Introduction
Lung cancer is the leading cause of
cancer-associated mortality, with 1 million patients worldwide each
year succumbing to this disease (1). The majority of lung cancer cases
(>80%) are non-small cell lung cancer (NSCLC), which consists of
large cell carcinoma, adenocarcinoma and squamous cell carcinoma.
However, ~30% of patients with NSCLC do not receive a specific
classification (2,3). A standard treatment approach for
patients with unresectable NSCLC is radiotherapy alone or combined
with chemotherapy, as this may improve overall survival and control
or reduce the lesion site, and prevent metastases (4). It has been reported that
radiation-induced pneumonitis in patients with NSCLC is correlated
with numerous factors (5). The
most extensively investigated factors include dose-volume
parameters to healthy lung tissue and selected cytokines (6); however, thus far none have been
demonstrated to be accurate enough to control the radiation dose to
prevent further damage. In addition, it is difficult to balance the
requirement for high-dose radiation to achieve adequate tumor
control with the need to limit the dose to healthy lung tissue in
patients with larger tumors (7). A
previous study demonstrated that inflammatory and fibrogenic
cytokines are critical in the development of pneumonitis as a
complication of radiotherapy (8).
Inflammation may be regulated by transforming growth factor β
(TGFβ), a pleiotropic cytokine that stimulates connective
tissue collagen formation and reduces degradation, thus resulting
in fibrosis (9). TGFβ has been
revealed to be markedly associated with damage to the lung
architecture (8). Previous studies
have identified TGFβ as a marker of patients at risk of developing
symptomatic pneumonitis (10,11).
In the majority of cells, differentiation,
proliferation and other functions are controlled by the
TGFβ1 gene. Numerous cellular processes in the
developing embryo and the adult organism, including homeostasis,
apoptosis, differentiation and growth are associated with the
TGFβ1 signaling pathway (12). As an important modulator of the
inflammatory response, TGFβ has been extensively investigated in
the irradiation-induced development of tissue fibrosis (13). Plasma levels of TGFβ1 have been
investigated as a predictor for lung injury induced by
radiotherapy. Human and animal studies have indicated that TGFβ
acts as primary regulator in the process of lung injury induced by
radiation (7,14,15).
Following administration of anti-TGFβ antibodies, TGFβ activation
was reduced and the inflammatory response decreased several weeks
after radiotherapy, further demonstrating that targeting the TGFβ
signaling pathway may be a potential strategy to prevent lung
injury induced by radiotherapy (16).
MicroRNAs (miRNAs) are endogenously encoded
single-stranded RNAs ~22 nucleotides in length, which are essential
in various pathological conditions (17). miRNAs bind specifically to target
messenger RNAs (mRNAs) and induce mRNA degradation or translational
repression, resulting in posttranscriptional gene suppression
(18). Various biological
processes, including immunity, inflammation, cellular development,
apoptosis, metabolism, differentiation and proliferation are
regulated by miRNAs (17–19). However, a single nucleotide
polymorphism (SNP; rs12976445) in pre-microRNA-125a may compromise
the mature processing of the miRNA, reducing miR-125a production
(20). Therefore, it has been
hypothesized that rs12976445 may be associated with the risk of
developing pneumonitis in patients with NSCLC treated with
radiotherapy, by affecting the production of miR-125a, and
consequently its target, TGFβ expression.
Materials and methods
Ethical considerations
The present study was conducted according to the
Declaration of Helsinki guidelines and the study protocol was
approved by the Human Ethics Committee of Shaanxi Friendship
Hospital (Xi'an, China). Written informed consent was obtained from
all participants prior to the study.
Patient eligibility and study
design
The present study was performed on patients with
NSCLC (n=1,023) who were admitted to the Oncology Department,
Shaanxi Friendship Hospital. All patients received chest computed
tomography (CT), pulmonary function tests, biochemical analysis, a
complete blood count and a physical examination, and a complete
medical history was taken (Table
I). The patients were recorded and treated in accordance with
the Radiation Therapy Oncology Group and the European Organization
for the Research and Treatment of Cancer guidelines. All patients
were followed up; assessments included chest CT, chest X-ray and a
physical examination. Assessments were performed at three-month
intervals for a period of two years following the completion of
radiotherapy, and subsequently at six-month intervals. Patients
were divided into two groups based on the presence or absence of
pneumonitis (pneumonitis positive, n=534; pneumonitis negative,
n=489). The criteria for enrollment in the present study included:
i) A diagnosis of NSCLC; ii) no history of surgery and
chemotherapy; iii) good heart, liver and kidney function; iv) no
distant metastasis; v) no history of myocardial infarction,
cerebral infarction or other critical illness in the previous six
months; vi) a life expectancy of at least six months; and vii) a
prescribed radiation dose of 50–70 Gy. Patients with any of the
following were excluded: i) A history of surgery or chemotherapy
for a thoracic tumor; ii) a history of severe pulmonary dysfunction
or pulmonary fibrosis; iii) a history of total or partial pulmonary
lobectomy; iv) poor general health; v) intolerance to radiation or
incomplete radiotherapy; vi) a diagnosis of asthma, serious chronic
bronchitis, emphysema or severe pulmonary infection; and vii) a
diagnosis of another serious disease, including myocardial
infarction and cerebral infarction within the previous six months.
Lung tissue samples (70) were obtained from 1,023 NSCLC patients:
[CC=36, 18 pneumonitis (+), 18, pneumonitis (−); CT=28, 14
pneumonitis (+), 14 pneumonitis (−); TT=6, 3 pneumonitis (+), 3
pneumonitis (−)] and genotyping for rs12976445 was conducted on
blood samples obtained from all patients.
 | Table I.Demographic and clinicopathological
features of the participants of the present study. |
Table I.
Demographic and clinicopathological
features of the participants of the present study.
| Parameter | Pneumonitis
(+) | Pneumonitis
(−) |
|---|
| Number | 534 | 489 |
| Sex (M:F) | 534 (276:258) | 489 (254:235) |
| Average age
(years) | 52.63±8.63 | 51.37±9.33 |
| Hypertension
(%) | 4.28 | 7.87 |
| Current smokers
(%) | 12.14 | 2.86 |
| Hyperlipidemia
(%) | 2.04 | 9.36 |
| Diabetes mellitus
(%) | 2.34 | 1.27 |
| Coronary heart
disease (%) | 7.68 | 6.12 |
| Body mass index
(kg/m2) | 20.25±1.41 | 21.03±0.97 |
Genotyping
The QIAamp DNA Blood Mini kit (Qiagen, Inc.,
Valencia, CA, USA) was used to extract genomic DNA from blood (200
µl) samples, according to the manufacturer's protocol. Sanger
sequencing using an ABI PRISM® 3100 Genetic Analyzer
(Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) was performed to determine the genotype of rs12976445.
Cell culture and transfection
A549 human lung carcinoma cells were obtained from
the American Type Culture Collection (Manassas, VA, USA), and were
cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 100 mg/ml streptomycin,
100 U/ml penicillin and 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) at 37°C in a 5% CO2 atmosphere.
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to perform transient cell transfections
of small RNAs or plasmids. miR-125a mimic/antagomir
(5′-CTATGTTTGAATGAGGCTTCAG-3′ and 5′-CGCGTCGCCGCGTGTTTAAACG-3′;
Sangon Biotech Co., Ltd., China) or TGFβ small interfering
(si)RNA (5′-CCCAGAACCAGGAGAAGAA-3′ and 5′-UUCUUCUCCUGGUUCUGGG-3′;
Sangon Biotech Co., Ltd., China) were transfected at a final
concentration of 100 nM. Briefly, the concentration of pcDNA3 was
16 µg/ml, the number of cells 2.0×105/ml and the
duration and temperature of the transfection was 6 h at 37°C. A
scramble control oligo sequence with no known target in the human
genome (5′-TGCACAATTTGATGCCGGTTTAGTAT-3′ and
5′-TTAAAATGCAGATGCTGAACTGGGAA-3′; Invitrogen; Thermo Fisher
Scientific, Inc.) served as a control.
Plasmid construction, target
prediction and luciferase assay
By scanning target gene prediction databases
(www.targetscan.org), the putative target
gene of the miR-125a were pooled from 3 databases. Experimental
validation on miR-125a and TGFβ was performed. The full-length cDNA
of the human TGFβ gene was amplified by polymerase chain
reaction (PCR) and was subcloned into a pcDNA3 vector (Invitrogen;
Thermo Fisher Scientific, Inc.) using the following cloning primer:
Forward, 5-ATGCCGCCCTCCGGGCTGCGGCTGCTG-3′ and reverse,
5′-TCAGCTGCACTTGCAGGAGCGCA-3′ (Sangon Biotech Co., Ltd., Shanghai,
China). The following primers were used to build the TGFβ
3′untranslated region (UTR) reporter plasmids (psiCHECK; Promega
Corporation, Madison, WI, USA): Forward,
5′-GGTCCCGCCCCGCCCCGCCCCGCCCCG-3′ and reverse,
5′-GGCCTGAACTACTATCTTTTA-3′. The predicted binding sequence
(www.mirdb.org) was mutated using QuikChange XL
Site-Directed Mutagenesis kit (Stratagene; Agilent Technologies,
Inc., Santa Clara, CA, USA). A549 cells were seeded onto 6-well
plates at a density of 5×105. Following transfection for
48 h, cells were lysed to determine reporter gene activity with the
Dual-Luciferase® Reporter assay system (Promega
Corporation, Madison, WI, USA), by measuring the proportion of
Renilla plasmid vs. firefly plasmid (10:1). All experiments
were performed in triplicate.
Western blot analysis
Cells were collected and lysed in radio
immunoprecipitation assay buffer [1 mM phenylmethylsulfonyl
fluoride, 0.1% sodium dodecyl sulfate (SDS), 1% sodium
deoxycholate, 1% NP-40, 10% glycerol, 1 mM dithiothreitol, 150 mM
NaCl and 50 mM Tris-HCl (pH 8.0)]. Cell lysates were cleared of
cell debris by centrifugation at 20,800 × g for 15 min at 4°C. The
proteins were extracted using a BCA Protein Assay kit and the
concentration was determined by ultraviolet absorption (21), prior to being separated by 12%
SDS-polyacrylamide gel electrophoresis with 35 µg protein loaded on
each lane and transferred onto a polyvinylidene difluoride membrane
(GE Healthcare Life Sciences, Chalfont, UK). Membranes were blocked
with 5% non-fat milk in Tris-buffered saline with 0.1% Tween-20 at
room temperature for 2 h. The membranes were subsequently probed
with primary antibodies against TGFβ (anti-mouse; 3711S; 1:10,000;
Cell Signaling Technology, Inc., Danvers, MA, USA) and β-actin
(anti-mouse; 4967S; 1:10,000; Cell Signaling Technology, Inc.).
Following three washes with TBST, membranes were incubated with
secondary antibody (Goat anti mouse-IgG-HRP, 1:10,000, Cell
Signaling Technology, Inc.). Signals were developed using an
Enhanced Chemiluminescence kit (Thermo Fisher Scientific, Inc.).
Immuno reactive bands were quantified individually by estimating
the number of pixels in ImageJ software version 1.4.1 (National
Institutes of Health, Bethesda, MD, USA).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) in
accordance with the manufacturer's protocol, later tested by the
following 50 µl PCR reaction system: 37.5 µl ddH2O, 10
mm dNTP 1 µl, 10×5 µl PCR buffer, 25 mm Mgcl 2 3 µl, upstream
primer 1 µl, downstream primers 1 µl, template cDNA 1 µl (100°C
water bath for 1 min, 0.5 µl Tag enzymes added over ice then 50 µl
liquid paraffin added followed by centrifugation at 11,180 × g at
4°C for 1 min. PCR conditions were: 94°C 1 min, 58°C 50 sec, 72°C
90 sec, 40 cycles and then 72°C for 10 min, followed by storage at
−20°C. RNAs were then reverse transcribed using the PrimeScript™
RT-PCR kit (Takara Biotechnology Co., Ltd., Dalian, China). qPCR
was performed on the resultant cDNA using the SYBR®
Green Master mix (Takara Biotechnology Co., Ltd.) to determine the
expression of miR-125a. PCR-master mix (45 µl) containing 2 µl of
enzyme-mix from the Qiagen OneStep RT-PCR kit (including the RT and
the hot-start Taq polymerase), 1X RT-PCR buffer (included in kit)
400 µM of each dNTP, 20U of Riblock RNase Inhibitor (Fermentas;
Thermo Fisher Scientific, Inc.) and 200 nM of each forward and
reverse primer was added to 5 µl of previously treated sample RNA.
The initial steps of the cycle program consisted of 30 min at 50°C
to produce enough specific initial DNA and 15 min at 95°C for the
activation of the Taq polymerase. Overall, 40 cycles of 15 sec at
95°C, 30 sec at 58°C and 1 min at 72°C were performed. Following a
final elongation step at 72°C for 10 min, the amplification product
was cooled at 8°C. The following primers were used (Sangon Biotech
Co., Ltd.): Forward, 5′-CGATGTCGTATATGCGTCGTATG-3′ and reverse,
5′-CGTAGTCGTCGTATGCTAGCGT-3′ for miR-125a; forward,
5′-CGTAGCTAGTCGTATGCTGA-3′ and reverse,
5′-CGATGTCGTAGGTCTAGCTGATGC-3′ for TGFβ. U6 forward,
5′-ATGACACGCAAATTCCCTCGAGGCGTGAAGCGTTCCATA-3′ and reverse,
5′-TATGGAACGCTTCACGCCTCGAGGGAATTTGCGTGTCAT-3′ small nuclear RNA
served as an internal control for miRNA and glyceraldehyde
3-phosphate dehydrogenase (GAPDH) forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-TGGTGAACGCCAGTGGA-3′ for
mRNA. Data was normalized to U6 and GAPDH using the
2−ΔΔCq method (22).
Statistical analysis
The relative expression of miRNA in each target was
expressed (mean ± standard deviation/standard error of the mean),
and the difference expression of miRNA between different groups was
compared with non-paired test or single factor variance analysis.
The Hardy-Weinberg equilibrium was tested using the Pearson's
Chi-square test (goodness-of-fit) for the SNP. Genotype
distribution differences between cohorts were examined using the
Pearson's χ2 test and logistic regression analysis. All
statistical analyses were two-sided and were performed in SPSS
software version 17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference. The
experiments were repeated three times.
Results
TGFβ is a target of miR-125a
Based on reports that TGFβ was functionally involved
in the development of pneumonitis in patients with lung cancer that
received radiotherapy, and that miR-125a negatively regulates
TGFβ, the present study aimed to investigate the molecular
mechanism underlying pneumonitis, a primary adverse effect of radio
therapy in the treatment of lung cancer, including the downstream
mediators and signaling pathways of miR-125a. Online miRNA target
prediction tools (http://www.targetscan.org) were used to search the
target gene of miR-125a; these identified TGFβ as a
candidate target gene in lung cancer cells with the ‘seed sequence’
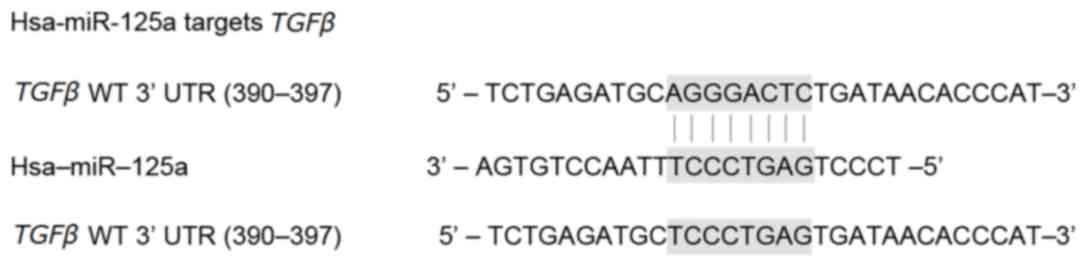
(390–397 bp) in the 3′UTR of TGFβ (Fig. 1).
To verify TGFβ as a direct target gene of
miR-125a, a dual-luciferase activity reporter assay was performed
on miR-125a-overexpressing lung cancer cells transfected with
wild-type TGFβ 3′UTR constructs or mutant TGFβ 3′UTR
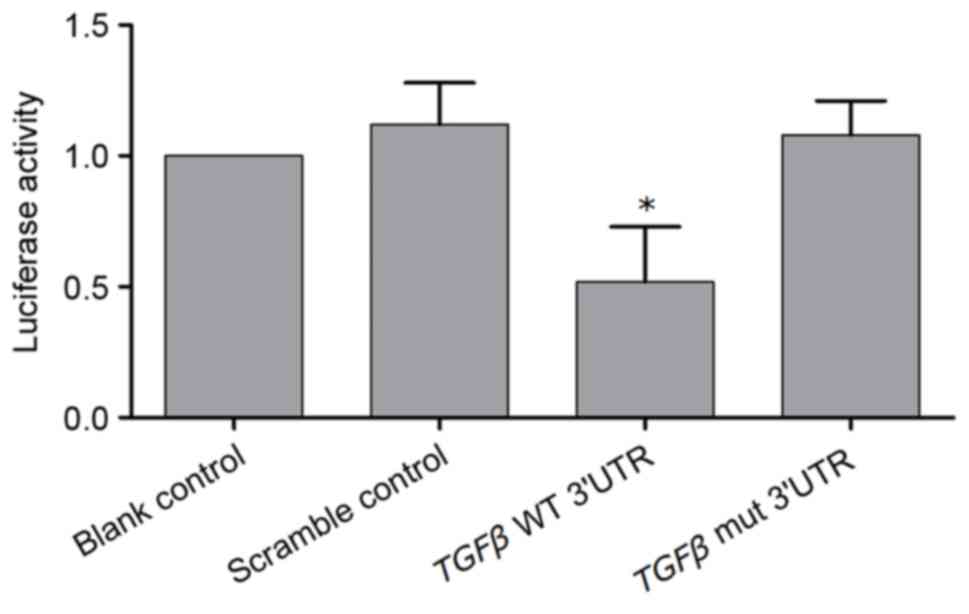
constructs. As presented in Fig.
2, compared with the scramble controls, cells transfected with
wild-type TGFβ 3′UTR constructs exhibited reduced relative
luciferase activity (P<0.05), whereas cells transfected with
mutant TGFβ 3′UTR constructs demonstrated comparable
luciferase activity (P>0.05), indicating that TGFβ was a
direct target of miR-125a and that the binding site is located at
390–397 bp of the 3′UTR of TGFβ.
Effects of rs12976445 on miR-125a
expression
Due to the negative regulatory association between
miR-125a and TGFβ, the effect of the rs12976445 polymorphism on the
interaction between miR-125a and TGFβ was investigated.
C-509T is located 509 bp upstream of the exon1 codon of the gene.
Tissue samples were collected from patients with lung cancer and
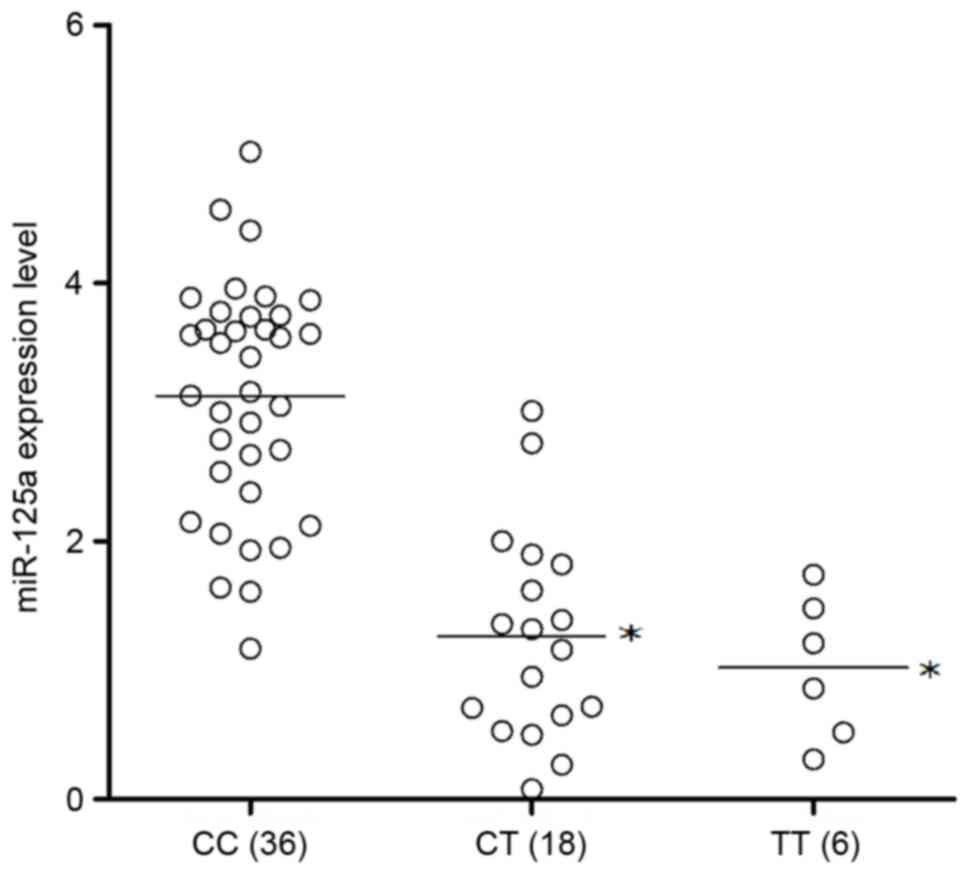
genotyped as CC (n=36), CT (n=18) or TT (n=6). The miR-125a
expression levels of the three genotypes were then analyzed. As
presented in Fig. 3, the
expression levels of miR-125a were significantly greater in the CC
genotype group compared with the genotype groups carrying the minor
allele, the CT and TT groups (P<0.05). These two groups
exhibited comparable miR-125a expression levels, suggesting that
the presence of the minor allele, T compromised the expression of
miR-125a.
To investigate the association between the
rs12976445 polymorphism and the risk of pneumonitis in patients
with lung cancer that received radio therapy, 534 lung cancer
patients with diagnosed pneumonitis and 489 lung cancer patients
without pneumonitis were enrolled in the present study. The
distributions of the rs12976445 polymorphism were in Hardy-Weinberg
equilibrium among the case group and controls. As the expression
levels of miR-125a were similar in the TT and CT groups, and
significantly reduced compared with the CC group, a dominant model
of the minor allele was indicated. Since the frequency of TT was
relatively low in the population assessed, the CT and TT groups
were combined. Significant differences were observed regarding
genotype distribution of rs12976445 between patients with and
without pneumonitis [odds ratio (OR)=1.43; 95% confidence interval
(CI)=1.13–1.94; P=0.03], as presented in Table II. This is despite the fact that
the polymorphism is not located in the binding site of miR-125a in
the 3′UTR of TGFβ, and the effect of the polymorphism on the
interaction between miR-125a and TGFβ may therefore be
attributed to an alteration in the secondary structure caused by
the presence of the minor allele, or linkage disequilibrium with
another variant that may directly cause the disruption between
miR-125a and TGFβ. Alternatively, the polymorphism may
compromise the transcription of TGFβ.
 | Table II.miR-125a genotype distribution in
lung cancer patients with and without pneumonitis. |
Table II.
miR-125a genotype distribution in
lung cancer patients with and without pneumonitis.
| Genotype | Pneumonitis (+)
(n=534) (%) | Pneumonitis (−)
(n=489) (%) | Adjusted OR (95%
CI) |
|---|
| CC | 363 (67.97) | 361 (73.82) |
|
| CT | 160 (29.96) | 122 (24.95) |
|
| TT | 11 (2.07) | 6 (1.23) |
|
| CT/TT | 171 (32.03) | 128 (26.18) | 1.43
(1.13–1.94) |
|
|
|
| P=0.03 |
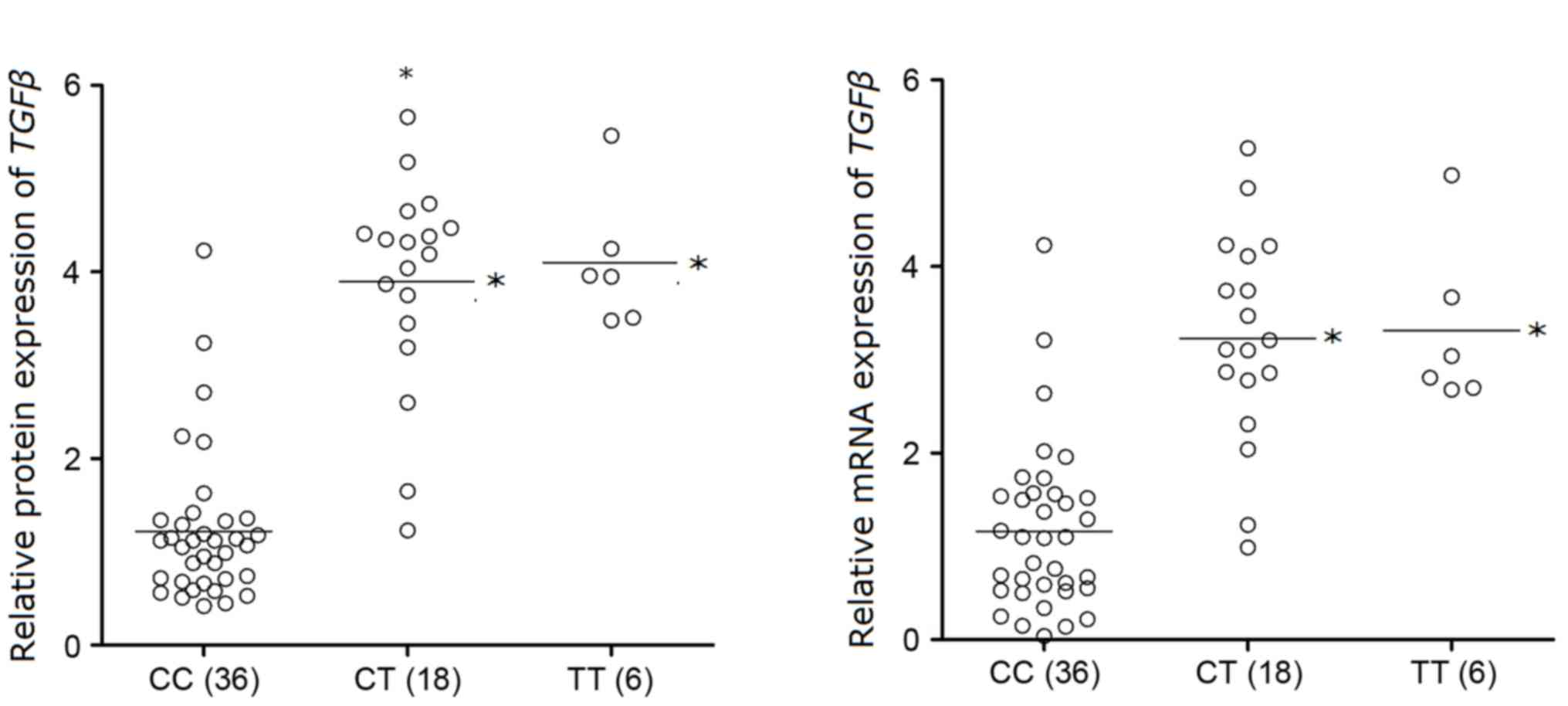
RT-qPCR and western blot analyses were performed to
investigate the mRNA and protein expression levels of TGFβ in each
rs12976445 polymorphism genotype group. As presented in Fig. 4, the mRNA and protein expression
levels of TGFβ were reduced in the CC genotype group, compared with
the minor-allele-carrying CT/TT genotype groups (P<0.05), which
had decreased expression levels of miR-125a. These findings further
support the hypothesis of the negative association between miR-125a
and TGFβ.
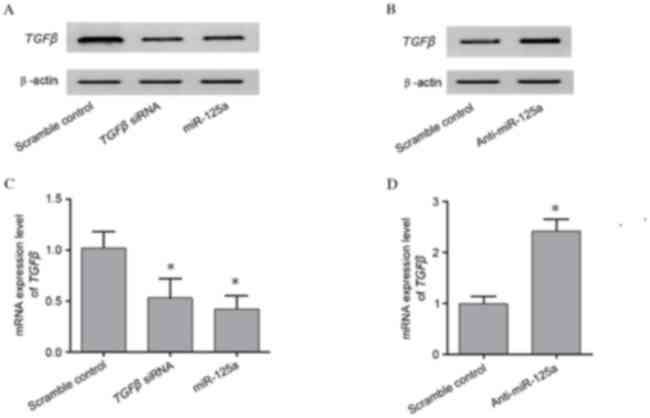
To investigate the interaction between miR-125a and
TGFβ, the mRNA and protein expression levels of TGFβ in A549
cells transfected with TGFβ siRNA, an miR-125a mimic, and
anti-miR-125a were analyzed and compared with the scramble
controls. TGFβ protein expression levels were reduced in cells
transfected with an miR-125a mimic or TGFβ siRNA, compared
with the scramble control (Fig.
5A). TGFβ protein expression levels were greater in cells
transfected with anti-miR-125a, compared with the scramble control
(Fig. 5B). A similar pattern was
observed in TGFβ mRNA expression levels (P<0.05; Fig. 5C and D). The above findings further
validate the negative association between miR-125a and its target
TGFβ.
Discussion
A common complication of lung radio therapy,
radiation-induced lung injury, including radiation fibrosis and
pneumonitis, is associated with a poor prognosis. Radio
therapy-induced pneumonitis may be predicted by pulmonary function
parameters in the clinic (23).
The infiltration of inflammatory cells into alveolar spaces and the
pulmonary interstitium is a characteristic of pneumonitis at the
cellular level. In the present study, bioinformatics tools were
used to identify potential binding sites of miR-125a in the 3′UTR
of TGFβ, and this was subsequently confirmed using a
dual-luciferase reporter system. In addition, tissue samples were
collected from patients with lung cancer and genotyped as CC
(n=36), CT (n=28) or TT (n=6). The expression levels of miR-125a
and TGFβ were determined in these samples, which exhibited
upregulated miR-125a and downregulated TGFβ protein and mRNA
expression levels in cells genotyped as CC compared with cells
carrying the minor allele, T.
It has previously been demonstrated that cytokines
are involved in the development of pneumonitis (24). TGFβ is involved in the
pathogenesis of radiation-induced pneumonitis; therefore, the
outcomes of patients with radiation-induced pneumonitis may be
predicted by TGFβ expression. As a ubiquitous profibrotic
and immunomodulatory cytokine, TGFβ is essential in tissue
responses to irradiation. It has been observed that rising plasma
levels of TGFβ1 during thoracic radio therapy is
associated with the subsequent occurrence of radiation-induced
pneumonitis (25–27). As a result, TGFβ has been
adopted as a sensitive plasma marker of radiation-induced
pneumonitis following thoracic irradiation. A previous animal study
demonstrated that anti-TGFβ antibodies decreased the levels of
TGFβ during thoracic irradiation, resulting in attenuation
of radiation-induced pneumonitis (28). In the present study, miR-125a was
identified as a regulator of TGFβ in A549 cells using in
silico analysis and a dual-luciferase reporter system.
Previous studies have demonstrated that the C-509T
SNP is associated with TGFβ gene promoter activity
enhancement, and the T allele of C-509T is associated with
increased serum levels of TGFβ. The serum level of
TGFβ in the CC genotype is decreased compared with the CT or
TT genotypes (29). In addition, a
previous study revealed that greater circulating TGFβ levels
during radiation therapy are correlated with poor prognosis of
locally advanced NSCLC (30).
Therefore, compared with those with TGFβ C-509T CT or TT
genotypes, patients with CC genotype may have improved survival due
to reduced circulating TGFβ levels during radio therapy.
Furthermore, various studies have implicated TGFβ in
radiation-induced lung injury; in particular, a persistently
elevated or rising level of TGFβ during the course of
thoracic radiation therapy has been associated with symptomatic
radiation pneumonitis (14,27).
Since miR-125a was identified as a regulator of TGFβ and
rs12976445 interferes with the mature processing of this miRNA,
reducing the miRNA expression levels, the present study examined
the effect of rs12976445 on the expression levels of miR-125a and
TGFβ. The expression levels of miR-125a were markedly
greater in the CC genotype group compared with those carrying the
minor allele, the CT and TT groups, which exhibited comparable
miR-125a expression levels, indicating that the T allele is
dominant.
The MIR125A gene, which encodes miR-125a, is
situated in a gene cluster on chromo some 19q13.41. miR-125a has
been reported to be involved in the pathogenesis of human diseases,
including verrucous carcinoma (31), ovarian cancer (32), gastric cancer (33), breast cancer (34) and systemic lupus erythematosus
(35). It has previously been
demonstrated that an SNP (rs12976445) in the precursor of miR-125a,
located in the stem loop of the molecule, may undermine the mature
processing of the miRNA, leading to a decrease in the production of
miR-125a (21). In the present
study, 534 lung cancer patients with diagnosed pneumonitis and 489
lung cancer patients without pneumonitis were recruited, and
significant differences were noted regarding genotype distribution
of rs12976445 between those with and without pneumonitis (OR=1.43;
95% CI=1.13–1.94; P=0.03).
In conclusion, the results of the present study
demonstrated that patients with radiation-induced pneumonitis have
decreased expression levels of miR-125a in lung tissue. Presence of
the minor allele of the rs12976445 polymorphism increased
expression levels of TGFβ by decreasing the expression
levels of miR-125a, and therefore may be associated with the
development of pneumonitis in patients with lung cancer receiving
radio therapy. These results suggested that rs12976445 may be a
potential biomarker to predict the risk of pneumonitis following
radio therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
H-YQ planned the study, collected and interpreted
the data, assembled the literature and wrote the manuscript. TY
collected and interpreted the data, and wrote the manuscript. J-FH
interpreted the data, assembled the literature and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
Declaration of Helsinki guidelines and the study protocol was
approved by the Human Ethics Committee of Shaanxi Friendship
Hospital.
Patient consent for publication
Written informed consent was obtained from all
participants prior to the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Midanik LT, Klatsky AL and Armstrong MA:
Changes in drinking behavior: Demographic, psychosocial, and
biomedical factors. Int J Addict. 25:599–619. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang WC, Shyh-Chang N, Yang H, Rai A,
Umashankar S, Ma S, Soh BS, Sun LL, Tai BC, Nga ME, et al: Glycine
decarboxylase activity drives non-small cell lung cancer
tumor-initiating cells and tumorigenesis. Cell. 148:259–272. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guan X, Yin M, Wei Q, Zhao H, Liu Z, Wang
LE, Yuan X, O'Reilly MS, Komaki R and Liao Z: Genotypes and
haplotypes of the VEGF gene and survival in locally advanced
non-small cell lung cancer patients treated with chemoradiotherapy.
BMC Cancer. 10:4312010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yin M, Liao Z, Yuan X, Guan X, O'Reilly
MS, Welsh J, Wang LE and Wei Q: Polymorphisms of the vascular
endothelial growth factor gene and severe radiation pneumonitis in
non-small cell lung cancer patients treated with definitive
radiotherapy. Cancer Sci. 103:945–950. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hildebrandt MA, Komaki R, Liao Z, Gu J,
Chang JY, Ye Y, Lu C, Stewart DJ, Minna JD, Roth JA, et al: Genetic
variants in inflammation-related genes are associated with
radiation-induced toxicity following treatment for non-small cell
lung cancer. PLoS One. 5:e124022010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vlasova MA and Moshkovskii SA: Molecular
interactions of acute phase serum amyloid A: Possible involvement
in carcinogenesis. Biochemistry (Mosc). 71:1051–1059. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao L, West BT, Hayman JA, Lyons S, Cease
K and Kong FM: High radiation dose may reduce the negative effect
of large gross tumor volume in patients with medically inoperable
early-stage non-small cell lung cancer. Int J Radiat Oncol Biol
Phys. 68:103–110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rübe CE, Wilfert F, Uthe D, König J, Liu
L, Schuck A, Willich N, Remberger K and Rübe C: Increased
expression of pro-inflammatory cytokines as a cause of lung
toxicity after combined treatment with gemcitabine and thoracic
irradiation. Radiother Oncol. 72:231–241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kong FM, Ao X, Wang L and Lawrence TS: The
use of blood biomarkers to predict radiation lung toxicity: A
potential strategy to individualize thoracic radiation therapy.
Cancer Control. 15:140–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fosslien E: Cancer morphogenesis: Role of
mitochondrial failure. Ann Clin Lab Sci. 38:307–329.
2008.PubMed/NCBI
|
|
11
|
Anscher MS, Kong FM, Marks LB, Bentel GC
and Jirtle RL: Changes in plasma transforming growth factor beta
during radiotherapy and the risk of symptomatic radiation-induced
pneumonitis. Int J Radiat Oncol Biol Phys. 37:253–258. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Burger A, Löffler H, Bamberg M and
Rodemann HP: Molecular and cellular basis of radiation fibrosis.
Int J Radiat Biol. 73:401–408. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hakenjos L, Bamberg M and Rodemann HP:
TGF-beta1-mediated alterations of rat lung fibroblast
differentiation resulting in the radiation-induced fibrotic
phenotype. Int J Radiat Biol. 76:503–509. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao L, Wang L, Ji W, Wang X, Zhu X,
Hayman JA, Kalemkerian GP, Yang W, Brenner D, Lawrence TS and Kong
FM: Elevation of plasma TGF-beta1 during radiation therapy predicts
radiation-induced lung toxicity in patients with non-small-cell
lung cancer: A combined analysis from Beijing and Michigan. Int J
Radiat Oncol Biol Phys. 74:1385–1390. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xue J, Li X, Lu Y, Gan L, Zhou L, Wang Y,
Lan J, Liu S, Sun L, Jia L, et al: Gene-modified mesenchymal stem
cells protect against radiation-induced lung injury. Mol Ther.
21:456–465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martin M, Lefaix J and Delanian S:
TGF-beta1 and radiation fibrosis: A master switch and a specific
therapeutic target? Int J Radiat Oncol Biol Phys. 47:277–290. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mendell JT: MicroRNAs: Critical regulators
of development, cellular physiology and malignancy. Cell Cycle.
4:1179–1184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lehmann TP, Korski K, Ibbs M, Zawierucha
P, Grodecka-Gazdecka S and Jagodziński PP: rs12976445 variant in
the pri-miR-125a correlates with a lower level of hsa-miR-125a and
ERBB2 overexpression in breast cancer patients. Oncol Lett.
5:569–573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beaven GH and Holida ER: Ultraviolet
absorption spectra of proteins and amino acids. Adv Prot Chem.
7:319–386. 1952. View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kocak Z, Borst GR, Zeng J, Zhou S, Hollis
DR, Zhang J, Evans ES, Folz RJ, Wong T, Kahn D, et al: Prospective
assessment of dosimetric/physiologic-based models for predicting
radiation pneumonitis. Int J Radiat Oncol Biol Phys. 67:178–186.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsoutsou PG and Koukourakis MI: Radiation
pneumonitis and fibrosis: Mechanisms underlying its pathogenesis
and implications for future research. Int J Radiat Oncol Biol Phys.
66:1281–1293. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Y, Williams J, Ding I, Hernady E, Liu
W, Smudzin T, Finkelstein JN, Rubin P and Okunieff P: Radiation
pneumonitis and early circulatory cytokine markers. Semin Radiat
Oncol. 12 1 Suppl 1:S26–S33. 2002. View Article : Google Scholar
|
|
26
|
Evans ES, Kocak Z, Zhou SM, Kahn DA, Huang
H, Hollis DR, Light KL, Anscher MS and Marks LB: Does transforming
growth factor-beta1 predict for radiation-induced pneumonitis in
patients treated for lung cancer? Cytokine. 35:186–192. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu XL, Huang H, Bentel G, Clough R, Jirtle
RL, Kong FM, Marks LB and Anscher MS: Predicting the risk of
symptomatic radiation-induced lung injury using both the physical
and biologic parameters V(30) and transforming growth factor beta.
Int J Radiat Oncol Biol Phys. 50:899–908. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anscher MS, Thrasher B, Rabbani Z, Teicher
B and Vujaskovic Z: Antitransforming growth factor-beta antibody
1D11 ameliorates normal tissue damage caused by high-dose
radiation. Int J Radiat Oncol Biol Phys. 65:876–881. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grainger DJ, Heathcote K, Chiano M,
Snieder H, Kemp PR, Metcalfe JC, Carter ND and Spector TD: Genetic
control of the circulating concentration of transforming growth
factor type beta1. Hum Mol Genet. 8:93–97. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao L, Ji W, Zhang L, Ou G, Feng Q, Zhou
Z, Lei M, Yang W and Wang L: Changes of circulating transforming
growth factor-beta1 level during radiation therapy are correlated
with the prognosis of locally advanced non-small cell lung cancer.
J Thorac Oncol. 5:521–525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Odar K, Boštjančič E, Gale N, Glavač D and
Zidar N: Differential expression of microRNAs miR-21, miR-31,
miR-203, miR-125a-5p and miR-125b and proteins PTEN and p63 in
verrucous carcinoma of the head and neck. Histopathology.
61:257–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cowden Dahl KD, Dahl R, Kruichak JN and
Hudson LG: The epidermal growth factor receptor responsive miR-125a
represses mesenchymal morphology in ovarian cancer cells.
Neoplasia. 11:1208–1215. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nishida N, Mimori K, Fabbri M, Yokobori T,
Sudo T, Tanaka F, Shibata K, Ishii H, Doki Y and Mori M:
MicroRNA-125a-5p is an independent prognostic factor in gastric
cancer and inhibits the proliferation of human gastric cancer cells
in combination with trastuzumab. Clin Cancer Res. 17:2725–2733.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iorio MV, Ferracin M, Liu CG, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao X, Tang Y, Qu B, Cui H, Wang S, Wang
L, Luo X, Huang X, Li J, Chen S and Shen N: MicroRNA-125a
contributes to elevated inflammatory chemokine RANTES levels via
targeting KLF13 in systemic lupus erythematosus. Arthritis Rheum.
62:3425–3435. 2010. View Article : Google Scholar : PubMed/NCBI
|