Introduction
Gastric cancer is one of the most common cancers
with a high prevalence worldwide (1). In total, >80% of diagnoses occur
when gastric cancer has already progressed to later stages of the
disease, which significantly impedes the effectiveness of therapy
in this highly aggressive disease. Thus, the prognosis of patients
with gastric cancer remains poor (2). Early diagnosis of gastric cancer and
effective treatment to prevent gastric cancer progression is
critical in improving the clinical management of gastric cancer.
Hence, the underlying molecular mechanisms of gastric cancer
require further investigation in order to develop successful
therapeutic strategies to improve patient survival.
Helicobacter pylori (H. pylori)
infection is an important oncogenic driver of gastric cancer. It
was reported in 2008 that an estimated 660,000 cases of cancer were
attributable to H. pylori infection, which is ~5.2% of all
cancer cases (3). Upon H.
pylori infection, a cascade of molecular alterations that may
ultimately lead to tumorigenesis is induced in gastric cells
(4). Long non-coding RNAs
(lncRNAs), which constitute a large portion of the human genome,
are increasingly identified as novel cancer regulators (5). They are typically >200 nucleotides
in length and modulate gene expression post-transcriptionally
(6). In gastric cancer, a number
of lncRNAs have been confirmed to be involved in cancer promotion
or suppression (5,7–9). The
lncRNAs HOX Transcript Antisense RNA and H9 have been identified as
potent gastric cancer inducers by increasing tumor cell
invasiveness and metastasis (10).
In addition, downregulation of the lncRNA Maternally Expressed 3 is
associated with poor survival in patients with gastric cancer
(11). Strategies to regulate
these lncRNAs may therefore be devised to impede gastric cancer
progression. Lnc-G protein subunit α transducin 1 (GNAT1)-1 is a
novel cancer suppressor (12). In
colorectal cancer, downregulation of lnc-GNAT1-1 is characteristic
of high risk patients with poor prognosis. Lnc-GNAT1-1 suppresses
colorectal cancer by modulating the Raf kinase inhibitor protein
(RKIP)-nuclear factor (NF)-κB-protein snail homolog 1 (Snail)
circuit (12). However, to the
best of our knowledge, the regulatory role of lnc-GNAT1-1 in
gastric cancer has not yet been reported. Understanding the role
and mechanism of lnc-GNAT1-1 in gastric cancer may contribute to
the discovery of novel diagnostic and therapeutic tools to improve
the clinical management of gastric cancer.
The present study focused on the role of lnc-GNAT1-1
in gastric cancer induced by H. pylori infection and aimed
to suggest potential strategies for gastric cancer treatment based
on lnc-GNAT1-1 regulation. The results of the present study may aid
in uncovering the molecular mechanism of H. pylori in
gastric cancer tumorigenesis and provide a gene therapy tool for
the treatment of this highly aggressive cancer.
Materials and methods
Cell culture
Normal gastric epithelial cells were obtained from
Wuhan Feiyi Group (Wuhan, China). Human gastric cancer cell lines
SGC-7901 and MKN45 were obtained from American Type Culture
Collection (ATCC; Manassas, VA, USA). All cell lines were
maintained in RPMI-1640 (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
at 37°C in a humidified atmosphere of 95% air and 5%
CO2.
Infection of cells with H. pylori and
cell transfection
Cell transfection was performed as previously
described (12). Human gastric
cancer cell lines SGC-7901 and MKN45, obtained from Procell Life
Science & Technology Co., Ltd. (Wuhan, China), were grown in
Minimum Essential Medium with Earle's salts (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) with 10% FBS in a 5% CO2
atmosphere at 37°C, and collected during the logarithmic growth
phase, and cells were seeded into 6-well plates at a density of
5×105 cells per well. Standard H. pylori (ATCC),
which contained the entire cytotoxin-associated gene pathogenicity
island (cagPAI), including cagA, grown in Brucella
broth (Shanghai Biomart Technology Co., Ltd., Shanghai, China) were
incubated with SGC-7901 and MKN45 cells to infect cells at a ratio
of bacteria to SGC-7901 and MKN45 cells of 100:1 and with a
multiplicity of infection of 10 (5×106 plaque forming
units). Isogenic mutants lacking cagPAI or cagA
(ATCC) were also inoculated into the gastric epithelial cells as
control measures. Following 24 h, total RNA was extracted from the
gastric epithelial cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and reverse
transcribed to cDNA using High-Capacity cDNA Reverse Transcription
Kit (Thermo Fisher Scientific, Inc.) under the following
temperature protocol: 55°C for 30 min and 85°C for 20 min.
Transfection was performed using a 2nd generational system using
293 cells. PCR was performed to amplify an EcoRI-EcoRI fragment
containing full-length lnc-GNAT1-1 cDNA using Phusion®
High-Fidelity DNA Polymerase (New England Biolabs, Inc., Ipswich,
MA, USA) using following thermocycling conditions: 98°C for 20 sec;
followed by 30 cycles of 98°C for 5 sec, 55°C for 10 sec and 72°C
for 30 sec; and then a final extension step at 72°C for 5 min.
Primers were directly provided by GenePharma Co., Ltd. (Shanghai,
China; sequences not available). This fragment was inserted into
EcoRI linearized pIRSE2-EGFP vectors (Clontech Laboratories, Inc.,
Mountainview, CA, USA) to establish lnc-GNAT1-1 expressing vectors.
Lipofectamine® 2000 reagent (cat. no. 11668-019;
Invitrogen, Thermo Fisher Scientific, Inc.) was then used to
transfect 10 nM vectors into 5×107 SGC-7901 and MKN45
cells. Empty pIRSE2-EGFP vectors were used as negative control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using
TRIzol® reagent (Thermo Fisher Scientific, Inc.).
First-strand cDNA was generated using a Reverse Transcription
system kit (Takara Biotechnology Co., Ltd., Dalian, China). RNA was
heated to 70°C for 5 min, chilled on ice for 5 min and then
incubated at 25°C for 5 min. Reagents within the Reverse
Transcription kit were used to polyadenylate all lcnRNAs prior to
cDNA conversion, as some of lncRNAs did not have poly A tails. qPCR
was performed using a SYBR-Green PCR kit (ABgene UK Ltd.; Thermo
Fisher Scientific, Inc.) in a StepOne System (Applied Biosystems;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. PCR thermocycling conditions used were as follows:
50°C for 60 sec; followed by 40 cycles of 95°C for 15 sec and 56°C
for 35 sec. The primer sequences used were as follows: lnc-GNAT1-1
forward 5′-ATGTGTCGCCAGGTTGCTGTA-3′, and reverse,
5′-CCGCTGAGGACTAGAGTAGC-3′; and GAPDH forward
5′-GCAAGAGCACAAGAGGAAGA-3′, GAPDH reverse,
5′-ACTGTGAGGAGGGGAGATTC-3′. GAPDH was used as an endogenous
control. RT-qPCR results were quantified with ABI Prism 7900HT
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and were
analyzed using the 2−ΔΔCq method (13).
Invasion assay
Invasion assays were performed using Transwell
invasion chambers coated with Matrigel (50 µl/filter; BD
Biosciences, Franklin Lakes, NJ, USA) according to the
manufacturer's instructions. A total of 2×104 cells were
transfected with pLV-Control (Ctrl) or pLV-lnc-GNAT1-1 and cultured
for 48 h prior to transfer to the upper Matrigel-coated invasion
chamber in a 1% fetal calf serum (FCS; Gibco; Thermo Fisher
Scientific, Inc.) and Dulbecco's modified Eagle's Medium/Nutrient
Mixture F-12 (DMEM/F12; Gibco; Thermo Fisher Scientific, Inc.).
DMEM/F12 containing 10% FCS (Sigma-Aldrich; Merck KGaA) was added
to the lower chamber. Following incubation for 24 h at 37°C with 5%
CO2, invasive cells in the lower chamber were stained
with 1% crystal violet (Sigma-Aldrich; Merck KGaA) for 30 min at
25°C. Cells were subsequently counted under a light microscope
(magnification, ×100). Assays were repeated six times.
Migration assay
Migration assays were performed in a 24-well
Transwell chamber system. Following transfection for 24 h, cells
were seeded in the upper chamber at 2×104 cells/ml in
0.1 ml serum-free DMEM/F12 media. Media supplemented with 10% FBS
was placed in the bottom well at a volume of 0.8 ml. Cells were
incubated for 24 h at 37°C with 5% CO2. Following this,
migrated cells in the lower chamber were stained with 1% crystal
violet for 30 min at 25°C. Cells were counted under a light
microscope (magnification, ×100). All experiments were repeated six
times over multiple days.
In vivo assay of tumor growth
A total of 8 of BALB/c nude mice (age, 6–8 weeks;
weight, 20–22 g; 4 male and 4 female) were purchased from Charles
River Laboratories, Inc. (Beijing, China) and housed at a
temperature of 25°C with a humidity of 50%, a 12/12 h light/dark
cycle and free access to food and water. The protocol of the
present study was approved by the Ethics Review Committee of The
First Hospital of Lanzhou University (Lanzhou, China). SGC-7901 or
MKN45 cells (1×107) that had been stably transfected
with lnc-GNAT1-1-expressing vectors were suspended in 100 µl PBS
and subcutaneously inoculated into the bilateral armpit, a thin
subcutaneous fat and muscle layer between the fore limb and trunk
of 4 BALB/c nude mice. An equivalent amount of SGC-7901 or MKN45
cells were injected into a further 4 BALB/c nude mice to generate a
control group, and the mice did not demonstrate any clinical signs
of tumor burden. The tumors were measured every 7 days following
implantation, and the volume of each tumor was calculated by length
× width2 × 0.5. At 4 weeks following inoculation, mice
were sacrificed and tumor tissues were collected and weighed.
Humane end-points were strictly observed. Mice exhibiting signs of
moderate to severe discomfort were euthanized. This was
accomplished by anesthetizing the animals with
2,2,2-tribromoethanol (0.25 ml/kg) (14) followed by cervical dislocation, in
accordance with the American Veterinary Medical Association
guidelines on euthanasia (15).
Analgesics were not used as they had the potential to affect the
experimental outcomes of the study.
Western blot analysis
Total protein from cell lysates of both tumor
samples and tissue samples from healthy mice were extracted using
1% SDS buffer (cat. no. P0013K; Beyotime Institute of
Biotechnology, Haimen, China), and protein concentration was then
determined using a BCA protein assay. Protein samples (40 µg/lane)
were separated by 10% SDS-PAGE gel and transferred onto
nitrocellulose membranes. Following blocking for 1 h at 4°C in 5%
non-fat milk and then washing with Tris-buffered saline with 10%
Tween 20, membranes were incubation with anti-β-catenin (1:1,000;
cat. no. 9582; Cell Signaling Technology, Inc., Danvers, MA, USA),
anti-Cyclin D (1:1,000; cat. no. sc-8396; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), anti-c-Myc (1:900; cat. no. sc-789; Santa
Cruz Biotechnology, Inc.) and anti-β-actin (1:2,000; cat. no.
ab8227; Abcam, Cambridge, UK) primary antibodies overnight at 4°C.
Following this, blots were incubated with anti-rabbit
IgG-horseradish peroxidase secondary antibodies (1:3,000; cat. no.
TA130024; OriGene Technologies, Inc., Rockville, MD, USA) for 1 h
at room temperature. Protein bands were visualized using an
enhanced chemiluminescent reagent (PerkinElmer, Inc., Waltham, MA,
USA) and quantified with ImageJ 1.37 software (version 1.37;
National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis was performed using SPSS 19.0 software (IBM
Corp., Armonk, NY, USA). One-way analysis of variance followed by
Tukey's post-hoc test was used to determine significant differences
between >2 groups. Comparisons between two groups were performed
using Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
H. pylori infection downregulates
lnc-GNAT1-1 expression in normal gastric and gastric cancer cell
lines
The effect of H. pylori infection on the
expression of lnc-GNAT1-1 in normal and gastric cancer cells was
investigated. The RNA expression of lnc-GNAT1-1 was detected by
RT-qPCR analysis. As shown in Fig.
1A, normal gastric GES cells had significantly lower
lnc-GNAT1-1 levels following H. pylori infection at a MOI of
1:1 and 10:1 (1:1, P=0.02 and 10:1, P=0.007; Fig. 1A). A higher MOI induced a greater
reduction in lnc-GNAT1-1 expression, indicating a dose-dependent
manner in this effect. The reduction in lnc-GNAT1-1 expression was
also observed in SGC-7901 cells (1:1, P=0.01 and 10:1, P=0.002;
Fig. 1B) and MKN45 cells (1:1,
P=0.02 and 10:1, P=0.004; Fig.
1C). The RNA expression of lnc-GNAT1-1 was significantly
decreased in SGC-7901 and MKN45 cells (P=0.009 and P=0.012,
respectively; Fig. 1D) compared
with normal gastric GES cell at the same MOI (10:1). Therefore, the
results revealed that normal gastric cells and gastric cancer cells
were subject to lnc-GNAT1-1 reduction upon H. pylori
infection; however, the lnc-GNAT1-1 reduction was more marked in
gastric cancer cells.
Ectopic lnc-GNAT1 overexpression
inhibits gastric cancer cell invasion and migration
To further confirm the role of lnc-GNAT1-1 in
gastric cells under H. pylori infection, lnc-GNAT1-1 was
overexpressed through transfection of a lnc-GNAT1-1 expression
vector. Lentiviral transfection was used to transfect lnc-GNAT1-1
expression vector to induce lnc-GNAT1-1 overexpression. RT-qPCR was
used to analyze lnc-GNAT1-1 expression in transfected SGC-7901 and
MNK45 cells, compared with that of untransfected cells. As
presented in Fig. 2, SGC-7901 and
MNK45 cells transfected with lnc-GNAT-1 had significantly higher
levels of lnc-GNAT1-1 (P=0.004).
In addition, the effects of lnc-GNAT1-1
overexpression on gastric cancer cell migration and invasion was
explored. The results revealed that lnc-GNAT1-1 overexpression
significantly reduced the migration and invasion ability of
SGC-7901 (Fig. 3A-C) and MNK45
cells (Fig. 3D-F).
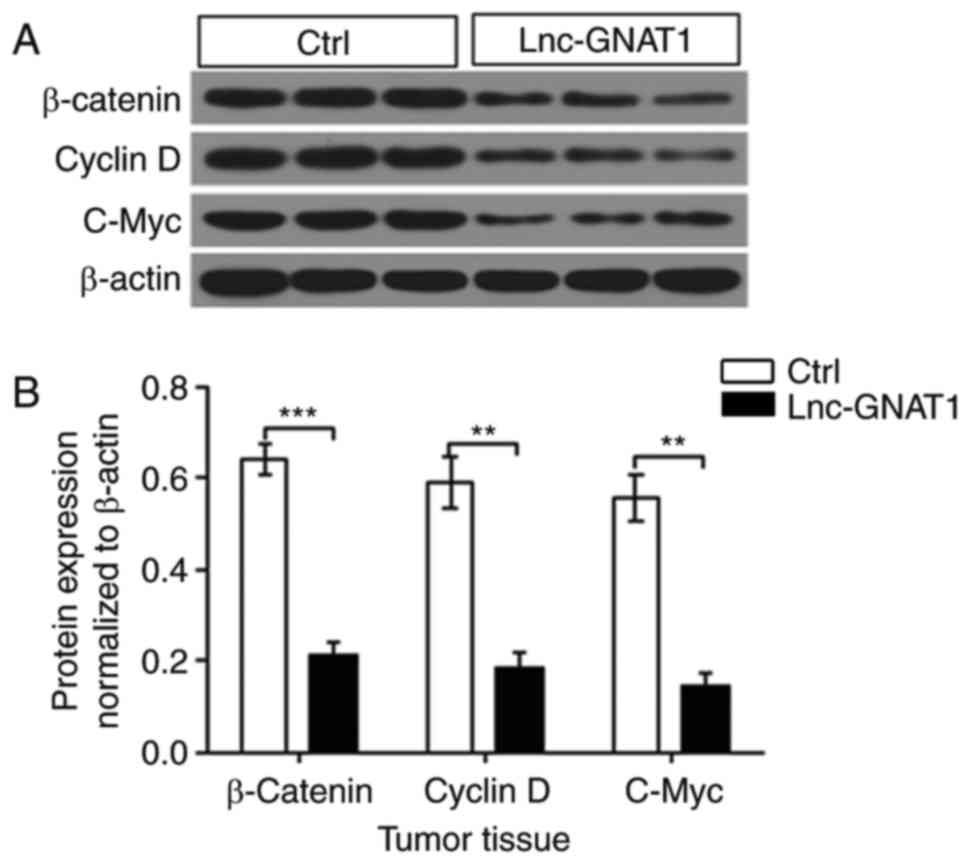
Lnc-GNAT1 downregulates Wnt/β-Catenin
pathway protein expression in gastric cancer cells infected with H.
pylori
To investigate the association between Wnt/β-catenin
signaling and tumor growth, the expression of key proteins in the
Wnt/β-catenin signaling pathway was evaluated. β-catenin, cyclin D
and c-Myc expression was significantly decreased in tumors
overexpressing lnc-GNAT1-1 (Fig.
4).
To elucidate the underlying mechanism of lnc-GNAT1-1
in inhibiting gastric cancer migration and invasion, the
association between lnc-GNAT1-1 overexpression and the
Wnt/β-catenin pathway was also investigated in gastric cancer cell
lines. Consistently, lnc-GNAT1-1 overexpression significantly
decreased Wnt/β-catenin pathway protein expression in SGC-7901
cells (Fig. 5A and B) and MNK45
cells (Fig. 5C and D). These
results indicated that lnc-GNAT1-1 may have the potential to be
developed as a cancer therapeutic that may impede cancer cell
migration through Wnt/β-catenin pathway protein expression
inhibition. Thus, the efficacy of lnc-GNAT1 overexpression in
attenuating tumor growth in vivo was further
investigated.
Overexpression of lnc-GNAT1-1 inhibits
tumor tissue growth in nude mice
MNK45 cells transfected with or without lnc-GNAT1-1
were used to construct gastric cancer tumor xenografts and tumor
growth was subsequently monitored. As shown in Fig. 6, tumor volume and tumor weight were
monitored 4 weeks following inoculation. A much slower rate of
volume increase was observed in gastric tumors derived from MNK45
cells overexpressing lnc-GNAT1-1 (Fig.
6A). As presented in Fig. 6B and
C, tumor size and tumor weight of lnc-GNAT1-1 overexpressing
tumors was also decreased when compared with the control group.
Taken together, these results indicated that
lnc-GNAT1-1 overexpression may impede gastric cancer
progression.
Discussion
It is well established that H. pylori
infection is an important risk factor in gastric cancer. H.
pylori is categorized as a group I carcinogen by the
International Agency for Research on Cancer (16). Therefore, substantial effort has
been devoted to reduce H. pylori infection in order to
decrease the incidence of gastric cancer; this has been successful
in developing countries (17).
Despite significant progress, chemotherapy and radiation therapy
are combined with surgery to improve therapeutic outcomes, and
advanced strategies and effective treatments are required for
patients with gastric cancer. At present, the highly invasive
gastrectomy remains the principle treatment for gastric cancer
(18) and more efficient
approaches in gastric cancer treatment must be developed.
Encouraged by progress in gene therapeutics for
various types of cancer (19), the
present study aimed to evaluate the role of an emerging cancer
regulating lncRNA, lnc-GNAT1-1, in gastric cancer. The results
revealed that lnc-GNAT1-1expression was significantly downregulated
by H. pylori infection. A previous report has demonstrated
that lnc-GNAT1-1 may act as a cancer suppressor by inhibiting
cancer cell invasion and migration (12), suggesting that decreased levels of
lnc-GNAT1-1 may bean indicator for high-risk gastric cancer.
In the present study, lnc-GNAT1-1 overexpression
induced by lentiviral transfection resulted in decreased cancer
cell invasion and migration in vitro. In vivo,
gastric tumor growth was also attenuated by lnc-GNAT1-1
overexpression, which demonstrated the function of lnc-GNAT1-1 in
gastric cancer. Compared with surgery, gene therapy strategies are
less invasive. In addition, as normal tissues express high
levelslnc-GNAT1-1 (12),
non-specific transfection of lnc-GNAT1-1 expressing vector
presumably would not induce toxic effects on normal tissues, making
it a potential tumor-specific treatment strategy.
The Wnt/β-catenin signaling pathway promotes the
growth of aggressive cancer cells (20). The present study identified the
Wnt/β-catenin signaling pathway as an underlying mechanism of
lnc-GNAT1-1 in gastric cancer cell regulation. Activation of the
Wnt/β-catenin pathway is a putative mechanism in the promotion of
cancer cell invasion, migration and dissemination (21). H. pylori infection has also
been associated with increased Wnt/β-catenin signaling to generate
gastric cancer cells with stem cell-like properties (22). However, to the best of our
knowledge, the association between lnc-GNAT1-1and the Wnt/β-catenin
signaling pathway has not been previously reported. Previous
findings have suggested that lnc-GNAT1-1 is associated with
RKIP-NF-κB-Snail pathway modulation (12). As Wnt/β-catenin and the
RKIP-NF-κB-Snail signaling pathways may contribute to malignant
cancer development, this finding may account for the therapeutic
potential of lnc-GNAT1-1 in inhibiting gastric cancer progression
(12). In the present study, it
was demonstrated that H. pylori infection significantly
downregulated the expression of lnc-GNAT-1. Furthermore,
lnc-GNAT1-1 overexpression was revealed to decrease the protein
expression associated with the Wnt/β-catenin pathway, suppress
tumor growth and suppress gastric cancer cell migration and
invasion abilities. However, the results of the present study
suggested that lnc-GNAT1-1 did not fully suppress Wnt/β-catenin
signaling, and thus there may be a requirement for other potent
Wnt/β-catenin signaling suppressors combined with potential
lnc-GNAT1-1 targeting therapies for the treatment of gastric
cancer. It was not investigated if lnc-GNAT1-1 overexpression may
be useful in the treatment of chemoresistant gastric cancer in the
present study. However, it is probable that chemoresistance may be
alleviated, as Wnt/β-catenin signaling has been reported to be
involved in the development of chemoresistance (23).
In conclusion, the present study demonstrated that
lnc-GNAT1-1 may have an important role in gastric cancer induced by
H. pylori infection. Upon infection, lnc-GNAT1-1 expression
was significantly downregulated, and lnc-GNAT1-1 overexpression
inhibited gastric cancer cell migration and invasion. In addition,
Wnt/β-catenin signaling pathway protein expression was reduced by
lnc-GNAT1-1 overexpression. Furthermore, tumor growth was slower in
mice inoculated with cells overexpressing lnc-GNAT1-1. Therefore,
gene therapy targeting lnc-GNAT1-1 may be a potential strategy for
gastric cancer suppression; however, further studies are required
to validate lnc-GNAT1-1 as a useful biomarker for gastric cancer
diagnosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Natural
Science Foundation of Gansu Province (grant nos. 1506RJZA255 and
1308JZA240-01), The Natural Science Foundation of China (grant no.
31570509) and The Science and Technology Program of Lanzhou City
(grant no. 2016-RC-57).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LL, TS, BL, LZ and XL were responsible for the
conception and design of the study. LL and XL performed the
experiments. LL, TS and BL analyzed and interpreted the data. LL
and BL drafted the article. LZ and XL were responsible for the
revision of the manuscript.
Ethics approval and consent to
participate
The protocol of the present study was approved by
the Ethics Review Committee of the First Hospital of Lanzhou
University (Lanzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:72016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu HS and Xiao HS: MicroRNAs as potential
biomarkers for gastric cancer. World J Gastroentero.
20:12007–12017. 2014. View Article : Google Scholar
|
|
3
|
Plummer M, Franceschi S, Vignat J, Forman
D and de Martel C: Global burden of gastric cancer attributable to
Helicobacter pylori. Int J Cancer. 136:487–490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bass AJ, Thorsson V, Shmulevich I,
Reynolds SM, Miller M, Bernard B, Hinoue T, Laird PW, Curtis C,
Shen H, et al: Comprehensive molecular characterization of gastric
adenocarcinoma. Nature. 513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xia T, Liao Q, Jiang X, Shao Y, Xiao B, Xi
Y and Guo J: Long noncoding rna associated-competing endogenous
rnas in gastric cancer. Sci Rep. 4:60882014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi X, Sun M, Wu Y, Yao Y, Liu H, Wu G,
Yuan D and Song Y: Post-transcriptional regulation of long
noncoding rnas in cancer. Tumour Biol. 36:503–513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu X, Sun M, Nie FQ, Ge YB, Zhang EB, Yin
DD, Kong R, Xia R, Lu KH, Li JH, et al: Lnc RNA HOTAIR functions as
a competing endogenous RNA to regulate HER2 expression by sponging
miR-331-3p in gastric cancer. Mol Cancer. 13:922014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song H, Sun W, Ye G, Ding X, Liu Z, Zhang
S, Xia T, Xiao B, Xi Y and Guo J: Long non-coding RNA expression
profile in human gastric cancer and its clinical significances. J
Transl Med. 11:2252013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao WJ, Wu HL, He BS, Zhang YS and Zhang
ZY: Analysis of long non-coding RNA expression profiles in gastric
cancer. World J Gastroentero. 19:3658–3664. 2013. View Article : Google Scholar
|
|
10
|
Li H, Yu B, Li J, Su L, Yan M, Zhu Z and
Liu B: Overexpression of lncRNA H19 enhances carcinogenesis and
metastasis of gastric cancer. Oncotarget. 5:2318–2329. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun M, Xia R, Jin F, Xu T, Liu Z, De W and
Liu X: Downregulated long noncoding RNA MEG3 is associated with
poor prognosis and promotes cell proliferation in gastric cancer.
Tumor Biol. 35:1065–1073. 2014. View Article : Google Scholar
|
|
12
|
Ye C, Shen Z, Wang B, Li Y, Li T, Yang Y,
Jiang K, Ye Y and Wang S: A novel long non-coding RNA lnc-GNAT1-1
is low expressed in colorectal cancer and acts as a tumor
suppressor through regulating RKIP-NF-κB-Snail circuit. J Exp Clin
Cancer Res. 35:1872016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vieira RP, Ossig A, Perez J, Grassi VG,
Petzhold CL, Peres AC, Costa JM and Lona LMF: Styrene atrp using
the new initiator 2,2,2-tribromoethanol: Experimental and
simulation approach. Polym Eng Sci. 55:2270–2276. 2015. View Article : Google Scholar
|
|
15
|
Mahady G, Pendland S, Yun G and Lu Z:
Turmeric (Curcuma longa) and curcumin inhibit the growth of
Helicobacter pylori, a group 1 carcinogen. Anticancer Res.
22:4179–4181. 2002.PubMed/NCBI
|
|
16
|
Lee YC, Chen THH, Chiu HM, Shun CT, Chiang
H, Liu TY, Wu MS and Lin JT: The benefit of mass eradication of
Helicobacter pylori infection: A community-based study of gastric
cancer prevention. Gut. 62:676–682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Japanese Gastric Cancer Association, .
Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric
Cancer. 14:113–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ibraheem D, Elaissari A and Fessi H: Gene
therapy and DNA delivery systems. Int J Pharm. 459:70–83. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mao J, Fan S, Ma W, Fan P, Wang B, Zhang
J, Wang H, Tang B, Zhang Q, Yu X, et al: Roles of Wnt/β-catenin
signaling in the gastric cancer stem cells proliferation and
salinomycin treatment. Cell Death Dis. 5:e10392014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yong X, Tang B, Xiao YF, Xie R, Qin Y, Luo
G, Hu CJ, Dong H and Yang SM: Helicobacter pylori upregulates Nanog
and Oct4 via Wnt/β-catenin signaling pathway to promote cancer stem
cell-like properties in human gastric cancer. Cancer Lett.
374:292–303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sagara N and Katoh M: Mitomycin C
resistance induced by TCF-3 overexpression in gastric cancer cell
line MKN28 is associated with DT-diaphorase down-regulation. Cancer
Res. 60:5959–5962. 2000.PubMed/NCBI
|
|
23
|
Noda T, Nagano H, Takemasa I, Yoshioka S,
Murakami M, Wada H, Kobayashi S, Marubashi S, Takeda Y, Dono K, et
al: Activation of Wnt/beta-catenin signalling pathway induces
chemoresistance to interferon-α/5-fluorouracil combination therapy
for hepatocellular carcinoma. Br J Cancer. 100:1647–1658. 2009.
View Article : Google Scholar : PubMed/NCBI
|