Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune
disorder. It affects the joints, to eventually result in joint
deformities (1), and influences
other parts of the body; symptoms include a low red blood cell
count, and inflammation around the lungs and heart (2). Age is a risk factor for RA, and RA is
considered a global health risk as the aging population increases
in a number of countries (3). At
present, RA treatments predominantly focus on reducing pain and
inflammation to improve patient quality of life (4).
Fibroblast-like synoviocytes are highly specialized
cells in the joint synovial membrane, which are considered to be
involved in the pathogenesis of RA (5). The synovial membrane is located
between the joint capsule and the joint cavity, which reduces
friction between the joint cartilage and supplies nutrients to the
surrounding cartilage (6).
Fibroblast-like synoviocytes are one of the two principal cell
types in the intima of the synovial membrane, and are essential for
internal joint homeostasis (7).
During RA progression, the physiology of fibroblast-like
synoviocytes is markedly altered; contact inhibition properties are
lost and excessive proliferation occurs. Furthermore, these cells
begin to secrete numerous pro-inflammatory cytokines, including
interleukin (IL)-1β, tumor necrosis factor (TNF)-α and IL-18, which
creates an inflammatory environment in the synovium and contributes
to the destruction of the joint (5,8).
The nuclear factor (NF)-κB/NLR family pyrin domain
containing 3 (NLRP3) signaling pathway is the central hub of the
inflammatory response, which mediates the transcription of a large
number of pro-inflammatory genes, including TNF-α, IL-1β, IL-6 and
IL-18 (9,10). Overactivation of the NF-κB pathway
results in multiple inflammatory diseases, including RA (11). Inhibition of NF-κB activity
markedly ameliorates the symptoms of RA (12).
Galangin is a natural flavonoid extracted from the
roots of Alpinia officinarum. In South Africa, this
traditional herb is used to treat infection (13). Galangin has exhibited multiple
beneficial properties, including anti-oxidative,
anti-proliferative, immunoprotective and cardioprotective effects
(14). Among these, the
anti-inflammatory properties of galangin have gained the attention
of researchers in the RA field (15–17).
As the potential of galangin in treating RA has
previously been demonstrated (18,19),
the present study attempted to investigate the mechanisms
underlying the protective effects of galangin in RA fibroblast-like
synoviocytes.
Materials and methods
Cell line and reagents
Primary human RA fibroblast-like synovium cells
(RAFSCs; cat. no. JNO17-929) were purchased from Guangzhou Jennio
Biotechnology Co., Ltd. (Guangzhou, China). Galangin was purchased
from Sichuan Weikeqi Biological Technology Co., Ltd. (Chengdu,
China). Lipopolysaccharide (LPS) was obtained from Beijing Solarbio
Science & Technology Co., Ltd. (Beijing, China). Primary
antibodies against inducible nitric oxide synthase (iNOS; cat. no.
ab3523) and cyclooxygenase (COX)-2 (cat. no. ab15191) were
purchased, including anti-NLRP3 (cat. no. ab4207; all Abcam,
Cambridge, UK), anti-apoptosis-associated speck-like protein
containing A (ASC; cat. no. sc-22514-R), anti-IL-1β (cat. no.
sc-1250), anti-pro-caspase-1 (cat. no. sc-514), anti-caspase-1
(cat. no. sc-56036), anti-phosphorylated (p)-NF-κB inhibitor α
(IκBα; cat. no. sc-8404), anti-IκBα (cat. no. sc-847), anti-p-NF-κb
(cat. no. sc-101749) and anti-NF-κB (cat. no. sc-109; all Santa
Cruz Biotechnology, Inc., Dallas, TX, USA). Horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG and fluorescent-labeled
(SABC-DyLight 488) secondary antibodies (cat. no. SA1033) were
purchased from Wuhan Boster Biological Technology, Ltd. (Wuhan,
China).
Cell culture
RAFSCs were cultured in complete medium [Dulbecco's
modified Eagle's medium (DMEM); Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA] containing 10% fetal calf serum
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), 100 U/ml
penicillin and 100 µg/ml streptomycin. Cells in the logarithmic
growth phase were used. Primary keratinocytes were incubated in
DMEM for 24 h at 37°C in the dark with 1 mg/ml freshly made
alanine, and Propionibacterium acnes (P. acnes) at a ratio
of 50:1 (P. acnes: keratinocytes) for starvation. Following
starvation, cells were cultured with LPS and different doses of
galangin. Nothing was added to the control group. LPS (1 µg/ml) in
combination with galangin (0, 1, 5 and 10 ng/ml) was added to each
group. Cells were incubated for 24 h at 37°C and subsequently
collected for further experimentation. All cells were adherent.
ELISA
RAFSCs were homogenized in PBS and centrifuged at
1,000 × g for 15 min at 4°C. Supernatant (1 ml) was subjected to
ELISA detection of IL-1β, TNF-α, IL-18, prostaglandin
(PG)E2, and nitric oxide (NO) levels using the
Quantikine ELISA kit (cat. no. DHD00B; R&D Systems, Inc.,
Minneapolis, MN, USA) and the PGE2 ELISA Correlate EIA™ kit (cat.
no. ab133021; Abcam), according to the manufacturers' protocols.
The absorbance of the samples at 450 nm was detected using a
microplate reader (Thermo Fisher Scientific, Inc.).
Measurement of superoxide dismutase
(SOD) activity and malondialdehyde (MDA) content
In order to investigate the antioxidant effect of
galangin, SOD activity and MDA content in RAFSCs was measured. At
the end of incubation for 24 h at 37°C, the cell solution was
centrifuged at 1,000 × g for 15 min at 4°C. Culture supernatant and
lysate was collected. SOD activity and MDA content was measured
using a Cell MDA assay kit purchased from Nanjing Jiancheng
Bioengineering Institute (cat. no. A003-1; Nanjing, China),
according to the manufacturer's protocol. The absorbance of SOD and
MDA was detected using a microplate reader (Thermo Fisher
Scientific, Inc.) at 550 and 450 nm, respectively.
Western blot analysis
Total protein was extracted from RAFSCs using 1% SDS
lysis buffer (Beyotime Institute of Biotechnology, Haimen, China),
and protein concentration was determined using a bicinchoninic acid
protein assay. Proteins (40 µg/lane) were loaded and separated by
12% SDS-PAGE and transferred onto polyvinylidene difluoride
membranes. Membranes were incubated with primary antibodies against
NLRP3 (1:800), ASC (1:1,000), IL-1β (1:900), pro-caspase-1
(1:1,000), caspase-1 (1:1,000), p-IκBα (1:1,200), IκBα (1:900),
anti-p-NF-κB (1:1,000), anti-NF-κB (1:1,000), anti-β-actin
(1:2,000; cat. no. ab8227; Abcam), anti-iNOS (1:800) and anti-COX-2
(1:900) at 4°C overnight, followed by incubation with
HRP-conjugated goat anti-rabbit IgG and fluorescent-labeled
secondary antibodies (1:10,000; Wuhan Boster Biological Technology,
Ltd.) at 37°C for 2 h. Enhanced chemiluminescence (Beyotime
Institute of Biotechnology) solution was added and the blots were
exposed on X-ray films in a dark room. Images were subsequently
captured, and Bandscan software version 5.0 (Glyko, Inc.; BioMarin
Pharmaceutical, Inc., Novato, CA, USA) was used for analysis of the
gel images.
Statistical analysis
All data are expressed as the mean ± standard
deviation (n=6), and statistical analysis was performed with SPSS
11.0 (SPSS Inc., Chicago, IL, USA). One-way analysis of variance
followed by Tukey's post-hoc test was used to determine significant
differences between multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Galangin inhibits LPS-induced IL-1β,
TNF-α, IL-18, PGE2 and NO expression levels in
RAFSCs
The effects of galangin on the expression of IL-1β,
TNF-α and IL-18 in RAFSCs were determined by ELISA. As presented in
Table I, the expression of these
cytokines was strongly induced by LPS (P<0.05), with an increase
of 4–7 fold. However, when cells were pre-treated with galangin and
LPS, IL-18, IL-1β and TNF-α expression was significantly inhibited
(P<0.05; Table I). When cells
were treated with 10 ng/ml galangin, IL-1β and TNF-α expression was
comparable to the control group; IL-18 expression was additionally
markedly inhibited, compared with the LPS only group. These
findings suggested that galangin inhibited LPS-induced IL-1β, TNF-α
and IL-18 expression in RAFSCs in a concentration-dependent manner,
highlighting its potential anti-inflammatory effects.
 | Table I.Expression levels of IL-1β, TNF-α,
IL-18, PGE2 and NO in rheumatoid arthritis fibroblast-like synovium
cells treated with LPS and/or galangin. |
Table I.
Expression levels of IL-1β, TNF-α,
IL-18, PGE2 and NO in rheumatoid arthritis fibroblast-like synovium
cells treated with LPS and/or galangin.
|
| Marker |
|---|
|
|
|
|---|
| Group | IL-1β | TNF-α | IL-18 | PGE2, ng/ml | NO, µmol/ml |
|---|
| Control |
65.21±3.12a–d |
29.47±1.28a–d |
35.47±9.64a–d |
0.11±0.08a–d |
35.74±3.56a,b |
| LPS only |
279.36±20.78b–d |
167.34±32.15b–d |
234.97±21.45b–d |
2.51±0.15b–d |
298.09±37.45b–d |
| LPS + 1 ng/ml
galangin |
135.27±12.34a,c,d |
82.39±19.85a,c,d |
135.65±11.67a,c,d |
1.45±0.26a,c,d |
68.17±28.57a,c,d |
| LPS + 5 ng/ml
galangin |
93.74±9.85a,b,d |
61.46±14.61a,b,d |
85.76±9.73a,b,d |
1.02±0.32a,b,d |
37.12±9.78a,b |
| LPS + 10 ng/ml
galangin |
76.51±15.67a–c |
39.27±5.8a–c |
62.21±4.38a–c |
0.39±0.18a–c |
34.37±7.62a,b |
Levels of PGE2 and NO in the RAFSC
culture supernatant were additionally detected by ELISA, in order
to investigate whether galangin regulated LPS-induced NO and
PGE2 production. As presented in Table I, PGE2 and NO levels
were significantly increased by LPS, compared with the control
group (P<0.05). Furthermore, PGE2 and NO levels were
suppressed with galangin treatment (P<0.05 vs. LPS only group).
These results demonstrated that galangin inhibited the LPS-induced
expression of PGE2 and NO in RAFSCs.
Galangin inhibits the LPS-induced
expression of iNOS and COX-2 in RAFSCs
Considering that galangin inhibited NO and PGE2
production, western blot analysis was performed in order to examine
whether these inhibitory effects were associated with iNOS and
COX-2 regulation in RAFSCs. As presented in Fig. 1, LPS significantly increased the
expression of iNOS and COX-2 (P<0.01). Pre-treatment with 1
ng/ml galangin decreased iNOS and COX-2 expression (P<0.05),
which was not significantly different when compared with the
control (P>0.05). At a dose of 5 or 10 ng/ml, the iNOS and COX-2
expression levels decreased below those of the control group
(P<0.05). Therefore, these results demonstrated that galangin
inhibited LPS-induced expressions of iNOS and COX-2 in RAFSCs.
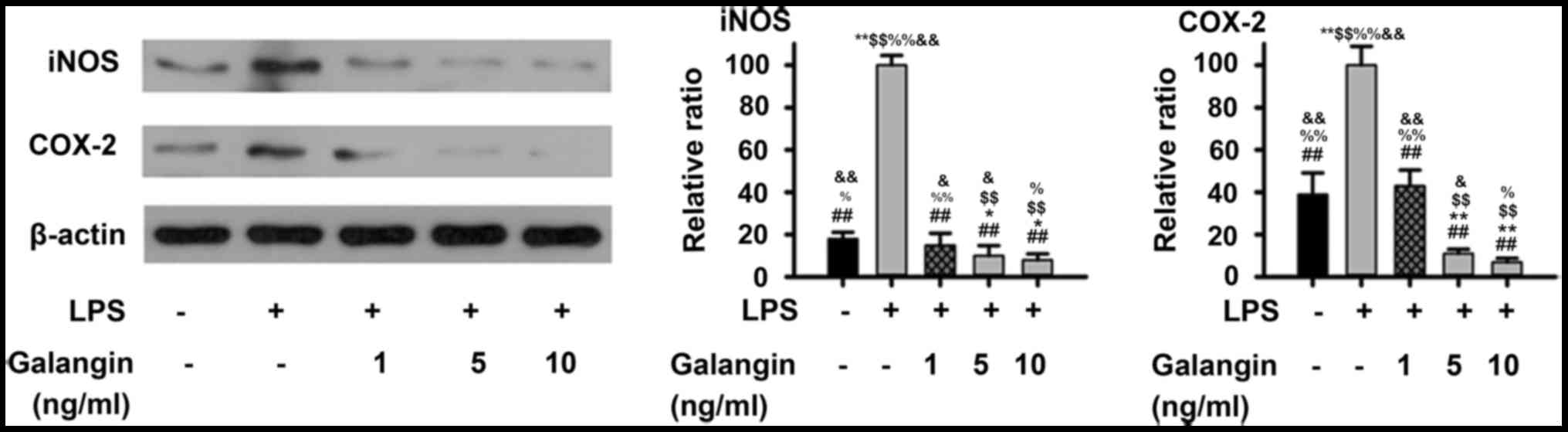 | Figure 1.Expression levels of iNOS and COX-2 in
RAFSCs treated with LPS and/or galangin. In the LPS group, cells
were treated with 1 µg/ml LPS only. In the galangin groups, cells
were treated with 1 µg/ml LPS and galangin at 1, 5 or 10 ng/ml. The
LPS group had significantly elevated levels of iNOS and COX-2
compared with the control group. Cells treated with galangin had
significantly lower levels, compared with the LPS group.
*P<0.05, **P<0.01 vs. control group; ##P<0.01
vs. LPS group; $$P<0.01 vs. LPS + galangin (1 ng/ml);
%P<0.05, %%P<0.01 vs. LPS + galangin (5
ng/ml); &P<0.05, &&P<0.01
vs. LPS + galangin (10 ng/ml). iNOS, inducible nitric oxide
synthase; COX-2, cyclooxygenase 2; RAFSCs, rheumatoid arthritis
fibroblast-like synovium cells; LPS, lipopolysaccharide. |
Galangin decreases SOD activity and
increases MDA content
It was demonstrated that LPS significantly decreased
SOD activity (P<0.05), and pre-treatment with galangin increased
SOD activity (P<0.05) (Fig. 2).
MDA content had an opposite tendency, with galangin decreasing the
levels of MDA. These findings provided evidence that galangin
exhibited antioxidative effects in RAFSCs.
Galangin suppresses the NF-κB/NLRP3
signaling pathway
The NF-κB/NLRP3 pathway, which upregulates multiple
pro-inflammatory cytokines, has been considered to be a key
signaling pathway in the progression of RA (12). In order to understand the
underlying mechanisms of the protective effects of galangin in
RAFSCs, the expression of multiple factors in the NF-κB/NLRP3
signaling pathway were measured by western blot analysis. The
expression of NLRP3, ASC, IL-1β, pro-caspase-1, caspase-1, p-IκBα
and p-NF-κB in RAFSCs was upregulated by LPS stimulation
(P<0.05) (Fig. 3).
Pre-treatment with galangin (1 ng/ml) decreased ASC,
pro-caspase-1/caspase-1, p-IκBα and p-NF-κB (P<0.05) expression;
however, it did not significantly decrease NLRP3 or IL-1β
expression. Pre-treatment with 5 or 10 ng/ml galangin significantly
attenuated the LPS-induced overexpression of all these factors
(P<0.05). These results indicated that galangin may have
protected RAFSCs by suppressing the NF-κB/NLRP3 signaling
pathway.
 | Figure 3.Expression of proteins associated with
the NF-κB/NLRP3 signaling pathway in RAFSCs treated with LPS and/or
galangin. (A) Western blot analysis. The LPS group had
significantly elevated levels of (B) NLRP3, (C) ASC, (D)
pro-caspase-1/caspase-1, (E) IL-1β, (F) p-IκBα and (G) p-NF-κB.
When RAFSCs were pre-treated with 1 ng/ml galangin, expression
levels of ASC, p-IκBα, p-NF-κB were significantly lower compared
with the LPS group, whereas when the cells were pre-treated with 5
or 10 ng/ml galangin, the expression of all proteins was
significantly lower, compared with the LPS group. *P<0.05,
**P<0.01 vs. control group; ##P<0.01 vs. LPS
group; $$P<0.01 vs. LPS + galangin (1 ng/ml);
%P<0.05, %%P<0.01 vs. LPS + galangin (5
ng/ml); &P<0.05, &&P<0.01
vs. LPS + galangin (10 ng/ml). NF-κB, nuclear factor-κB; NLRP3, NLR
family pyrin domain containing 3; RAFSCs, rheumatoid arthritis
fibroblast-like synovium cells; LPS, lipopolysaccharide; ASC,
apoptosis-associated speck-like protein containing A; IL-1β,
interleukin-1β; p-, phosphorylated; IκBα, NF-κB inhibitor α. |
Discussion
RA is a systemic immune and inflammatory disease.
For the majority of patients, RA is a progressive, life-long
disease that shortens life expectancy by 3–20 years (20). Unfortunately, despite extensive
research, RA pathogenesis remains unclear, and there is currently
no cure for this disease (21).
Bacterial LPS is capable of eliciting a strong
immune response in vitro and in vivo, and is
frequently used to induce symptoms of RA (22). IL-18, IL-1β and TNF-α are
pro-inflammatory cytokines that have pivotal roles in RA, and are
frequently used as inflammatory markers (23,24).
Furthermore, a number of factors downstream of proinflammatory
cytokines are responsible for creating an inflammatory environment
around the joint. For example, NO is a free radical that is
generated enzymatically by the cytokine-induced iNOS pathway and
contributes to the pathogenesis of arthritis (25). In addition, one tissue specific
isoform of COX-2 produces PGE2, which aggravates
synovial inflammation by increasing local blood flow and
vasopermeability (26).
Furthermore, TNF-α and other pro-inflammatory cytokines enhance the
production of COX-2 and PGE2 (27). All these factors were considered as
incentives in RA.
Therefore, the present study investigated the
inhibitory effect of galangin on the LPS-induced increase in IL-1β,
TNF-αIL-18, PGE2, NO, iNOS and COX-2 expression levels
in RAFSCs. The results indicated that galangin significantly
inhibited the release of IL-1β, TNF-α, IL-18, PGE2, NO
into the medium, in addition to iNOS and COX-2. The observed
reduction in NO production and PGE2 release when cells
were pretreated with galangin may have resulted from the
transcriptional suppression of iNOS and COX-2. However, a
limitation of the present study was that the effect of treatment
with galangin on cell survival and apoptosis was not evaluated.
Free radicals cause damage to cellular components
and contribute to the development of numerous inflammatory diseases
(28). SOD is an enzyme that
reduces the damage of superoxides and MDA is a marker for oxidative
stress; the expression of these molecules is negatively and
positively associated with RA symptoms, respectively (29). In the present study, it was
revealed that galangin decreased LPS-induced cytokine expression in
the RAFSC culture supernatant, and therefore may have protected
cells from cytokine-induced damage. The antioxidative effects of
galangin were also investigated. Galangin exhibited significant
antioxidant effects, as evidenced by the increased SOD activity and
lower MDA content detected. Cells treated with 1 or 5 ng/ml
galangin had significant differences in expression compared with
the LPS only treatment group, in SOD activity and MDA content. The
increase in SOD activity suggested that galangin enhanced the
antioxidant capability of the cells, while the decrease in MDA
content indicated that galangin may have reduced lipid membrane
oxidation by scavenging free radicals. Although SOD activity and
MDA content may be sufficient to draw the conclusion that galangin
had antioxidative effect on RAFSCs, in vivo experiments to
visualize cellular ROS with molecular dye or other methods may
further elucidate the function of galangin in reducing the
inflammatory response. Therefore, further studies in vivo
are required in the future.
A previous study suggested that galangin prevents
osteoclastic bone destruction and osteoclastogenesis in osteoclast
precursors, and additionally in collagen-induced arthritis mice,
without toxicity via attenuation of TNF superfamily member
11-induced activation of the mitogen activated protein kinase
(MAPK)8, MAPK14 and NF-κB signaling pathways (18). In the present study, it was
determined that the protective effects of galangin in RAFSCs were
likely due to NF-κB/NLRP3 signaling pathway downregulation.
As a result, it was concluded that galangin
suppressed pro-inflammatory signaling in fibroblast-like
synoviocytes in vitro, and that inhibition of the
NF-κB/NLRP3 pathway was a key mechanism in this protective effect.
Therefore, galangin may provide a novel direction for the
development of RA therapies in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the current
study are included in this published article.
Authors' contributions
QF, YG, HZ, ZW and JW were responsible for the
conception and design of the study. QF, YG and HZ performed the
experiments, and analyzed and interpreted the data. QF drafted the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chang X, He H, Zhu L, Gao J, Wei T, Ma Z
and Yan T: Protective effect of apigenin on Freund's complete
adjuvant-induced arthritis in rats via inhibiting P2×7/NF-κB
pathway. Chem Biol Interact. 236:41–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Majithia V and Geraci SA: Rheumatoid
arthritis: Diagnosis and management. Am J Med. 120:936–939. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regional mortality from 235 causes of death for 20
age groups in 1990 and 2010: A systematic analysis for the Global
burden of disease study 2010. Lancet. 380:2095–2128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saag KG, Teng GG, Patkar NM, Anuntiyo J,
Finney C, Curtis JR, Paulus HE, Mudano A, Pisu M, Elkins-Melton M,
et al: American college of rheumatology 2008 recommendations for
the use of nonbiologic and biologic disease-modifying antirheumatic
drugs in rheumatoid arthritis. Arthritis Rheum. 59:762–784. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartok B and Firestein GS: Fibroblast-like
synoviocytes: Key effector cells in rheumatoid arthritis. Immunol
Rev. 233:233–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Sousa EB, Casado PL, Moura Neto V,
Duarte ME and Aguiar DP: Synovial fluid and synovial membrane
mesenchymal stem cells: Latest discoveries and therapeutic
perspectives. Stem Cell Res Ther. 5:1122014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang SK, Gu Z and Brenner MB:
Fibroblast-like synoviocytes in inflammatory arthritis pathology:
The emerging role of cadherin-11. Immunol Rev. 233:256–266. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Firestein GS: Evolving concepts of
rheumatoid arthritis. Nature. 423:356–361. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simmonds RE and Foxwell BM: Signalling,
inflammation and arthritis: NF-kappaB and its relevance to
arthritis and inflammation. Rheumatology (Oxford). 47:584–590.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baldwin AS Jr: The NF-kappa B and I kappa
B proteins: New discoveries and insights. Annu Rev Immunol.
14:649–683. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tak PP and Firestein GS: NF-kappaB: A key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamamoto Y and Gaynor RB: Therapeutic
potential of inhibition of the NF-kappaB pathway in the treatment
of inflammation and cancer. J Clin Invest. 107:135–142. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van Vuuren SF: Antimicrobial activity of
South African medicinal plants. J Ethnopharmacol. 119:462–472.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bestwick CS and Milne L: Influence of
galangin on HL-60 cell proliferation and survival. Cancer Lett.
243:80–89. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sivakumar AS and Anuradha CV: Effect of
galangin supplementation on oxidative damage and inflammatory
changes in fructose-fed rat liver. Chem Biol Interact. 193:141–148.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim HH, Bae Y and Kim SH: Galangin
attenuates mast cell-mediated allergic inflammation. Food Chem
Toxicol. 57:209–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zha WJ, Qian Y, Shen Y, Du Q, Chen FF, Wu
ZZ, Li X and Huang M: Galangin abrogates ovalbumin-induced airway
inflammation via negative regulation of NF-kappaB. Evid Based
Complement Alternat Med. 2013:7676892013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huh JE, Jung IT, Choi J, Baek YH, Lee JD,
Park DS and Choi DY: The natural flavonoid galangin inhibits
osteoclastic bone destruction and osteoclastogenesis by suppressing
NF-κB in collagen-induced arthritis and bone marrow-derived
macrophages. Eur J Pharmacol. 698:57–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen S: Natural products triggering
biological targets-a review of the anti-inflammatory phytochemicals
targeting the arachidonic acid pathway in allergy asthma and
rheumatoid arthritis. Curr Drug Targets. 12:288–301. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wasserman AM: Diagnosis and management of
rheumatoid arthritis. Am Fam Physician. 84:1245–1252.
2011.PubMed/NCBI
|
|
21
|
Kapoor S, Fitzpatrick M, Clay E, Bayley R,
Wallace GR and Young SP: Metabolomics in the analysis of
inflammatory diseases. Roessner U: Metabolomics. Rijeka (HR):
InTech. Chapter 112012.
|
|
22
|
Yoshino S and Ohsawa M: The role of
lipopolysaccharide injected systemically in the reactivation of
collagen-induced arthritis in mice. Br J Pharmacol. 129:1309–1314.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feldmann M, Brennan FM and Maini RN: Role
of cytokines in rheumatoid arthritis. Annu Rev Immunol. 14:397–440.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gracie JA, Forsey RJ, Chan WL, Gilmour A,
Leung BP, Greer MR, Kennedy K, Carter R, Wei XQ, Xu D, et al: A
proinflammatory role for IL-18 in rheumatoid arthritis. J Clin
Invest. 104:1393–1401. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grabowski PS, Wright PK, Van't Hof RJ,
Helfrich MH, Ohshima H and Ralston SH: Immunolocalization of
inducible nitric oxide synthase in synovium and cartilage in
rheumatoid arthritis and osteoarthritis. Br J Rheumatol.
36:651–655. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schleimer RP: Inflammation: Basic
principles and clinical correlates edited by John Gallin, Ira
Goldstein and Ralph Snyderman, Raven Press, $219.00 (xvii + 995
pages) ISBN 0 88167 344 7. Immunol Today. 9:327, 19871988.
|
|
27
|
Anderson GD, Hauser SD, McGarity KL,
Bremer ME, Isakson PC and Gregory SA: Selective inhibition of
cyclooxygenase (COX)-2 reverses inflammation and expression of
COX-2 and interleukin 6 in rat adjuvant arthritis. J Clin Invest.
97:2672–2679. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hadjigogos K: The role of free radicals in
the pathogenesis of rheumatoid arthritis. Panminerva Med. 45:7–13.
2003.PubMed/NCBI
|
|
29
|
Mansour RB, Lassoued S, Gargouri B, El
Gaïd A, Attia H and Fakhfakh F: Increased levels of autoantibodies
against catalase and superoxide dismutase associated with oxidative
stress in patients with rheumatoid arthritis and systemic lupus
erythematosus. Scand J Rheumatol. 37:103–108. 2008. View Article : Google Scholar : PubMed/NCBI
|

















