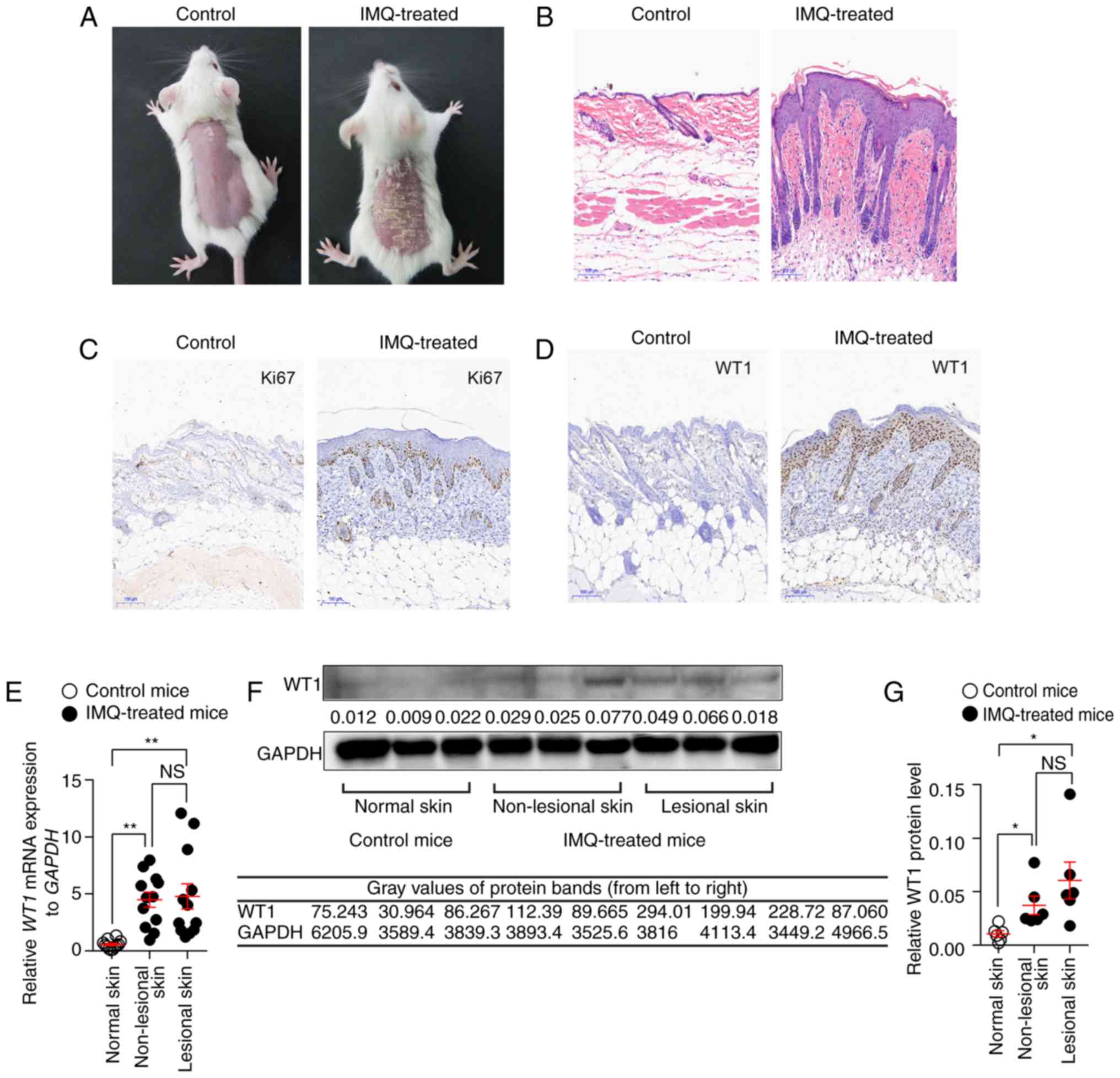 | Figure 2.WT1 expression is elevated in
non-lesional and lesional skins of the IMQ-induced psoriasis-like
mouse model. IMQ cream was painted on the shaved backs of BALB/c
mice for 7 consecutive days. (A) Phenotypic presentation and (B)
hematoxylin and eosin staining of the skin lesions from IMQ-treated
mice. Immunostaining of (C) Ki67 or (D) WT1 in skin lesions derived
from IMQ-treated mice and normal skin samples derived from control
mice. Magnifications, ×100. (E) The mRNA expression of WT1 in
non-lesional (n=12) and lesional skins (n=12) from IMQ-treated mice
and normal skin samples (n=12) derived from control mice
(non-lesional skin vs. normal skin, P=0.0020; lesional skin vs.
normal skin, P=0.0010; non-lesional skin vs. lesional skin,
P=0.9590). (G) The protein expression of WT1 in non-lesional and
lesional skins from IMQ-treated mice (n=6) and normal skin samples
derived from control mice (n=6) was assessed. Representative image
of the (F) western blotting and (G) statistical analysis of the
data for WT1 protein expression (non-lesional skin vs. normal skin,
P=0.0150; lesional skin vs. normal skin, P=0.0172; non-lesional
skin vs. lesional skin, P=0.2552). Data (A-D and F) are
representative of at least three independent experiments with three
to six samples per group. Data (E and G) are pooled from two
independent experiments. Data are presented as the mean ± standard
error of the mean. *P<0.05, **P<0.01. NS, not significant.
One-way analysis of variance with Bonferroni post hoc test (E and
G) was used. IMQ, imiquimod; WT1, Wilms' tumor 1. |
Overexpressing WT1 in keratinocytes
promotes proliferation and inhibits apoptosis
Abnormal proliferation and apoptosis of
keratinocytes is a pathological hallmark of psoriasis (26). To examine the role of increased WT1
in the pathogenesis of PV, HaCaT cells were transfected with a WT1
overexpression plasmid or a control plasmid. The results of the
western blot analysis demonstrated that the WT1 protein was
successfully overexpressed in the WT1 expression
plasmid-transfected cells (Fig.
3A). A CCK-8 assay was performed to detect the proliferation of
HaCaT cells. The results demonstrated that WT1 overexpression
promoted the proliferation of HaCaT cells, particularly on days 4
and 5 following transfection (Fig.
3B). Apoptosis analysis was subsequently performed. The results
of the flow cytometry revealed that the proportion of
Annexin-positive and propidium iodide-negative cells, which
represent early apoptotic cells, was significantly decreased in the
WT1 plasmid transfection group compared with the control group
(Fig. 3C and D).
 | Figure 3.Overexpressing WT1 in keratinocytes
promotes proliferation and inhibits apoptosis. (A) HaCaT cells were
transfected with a WT1 overexpression or control plasmid for 48 h,
and the protein expression of WT1 was detected. (B) HaCaT cells
were transfected with a WT1 overexpression or control plasmid, and
the cells were collected on the indicated days to detect the
proliferation status using a Cell Counting Kit-8 assay (n=6; D3,
P=0.0216; D4, P=0.0356; D5, P=0.0188). (C) HaCaT cells were
transfected with a WT1 overexpression or control plasmid, and the
cells were harvested after 48 h for the apoptosis assay (n=6). (D)
Statistical analysis of the data is presented (P=0.0003). All
experiments were repeated a minimum of three times. Data are
presented as the mean ± standard error of the mean. *P<0.05,
***P<0.001 vs. plasmid ctr. Two-tailed unpaired Student's
t-tests (B and D) were used. WT1, Wilms' tumor 1; D, day; ctr,
control; FITC, fluorescein isothiocyanate; PI, propidium iodide;
OD, optical density. |
Knockdown of WT1 in keratinocytes
inhibits proliferation and promotes apoptosis
To further investigate the functional mechanism of
WT1 in the pathogenesis of psoriasis, WT1 expression was knocked
down in HaCaT cells using siRNA. The western blot analysis
demonstrated that WT1 expression was significantly inhibited in the
HaCaT cells transfected with WT1 siRNA compared with the negative
control (Fig. 4A). The transfected
HaCaT cells were harvested at different time points for the CCK-8
assay. The results revealed that the proliferation ability of HaCaT
cells was significantly decreased in the WT1 siRNA-transfected
group compared with the control group (Fig. 4B). In addition, the effect of WT1
knockdown on the apoptosis of HaCaT cells was detected by flow
cytometry, and it was observed that the proportion of early
apoptotic HaCaT cells was clearly increased in the WT1
siRNA-transfected group compared with the control siRNA group
(Fig. 4C and D).
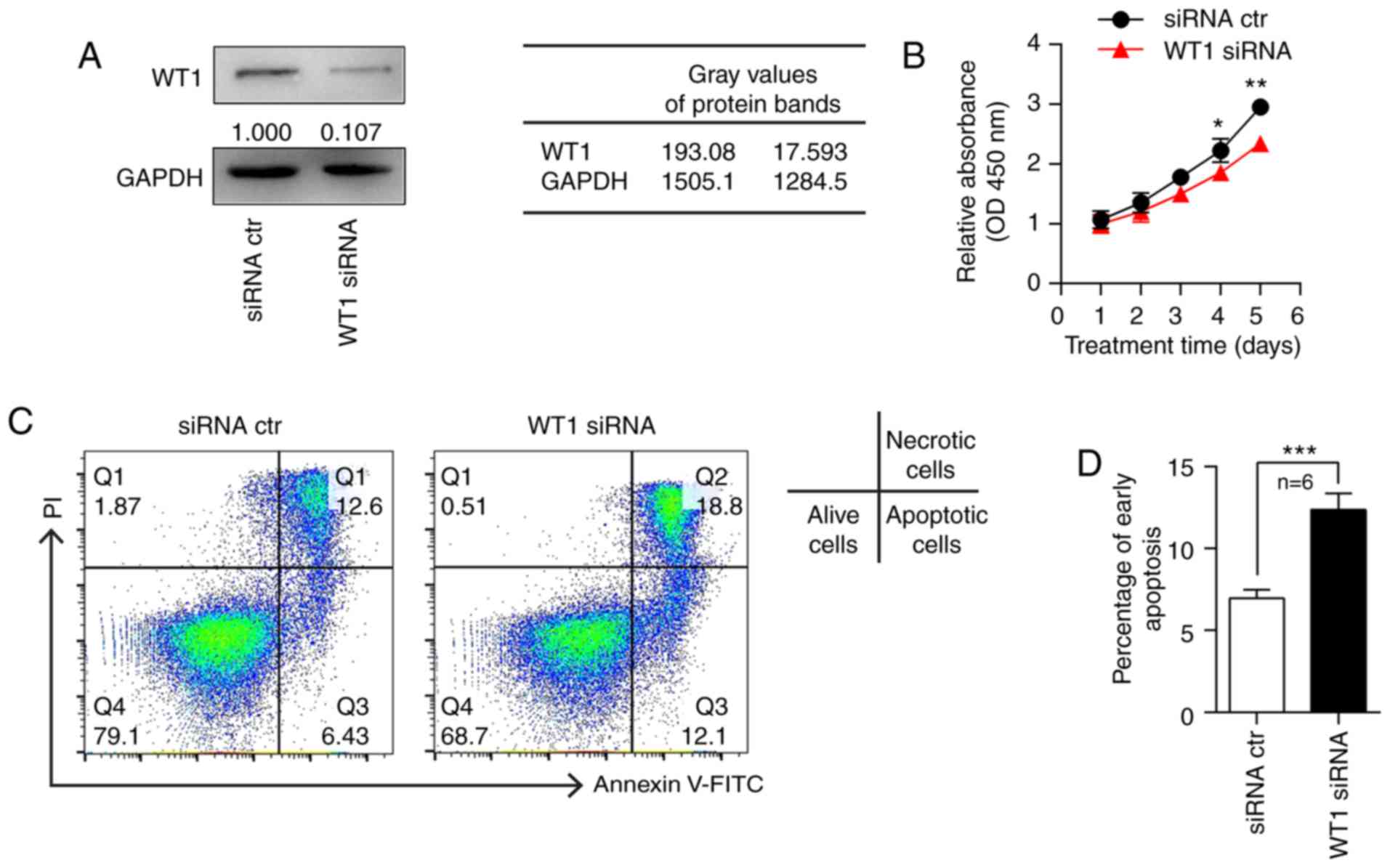 | Figure 4.Knockdown of WT1 in keratinocytes
inhibits proliferation and promotes apoptosis. (A) HaCaT cells were
transfected with WT1 siRNA or control siRNA for 48 h, and the
protein expression of WT1 was detected. (B) HaCaT cells were
transfected with WT1 siRNA or control siRNA, and the cells were
collected on the indicated days to detect the proliferation status
by Cell Counting Kit-8 assay (n=6; D4, P=0.0454; D5, P=0.0021). (C)
HaCaT cells were transfected with WT1 or control siRNA, then the
cells were harvested after 48 h for the apoptosis assay (n=6). (D)
Statistical analysis of the data is presented (P=0.0007). All
experiments were repeated a minimum of three times. Data are
presented as the mean ± standard error of the mean. *P<0.05,
**P<0.01, ***P<0.001 vs. siRNA ctr. Two-tailed unpaired
Student's t-tests (B and D) were used. WT1, Wilms' tumor 1; siRNA,
small interfering RNA; D, day; ctr, control; FITC, fluorescein
isothiocyanate; PI, propidium iodide; OD, optical density. |
WT1 expression is induced by
proinflammatory factors
It has been previously demonstrated that epidermal
keratinocytes are responsive to immune cell-derived cytokines,
including IFNs, IL-17 and IL-22, which are increased in the skin
lesions of psoriasis (27). To
investigate the possible mechanisms associated with WT1
upregulation, HaCaT cells were stimulated with IL-17A, IFN-γ and
IL-22. The results of RT-qPCR demonstrated that cytokines at higher
concentrations, including IFN-γ, IL-17A and IL-22, promoted WT1
mRNA expression (Fig. 5). These
results indicated that the overexpression of WT1 may be due to the
stimulation of proinflammatory factors in the local skin lesions of
patients with psoriasis.
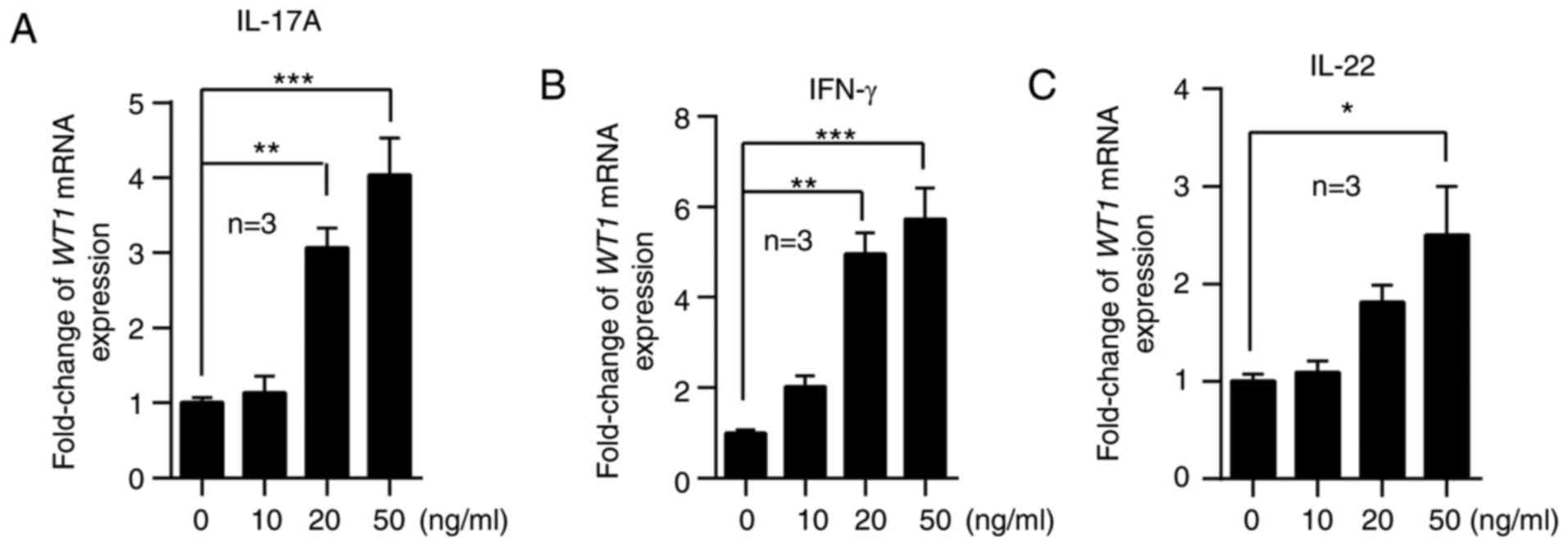 | Figure 5.WT1 expression is induced by
proinflammatory factors. HaCaT cells were stimulated with 0, 10, 20
or 50 ng/ml (A) IL-17A, (B) IFN-γ and (C) IL-22 for 24 h. The cells
were collected and analyzed to detect the mRNA expression of WT1
(n=3). Compared with the control group, the mRNA expression of WT1
was increased in HaCaT cells stimulated with IL-17A (20 vs. 0
ng/ml, P=0.003; 50 vs. 0 ng/ml, P=0.0001), IFN-γ (20 vs. 0 ng/ml,
P=0.001; 50 vs. 0 ng/ml, P=0.0001) and IL-22 (50 vs. 0 ng/ml,
P=0.011). All experiments were repeated a minimum of three times.
The data are presented as the mean ± standard error of the mean.
*P<0.05, **P<0.01, ***P<0.001. One-way analysis of
variance with Dunnett's post hoc test was used. WT1, Wilms' tumor
1; IFN, interferon; IL, interleukin. |
Discussion
Excessive proliferation and the abnormal apoptosis
of keratinocytes serve an important role in the formation of
psoriatic skin lesions (28).
Numerous cellular growth and metabolism-associated genes have been
reported to be dysregulated in psoriasis skin lesions. The WT1
gene, frequently mutated in numerous cancer types, encodes
transcription factor WT1, which regulates apoptosis and cell cycle
progression (29–31). In normal tissues, WT1 is an
important regulator of cell growth and development (32). Accumulating evidence has
demonstrated that WT1 has an oncogenic function in tumorigenesis.
WT1 knockdown by a WT1 antisense oligomer or WT1 specific short
hairpin RNA inhibits the growth of cancer cells expressing WT1
(33–35). Furthermore, overexpression of WT1
promotes cell growth, migration and invasion, and also inhibits
cellular apoptosis under certain conditions (36). However, there are no reports about
WT1 mutation in psoriasis as yet, to the best of our knowledge, and
there is poor understanding as to whether the dysregulation of WT1
is involved in the abnormal growth of keratinocytes observed in
psoriasis.
The present study demonstrated that the expression
of WT1 was low in normal skin and significantly upregulated in
non-lesional and lesional skins from patients with psoriasis and
the IMQ-induced psoriasis-like mouse model, suggesting that the
alteration in WT1 gene expression may be involved in the early
pathogenesis of psoriasis. The expression of WT1 was also
positively correlated with PASI scores. In addition, the increased
WT1 was limited to the epidermis of psoriatic skin, and it promoted
the proliferation and inhibited the apoptosis of keratinocytes. WT1
is frequently involved in the regulation of cell growth and
apoptosis through different target genes and signaling pathways,
including C-myc (37), cellular
tumor antigen p53-mediated cellular apoptosis (38), and the mitogen activated protein
kinase and Janus kinase-signal transducer and activator of
transcription signaling pathways (39). The majority of these target genes
and signaling pathways are also involved in the pathogenesis of
psoriasis (40–42). A previous study reported that WT1
mediates keratinocyte growth factor (KGF) signaling in breast
cancer cells, which promotes DNA synthesis, cell proliferation and
migration (43). KGF has been
demonstrated to be upregulated in the upper dermis of psoriatic
skin and its expression is correlated with keratinocyte growth
(44). Therefore, it was
speculated that WT1 promotes keratinocyte proliferation by
regulating the downstream KGF signaling pathway in patients with
psoriasis.
Although the initial events triggering a psoriatic
lesion remain unclear, pro-inflammatory cytokines, including
IL-17A, IL-22 and IFN-γ, may drive keratinocyte hyperproliferation
and aberrant differentiation in psoriasis. A previous study
suggested that the average protein expression levels of IL-17A,
IFN-γ and IL-22 in the cell culture supernatant of psoriatic
CD4+ T cells are on the order of magnitude ng/ml
(45). In accordance with the
literature (46), HaCaT cells were
with 0, 10, 20 or 50 ng/ml IL-17A, IFN-γ and IL-22 to examine their
effect on the expression of WT1. It was demonstrated that these
inflammatory cytokines were capable of inducing the overexpression
of WT1 to varying degrees. It is widely accepted that the
activation of nuclear factor (NF)-κB transcription factors, which
are crucial mediators involved in the pathogenesis of psoriasis
(47), is a common downstream
event following the stimulation of each of these cytokines
(48–50). The present results provide a
possible novel mechanism through which inflammatory cytokines may
stimulate the upregulation of WT1 in psoriatic skin. This process
may be mediated by NF-κB signaling, which serves an essential role
in cell cycle regulation in the pathogenesis of psoriasis.
In conclusion, the results of the present study
demonstrated that inflammatory cytokines induced the overexpression
of WT1, which mediated the excessive proliferation and inhibited
the apoptosis of keratinocytes in psoriasis. To the best of our
knowledge, the present study is the first to focus on the
expression and role of WT1 in psoriasis, and a potential novel
factor associated with the pathogenesis of psoriasis has been
revealed. However, further research is required to confirm these
findings and investigate the possible molecular mechanisms of WT1
in psoriasis.
Acknowledgements
The authors thank Professor Zhang Janzhong
(Department of Dermatology, Peking University People's Hospital)
and Professor Zheng Min (Department of Dermatology, The Second
Affiliated Hospital, Zhejiang University School of Medicine) for
their advice on the experimental design.
Funding
The present study was supported by: The National
Science Fund for Excellent Young Scholars (grant no. 81522038) and
the Project of Innovation-driven Plan of Central South University
(grant no. 2016CX029) to MZ; the National Natural Science
Foundation of China (grant no. 81573051) to YS; the Key Program of
National Natural Science Foundation of China (grant no. 81430074)
to QL; the Fundamental Research Funds for the Central Universities
of Central South University (grant no. 2016zzts143) to RW; and the
National Natural Science Foundation of China (grant no. 81502732)
to YL.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
RW performed most of the experiments, analyzed the
data and wrote the manuscript. YLiao performed the cell
transfection. WS, YLiu and YS collected the clinical samples,
evaluated the PASI score of patients and analyzed the correlation
between WT1 expression and PASI score. JZ, MZhe and GC critically
revised the manuscript, and provided technical support and
suggestions. QL and MZha designed and supervised the study.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Second Xiangya Hospital of Central South
University (Changsha, China). Written informed consent was obtained
from all subjects. All procedures involving animals were approved
and supervised by the Animal Care and Use Committee of the Second
Xiangya Medical School of Central South University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schön MP and Boehncke WH: Psoriasis. N
Engl J Med. 352:1899–1912. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lowes MA, Bowcock AM and Krueger JG:
Pathogenesis and therapy of psoriasis. Nature. 445:866–873. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boehncke WH and Schön MP: Psoriasis.
Lancet. 386:983–994. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suárez-Fariñas M, Li K, Fuentes-Duculan J,
Hayden K, Brodmerkel C and Krueger JG: Expanding the psoriasis
disease profile: Interrogation of the skin and serum of patients
with moderate-to-severe psoriasis. J Invest Dermatol.
132:2552–2564. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tan NS, Michalik L, Noy N, Yasmin R, Pacot
C, Heim M, Flühmann B, Desvergne B and Wahli W: Critical roles of
PPAR beta/delta in keratinocyte response to inflammation. Genes
Dev. 15:3263–3277. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Croxford AL, Karbach S, Kurschus FC,
Wörtge S, Nikolaev A, Yogev N, Klebow S, Schüler R, Reissig S,
Piotrowski C, et al: IL-6 regulates neutrophil microabscess
formation in IL-17A-driven psoriasiform lesions. J Invest Dermatol.
134:728–735. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uyemura K, Yamamura M, Fivenson DF, Modlin
RL and Nickoloff BJ: The cytokine network in lesional and
lesion-free psoriatic skin is characterized by a T-helper type 1
cell-mediated response. J Invest Dermatol. 101:701–705. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zaba LC, Suárez-Fariñas M, Fuentes-Duculan
J, Nograles KE, Guttman-Yassky E, Cardinale I, Lowes MA and Krueger
JG: Effective treatment of psoriasis with etanercept is linked to
suppression of IL-17 signaling, not immediate response TNF genes. J
Allergy Clin Immunol. 124:1022–1110.e1-e395. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schön M, Behmenburg C, Denzer D and Schön
MP: Pathogenic function of IL-1 beta in psoriasiform skin lesions
of flaky skin (fsn/fsn) mice. Clin Exp Immunol. 123:505–510. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gisondi P, Gubinelli E, Cocuroccia B and
Girolomoni G: Targeting tumor necrosis factor-alpha in the therapy
of psoriasis. Curr Drug Targets Inflamm Allergy. 3:175–183. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Green LM, Wagner KJ, Campbell HA, Addison
K and Roberts SG: Dynamic interaction between WT1 and BASP1 in
transcriptional regulation during differentiation. Nucleic Acids
Res. 37:431–440. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park S, Tomlinson G, Nisen P and Haber DA:
Altered trans-activational properties of a mutated WT1 gene product
in a WAGR-associated Wilms' tumor. Cancer Res. 53:4757–4760.
1993.PubMed/NCBI
|
|
13
|
Bruening W, Gros P, Sato T, Stanimir J,
Nakamura Y, Housman D and Pelletier J: Analysis of the 11p13 Wilms'
tumor suppressor gene (WT1) in ovarian tumors. Cancer Invest.
11:393–399. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Silberstein GB, Van Horn K, Strickland P,
Roberts CT Jr and Daniel CW: Altered expression of the WT1 wilms
tumor suppressor gene in human breast cancer. Proc Natl Acad Sci
USA. 94:pp. 8132–8137. 1997; View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oji Y, Yano M, Nakano Y, Abeno S,
Nakatsuka S, Ikeba A, Yasuda T, Fujiwara Y, Takiguchi S, Yamamoto
H, et al: Overexpression of the Wilms' tumor gene WT1 in esophageal
cancer. Anticancer Res. 24:3103–3108. 2004.PubMed/NCBI
|
|
16
|
Keilholz U, Menssen HD, Gaiger A, Menke A,
Oji Y, Oka Y, Scheibenbogen C, Stauss H, Thiel E and Sugiyama H:
Wilms' tumour gene 1 (WT1) in human neoplasia. Leukemia.
19:1318–1323. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oji Y, Nakamori S, Fujikawa M, Nakatsuka
S, Yokota A, Tatsumi N, Abeno S, Ikeba A, Takashima S, Tsujie M, et
al: Overexpression of the Wilms' tumor gene WT1 in pancreatic
ductal adenocarcinoma. Cancer Sci. 95:583–587. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao SM, Yang JJ, Chen CQ, Chen JJ, Ye LP,
Wang LY, Wu JB, Xing CY and Yu K: Pure curcumin decreases the
expression of WT1 by upregulation of miR-15a and miR-16-1 in
leukemic cells. J Exp Clin Cancer Res. 31:272012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Loeb DM and Sukumar S: The role of WT1 in
oncogenesis: Tumor suppressor or oncogene? Int J Hematol.
76:117–126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hohenstein P and Hastie ND: The many
facets of the Wilms' tumour gene, WT1. Hum Mol Genet 15 Spec No.
2:R196–R201. 2006. View Article : Google Scholar
|
|
21
|
Huff V: Wilms' tumours: About tumour
suppressor genes, an oncogene and a chameleon gene. Nat Rev Cancer.
11:111–121. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Armstrong AW, Parsi K, Schupp CW, Mease PJ
and Duffin KC: Standardizing training for psoriasis measures:
Effectiveness of an online training video on Psoriasis Area and
Severity Index assessment by physician and patient raters. JAMA
Dermatol. 149:577–582. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Malkic Salihbegovic E, Hadzigrahic N and
Cickusic AJ: Psoriasis and metabolic syndrome. Med Arch. 69:85–87.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van der Fits L, Mourits S, Voerman JS,
Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens
EP and Lubberts E: Imiquimod-induced psoriasis-like skin
inflammation in mice is mediated via the IL-23/IL-17 axis. J
Immunol. 182:5836–5845. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mak RK, Hundhausen C and Nestle FO:
Progress in understanding the immunopathogenesis of psoriasis.
Actas Dermosifiliogr. 100 Suppl 2:S2–S13. 2009. View Article : Google Scholar
|
|
27
|
Nestle FO, Kaplan DH and Barker J:
Psoriasis. N Engl J Med. 361:496–509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ragaz A and Ackerman AB: Evolution,
maturation, and regression of lesions of psoriasis. New
observations and correlation of clinical and histologic findings.
Am J Dermatopathol. 1:199–214. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scharnhorst V, Dekker P, van der Eb AJ and
Jochemsen AG: Internal translation initiation generates novel WT1
protein isoforms with distinct biological properties. J Biol Chem.
274:23456–23462. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang L, Han Y, Suarez Saiz F and Minden
MD: A tumor suppressor and oncogene: The WT1 story. Leukemia.
21:868–876. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Scharnhorst V, van der Eb AJ and Jochemsen
AG: WT1 proteins: Functions in growth and differentiation. Gene.
273:141–161. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wagner KD, Cherfils-Vicini J, Hosen N,
Hohenstein P, Gilson E, Hastie ND, Michiels JF and Wagner N: The
Wilms' tumour suppressor Wt1 is a major regulator of tumour
angiogenesis and progression. Nat Commun. 5:58522014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Algar EM, Khromykh T, Smith SI, Blackburn
DM, Bryson GJ and Smith PJ: A WT1 antisense oligonucleotide
inhibits proliferation and induces apoptosis in myeloid leukaemia
cell lines. Oncogene. 12:1005–1014. 1996.PubMed/NCBI
|
|
34
|
Yamagami T, Sugiyama H, Inoue K, Ogawa H,
Tatekawa T, Hirata M, Kudoh T, Akiyama T, Murakami A and Maekawa T:
Growth inhibition of human leukemic cells by WT1 (Wilms tumor gene)
antisense oligodeoxynucleotides: Implications for the involvement
of WT1 in leukemogenesis. Blood. 87:2878–2884. 1996.PubMed/NCBI
|
|
35
|
Tatsumi N, Oji Y, Tsuji N, Tsuda A,
Higashio M, Aoyagi S, Fukuda I, Ito K, Nakamura J, Takashima S, et
al: Wilms' tumor gene WT1-shRNA as a potent apoptosis-inducing
agent for solid tumors. Int J Oncol. 32:701–711. 2008.PubMed/NCBI
|
|
36
|
Xu C, Wu C, Xia Y, Zhong Z, Liu X, Xu J,
Cui F, Chen B, Røe OD, Li A and Chen Y: WT1 promotes cell
proliferation in non-small cell lung cancer cell lines through
up-regulating cyclin D1 and p-pRb in vitro and in vivo. PLoS One.
8:e688372013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hewitt SM, Hamada S, McDonnell TJ,
Rauscher FJ III and Saunders GF: Regulation of the proto-oncogenes
bcl-2 and c-myc by the Wilms' tumor suppressor gene WT1. Cancer
Res. 55:5386–5389. 1995.PubMed/NCBI
|
|
38
|
Maheswaran S, Englert C, Bennett P,
Heinrich G and Haber DA: The WT1 gene product stabilizes p53 and
inhibits p53-mediated apoptosis. Genes Dev. 9:2143–2156. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li X, Li Y, Yuan T, Zhang Q, Jia Y, Li Q,
Huai L, Yu P, Tian Z, Tang K, et al: Exogenous expression of WT1
gene influences U937 cell biological behaviors and activates MAPK
and JAK-STAT signaling pathways. Leuk Res. 38:931–939. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim BH, Lee JM, Jung YG, Kim S and Kim TY:
Phytosphingosine derivatives ameliorate skin inflammation by
inhibiting NF-κB and JAK/STAT signaling in keratinocytes and mice.
J Invest Dermatol. 134:1023–1032. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Moorchung N, Vasudevan B, Dinesh Kumar S
and Muralidhar A: Expression of apoptosis regulating proteins p53
and bcl-2 in psoriasis. Indian J Pathol Microbiol. 58:423–426.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Casado M, Martin M, Muñoz A and Bernal J:
Vitamin D3 inhibits proliferation and increases c-myc expression in
fibroblasts from psoriatic patients. J Endocrinol Invest.
21:520–525. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zang XP, Pento JT and Tari AM: Wilms'
tumor 1 protein and focal adhesion kinase mediate keratinocyte
growth factor signaling in breast cancer cells. Anticancer Res.
28:133–137. 2008.PubMed/NCBI
|
|
44
|
Kovacs D, Falchi M, Cardinali G, Raffa S,
Carducci M, Cota C, Amantea A, Torrisi MR and Picardo M:
Immunohistochemical analysis of keratinocyte growth factor and
fibroblast growth factor 10 expression in psoriasis. Exp Dermatol.
14:130–137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu R, Zeng J, Yuan J, Deng X, Huang Y,
Chen L, Zhang P, Feng H, Liu Z, Wang Z, et al: MicroRNA-210
overexpression promotes psoriasis-like inflammation by inducing Th1
and Th17 cell differentiation. J Clin Invest. 128:2551–2568. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yan S, Xu Z, Lou F, Zhang L, Ke F, Bai J,
Liu Z, Liu J, Wang H, Zhu H, et al: NF-κB-induced microRNA-31
promotes epidermal hyperplasia by repressing protein phosphatase 6
in psoriasis. Nat Commun. 6:76522015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Goldminz AM, Au SC, Kim N, Gottlieb AB and
Lizzul PF: NF-κB: An essential transcription factor in psoriasis. J
Dermatol Sci. 69:89–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hang do TT, Song JY, Kim MY, Park JW and
Shin YK: Involvement of NF-κB in changes of IFN-γ-induced
CIITA/MHC-II and iNOS expression by influenza virus in macrophages.
Mol Immunol. 48:1253–1262. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu Y, Zhu L, Liu L, Zhang J and Peng B:
Interleukin-17A stimulates migration of periodontal ligament
fibroblasts via p38 MAPK/NF-κB-dependent MMP-1 expression. J Cell
Physiol. 229:292–299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gelebart P, Zak Z, Dien-Bard J, Anand M
and Lai R: Interleukin 22 signaling promotes cell growth in mantle
cell lymphoma. Transl Oncol. 4:9–19. 2011. View Article : Google Scholar : PubMed/NCBI
|



















