Introduction
Mature brain-derived neurotrophic factor (mBDNF), as
well as its precursor proBDNF are widely distributed in the central
nervous system (CNS) as a member of the neurotrophin family (NTs).
The current view is generally believed that NTs has a protective
effect on neurons (1).
Interestingly, mature NTs (for example, mBDNF) are not the primary
gene products. They are removed from a relatively large
neurotrophic factor precursor (for example, proBDNF). The full
length protein of proBDNF is about 35 kDa. After shearing, a mature
molecule (mBDNF) with a length of about 13.5 kDa and a precursor
fragment (pre-domain) of about 20 kDa are obtained. Usually,
proBDNF has two processes to be cut by enzyme: One is cleaved
endogenously and then secreted to the extracellular matrix
(2), the other is directly
secreted to the extracellular domain, and then modified in the
extracellular environment (3).
Deffer from the mBDNF, which promotes neuron
survival and regeneration, which are important to functional
recovery after injury to the nerve system (4), no one mentioned whether the exogenous
proBDNF may have certain functions. People thought proBDNF was just
a middle product. In 2001, Lee et al reported the inhibitory
effect of proBDNF on neurons (5).
Beattie found that full-length proNGF secreted into the
extracellular binding to p75 neurotrophin receptor (p75NTR)
mediated apoptosis in neurons and glial cells (6). In particular, our previous study
found that the inhibitory effect of proBDNF on cells was induced
only after nerve injury. After spinal cord injury, proBDNF could
inhibit the regeneration of axons (7).
In our previous study, we have shown that endogenous
proBDNF can inhibit macrophage infiltration and disturb
demyelination and remyelination after SCI (7). This indicates that proBDNF may affect
cells other than neurons during post-injury repair.
The present study focused on the functions of
proBDNF in proliferation and migration of oligodendroglia. We
observed that proBDNF can inhibit proliferation and migration of
OLN-93 cells, a permanent oligodendroglia cell line. Moreover,
anti-proBDNF treatment could be effective in protecting cells from
apoptosis and in promoting cell proliferation and migration.
Therefore, blocking proBDNF may be a therapeutic target for
traumatic injuries in CNS.
Materials and methods
Cell culture and maintenance
The OLN-93 oligodendroglia cell line was utilized in
this study. OLN-93 cells are known to express several
oligodendroglial markers; however, they do not exhibit
characteristics of astrocytes (8).
Cells were incubated at 37°C and 5% CO2 in Dulbecco's
modified Eagle's medium (DMEM)/F12 with 10% fetal bovine serum
(FBS; both Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Growth medium was changed twice a week. When the cells
reached 70% confluency, they were digested with 1% trypsin/EDTA
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 2 min. FBS
was added to stop the digestion once the cells were round and
floating. Cells were then seeded in plates or flasks and maintained
in an incubator.
One subset of cells was directly fixed for
fluorescent immunohistochemical staining using primary antibodies
against proBDNF, p75NTR and sortilin, whereas another subset of
cells was treated with serial concentrations of recombinant proBDNF
(1, 3, 10, 30, 100 ng/ml); bovine serum albumin (BSA; 100 ng/ml)
treatment was used as a control. Meanwhile, sheep anti-proBDNF
antibody (5, 10 µg/ml), monoclonal proBDNF antibody (PB192E; 100
ng/ml), mouse anti-p75NTR antibody (10 µg/ml; 9,650 from Moses
Chao), recombinant p75NTR extracellular domain-human FC fusion
protein (p75NTRECD-fc; 3 µg/ml), and normal IgG (10 µg/ml) were
also administrated in in vitro observations.
p75NTRECD-fc and the recombinant proBDNF with
harbouring an RR-AA mutation on the cleavage site were produced as
previously described and characterized by Fan et al
(9). Sheep anti-proBDNF antibody
is able to specifically recognise proBDNF; however, it cannot
recognise mature BDNF and other NTs (7,8).
Cell viability, proliferation, migration and apoptosis were
determined using the MTT assay, BrdU staining, scratch assay and
activated caspase-3 staining respectively.
Immunocytochemistry
First, cells were seeded at 105
cells/well on a pre-treated glass coverslip in a 24-well plate.
When they reached 70% confluency, 4% paraformaldehyde (PFA) was
added to fix the cells at room temperature for 10 min. The fixed
cells were subsequently subjected to a PBS wash three times before
being immersed in blocking buffer (2% BSA, 0.5% Triton X-100, 0.1%
Tween-20, 5% donkey serum) at room temperature for 60 min. Rabbit
anti-p75 (1:1,000; Abcam, Cambridge, UK), sheep anti-proBDNF (5
µg/ml; University of South Australia, Adelaide, Australia) and
rabbit anti-sortilin (1:1,000; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) antibodies were then introduced as primary
antibodies and incubated with the cells at 4°C for overnight. Then,
after washing the cells three times PBST, sheep anti-rabbit IgG CY3
(1:1,000; Invitrogen; Thermo Fisher Scientific, Inc.) and donkey
anti-sheep IgG CY3 (1:1,000; EMD Millipore, Billerica, MA, USA)
were applied as secondary antibodies. In addition, DAPI (1:1,000;
Sigma-Aldrich; Merck KGaA) was diluted in the antibody solution to
serve as a nuclear dye. Subsequently, cells were incubated with
corresponding secondary antibody and DAPI at room temperature for
60 min. Finally, after washing the cells three times, fluorescent
images of the cells were captured using an Olympus BX-50 (Olympus
Corp., Tokyo, Japan) fluorescent microscope equipped with a CCD
camera.
Western blot analysis
Cells were seeded at 106 cells/well in a
6-well plate, incubated at 37°C and 5% CO2 and
subsequently lysed using RIPA buffer [20 mM Tris-HCl (pH 7.5), 150
mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium
deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate,
1 mM Na3VO4, 1 mM phenylmethylsulphonyl
fluoride, 1 µg/ml leupeptin] when they reached a confluency of 70%.
After sonication and centrifugation to remove cell debris, the
lysate was loaded onto a sodium dodecyl sulphate polyacrylamide
gel, containing stacking (0.5%) and a separating gel (10%). On
completion of electrophoresis, proteins were transferred onto a
nitrocellulose membrane (GE Healthcare, Chicago, IL, USA) using the
semi-dry method (Bio-Rad, Berkeley, CA, USA) at 20 V, 20 min. The
membrane was then washed with PBS three times and blocked with a
blocking buffer (5% skim milk in PBS) for 1 h at room temperature.
Rabbit anti-p75 (1:1,000; Abcam), sheep anti-proBDNF (5 µg/ml;
University of South Australia) and rabbit anti-sortilin (1:1,000;
Sigma-Aldrich; Merck KGaA) antibodies were subsequently added as
primary antibodies, followed by incubation at 4°C for overnight.
Before and after triple washes with PBST, goat anti-rabbit IgG-HRP
(1:3,000) and goat-anti-sheep IgG-HRP (1:3,000; both Sigma-Aldrich;
Merck KGaA) were co-incubated with the membrane for 1 h at room
temperature. ECL substrate solutions A & B (GE Healthcare) were
mixed and added onto the membrane in order to observe any potential
fluorescent protein bands. Finally, the film was developed in a
dark room to obtain results.
Cell viability assay
Cells were seeded at 104 cells/well in a
96-well plate, followed by incubation at 37°C and 5% CO2
for overnight to allow the cells to attach to the bottom of the
wells. After washing the cells with PBS three times, the cells were
cultured in a serial concentration of proBDNF (1, 3, 10, 30, 100
ng/ml) or in a serial concentration of proBDNF and monoclonal
proBDNF antibody PB192E (100 ng/ml) in an FBS-free medium. BSA (100
ng/ml) treatment was used as a control. After 20 h, 10 µl of MTT
solution (5 mg/ml in PBS; Sigma-Aldrich-Aldrich; Merck KGaA) was
added to each well, followed by incubation for an additional 4 h.
Subsequently, 100 µl of filter-sterilized solubilisation solution
(10% SDS in 0.01 M HCl) was added to each well and incubated
overnight at 37°C to dissolve the insoluble purple formazan product
in order to produce a coloured solution. Finally, the optical
density (OD) of the solution in each well was measured at 595 nm
using a multi-well scanning spectrophotometer (Bio-Rad Model 2550
EIA Reader); a wavelength of 620 nm was used as a reference.
BrdU proliferation assay
Cells were at first seeded at 5×105 cells
per well on a pre-treated glass coverslip in a 24-well plate.
Approximately 200 µl of BrdU (80 µM BrdU-medium solution;
Sigma-Aldrich; Merck KGaA) was then added to each well, followed by
incubation at 37°C and 5% CO2 for 24 h. The cells were
then fixed in 4% PFA and washed with PBS three times. After a 1 h
incubation in HCl and neutralization in boric buffer (pH 8.0–8.5),
the cells were blocked using a blocking buffer (2% BSA, 0.5% Triton
X-100, 0.1% Tween-20, 5% donkey serum). An anti-BrdU antibody
(1:1,000; DSHB; University of Iowa, Iowa City, USA) was incubated
with the cells as a primary antibody. Then, after washing the cells
with PBST three times, cells were subjected to secondary antibody
of goat anti-mouse IgG CY3 (1:1,000; Invitrogen; Thermo Fisher
Scientific, Inc.) and DAPI (1:1,000; Sigma-Aldrich; Merck KGaA).
After washing the cells three times, the number of
BrdU/DAPI-positive cells was counted at five random fields per
slice at magnification, ×10 under a fluorescence microscope.
Scratch assay
The scratch assay was performed as previously
described (10). Briefly, OLN-93
cells cultured in a 6-well plate were wounded using a sterile P20
pipette tip, followed by washing with culture medium to remove cell
debris. The cells were then treated with 1, 3, 10, 30, and 100
ng/ml proBDNF and 100 ng/ml BSA for 24 or 48 h, respectively.
Subsequently, cells were fixed by 4% PFA for 10 min then stained by
0.05% crystal violet for 30 min. After washed by PBS, phase
contrast images were captured in 6 different fields of every wound
using an inverted microscope (Olympus IX-71; Olympus Corp.). Cells
that migrated into the wound space were counted in the captured
image. The experiments were performed in triplicates.
Cell apoptosis assay
OLN-93 cells at 5×104 cells per well were
seeded in 24-well plates. When the cells reached 90% confluency,
they were washed three times with PBS and treated with proBDNF,
proBDNF antibody and BSA consecutively at 37°C and 5%
CO2 for 24 h. After treatment, cells were fixed in 4%
PFA at room temperature for 10 min and then washed with PBS before
being blocked using a blocking buffer (2% BSA, 0.5% Triton X-100,
0.1% Tween-20, 5% donkey serum). Next, rabbit anti-activated
caspase-3 (1:1,000; EMD Millipore) was co-incubated with the cells
at 4°C for overnight. Then, sheep anti-rabbit IgG FITC (1:1,000;
Abcam) and DAPI (1:1,000; Sigma-Aldrich; Merck KGaA) were applied
and co-incubated with the cells at room temperature for 1 h. After
washing the cells 3 times, fluorescent images of the cells were
captured using an Olympus BX-50 fluorescent microscope.
Data acquisition and statistical
analysis
Five visual fields were randomly selected from
superior, inferior and central parts as well as from left-hand and
right-hand sides of each slide for fluorescence microscopy
analysis. Images at magnifications of ×10, ×20 and ×60 were
obtained using an Olympus BX-50 fluorescent microscope.
The total number of cells and positively-stained
cells were counted using Image J. Quantitative analysis was
performed for all experiments using SPSS for Windows v. 13.0. All
data are presented as mean ± standard error of the mean (SEM). Data
were analysed using one-way analysis of variance (ANOVA), followed
by Tukey's post-hoc tests, where appropriate, or t-test for paired
groups. P<0.05 was considered to indicate a statistically
significant difference. All experiments were repeated at least 3
times.
Results
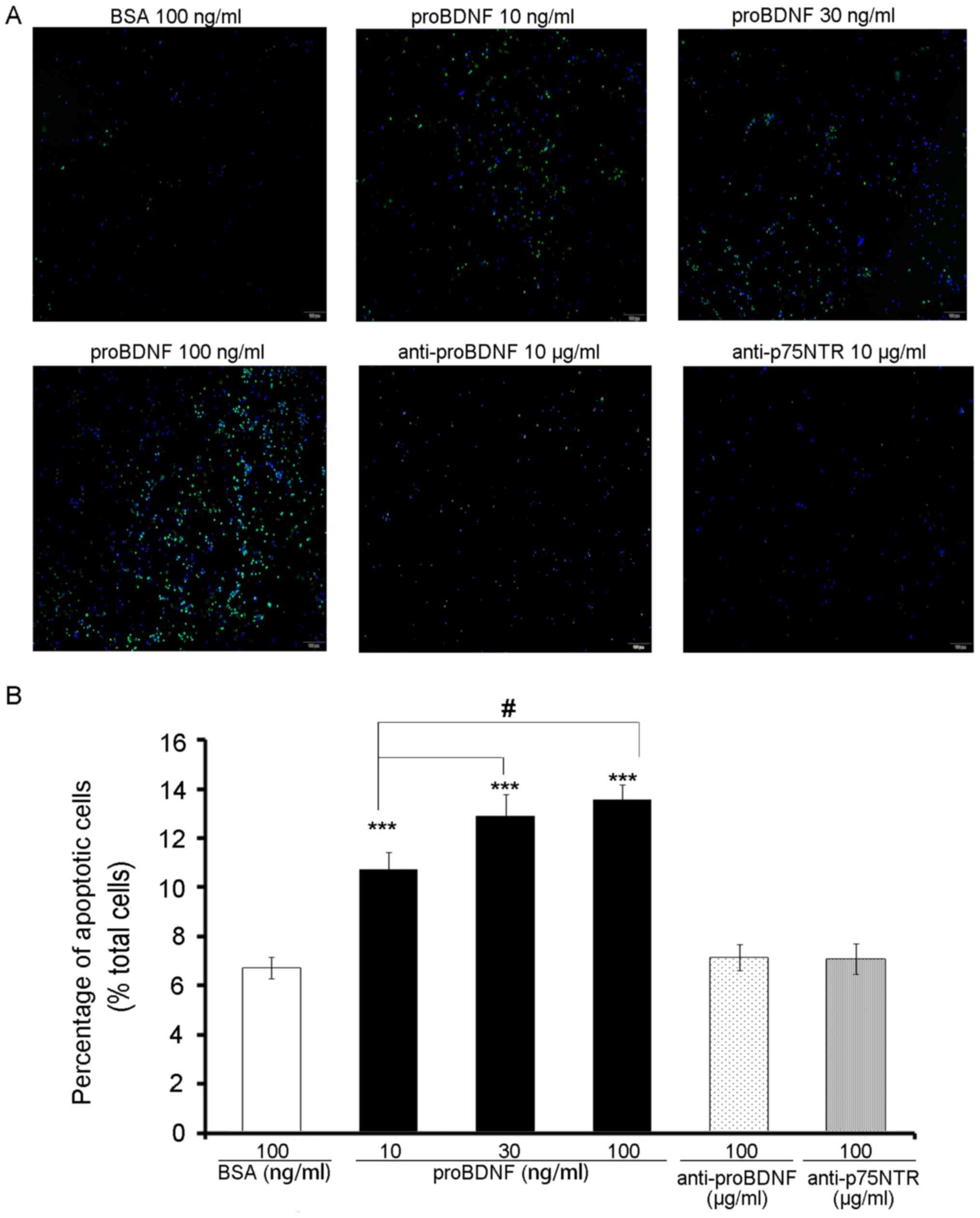
OLN-93 cells express proBDNF and its
receptors
OLN-93 cells were maintained in DMED/F12 1:1 medium.
In the presence of 10% FBS, cell proliferation occurred at a high
rate (8). All the cells expressed
proBDNF, p75NTR and sortilin as shown by fluorescent
immunocytochemistry (Fig. 1A-C).
The typical oligodendroglia cell marker NG2 (Fig. 1D), which was also used to label
oligodendrocytes in spinal cord was also expressed (11). Cell growth was observed to be
density-dependent. Cells were mainly bipolar with long cellular
extensions at lower confluency; they formed large clumps
interconnected with long, thin cellular processes at higher
confluency (Fig. 1E). Moreover,
Western blot analysis using the cell lysate showed bands of
proBDNF, p75NTR and sortilin at the corresponding molecular weights
(Fig. 1F). These results indicated
that OLN-93 cells expressed endogenous proBDNF and its receptors
p75NTR and sortilin.
 | Figure 1.OLN-93 cells express p75NTR,
sortilin, proBDNF and NG2. Immunohistochemistry analysis indicated
that cultured OLN-93 cells were positively stained with (A) p75NTR,
(B) sortilin (C), proBDNF and (D) NG2, which is a typical marker of
OPCs (magnification, ×60). (E) Cellular morphology of OLN-93 cells
after 7 days culture (magnification, ×20). (F) Western blot was
analysis employed to examine the expression of p75NTR, sortilin and
proBDNF. p75NTR, p75 neurotrophin receptor; BDNF, brain-derived
neurotrophic factor; OPC, oligodendrocyte precursor cell. |
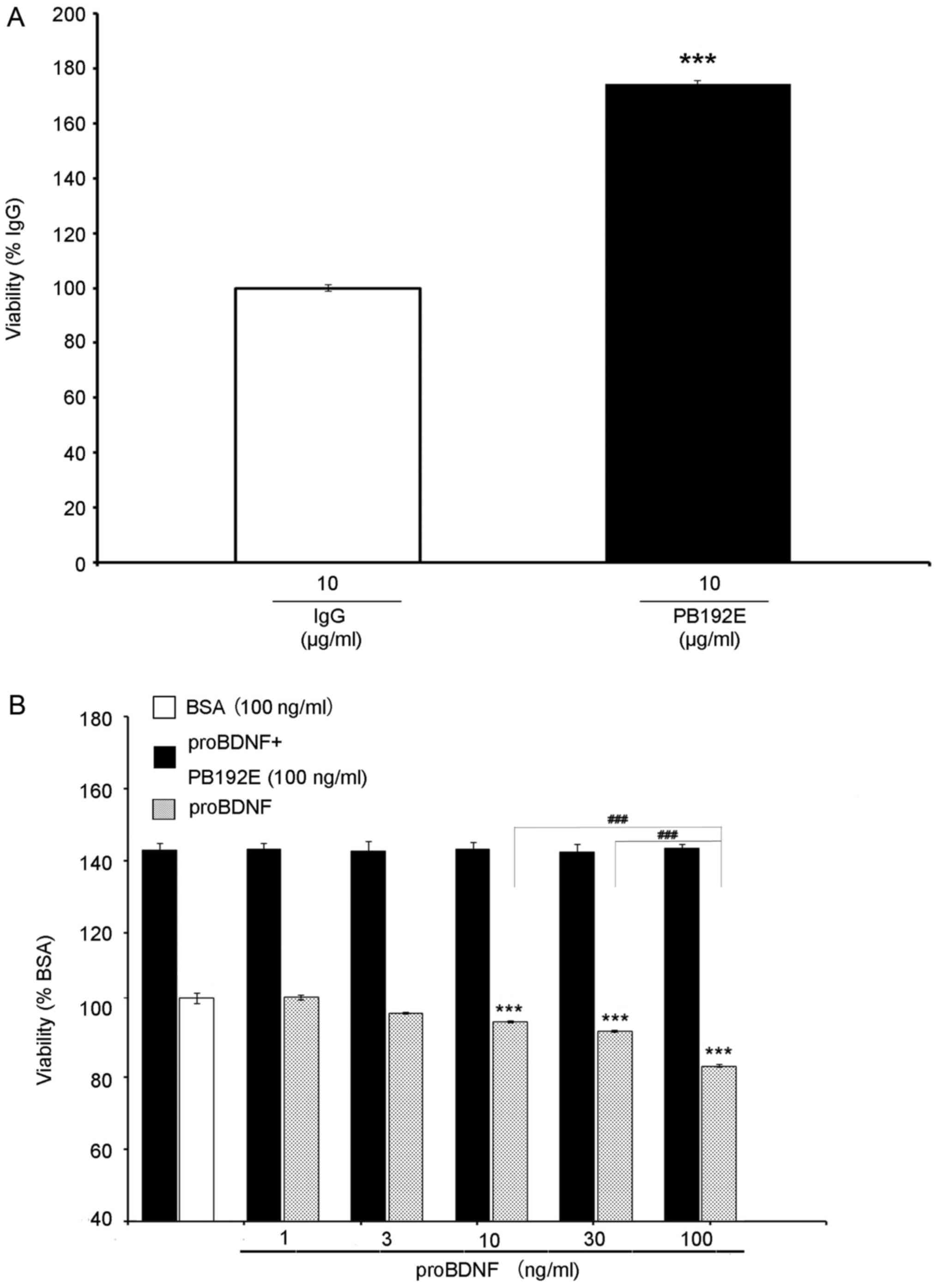
proBDNF inhibits OLN-93 cell
viability
To examine the effect of proBDNF on OLN-93 cells, we
used a monoclonal proBDNF antibody (PB192E), raised against the
pro-domain of BDNF, to neutralise the effects of proBDNF. Cells
treated with PB192E at 10 µg/ml were more active than those treated
with normal IgG (Fig. 2A),
indicating that endogenous proBDNF may have inhibited the cell
growth.
MTT assay revealed that proBDNF imparts a toxic
effect on cell viability. Treated with serial concentrations of
proBDNF (1, 3, 10, 30, 100 ng/ml), the cell growth curve
demonstrated a significant growth inhibition at 10 ng/ml, with the
highest growth inhibition at 100 ng/ml (Fig. 2B) (P=0.000904), indicating a
dose-dependent response.
Then, we treated the cells with serial
concentrations of proBDNF (1, 3, 10, 30, 100 ng/ml) and PB192E
antibody (100 ng/ml). Cells in this group had significantly higher
bioactivity than those in the BSA and proBDNF-treated groups
(Fig. 2B) (P=0.000194), indicating
that PB192E antibody can neutralise exogenous proBDNF as well.
proBDNF inhibits, but anti-proBDNF
promotes, OLN-93 cell proliferation
Quantitative assessment of the proliferative
activities in OLN-93 cells were performed (Fig. 3). BrdU/DAPI double-labelling was
applied to the OLN-93 cell proliferation assay (Fig. 3C). The proliferating cells were
BrdU+/DAPI+, whereas the non-proliferating
ones were only DAPI+. The percentage of
BrdU+/DAPI+ cells in the proBDNF-treated
groups was significantly lower than that in the BSA group,
illustrating a dose-dependent inhibition (Fig. 3A) (P=0.0206 in 10; P=0.0420 in 100
ng/ml; P=0.000126 in 30 ng/ml).
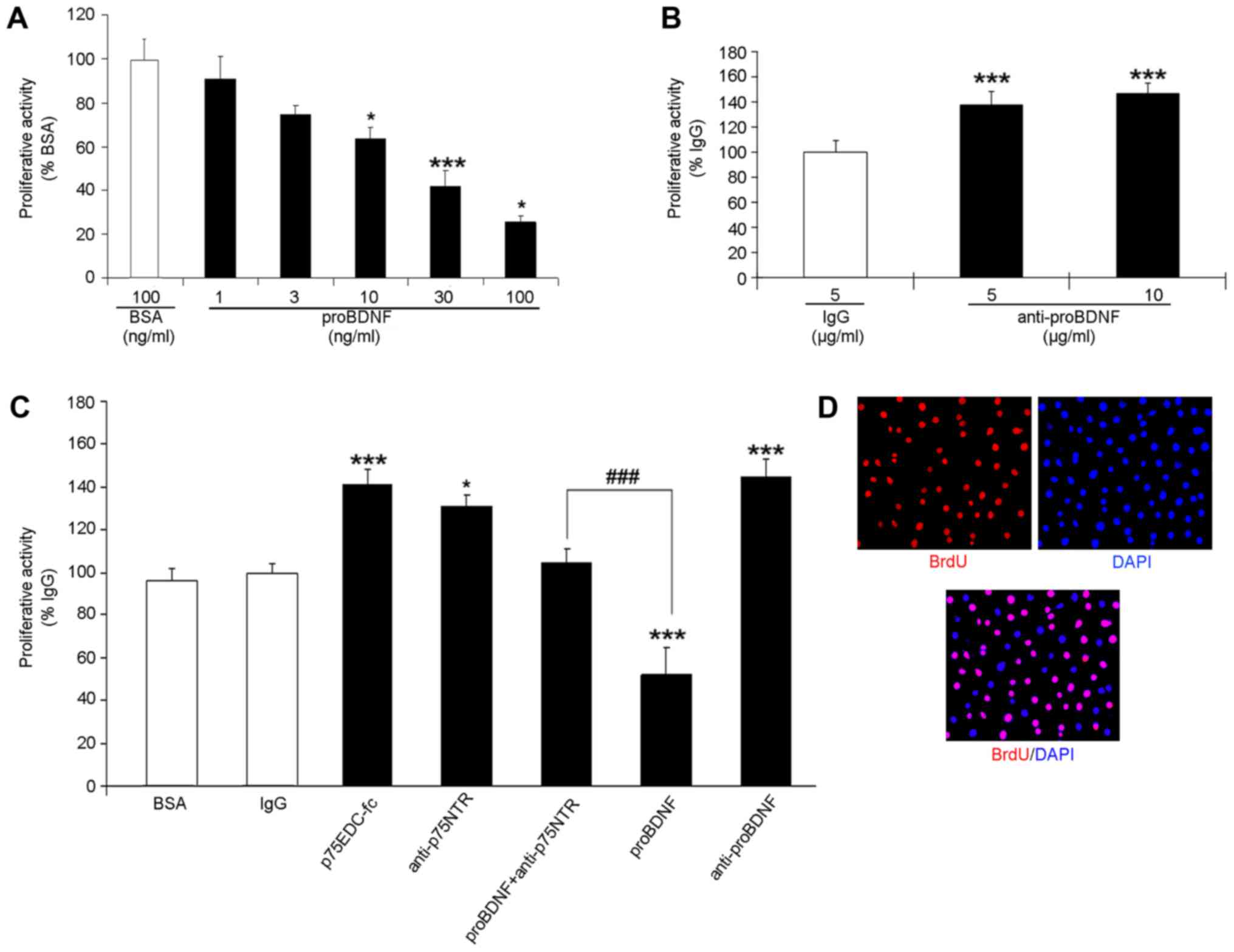 | Figure 3.Quantitative assessment of the
proliferative activities in OLN-93 cells. (A) Cells were treated
with serial concentration of proBDNF at 0, 1, 3, 10, 30, 100 ng/ml;
ANOVA, F=21.603 (B) Cells were treated with 5 and 10 µg/ml proBDNF
antibody. ANOVA, F=20.144 (C) Cells were treated with p75NTRecd-FC,
p75NTR antibody, respectively, and proBDNF + p75NTR antibody. The
activities were measured against the BSA group and calculated as
the % activity of BSA. ANOVA F=195.301. *P<0.05, ***P<0.001
vs. the BSA group, ###P<0.001 vs. proBDNF treated
group. (D) Photomicrograph of cultured OLN-93 cells, which have
been immunostained with BrdU (red) and DAPI (blue) (magnification,
×40). BSA, bovine serum albumin; BDNF, brain-derived neurotrophic
factor; ANOVA, one-way analysis of variance. |
To confirm our results, we used polyclonal sheep
anti-proBDNF antibodies to neutralise endogenous proBDNF. We found
that cells treated with polyclonal antibodies showed higher
proliferative activities than those treated with normal sheep IgG
(Fig. 3B) (P=0.000105). These
experiments indicate that OLN-93 cells secrete endogenous proBDNF,
which inhibits their growth.
Inhibitory effect of proBDNF on OLN-93
cell proliferation is blocked by soluble p75 receptor body or p75
antibodies
To investigate the possible pathway by which proBDNF
exerts an inhibitory on OLN-93 cells, recombinant fusion molecule
of p75NTRECD-fc and p75NTR functional antibody were utilized. These
have been used in our previous study as competitive inhibitors to
block p75NTR signal transduction extracellularly (12). In this study, we treated OLN-93
cells with 3 µg/ml of p75NTRECD-fc protein and 10 µg/ml of
anti-p75NTR for 24 h. Then, BrdU/DAPI double-labelling was
conducted to check the proliferative activities after 24 h of
incubation. Under both the treatments, the percentage of
proliferating cells significantly increased in comparison with
those in the normal sheep IgG control group (Fig. 3C) (P=0.00206 in p75NTRECD-fc;
P=0.0151 in anti-p75NTR).
Moreover, we treated OLN-93 cells with proBDNF and
p75NTR antibody. The cells were co-incubated with 100 ng/ml of
proBDNF and 10 µg/ml of p75NTR antibody for 24 h. Treated cells had
better proliferative activities than those in the proBDNF group
(Fig. 3C) (P=0.000103) but had no
significant differences in comparison with those in the BSA group
(Fig. 3C) (P=0.264). This
indicated that proBDNF can inhibit OLN-93 cell proliferation via
the p75NTR pathway and that proBDNF can be blocked by disruption of
p75NTR signal transduction with the soluble receptor body or
antibody.
proBDNF inhibits OLN-93 cell
migration
Both proliferation and migration are critical steps
in the regeneration and repair after CNS injury. To investigate the
function of proBDNF in OLN-93 cell migration, the scratch assay was
used. After scratching, a cell-free wound region was generated in
6-well culture plates (Fig. 4Aa, d,
g). Serial concentrations of proBDNF (10, 30, and 100 ng/ml)
and proBDNF antibody (5 and 10 µg/ml) were used to treat the cells.
After cell growth for 24 and 48 h, a number of cells were observed
migrating from the edges into the cell-free scratch region
(Fig. 4A). Cells treated with
proBDNF showed lower migration activity. A higher concentration of
proBDNF resulted in the migration of fewer cells, indicating a
dose-dependent inhibition of cell migration by proBDNF (Fig. 4B and C) (P=0.0112 in 10 ng/ml;
P=0.000104 in 30 ng/ml; P=0.000229 in 100 ng/ml).
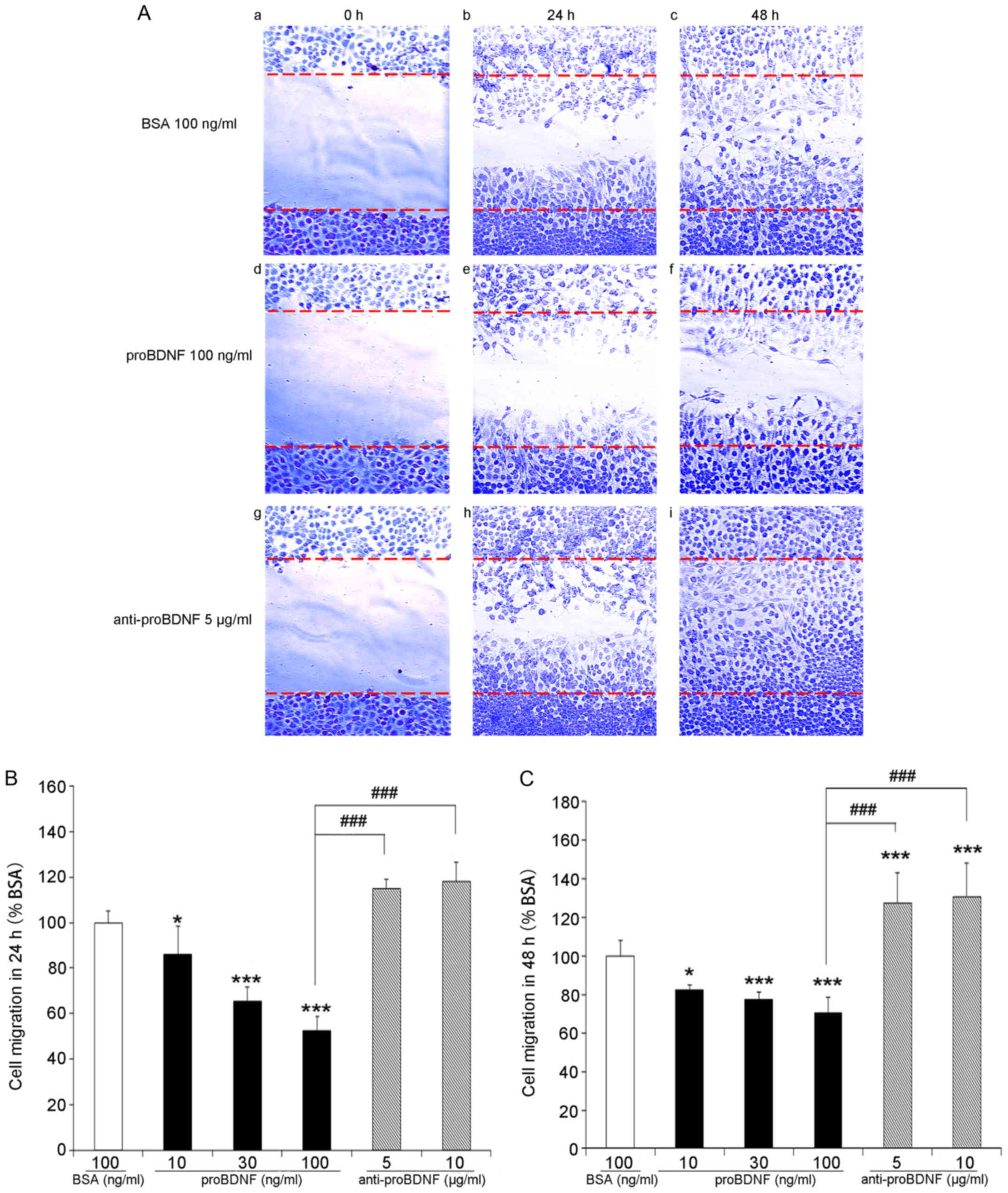 | Figure 4.Scratch assay of OLN-93 cells in
vitro. (A) Photomicrographs of cell migration to the scratch
zone at different time points (magnification, ×20). (a-c) control
group without any treatment at 0, 24, 48 h. (d-f) proBDNF treatment
group with 100 ng/ml proBDNF at 0, 24, 48 h. (g-i) proBDNF antibody
treatment group with 5 µg/ml proBDNF antibody at 0, 24, 48 h. (B
and C) Quantitative assessment of cells migrated into scratched
zone. For each condition, three representative fields within the
scratch were photographed. Data are presented as a percentage of
cells migration into scratch zone in culture medium group (control,
100%) AVONA F=298.336 (B); F=280.230 (C). *P<0.05, ***P<0.001
vs. BSA (100%); ###P<0.001 as indicated. BSA, bovine
serum albumin; BDNF, brain-derived neurotrophic factor; ANOVA,
one-way analysis of variance. |
In comparison with the proBDNF group, more cells
migrated into the scratch wound region in the proBDNF antibody (5
or 10 µg/ml) treatment group; however, the population of migrated
cells between different anti-proBDNF concentrations showed no
statistical differences (Fig. 4B and
C) (P=0.24 in 24 h; P=0.50 in 48 h).
proBDNF induces OLN-93 cell
apoptosis
As a member of the cysteine-aspartic acid protease
family, caspase-3 is activated when cell apoptosis occurs (13). In this study, we applied the
activated caspase-3 antibody to observe apoptosis of OLN-93 cells
that were treated with proBDNF (10, 30, 100 ng/ml) and BSA (100
ng/ml) (Fig. 5A).
As expected, the percentage of apoptotic cells in
the proBDNF group was significantly higher than that in the BSA
group (Fig. 5B) (P=0.0000384).
Moreover, with an increase in the dose of proBDNF, more cells were
found to be caspase-3-positive (Fig.
5B) (P=0.024).
Discussion
Mature BDNF promotes neural differentiation and
survival (14) and also plays an
important role in proliferation, migration and myelination of
oligodendrocytes (15). However,
the BDNF precursor, proBDNF, is involved in inhibitory activities
in the CNS such as promoting neuron apoptosis, inhibiting neurite
outgrowth and cell proliferation and survival via p75NTR and
co-receptor sortilin (16–19). The effects of proBDNF on
oligodendrocyte have remained unclear. According to our studies,
the inhibitory effect of proBDNF on OLN-93 cells is dose-dependent
and can be blocked by the proBDNF antibody (Fig. 2). These results indicated the
involvement of exogenous and endogenous proBDNF in the viability of
oligodendrocyte-like cells.
Endogenous and exogenous proBDNF suppressed OLN-93
proliferation and migration. In our previous studies, we observed
that proBDNF inhibited the migration of ED1+ macrophages
after SCI (7) and of granule cells
in the developing cerebella (20).
This result suggests that similar to neurons, proBDNF may exert
negative effects on the migration of non-neuronal cells. In order
to investigate this, the scratch assay was performed. Under a
standardized wound condition and same incubation period, the
migration was inhibited by proBDNF in a dose-dependent manner.
Higher concentrations of proBDNF had higher inhibitory activities
on cell migration, while treatment with proBDNF antibody
facilitated the migration (Fig.
4). Further, OLN-93 proliferation, as investigated by BrdU
staining, showed similar results (Fig.
3). These results indicate that proBDNF may be a mitosis
suppressor, which prevents the proliferation and migration of
oligodendrocyte precursor cell (OPC)s after SCI, and that its
detrimental effects can be inhibited by proBDNF antibodies.
Both mBDNF and proBDNF mediate their biological
activities by binding to the cell surface receptors. It is a common
phenomenon that the receptors which combine with mNTs always may
combine with proNTs; even though to mediate a different signal. The
four main related receptors are tyrosine kinase receptor (Trk)
(21), p75NTR (22) tumor necrosis factor (TNF) (23) and sortilin (24). Because of the difference in 3D
structure, proNTs cannot combine with the Trk receptor (25), however, it has a higher affinity to
p75NTR and sortilin (26).
P75NTR, as a receptor, can be combined with all
members of the NTs family. When combined with mNTs, P75NTR shows
protective effects on cells, which can promote neuronal survival,
myelination and migration. When combined with pro-NTs, P75NTR
mainly mediates the apoptosis of cell. It is significant that
although the Trk receptor binds to mNTs can mediate cell survival
(27), when p75NTR and Trk are
both on the cell surface, p75NTR plays a dominant role in
apoptosis. This may be due to the stronger affinity of p75NTR to
the pro-NTs. It can form a high affinity receptor complex and
conduct the apoptosis signal. In this process, sortilin has played
a key synergy.
Sortilin is a transmembrane protein that belongs to
the Vps10p fragment receptor family (28). It has a high affinity for proBDNF
and p75NTR. In fact, sortilin exists in the way of the co-receptor
in the reaction between pro-NTs and p75NTR (29). Sortilin can form a stable
sortilin/p75NTR complex with p75NTR. In the effect of pro-NTs, the
affinity of the compound is 10 times higher than that of mNTs,
indicating that the sortilin/p75NTR complex is more inclined to
combine with proBDNF rather than mBDNF. Further studies also found
that pro-NTs binding to sortilin/p75NTR showed stronger stability
to proteolysis and denaturation, and more difficult to decompose
and destroy (24). Therefore,
sortilin can protect pro-NTs from decomposition and destroy, thus
prolonging the time of action of pro-NTs and intensifying the
apoptosis process of cells.
In the present study, we found that exogenous
proBDNF promotes cell apoptosis in a dose-dependent manner
(Fig. 5), indicating that proBDNF
induces OLN-93 cell apoptosis. In order to investigate the presence
of the p75NTR signal transduction pathway in OLN-93 cells, p75NTR
functional antibody and recombinant fusion molecule of p75NTRECD-fc
were used to inhibit the functions of p75NTR (Fig. 3D). As expected, the obtained
results showed specific suppression of proBDNF when p75NTR was
blocked, suggesting that the functions of proBDNF are mediated via
the p75NTR signal transduction pathway. Considering the important
role of oligodendrocytes in vivo, these data suggest that
the proBDNF/p75NTR pathway is essential for the functions of
oligodendrocytes after CNS injury.
Mature oligodendrocytes are located in the outer rim
of the grey matter in the spinal cord (30). After CNS injury and cell loss, cell
migration of proliferative OPCs can be observed (31). Our previous studies have shown that
proBDNF inhibits the migration of cerebellar granule cells during
development (18) in addition to
inhibiting macrophage infiltration in the injured spinal cord
(7). In this study, the scratch
assay performed using OLN-93 cells showed that proBDNF inhibits
cell migration in vitro (Fig.
5).
In summary, we demonstrated that exogenous and
endogenous proBDNF negatively regulates the proliferation and
migration of OPC-like cells in vitro. Antibodies raised
against the BDNF pro-domain or p75NTR pathway blocker p75NTRECD-fc
effectively suppressed the functions of proBDNF and facilitated
cell proliferation and migration as well as reduced the apoptosis
of OPC-like cells, indicating their therapeutic value for
functional recoveries after SCI. However, additional in vivo
studies are needed; our research group has been actively conducting
further investigations in this field.
Acknowledgements
The authors would like to thank Dr Moses Chao from
New York University School of Medicine, (New York, NY, USA) for
providing the mouse antibody to p75NTR (9650).
Funding
The present study was supported by Australian
National Health and Medical Research Council (grant nos. 375109 and
595937) to XFZ., the National Natural Science Foundation of China
(grant no. 81070982) and Tianjin Natural Science Foundation (grant
no. 10JCZDJCI18800) to SQF
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XFZ and SF conceived the study. SL conducted the
analyses. WG, HZ, JHZ and LT also analysed the data. All authors
contributed to the writing and revisions of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BDNF
|
brain-derived neurotrophic factor
|
|
p75NTRECD-fc
|
p75NTR extracellular domain-human FC
fusion protein
|
|
SCI
|
spinal cord injury
|
|
CNS
|
central nervous system
|
|
OPC
|
oligodendrocyte precursor cell
|
References
|
1
|
Zhou XF, Song XY, Zhong JH, Barati S, Zhou
FH and Johnson SM: Distribution and localization of
pro-brain-derived neurotrophic factor-like immunoreactivity in the
peripheral and central nervous system of the adult rat. J
Neurochem. 91:704–715. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Provenzano MJ, Minner SA, Zander K, Clark
JJ, Kane CJ, Green SH and Hansen MR: p75(NTR) expression and
nuclear localization of p75(NTR) intracellular domain in spiral
ganglion Schwann cells following deafness correlate with cell
proliferation. Mol Cell Neurosci. 47:306–315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Banik NL, Powers JM and Hogan EL: The
effects of spinal cord trauma on myelin. J Neuropathol Exp Neuro.
39:232–244. 1980. View Article : Google Scholar
|
|
4
|
Song XY, Li F, Zhang FH, Zhong JH and Zhou
XF: Peripherally-derived BDNF promotes regeneration of ascending
sensory neurons after spinal cord injury. PLoS One. 3:e17072008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee R, Kermani P, Teng KK and Hempstead
BL: Regulation of cell survival by secreted proneurotrophins.
Science. 294:1945–1948. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beattie MS, Harrington AW, Lee R, Kim JY,
Boyce SL, Longo FM, Bresnahan JC, Hempstead BL and Yoon SO: ProNGF
induces p75-mediated death of oligodendrocytes following spinal
cord injury. J Neuron. 36:375–386. 2002. View Article : Google Scholar
|
|
7
|
Wong I, Liao H, Bai X, Zaknic A, Zhong J,
Guan Y, Li HY, Wang YJ and Zhou XF: ProBDNF inhibits infiltration
of ED1+ macrophages after spinal cord injury. Brain Behav Immun.
24:585–597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Richter-Landsberg C and Heinrich M:
OLN-93: A new permanent oligodendroglia cell line derived from
primary rat brain glial cultures. J Neurosci Res. 45:161–173. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fan YJ, Wu LL, Li HY, Wang YJ and Zhou XF:
Differential effects of pro-BDNF on sensory neurons after sciatic
nerve transection in neonatal rats. Eur J Neurosci. 27:2380–2390.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: a convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Trotter J, Karram K and Nishiyama A: NG2
cells: Properties, progeny and origin. Brain Res Rev. 63:72–82.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang YJ, Wang X, Lu JJ, Li QX, Gao CY, Liu
XH, Sun Y, Yang M, Lim Y, Evin G, et al: p75NTR regulates Abeta
deposition by increasing Abeta production but inhibiting Abeta
aggregation with its extracellular domain. J Neurosci.
31:2292–2304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salvesen GS: Caspases: Opening the boxes
and interpreting the arrows. Cell Death Differ. 9:3–5. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Henderson CE, Camu W, Mettling C, Gouin A,
Poulsen K, Karihaloo M, Rullamas J, Evans T, McMahon SB, Armanini
MP, et al: Neurotrophins promote motor neuron survival and are
present in embryonic limb bud. Nature. 363:266–270. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Von Dran MW, Singh H, Honeywell JZ and
Dreyfus CF: Levels of BDNF impact oligodendrocyte lineage cells
following a cuprizone lesion. J Neurosci. 31:14182–14190. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arnett MG, Ryals JM and Wright DE:
Pro-NGF, sortilin, and p75NTR: Potential mediators of
injury-induced apoptosis in the mouse dorsal root ganglion. Brain
Res. 1183:32–42. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen LW, Yung KK, Chan YS, Shum DK and
Bolam JP: The proNGF-p75NTR-sortilin signalling complex as new
target for the therapeutic treatment of Parkinson's disease. CNS
Neurol Disord Drug Targets. 7:512–523. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu ZQ, Li J, Deng J, Jiang XJ and Zhou HD:
Effects of proBDNF on cell proliferation and differentiation in
hippocampal dentate gyrus in Alzheimer' disease rat model. Zhonghua
Yi Xue Za Zhi. 90:1353–1356. 2010.(In Chinese). PubMed/NCBI
|
|
19
|
Sun Y, Lim Y, Li F, Liu S, Lu JJ,
Haberberger R, Zhong JH and Zhou XF: ProBDNF collapses neurite
outgrowth of primary neurons by activating RhoA. PLoS One.
7:e358832012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu ZQ, Sun Y, Li HY, Lim Y, Zhong JH and
Zhou XF: Endogenous proBDNF is a negative regulator of migration of
cerebellar granule cells in neonatal mice. Eur J Neurosci.
33:1376–1384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang EJ and Reichardt LF: Trk receptors:
Roles in neuronal signal transduction. Annu Rev Biochem.
72:609–642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoon SO, Casaccia-Bonnefil P, Carter B and
Chao MV: Competitive signaling between TrkA and p75 nerve growth
factor receptors determines cell survival. J Neurosci.
18:3273–3281. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johansson M, Jönsson M, Norrgård O and
Forsgren S: New aspects concerning ulcerative colitis and colonic
carcinoma: Analysis of levels of neuropeptides, neurotrophins, and
TNFalpha/TNF receptor in plasma and mucosa in parallel with
histological evaluation of the intestine. Inflamm Bowel Dis.
14:1331–1340. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Teng HK, Teng KK, Lee R, Wright S, Tevar
S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, et al: ProBDNF
induces neuronal apoptosis via activation of a receptor complex of
p75NTR and sortilin. J Neurosci. 25:5455–5463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu GY, Hughes MG, Ye Z, Hulsebosch CE and
McAdoo DJ: Concentrations of glutamate released following spinal
cord injury kill oligodendrocytes in the spinal cord. Exp Neurol.
187:329–336. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng D, Kim T, Ozkan E, Light M, Torkin R,
Teng KK, Hempstead BL and Garcia KC: Molecular and structural
insight into proNGF engagement of p75NTR and sortilin. J Mol Biol.
396:967–984. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Moheban DB, Conway BR,
Bhattacharyya A and Segal RA: Cell surface Trk receptors mediate
NGF-induced survival while internalized receptors regulate
NGF-induced differentiation. J Neurosci. 20:5671–5678. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nykjaer A, Lee R, Teng KK, Jansen P,
Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow
TE, et al: Sortilin is essential for proNGF-induced neuronal cell
death. Nature. 427:843–848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Volosin M, Song W, Almeida RD, Kaplan DR,
Hempstead BL and Friedman WJ: Interaction of survival and death
signaling in basal forebrain neurons: Roles of neurotrophins and
proneurotrophins. J Neurosci. 26:7756–7766. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Horner PJ, Power AE, Kempermann G, Kuhn
HG, Palmer TD, Winkler J, Thal LJ and Gage FH: Proliferation and
differentiation of progenitor cells throughout the intact adult rat
spinal cord. J Neurosci. 20:2218–2228. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McTigue DM, Horner PJ, Stokes BT and Gage
FH: Neurotrophin-3 and brain-derived neurotrophic factor induce
oligodendrocyte proliferation and myelination of regenerating axons
in the contused adult rat spinal cord. J Neurosci. 18:5354–5365.
1998. View Article : Google Scholar : PubMed/NCBI
|